High Seroprevalence of Toxoplasma gondii and Neospora caninum Infections Among Goats in Mexico Is Associated with Climatic, Environmental, and Risk Factors
Abstract
1. Introduction
2. Materials and Methods
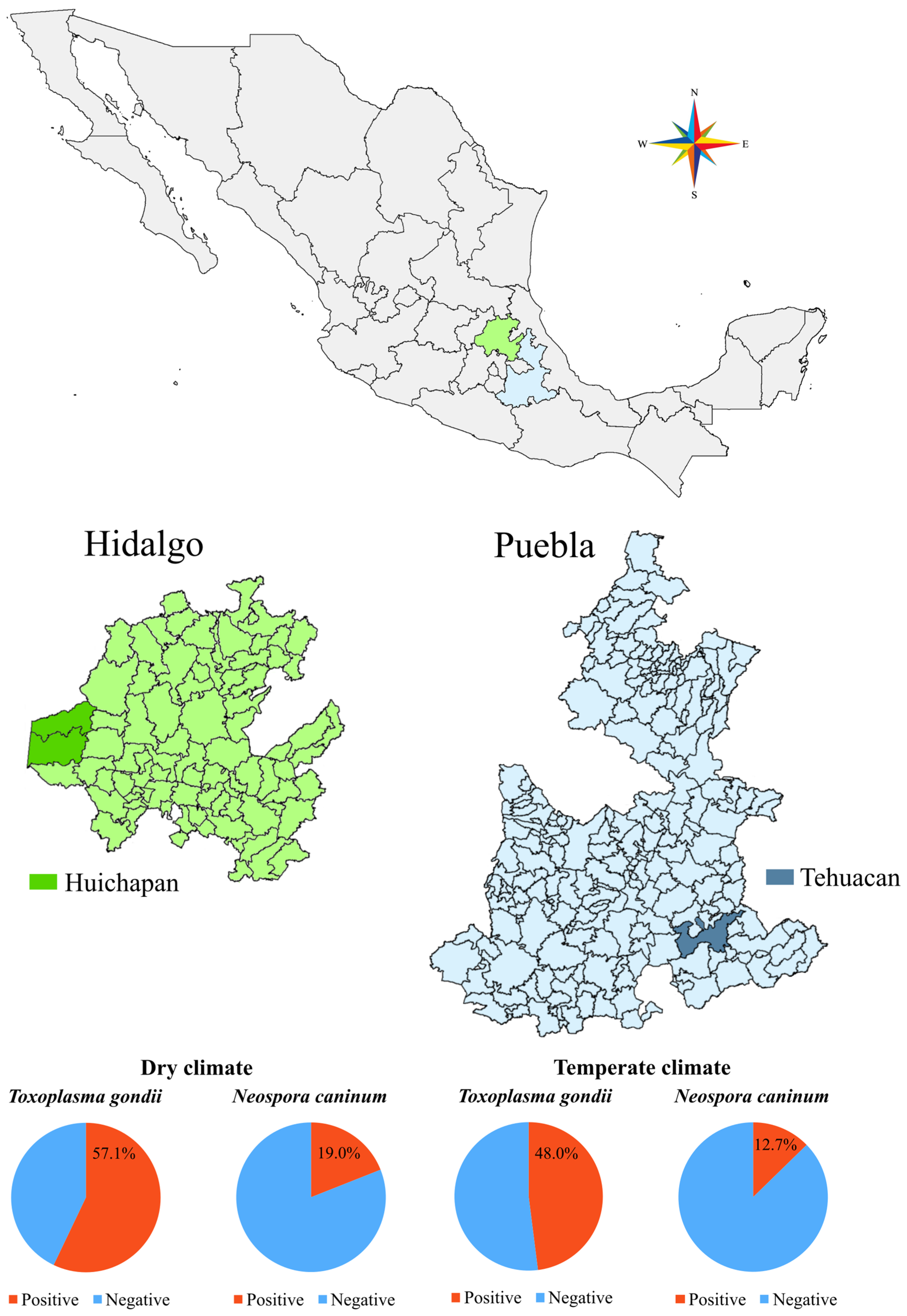
2.1. Study Area and Sample Collection
2.2. Remotely Sensed Climatic Data
2.3. Serological Examination of T. gondii and N. caninum
2.4. Statistical Analysis
3. Results
3.1. Seroprevalence of T. gondii in Goats
3.2. Risk Factors of T. gondii in a Temperate Climate
3.3. Risk Factors of T. gondii in a Dry Climate
3.4. Risk Factors of T. gondii in Both Dry and Temperate Climates
3.5. Seroprevalence of N. caninum in Goats
3.6. Risk Factors of N. caninum in a Temperate Climate
3.7. Risk Factors of N. caninum in a Dry Climate
3.8. Risk Factors of N. caninum in Both Dry and Temperate Climates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindsay, D.S.; Dubey, J.P. Neosporosis, toxoplasmosis, and sarcocystosis in ruminants: An update. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Hebbar, B.K.; Roy, M.; Mitra, P.; Chavhan, K.; Chaudhari, S.; Shinde, S.; Deshmukh, A.S. Seroprevalence, risk factors, and serological cross-reactivity for diagnosis of Toxoplasma gondii and Neospora caninum infections in goats in India. Microb. Pathog. 2022, 173, 105780. [Google Scholar] [CrossRef] [PubMed]
- Reichel, M.P.; Ayanegui-Alcérreca, M.A.; Gondim, L.F.; Ellis, J.T. What is the global economic impact of Neospora caninum in cattle–the billion dollar question. Int. J. Parasitol. 2013, 43, 133–142. [Google Scholar] [CrossRef]
- Freyre, A.; Bonino, J.; Falcon, J.; Castells, D.; Correa, O.; Casaretto, A. The incidence and economic significance of ovine toxoplasmosis in Uruguay. Vet. Parasitol. 1997, 73, 13–15. [Google Scholar] [CrossRef]
- Wei, X.-Y.; Gong, Q.-L.; Zeng, A.; Wang, W.; Wang, Q.; Zhang, X.-X. Seroprevalence and risk factors of Toxoplasma gondii infection in goats in China from 2010 to 2020: A systematic review and meta-analysis. Prev. Vet. Med. 2021, 186, 105230. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.A.; Reis, S.S.; de Sousa, M.L.; da Silva Moraes, E.; Garcia, J.L.; Nascimento, T.V.C.; da Cunha, I.A.L. A systematic literature review and meta-analysis of risk factors for Neospora caninum seroprevalence in goats. Prev. Vet. Med. 2020, 185, 105176. [Google Scholar] [CrossRef]
- Pozio, E. How globalization and climate change could affect foodborne parasites. Exp. Parasitol. 2020, 208, 107807. [Google Scholar] [CrossRef]
- Gilot-Fromont, E.; Lélu, M.; Dardé, M.-L.; Richomme, C.; Aubert, D.; Afonso, E.; Mercier, A.; Gotteland, C.; Villena, I. The Life Cycle of Toxoplasma gondii. Toxoplasm. Recent. Adv. 2012, 1. [Google Scholar] [CrossRef]
