1. Introduction
Sclerotinia sclerotiorum is a soilborne fungal pathogen, a representative species of the Sclerotineaceae family in Ascomycota [
1,
2]. It is one of the most devastating plant pathogens that causes substantial damage to major crops, including canola, vegetables, various beans, and sunflowers in Canada, the USA, and other countries [
3,
4]. Its widespread impact has resulted in substantial economic losses worldwide [
1,
3,
5]. Traditional control methods are largely ineffective due to the pathogen’s broad host range and the general lack of resistance in crop cultivars. A key feature of
S. sclerotiorum is the formation of sclerotia–a dormant structure encased in melanized surface layers [
5,
6]. Sclerotia serve two functions: acting as overwintering structures that allow the fungus to survive in unfavorable conditions and playing a crucial role in reproduction [
1]. Sclerotia can germinate as mycelia, leading to direct plant infection. They can also produce apothecia that can release windborne ascospores to infect nearby hosts [
1,
7]. During infection, like other fungal pathogens, it can form complex appressoria structures for host cell wall penetration [
1]. Despite its agricultural significance, the molecular mechanisms underlying
S. sclerotiorum’s ability to sense extracellular stimuli, produce key developmental structures such as sclerotia, apothecia and appressoria, and interact with plant hosts remain largely unknown.
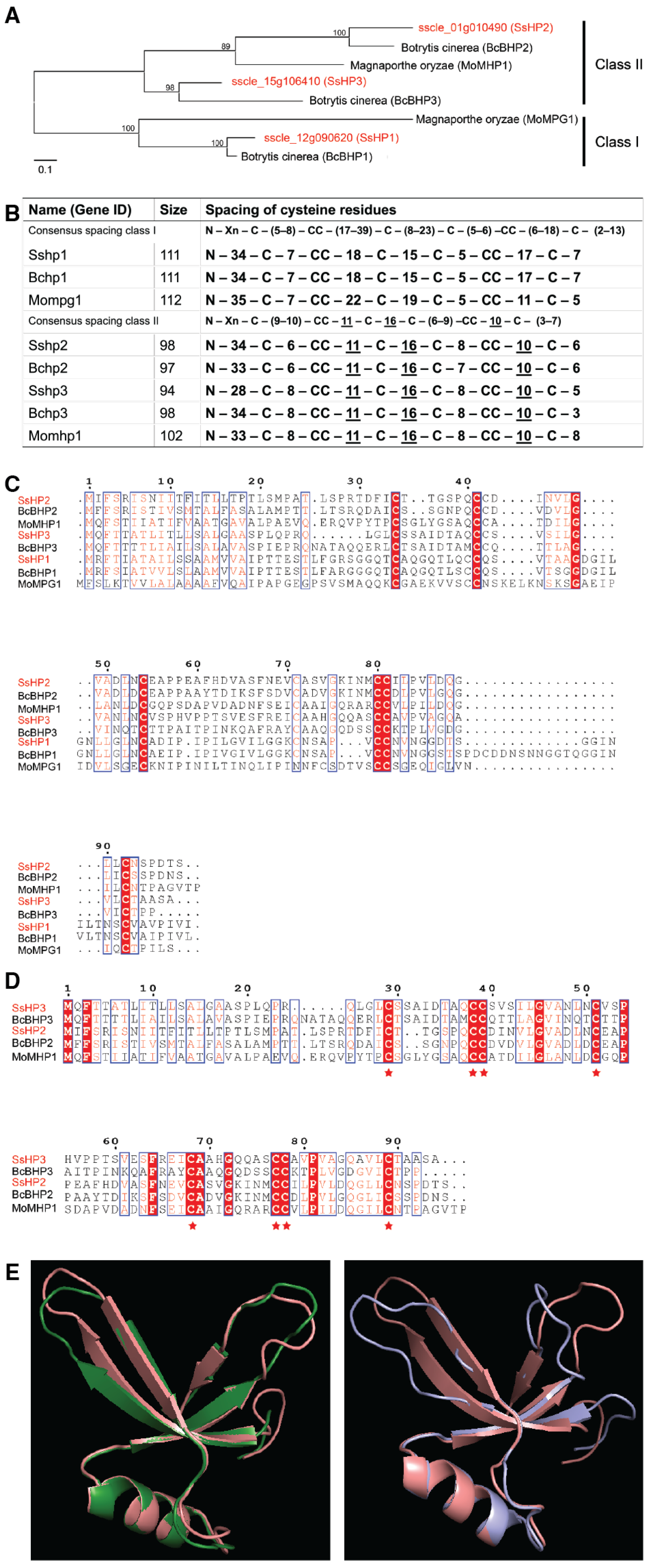
Hydrophobins (HPs) are small, secreted, hydrophobic proteins widely distributed in mycelial fungi [
8,
9]. They can self-assemble into robust, highly amphipathic polymeric monolayers [
9,
10,
11]. HPs are characterized by the presence of eight conserved cysteine (Cys) residues, separated into two classes based on their amino acid sequences, hydropathy profiles, and solvent solubility. Class I HPs, generally longer, are commonly found in both ascomycetes and basidiomycetes, whereas class II HPs are more conserved and limited to ascomycete [
12,
13]. Class I HPs are able to assemble into a stable structure, known as rodlet layer, and they are only soluble in strong acids, such as trifluoroacetic acid (TFA) or formic acid (FA) [
9]. In contrast, class II HPs cannot form the fibrillar rodlet morphology and can be dissolved in organic solvents and detergents such as 2% sodium dodecyl sulphate (SDS) or 60% ethanol [
9,
12].
