Role of S1PR1 in Modulating Airway Epithelial Responses to Pseudomonas aeruginosa in Cystic Fibrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Bacterial Strain and pwCF
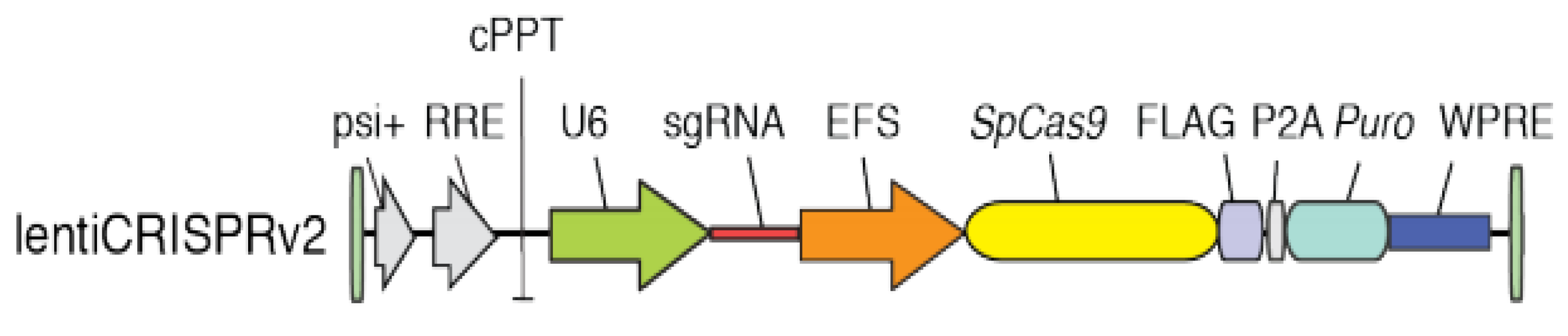
2.3. Generation and Sequence of S1PR1 Mutant Cells by CRISPR/Cas9
2.4. Bacterial Exoproducts Preparation
2.5. Cell Stimulation
2.6. Cytotoxicity and ELISA
2.7. Immunostaining Against S1PR1
2.8. Statistics
3. Results
3.1. Generation of Mutant Cell Lines for S1PR1 Through CRISPR/Cas9 Genetic Editing
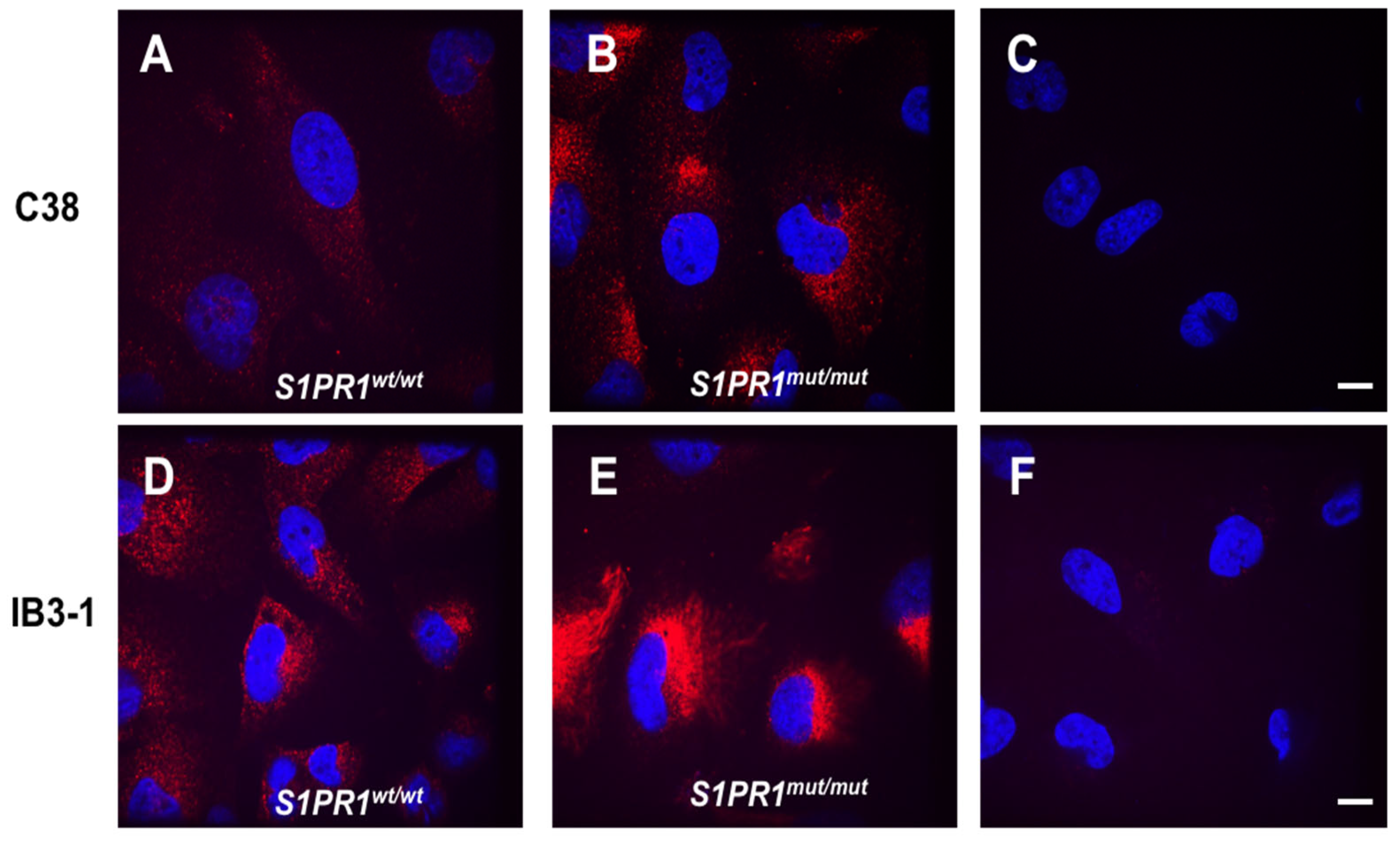
3.2. Immunolocalization of S1PR1 and Its Mutant Form in the Presence or Absence of CFTR
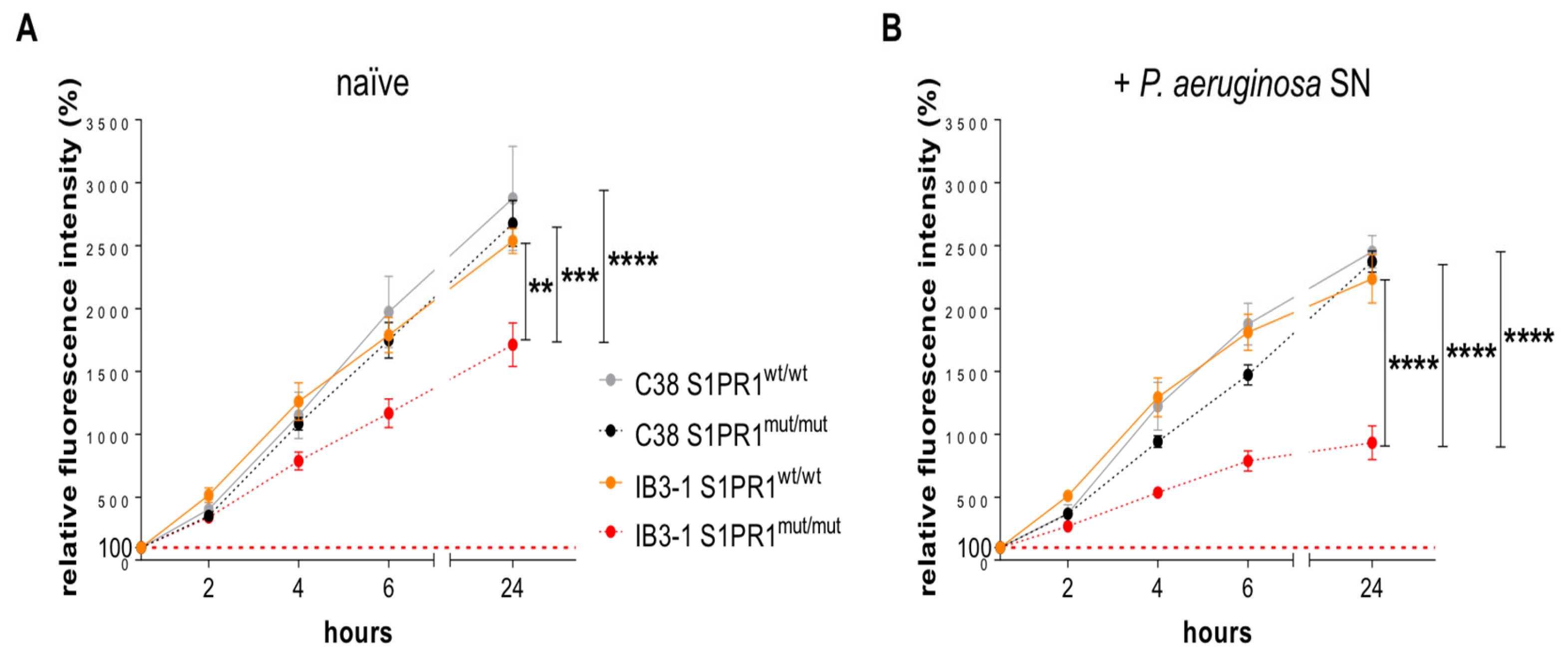
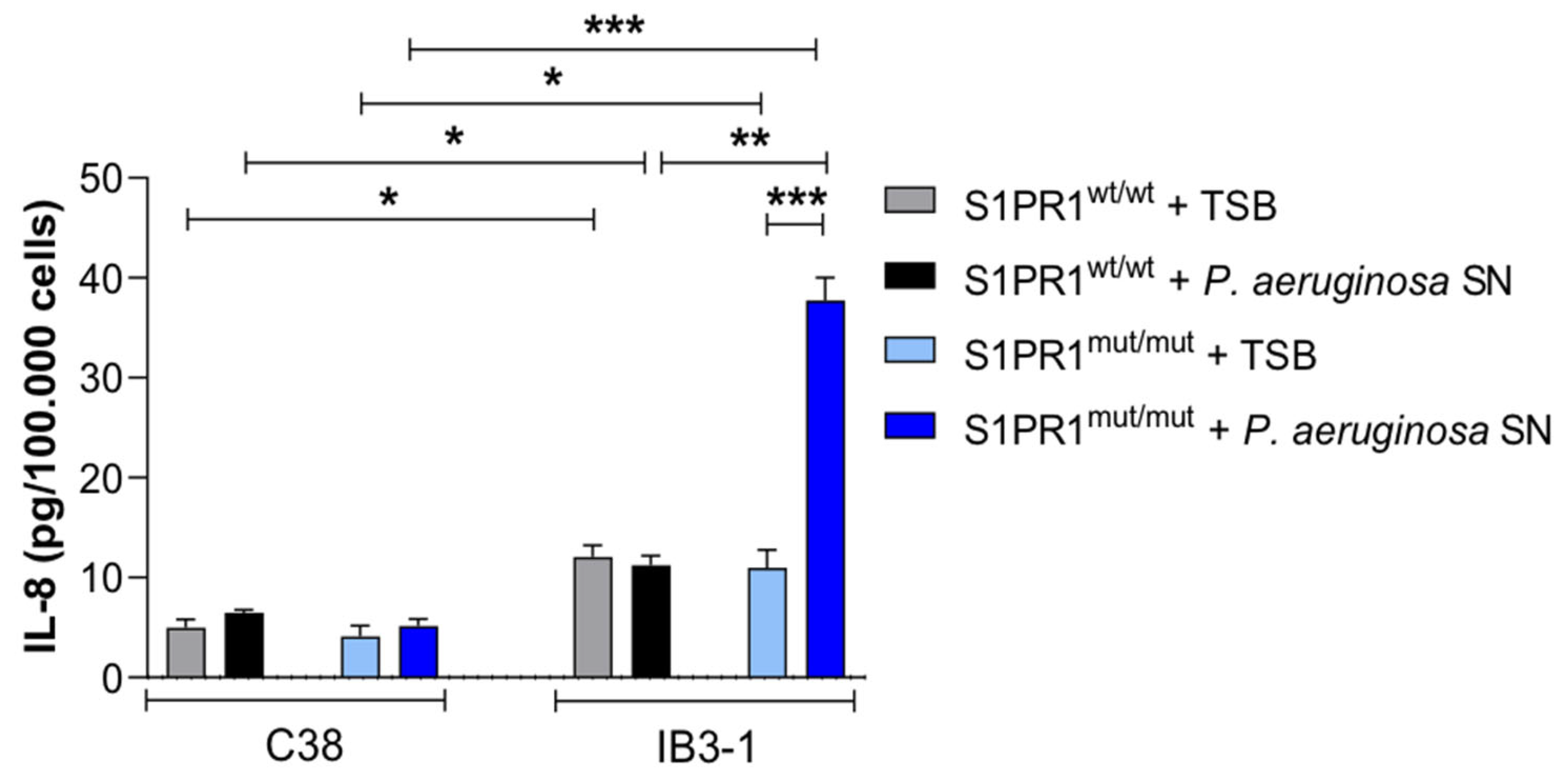
3.3. Response of IB3-1 and C38 Cell Lines Carrying S1PR1 or Its Mutant to Stimulation with P. aeruginosa Exoproducts
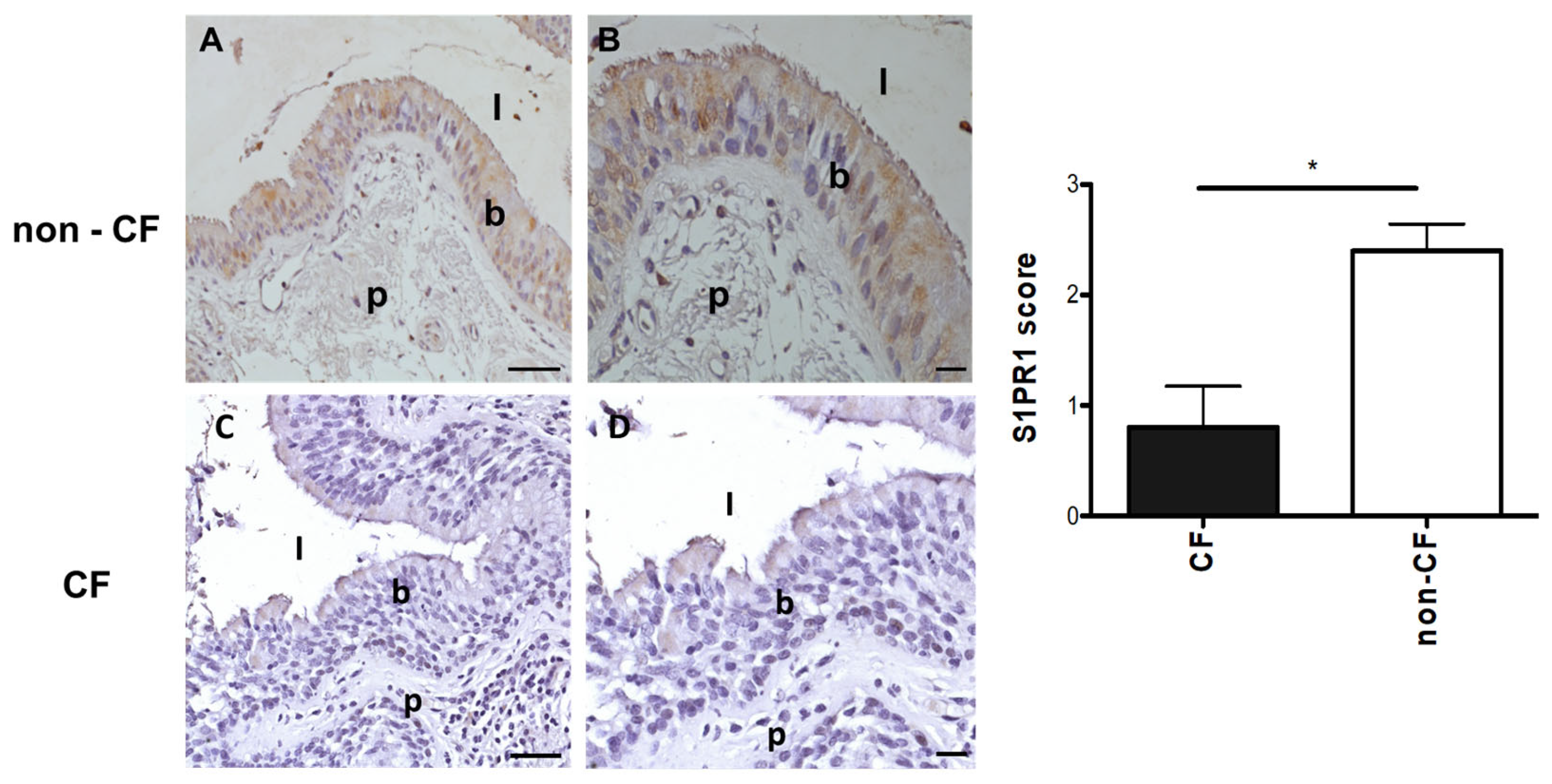
3.4. Protein Expression of S1PR1 in Lung Tissues of pwCF and Non-CF Individuals
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BSA | bovine serum albumin |
| CF | cystic fibrosis |
| CFTR | cystic fibrosis transmembrane conductance regulator |
| DAPI | 4,6-Diamidino-2-phenylindole dihydrochloride |
| IL-8 | interleukin-8 |
| MOI | multiplicity of infection |
| OD | optical density |
| O/N | overnight |
| PBS | phosphate-buffered saline |
| PFA | paraformaldehyde |
| pwCF | people with CF |
| S1PR1 | sphingosine 1-phosphate receptor 1 |
| SEM | standard errors of the means |