- Meerburg, B.G.; Kijlstra, A. Changing climate-changing pathogens: Toxoplasma gondii in North-Western Europe. Parasitol. Res. 2009, 105, 17–24. [Google Scholar] [CrossRef]
- Rostami, A.; Riahi, S.M.; Esfandyari, S.; Habibpour, H.; Mollalo, A.; Mirzapour, A.; Behniafar, H.; MohammadiMoghadam, S.; Kyvanani, N.A.; Aghaei, S.; et al. Geo-climatic factors and prevalence of chronic toxoplasmosis in pregnant women: A meta-analysis and meta-regression. Environ. Pollut. 2021, 288, 117790. [Google Scholar] [CrossRef]
- Rinaldi, L.; Fusco, G.; Musella, V.; Veneziano, V.; Guarino, A.; Taddei, R.; Cringoli, G. Neospora caninum in pastured cattle: Determination of climatic, environmental, farm management and individual animal risk factors using remote sensing and geographical information systems. Vet. Parasitol. 2005, 128, 219–230. [Google Scholar] [CrossRef]
- Villa-Mancera, A.; Reynoso-Palomar, A. High prevalence, potential economic impact, and risk factors of Fasciola hepatica in dairy herds in tropical, dry and temperate climate regions in Mexico. Acta Trop. 2019, 193, 169–175. [Google Scholar] [CrossRef]
- Rodrigues, A.A.; Reis, S.S.; da Silva Moraes, E.; do Nascimento Araújo, E.M.A.; de Moura Zanine, A.; Nascimento, T.V.C.; Garcia, J.L.; da Cunha, I.A.L. A systematic literature review and meta-analysis of Toxoplasma gondii seroprevalence in goats. Acta Trop. 2022, 230, 106411. [Google Scholar] [CrossRef] [PubMed]
- García, A.E. Modificaciones al Sistema de Clasificación Climática de Köppen, 5th ed.; Instituto de Geografía, Universidad Nacional Autónoma de México (UNAM): Ciudad de México, Mexico, 2004; pp. 1–92. [Google Scholar]
- Sandholt, I.; Rasmussen, K.; Andersen, J. A simple interpretation of the surface temperature/vegetation index space for assessment of surface moisture status. Remote Sens. Environ. 2002, 79, 213–224. [Google Scholar] [CrossRef]
- Rogan, W.J.; Gladen, B. Estimating prevalence from the results of a screening test. Am. J. Epidemiol. 1978, 107, 71–76. [Google Scholar] [CrossRef]
- Ahaduzzaman, M.; Hasan, T. Seroprevalence of Toxoplasma gondii infection in sheep and goats from different geographical regions of the world: Systematic review and meta-analysis. Transbound. Emerg. Dis. 2022, 69, 3790–3822. [Google Scholar] [CrossRef]
- Celi, K.; Guzmán, L.; Rey-Valeirón, C. Apicomplexans in goat: Prevalence of Neospora caninum, Toxoplasma gondii, Cryptosporidium spp., Eimeria spp. and risk factors in farms from Ecuador. Animals 2022, 12, 2224. [Google Scholar] [CrossRef] [PubMed]
- Gazzonis, A.L.; Veronesi, F.; Di Cerbo, A.R.; Zanzani, S.A.; Molineri, G.; Moretta, I.; Moretti, A.; Piergili Fioretti, D.; Invernizzi, A.; Manfredi, M.T. Toxoplasma gondii in small ruminants in Northern Italy—Prevalence and risk factors. Ann. Agric. Environ. Med. 2015, 22, 62–68. [Google Scholar] [CrossRef]
- Khalife, S.; Moubayed, S.; Mitri, R.; Geitani, R.; El Safadi, D. Seroprevalence and risk assessment of Toxoplasma gondii infection in sheep and goats in North and Beqaa governorates of Lebanon. Vet. World 2022, 15, 2180–2185. [Google Scholar] [CrossRef]
- Chiang, S.-H.; Huang, H.H.; Chou, C.-C.; Chu, C.-S.; Shih, W.-L.; Lai, J.-M.; Lin, H.-C.; Yang, W.-C.; Lee, H.-H.; Tsai, Y.-C. Epidemiological survey of Toxoplasma gondii and Neospora caninum infections in dairy goats in Central-Southern Taiwan. J. Vet. Med. Sci. 2020, 82, 1537–1544. [Google Scholar] [CrossRef]
- Hu, X.-H.; Xie, S.-C.; Liang, Q.-L.; Sun, L.-X.; Li, Z.; Yang, J.-F.; Zhu, X.-Q.; Zou, F.-C.; He, J.-J. Seroprevalence and risk factors of Toxoplasma gondii and Neospora caninum infection in black goats in Yunnan Province, Southwestern China. Front. Vet. Sci. 2022, 9, 975238. [Google Scholar] [CrossRef]
- Gazzonis, A.L.; Zanzani, S.A.; Villa, L.; Manfredi, M.T. Toxoplasma gondii in naturally infected goats: Monitoring of specific IgG levels in serum and milk during lactation and parasitic DNA detection in milk. Prev. Vet. Med. 2019, 170, 104738. [Google Scholar] [CrossRef]
- Dahmane, A.; Almeida, D.; Reghaissia, N.; Baroudi, D.; Samari, H.; Abdelli, A.; Laatamna, A.; Mesquita, J.R. Seroprevalence assessment and risk factor analysis of Toxoplasma gondii infection in goats from Northeastern Algeria. Animals 2024, 14, 883. [Google Scholar] [CrossRef] [PubMed]
- Basso, W.; Holenweger, F.; Schares, G.; Müller, N.; Campero, L.M.; Ardüser, F.; Moore-Jones, G.; Frey, C.F.; Zanolari, P.J. Toxoplasma gondii and Neospora caninum infections in sheep and goats in Switzerland: Seroprevalence and occurrence in aborted foetuses. Food Waterborne Parasitol. 2022, 28, e00176. [Google Scholar] [CrossRef]
- Bachan, M.; Deb, A.R.; Maharana, B.R.; Sudhakar, N.; Sudan, V.; Saravanan, B.; Tewari, A.K. High seroprevalence of Toxoplasma gondii in goats in Jharkhand state of India. Vet. Parasitol. Reg. Stud. Rep. 2018, 12, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Selim, A.; Marzok, M.; Alshammari, A.; Al-Jabr, O.A.; Salem, M.; Wakid, M.H. Toxoplasma gondii infection in Egyptian domestic sheep and goats: Seroprevalence and risk factors. Trop. Anim. Health Prod. 2023, 55, 182. [Google Scholar] [CrossRef]
- Chettih, I.; Abdellaoui, L.; Mekroud, M.; Dahmani, A.; Nabi, M.; Omar, K.H.; Touhami, N.A.K.; Dahmani, H.; Bouasla, L.; Ouchetati, I. Seroprevalence and risk factors of Toxoplasma gondii infection among goats in Algeria. Comp. Immunol. Microbiol. Infect. Dis. 2024, 110, 102201. [Google Scholar] [CrossRef] [PubMed]