| sgRNA | single guide RNA |
| SN | supernatant |
| TSB | tryptic soy broth |
References
- Shteinberg, M.; Haq, I.J.; Polineni, D.; Davies, J.C. Cystic fibrosis. Lancet 2021, 397, 2195–2211. [Google Scholar] [CrossRef] [PubMed]
- Lund-Palau, H.; Turnbull, A.R.; Bush, A.; Bardin, E.; Cameron, L.; Soren, O.; Wierre-Gore, N.; Alton, E.W.; Bundy, J.G.; Connett, G.; et al. Pseudomonas aeruginosa infection in cystic fibrosis: Pathophysiological mechanisms and therapeutic approaches. Expert Rev. Respir. Med. 2016, 10, 685–697. [Google Scholar] [CrossRef]
- Gibson, R.L.; Burns, J.L.; Ramsey, B.W. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2003, 168, 918–951. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.; Allen, L.; Carr, S.B.; Davies, G.; Downey, D.; Egan, M.; Forton, J.T.; Gray, R.; Haworth, C.; Horsley, A.; et al. Future therapies for cystic fibrosis. Nat. Commun. 2023, 14, 693. [Google Scholar] [CrossRef]
- Cutting, G. Cystic fibrosis genetics: From molecular understanding to clinical application. Nat. Rev. Genet. 2015, 16, 45–56. [Google Scholar] [CrossRef]
- Cutting, G.R. Modifier genes in Mendelian disorders: The example of cystic fibrosis. Ann. N. Y. Acad. Sci. 2010, 1214, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Knowles, M.; Drumm, M. The influence of genetics on cystic fibrosis phenotypes. Cold Spring Harb. Perspect. Med. 2012, 2, a009548. [Google Scholar] [CrossRef]