- Irshad, A.; Noreen, S.; Khan, W.; Rasool, A. Sero-epidemiology, Spatial Distribution and Phylogenetic Analysis of Toxoplasma gondii in Goats of Malakand Division of Pakistan. Pak. Vet. J. 2024, 44, 2074–7764. [Google Scholar]
- Khan, M.Y.; Barlaam, A.; Gazzonis, A.L.; Ferrari, N.; Giangaspero, A. Seroprevalence and risk factors of Toxoplasma gondii infection in goats from South Punjab Province, Pakistan. Vet. Parasitol. Reg. Stud. Rep. 2024, 50, 101018. [Google Scholar] [CrossRef]
- Vitela-Mendoza, I.; Palacios-García, N.; Cruz-Vázquez, C.; Medina-Esparza, L.; Hernández-Rangel, J. Epidemiology of Toxoplasma gondii and Neospora caninum infection in goats from Aguascalientes, Mexico. J. Parasitol. 2023, 109, 588–591. [Google Scholar] [CrossRef]
- Masombuka, M.; Mphuthi, M.B.; Ngoshe, Y.B.; Mokolopi, G.; Gcebe, N. Seroprevalence and risk factors of Toxoplasma gondii in sheep and goats of North West Province, South Africa. BMC Vet. Res. 2024, 20, 120. [Google Scholar] [CrossRef]
- Dubey, J.; Schares, G.; Ortega-Mora, L. Epidemiology and control of neosporosis and Neospora caninum. Clin. Microbiol. Rev. 2007, 20, 323–367. [Google Scholar] [CrossRef]
- Shapiro, K.; Bahia-Oliveira, L.; Dixon, B.; Dumetre, A.; de Wit, L.A.; VanWormer, E.; Villena, I. Environmental transmission of Toxoplasma gondii: Oocysts in water, soil and food. Food Waterborne Parasitol. 2019, 15, e00049. [Google Scholar] [CrossRef] [PubMed]
- Charron, D.F.; Thomas, M.K.; Waltner-Toews, D.; Aramini, J.J.; Edge, T.; Kent, R.A.; Maarouf, A.R.; Wilson, J. Vulnerability of waterborne diseases to climate change in Canada: A review. J. Toxicol. Environ. Health Part A 2004, 67, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- VanWormer, E.; Fritz, H.; Shapiro, K.; Mazet, J.A.; Conrad, P.A. Molecules to modeling: Toxoplasma gondii oocysts at the human–animal–environment interface. Comp. Immunol. Microbiol. Infect. Dis. 2013, 36, 217–231. [Google Scholar] [CrossRef]
- Lindsay, D.S.; Dubey, J. Long-term survival of Toxoplasma gondii sporulated oocysts in seawater. J. Parasitol. 2009, 95, 1019–1020. [Google Scholar] [CrossRef]
- Messier, V.; Levesque, B.; Proulx, J.F.; Rochette, L.; Libman, M.D.; Ward, B.J.; Serhir, B.; Couillard, M.; Ogden, N.H.; É, D. Seroprevalence of Toxoplasma gondii among Nunavik Inuit (Canada). Zoonoses Public. Health 2009, 56, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Liang, L.-J.; Zheng, K.-Y.; Zhu, X.-Q. Impact of environmental factors on the emergence, transmission and distribution of Toxoplasma gondii. Parasites Vectors 2016, 9, 137. [Google Scholar] [CrossRef]
- Díaz, P.; Cabanelas, E.; Díaz, J.M.; Viña, M.; Béjar, J.P.; Pérez-Creo, A.; Prieto, A.; López, C.M.; Panadero, R.; Fernández, G.; et al. Seroprevalence of Toxoplasma gondii and Neospora caninum in goats from north-western Spain. Ann. Agric. Environ. Med. 2016, 23, 587–590. [Google Scholar] [CrossRef]
- Gazzonis, A.L.; Garcia, G.A.; Zanzani, S.A.; Mora, L.M.O.; Invernizzi, A.; Manfredi, M.T. Neospora caninum infection in sheep and goats from north-eastern Italy and associated risk factors. Small Rumin. Res. 2016, 140, 7–12. [Google Scholar] [CrossRef]
- Luo, H.; Li, K.; Zhang, H.; Wu, B.; Wang, J.; Shahzad, M.; Tu, Y.; Song, X.; Sun, S. Seroepidemiology of Toxoplasma gondii and Neospora caninum infections in goats in Hubei province, China. Trop. Biomed. 2016, 33, 285–289. [Google Scholar] [PubMed]
- Gharekhani, J.; Yakhchali, M.; Esmaeilnejad, B.; Mardani, K.; Majidi, G.; Sohrabei, A.; Berahmat, R.; Hazhir Alaei, M. Seroprevalence and risk factors of Neospora caninum and Toxoplasma gondii in small ruminants in Southwest of Iran. Arch. Razi Inst. 2018, 73, 305–310. [Google Scholar] [PubMed]
- López-Gatius, F.; García-Ispierto, I.; Santolaria, P.; Yániz, J.; López-Béjar, M.; Nogareda, C.; Almería, S. Relationship between Rainfall and Neospora caninum-associated abortion in two dairy herds in a dry environment. J. Vet. Med. Ser. B 2005, 52, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Alcalá-Gómez, J.; Medina-Esparza, L.; Quezada-Tristán, T.; Alcalá-Gómez, G. Impact of environmental factors on Neospora caninum and Toxoplasma gondii infection in breeding ewes from western Mexico. Int. J. Biometeorol. 2025, 69, 441–448. [Google Scholar] [CrossRef]
- Waap, H.; Bärwald, A.; Nunes, T.; Schares, G. Seroprevalence and Risk Factors for Toxoplasma gondii and Neospora caninum in Cattle in Portugal. Animals 2022, 12, 2080. [Google Scholar] [CrossRef]
- Schares, G.; Bärwald, A.; Staubach, C.; Ziller, M.; Klöss, D.; Wurm, R.; Rauser, M.; Labohm, R.; Dräger, K.; Fasen, W. Regional distribution of bovine Neospora caninum infection in the German state of Rhineland-Palatinate modelled by Logistic regression. Int. J. Parasitol. 2003, 33, 1631–1640. [Google Scholar] [CrossRef]
| Risk Factors | No. Tested | Positive No. | Prevalence (%) | Odds Ratio | 95% CI | p-Value | |
|---|---|---|---|---|---|---|---|
| Toxoplasma gondii | Age, year | ||||||
| ≤1 | 87 | 20 | 23.0 | 1.000 | |||
| 1–2 | 88 | 20 | 22.7 | 0.113 | 0.062–0.205 | <0.001 | |
| ≥2 | 179 | 130 | 72.6 | 0.111 | 0.061–0.201 | <0.001 | |
| Sex | |||||||
| Male | 105 | 64 | 61.0 | 1.000 | |||
| Female | 249 | 106 | 42.6 | 2.106 | 1.322–3.355 | 0.002 | |
| Abortion | |||||||
| No | 186 | 41 | 22.0 | 1.000 | |||
| Yes | 168 | 129 | 76.8 | 0.085 | 0.052–0.141 | <0.001 | |
| Presence of Cats | |||||||
| No | 143 | 53 | 37.1 | 1.000 | |||
| Yes | 211 | 117 | 55.5 | 0.473 | 0.306–0.731 | 0.001 | |