- Lore, N.I.; Iraqi, F.A.; Bragonzi, A. Host genetic diversity influences the severity of Pseudomonas aeruginosa pneumonia in the Collaborative Cross mice. BMC Genet. 2015, 16, 106. [Google Scholar] [CrossRef]
- Lore, N.I.; Sipione, B.; He, G.; Strug, L.J.; Atamni, H.J.; Dorman, A.; Mott, R.; Iraqi, F.A.; Bragonzi, A. Collaborative Cross Mice Yield Genetic Modifiers for Pseudomonas aeruginosa Infection in Human Lung Disease. mBio 2020, 11. [Google Scholar] [CrossRef]
- Bryan, A.M.; Del Poeta, M. Sphingosine-1-phosphate receptors and innate immunity. Cell. Microbiol. 2018, 20, e12836. [Google Scholar] [CrossRef] [PubMed]
- Rosen, H.; Gonzalez-Cabrera, P.J.; Sanna, M.G.; Brown, S. Sphingosine 1-phosphate receptor signaling. Annu. Rev. Biochem. 2009, 78, 743–768. [Google Scholar] [CrossRef]
- O’Sullivan, C.; Dev, K.K. The structure and function of the S1P1 receptor. Trends Pharmacol. Sci. 2013, 34, 401–412. [Google Scholar] [CrossRef]
- Aoki, M.; Aoki, H.; Ramanathan, R.; Hait, N.C.; Takabe, K. Sphingosine-1-Phosphate Signaling in Immune Cells and Inflammation: Roles and Therapeutic Potential. Mediat. Inflamm. 2016, 2016, 8606878. [Google Scholar] [CrossRef]
- Allende, M.L.; Zhou, D.; Kalkofen, D.N.; Benhamed, S.; Tuymetova, G.; Borowski, C.; Bendelac, A.; Proia, R.L. S1P1 receptor expression regulates emergence of NKT cells in peripheral tissues. FASEB J. 2008, 22, 307–315. [Google Scholar] [CrossRef]
- Obinata, H.; Gutkind, S.; Stitham, J.; Okuno, T.; Yokomizo, T.; Hwa, J.; Hla, T. Individual variation of human S1P(1) coding sequence leads to heterogeneity in receptor function and drug interactions. J. Lipid Res. 2014, 55, 2665–2675. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ma, S.F.; Wade, M.S.; Flores, C.; Pino-Yanes, M.; Moitra, J.; Ober, C.; Kittles, R.; Husain, A.N.; Ford, J.G.; et al. Functional variants of the sphingosine-1-phosphate receptor 1 gene associate with asthma susceptibility. J. Allergy Clin. Immunol. 2010, 126, 241–249, 249 e241-243. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Fu, R.; Tai, J.; Tian, Z.; Yuan, X.; Chen, Y.; Wang, M.; Jiang, H.; Ji, M.; Lai, F.; et al. S1PR1 serves as a viable drug target against pulmonary fibrosis by increasing the integrity of the endothelial barrier of the lung. Acta Pharm. Sin. B 2023, 13, 1110–1127. [Google Scholar] [CrossRef] [PubMed]
- Maceyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef]
- Malik, F.A.; Meissner, A.; Semenkov, I.; Molinski, S.; Pasyk, S.; Ahmadi, S.; Bui, H.H.; Bear, C.E.; Lidington, D.; Bolz, S.S. Sphingosine-1-Phosphate Is a Novel Regulator of Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Activity. PLoS ONE 2015, 10, e0130313. [Google Scholar] [CrossRef]
- Bragonzi, A.; Paroni, M.; Nonis, A.; Cramer, N.; Montanari, S.; Rejman, J.; Di Serio, C.; Doring, G.; Tummler, B. Pseudomonas aeruginosa microevolution during cystic fibrosis lung infection establishes clones with adapted virulence. Am. J. Respir. Crit. Care Med. 2009, 180, 138–145. [Google Scholar] [CrossRef]