| Rearing system | |||||||
| Intensive | 39 | 17 | 43.6 | 1.000 | |||
| Semi-Intensive | 315 | 153 | 48.6 | 0.818 | 0.419–1.600 | 0.557 | |
| Climate and environment | |||||||
| NDVI | 354 | 170 | 48.0 | 1.000 | 1.000–1.000 | 0.867 | |
| LST Day | 354 | 170 | 48.0 | 1.000 | 0.999–1.002 | 0.710 | |
| LST Noche | 354 | 170 | 48.0 | 1.001 | 0.997–1.006 | 0.555 | |
| Rainfall | 354 | 170 | 48.0 | 0.230 | 0.000–188.964 | 0.668 | |
| Elevation | 354 | 170 | 48.0 | 1.000 | 0.999–1.001 | 0.391 | |
| Neospora caninum | Age, year | ||||||
| ≤1 | 87 | 10 | 11.5 | 1.000 | |||
| 1–2 | 88 | 1 | 1.1 | 0.554 | 0.260–1.181 | 0.126 | |
| ≥2 | 179 | 34 | 19.0 | 0.049 | 0.007–0.364 | 0.003 | |
| Sex | |||||||
| Male | 105 | 13 | 12.4 | 1.000 | |||
| Female | 249 | 32 | 9.2 | 0.958 | 0.481–1.909 | 0.903 | |
| Abortion | |||||||
| No | 186 | 25 | 13.4 | 1.000 | |||
| Yes | 168 | 20 | 11.9 | 0.870 | 0.464–1.632 | 0.665 | |
| Presence of Dogs | |||||||
| No | 143 | 10 | 7.0 | 1.000 | |||
| Yes | 211 | 35 | 16.6 | 0.222 | 0.106–0.464 | <0.001 | |
| Rearing system | |||||||
| Intensive | 39 | 4 | 10.3 | 1.000 | |||
| Semi-Intensive | 315 | 41 | 13.0 | 0.764 | 0.258–2.261 | 0.626 | |
| Climate and environment | |||||||
| NDVI | 354 | 45 | 12.7 | 1.000 | 1.000–1.000 | 0.867 | |
| LST Day | 354 | 45 | 12.7 | 1.000 | 0.999–1.002 | 0.710 | |
| LST Noche | 354 | 45 | 12.7 | 1.001 | 0.997–1.006 | 0.555 | |
| Rainfall | 354 | 45 | 12.7 | 0.230 | 0.000–188.964 | 0.668 | |
| Elevation | 354 | 45 | 12.7 | 1.000 | 0.999–1.001 | 0.391 |
| Risk Factors | No. Tested | Positive No. | Prevalence (%) | Odds Ratio | 95% CI | p-Value | |
|---|---|---|---|---|---|---|---|
| Toxoplasma gondii | Age, year | ||||||
| ≤1 | 77 | 11 | 14.3 | 1.000 | |||
| 1–2 | 68 | 41 | 60.3 | 0.038 | 0.018–0.084 | <0.001 | |
| ≥2 | 128 | 104 | 81.3 | 0.350 | 0.181–0.677 | 0.002 | |
| Sex | |||||||
| Male | 117 | 70 | 59.8 | 1.000 | |||
| Female | 156 | 86 | 55.1 | 0.893 | 0.550–1.449 | 0.646 | |
| Abortion | |||||||
| No | 117 | 22 | 18.8 | 1.000 | |||
| Yes | 156 | 134 | 85.9 | 26.302 | 13.775–50.220 | <0.001 | |
| Presence of Cats | |||||||
| No | 93 | 45 | 48.4 | 1.000 | |||
| Yes | 180 | 111 | 61.7 | 0.583 | 0.351–0.966 | 0.036 | |
| Rearing system | |||||||
| Intensive | 245 | 137 | 55.9 | 1.000 | |||
| Semi-Intensive | 28 | 19 | 67.9 | 0.601 | 0.261–1.381 | 0.230 | |
| Climate and environment | |||||||
| NDVI | 273 | 156 | 57.1 | 1.000 | 1.000–1.001 | 0.253 | |
| LST Day | 273 | 156 | 57.1 | 0.991 | 0.974–1.008 | 0.304 | |
| LST Noche | 273 | 156 | 57.1 | 0.998 | 0.974–1.022 | 0.849 | |
| Rainfall | 273 | 156 | 57.1 | 0.330 | 0.000–16.658 | 0.864 | |
| Elevation | 273 | 156 | 57.1 | 1.000 | 1.000–1.001 | 0.360 | |
| Neospora caninum | Age, year | ||||||