- Zeitlin, P.; Lu, L.; Rhim, J.; Cutting, G.; Stetten, G.; Kieffer, K.A.; Craig, R.; Guggino, W.B. A cystic fibrosis bronchial epithelial cell line: Immortalization by adeno-12-SV40 infection. Am. J. Respir. Cell Mol. Biol. 1991, 4, 313–319. [Google Scholar] [CrossRef]
- Sanjana, N.E.; Shalem, O.; Zhang, F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 2014, 11, 783–784. [Google Scholar] [CrossRef]
- Lorè, N.; Cigana, C.; De Fino, I.; Riva, C.; Juhas, M.; Schwager, S.; Eberl, L.; Bragonzi, A. Cystic Fibrosis-Niche Adaptation of Pseudomonas aeruginosa Reduces Virulence in Multiple Infection Hosts. PLoS ONE 2012, 7, e35648. [Google Scholar] [CrossRef] [PubMed]
- Longhin, E.M.; El Yamani, N.; Runden-Pran, E.; Dusinska, M. The alamar blue assay in the context of safety testing of nanomaterials. Front. Toxicol. 2022, 4, 981701. [Google Scholar] [CrossRef] [PubMed]
- Corvol, H.; Blackman, S.M.; Boelle, P.Y.; Gallins, P.J.; Pace, R.G.; Stonebraker, J.R.; Accurso, F.J.; Clement, A.; Collaco, J.M.; Dang, H.; et al. Genome-wide association meta-analysis identifies five modifier loci of lung disease severity in cystic fibrosis. Nat. Commun. 2015, 6, 8382. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.H.; Gallins, P.J.; Pace, R.G.; Dang, H.; Aksit, M.A.; Blue, E.E.; Buckingham, K.J.; Collaco, J.M.; Faino, A.V.; Gordon, W.W.; et al. Genetic Modifiers of Cystic Fibrosis Lung Disease Severity: Whole-Genome Analysis of 7,840 Patients. Am. J. Respir Crit. Care Med. 2023, 207, 1324–1333. [Google Scholar] [CrossRef]
- Tsai, H.C.; Han, M.H. Sphingosine-1-Phosphate (S1P) and S1P Signaling Pathway: Therapeutic Targets in Autoimmunity and Inflammation. Drugs 2016, 76, 1067–1079. [Google Scholar] [CrossRef]
- Gaire, B.P.; Lee, C.H.; Sapkota, A.; Lee, S.Y.; Chun, J.; Cho, H.J.; Nam, T.G.; Choi, J.W. Identification of Sphingosine 1-Phosphate Receptor Subtype 1 (S1P(1)) as a Pathogenic Factor in Transient Focal Cerebral Ischemia. Mol. Neurobiol. 2018, 55, 2320–2332. [Google Scholar] [CrossRef]
- Pan, S.; Mi, Y.; Pally, C.; Beerli, C.; Chen, A.; Guerini, D.; Hinterding, K.; Nuesslein-Hildesheim, B.; Tuntland, T.; Lefebvre, S.; et al. A monoselective sphingosine-1-phosphate receptor-1 agonist prevents allograft rejection in a stringent rat heart transplantation model. Chem. Biol. 2006, 13, 1227–1234. [Google Scholar] [CrossRef]
- Bravo, G.A.; Cedeno, R.R.; Casadevall, M.P.; Ramio-Torrenta, L. Sphingosine-1-Phosphate (S1P) and S1P Signaling Pathway Modulators, from Current Insights to Future Perspectives. Cells 2022, 11, 2058. [Google Scholar] [CrossRef]
- Chavez, A.; Schmidt, T.T.; Yazbeck, P.; Rajput, C.; Desai, B.; Sukriti, S.; Giantsos-Adams, K.; Knezevic, N.; Malik, A.B.; Mehta, D. S1PR1 Tyr143 phosphorylation downregulates endothelial cell surface S1PR1 expression and responsiveness. J. Cell Sci. 2015, 128, 878–887. [Google Scholar] [CrossRef]