| ≤1 | 77 | 26 | 33.8 | 1.000 | |||
| 1–2 | 68 | 2 | 2.9 | 2.209 | 1.155–4.224 | 0.017 | |
| ≥2 | 128 | 24 | 18.8 | 0.131 | 0.030–0.574 | 0.007 | |
| Sex | |||||||
| Male | 117 | 16 | 13.7 | 1.000 | |||
| Female | 156 | 36 | 23.1 | 0.528 | 0.277–1.007 | 0.053 | |
| Abortion | |||||||
| No | 117 | 32 | 27.4 | 1.000 | |||
| Yes | 156 | 20 | 12.8 | 2.560 | 1.376–4.763 | 0.003 | |
| Presence of Dogs | |||||||
| No | 93 | 16 | 17.2 | 1.000 | |||
| Yes | 180 | 36 | 20.0 | 0.528 | 0.277–1.007 | 0.053 | |
| Rearing system | |||||||
| Intensive | 245 | 46 | 18.8 | 1.000 | |||
| Semi-Intensive | 28 | 6 | 21.4 | 0.848 | 0.325–2.209 | 0.753 | |
| Climate and environment | |||||||
| NDVI | 273 | 52 | 19.0 | 1.000 | 1.000–1.001 | 0.703 | |
| LST Day | 273 | 52 | 19.0 | 0.993 | 0.971–1.015 | 0.518 | |
| LST Noche | 273 | 52 | 19.0 | 0.987 | 0.957–1.018 | 0.395 | |
| Rainfall | 273 | 52 | 19.0 | 0.21 | 0.000–12.465 | 0.715 | |
| Elevation | 273 | 52 | 19.0 | 1.000 | 1.000–1.001 | 0.428 |
| Risk Factors | No. of Goats | Positive No. | Prevalence (%) | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|---|---|---|
| Age, year | ||||||
| ≤1 | 164 | 31 | 18.9 | 1.000 | ||
| 1–2 | 156 | 61 | 41.0 | 0.073 | 0.045–0.113 | <0.001 |
| ≥2 | 307 | 234 | 76.2 | 0.200 | 0.132–0.303 | <0.001 |
| Sex | ||||||
| Male | 222 | 129 | 58.1 | 1.000 | ||
| Female | 405 | 197 | 48.6 | 1.465 | 1.053–2.038 | 0.024 |
| Abortion | ||||||
| No | 303 | 63 | 20.8 | 1.000 | ||
| Yes | 324 | 263 | 81.2 | 16.425 | 11.086–24.335 | <0.001 |
| Presence of Cats | ||||||
| No | 301 | 133 | 44.2 | 1.000 | ||
| Yes | 326 | 193 | 59.2 | 1.970 | 1.419–2.734 | <0.001 |
| Rearing system | ||||||
| Intensive | 284 | 154 | 54.2 | 1.000 | ||
| Semi-Intensive | 343 | 172 | 50.1 | 1.178 | 0.859–1.614 | 0.309 |
| Climate and environment | ||||||
| NDVI | 627 | 326 | 52.0 | 1.000 | 1.000–1.000 | 0.280 |
| LST Day | 627 | 326 | 52.0 | 1.001 | 1.000–1.002 | 0.062 |
| LST Noche | 627 | 326 | 52.0 | 1.000 | 0.996–1.004 | 0.890 |
| Rainfall | 627 | 326 | 52.0 | 0.019 | 0.001–0.718 | 0.032 |
| Elevation | 627 | 326 | 52.0 | 0.999 | 0.998–1.00 | 0.078 |
| State, Climate regions | ||||||
| Hidalgo, Dry (Bs) | 273 | 156 | 57.1 | |||
| Puebla, Temperate (Cw) | 354 | 170 | 48.0 | 1.443 | 1.050–1.983 | 0.024 |
| Risk Factors | No. Tested | Positive No. | Prevalence (%) | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|---|---|---|
| Age, year | ||||||
| ≤1 | 164 | 36 | 22.0 | 1.000 | ||
| 1–2 | 156 | 3 | 1.9 | 1.207 | 0.757–1.927 | 0.429 |
| ≥2 | 307 | 58 | 18.9 | 0.084 | 0.026–0.273 | <0.001 |
| Sex | ||||||
| Male | 222 | 29 | 13.1 | 1.000 | ||
| Female | 405 | 68 | 16.8 | 0.745 | 0.466–1.191 | 0.218 |
| Abortion | ||||||
| No | 303 | 57 | 18.8 | 1.000 | ||
| Yes | 324 | 40 | 12.3 | 1.645 | 1.061–2.551 | 0.026 |
| Presence of Dogs | ||||||
| No | 236 | 27 | 11.4 | 1.000 | ||
| Yes | 391 | 70 | 17.9 | 0.340 | 0.210–0.546 | <0.001 |
| Rearing system | ||||||
| Intensive | 284 | 50 | 17.6 | 1.000 | ||
| Semi-Intensive | 343 | 47 | 13.7 | 1.346 | 0.872–2.076 | 0.179 |
| Climate and environment | ||||||
| NDVI | 627 | 97 | 15.5 | 1.000 | 1.000–1.001 | 0.288 |
| LST Day | 627 | 97 | 15.5 | 1.001 | 1.000–1.002 | 0.154 |
| LST Noche | 627 | 97 | 15.5 | 0.999 | 0.993–1.004 | 0.647 |
| Rainfall | 627 | 97 | 15.5 | 0.010 | 0.000–1.625 | 0.076 |
| Elevation | 627 | 97 | 15.5 | 0.999 | 0.998–1.001 | 0.273 |
| State, Climate regions | ||||||
| Hidalgo, Dry (Bs) | 273 | 52 | 19.0 | |||
| Puebla, Temperate (Cw) | 354 | 45 | 12.7 | 1.616 | 1.046–2.496 | 0.031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villa-Mancera, A.; Vargas-Tizatl, E.; Robles-Robles, J.M.; Utrera-Quintana, F.; Olivares-Pérez, J.; Olmedo-Juárez, A.; Córdova-Izquierdo, A.; González-Garduño, R.; Ponce-Covarrubias, J.L.; Rivero-Perez, N.; et al. High Seroprevalence of Toxoplasma gondii and Neospora caninum Infections Among Goats in Mexico Is Associated with Climatic, Environmental, and Risk Factors. Pathogens 2025, 14, 1170. https://doi.org/10.3390/pathogens14111170
Villa-Mancera A, Vargas-Tizatl E, Robles-Robles JM, Utrera-Quintana F, Olivares-Pérez J, Olmedo-Juárez A, Córdova-Izquierdo A, González-Garduño R, Ponce-Covarrubias JL, Rivero-Perez N, et al. High Seroprevalence of Toxoplasma gondii and Neospora caninum Infections Among Goats in Mexico Is Associated with Climatic, Environmental, and Risk Factors. Pathogens. 2025; 14(11):1170. https://doi.org/10.3390/pathogens14111170
Chicago/Turabian StyleVilla-Mancera, Abel, Eunice Vargas-Tizatl, José Manuel Robles-Robles, Fernando Utrera-Quintana, Jaime Olivares-Pérez, Agustín Olmedo-Juárez, Alejandro Córdova-Izquierdo, Roberto González-Garduño, José Luis Ponce-Covarrubias, Nallely Rivero-Perez, and et al. 2025. "High Seroprevalence of Toxoplasma gondii and Neospora caninum Infections Among Goats in Mexico Is Associated with Climatic, Environmental, and Risk Factors" Pathogens 14, no. 11: 1170. https://doi.org/10.3390/pathogens14111170
APA StyleVilla-Mancera, A., Vargas-Tizatl, E., Robles-Robles, J. M., Utrera-Quintana, F., Olivares-Pérez, J., Olmedo-Juárez, A., Córdova-Izquierdo, A., González-Garduño, R., Ponce-Covarrubias, J. L., Rivero-Perez, N., Patricio-Martínez, F., & Campos-García, H. (2025). High Seroprevalence of Toxoplasma gondii and Neospora caninum Infections Among Goats in Mexico Is Associated with Climatic, Environmental, and Risk Factors. Pathogens, 14(11), 1170. https://doi.org/10.3390/pathogens14111170