- Dalemans, W.; Barbry, P.; Champigny, G.; Jallat, S.; Dott, K.; Dreyer, D.; Crystal, R.G.; Pavirani, A.; Lecocq, J.P.; Lazdunski, M. Altered chloride ion channel kinetics associated with the delta F508 cystic fibrosis mutation. Nature 1991, 354, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ramjeesingh, M.; Reyes, E.; Jensen, T.; Chang, X.; Rommens, J.M.; Bear, C.E. The cystic fibrosis mutation (delta F508) does not influence the chloride channel activity of CFTR. Nat. Genet. 1993, 3, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Galli, F.; Battistoni, A.; Gambari, R.; Pompella, A.; Bragonzi, A.; Pilolli, F.; Iuliano, L.; Piroddi, M.; Dechecchi, M.C.; Cabrini, G.; et al. Oxidative stress and antioxidant therapy in cystic fibrosis. Biochim. Biophys. Acta 2012, 1822, 690–713. [Google Scholar] [CrossRef] [PubMed]
- Dobi, D.; Loberto, N.; Bassi, R.; Pistocchi, A.; Lunghi, G.; Tamanini, A.; Aureli, M. Cross-talk between CFTR and sphingolipids in cystic fibrosis. FEBS Open Bio 2023, 13, 1601–1614. [Google Scholar] [CrossRef]
- Sonnino, S.; Prinetti, A.; Mauri, L.; Chigorno, V.; Tettamanti, G. Dynamic and structural properties of sphingolipids as driving forces for the formation of membrane domains. Chem. Rev. 2006, 106, 2111–2125. [Google Scholar] [CrossRef]
| sgRNA ID | Target Sequence |
|---|---|
| W3 | CGTAGTCAGAGACCGAGCTG |
| W6 | GATGGCGAGGAGACTGAACA |
| W9 | ATATAGTGCTTGTGGTAGAG |
| Individual | Pathology | CFTR Mutations |
|---|---|---|
| BE 55 | CF | F508del/F508del |
| BE 60 | CF | F508del/R1162X |
| BE 68 | CF | F508del/F508del |
| BE 73 | CF | F508del/F508del |
| BE 79 | CF | F508del/F508del |
| BE 49 | non-CF | - |
| BE 50 | non-CF | - |
| BE 65 | non-CF | - |
| BE 66 | non-CF | - |
| BE 67 | non-CF | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cigana, C.; Caslini, C.; Migliara, A.; Alcala’-Franco, B.; Veschetti, L.; Lorè, N.I.; Lombardo, A.; Bragonzi, A. Role of S1PR1 in Modulating Airway Epithelial Responses to Pseudomonas aeruginosa in Cystic Fibrosis. Pathogens 2025, 14, 1146. https://doi.org/10.3390/pathogens14111146
Cigana C, Caslini C, Migliara A, Alcala’-Franco B, Veschetti L, Lorè NI, Lombardo A, Bragonzi A. Role of S1PR1 in Modulating Airway Epithelial Responses to Pseudomonas aeruginosa in Cystic Fibrosis. Pathogens. 2025; 14(11):1146. https://doi.org/10.3390/pathogens14111146
Chicago/Turabian StyleCigana, Cristina, Claudia Caslini, Alessandro Migliara, Beatriz Alcala’-Franco, Laura Veschetti, Nicola Ivan Lorè, Angelo Lombardo, and Alessandra Bragonzi. 2025. "Role of S1PR1 in Modulating Airway Epithelial Responses to Pseudomonas aeruginosa in Cystic Fibrosis" Pathogens 14, no. 11: 1146. https://doi.org/10.3390/pathogens14111146
APA StyleCigana, C., Caslini, C., Migliara, A., Alcala’-Franco, B., Veschetti, L., Lorè, N. I., Lombardo, A., & Bragonzi, A. (2025). Role of S1PR1 in Modulating Airway Epithelial Responses to Pseudomonas aeruginosa in Cystic Fibrosis. Pathogens, 14(11), 1146. https://doi.org/10.3390/pathogens14111146