HPs play essential roles in fungi by forming self-assembled amphipathic layers that lower surface tension, enabling aerial hyphae to breach the air-water interface [
14,
15,
16]. They also render spores hydrophobic, promoting efficient dispersal, protecting against external environment, and facilitating surface attachment to surfaces—an important prerequisite for infection and germination [
15,
16,
17,
18]. Many fungi produce multiple HPs that function across different stages of the life cycle. For instance, the basidiomycete
Schizophyllum commune encodes four HPs (SC1,SC3,SC4, and SC6) that are crucial for fruiting body development and for enabling hyphae to penetrate water or soil surfaces [
11,
19,
20]. Among them, the well-characterized SC3 also contributes to the cell wall matrix, influencing its composition [
21]. In ascomycete
Aspergillus nidulans, six HPs (RodA and DewA–DewE) collectively mediate amphipathic layer formation and spore hydrophobicity, with RodA playing the dominant role [
22]. Beyond development, HPs also participate in symbiotic interactions, including lichen symbiosis and the ectomycorrhizal association between
Tricholoma terreum and its host trees, largely in the pine family, but also the spruces [
23,
24].
HPs have also been studied in several plant fungal pathogens, where they play essential roles in various aspects of fungal biology. In the model plant pathogen
Magnaporthe oryzae, a class I HP (MPG1) and a class II HP (MHP1) have been identified. These proteins are involved in surface hydrophobicity, conidiation, appressorium development, as well as pathogenicity [
25,
26]. MPG1 is particularly important for host recognition, mediating morphogenetic signals that trigger appressorium differentiation [
25,
27]. Mutants with
MPG1 disruption fail to form the rodlet layer on conidial walls and exhibit a water wettable phenotype; whereas
MHP1 deletion mutants only display detergent wettable phenotype [
25,
26]. These finding suggested that MoMPG1 may specifically be responsible for rodlet layer formation in
M. oryzae, while MoMHP1 may support hydrophobicity through a rodlet-independent mechanism. In fungal corn pathogen
Fusarium verticillioides, five
HP genes have been identified. Among them,
HYD1 and
HYD2 are essential for the structural integrity of the long aerial chains of microconidia, a distinctive feature of this pathogen. However, none of these five HPs are essential for corn seedling infection [
28]. Similarly, in the wilt fungus
Verticillium dahlia, the class II HP VDH1 is required for its fungal development, including microsclerotia development and spore viability, but is not necessary for the initiation of disease in tomatoes [
29].
In the airborne plant pathogen
Botrytis cinerea, three
HP genes have been studied. Individually, these genes are not required for conidia and hyphae hydrophobicity and are not involved in its asexual development or pathogenicity [
30]. However, double (
bhp1/bhp2) and triple knock-out mutants exhibit abnormal apothecia development when used as maternal parents (sclerotia), suggesting the importance of HPs in fungal sexual development, particularly in maternal tissues [
13].
S. sclerotiorum is closely related to
B. cinerea, and are both in the Sclerotineaceae family. It also contains three
HP genes in its genome [
30]. However, the biological functions of these genes in
S. sclerotiorum remain largely unknown. In this study, we classified and characterized
HP genes in
S. sclerotiorum, including
SsHP1 in class I, and
SsHP2 and
SsHP3 as class II members. Phenotypic analyses of HP mutants revealed that
SsHP1 plays a role in sclerotia development, while
SsHP2 and
SsHP3 are essential for proper compound appressoria function and virulence. Double mutant
Sshp2 Sshp3 exhibited pronounced defects in both appressoria development and virulence, indicating overlapping and critical roles in infection structure formation and host colonization. In addition, all HPs in
S. sclerotiorum contribute to cell wall integrity, composition, and resistance to various abiotic stresses to different extents. Interestingly, these functions contrast with those reported in
B. cinerea, where HPs are dispensable for virulence but required for sexual development, suggesting an evolutionary divergence of HP function even between closely related fungi. Taken together, our findings provide new insights into the multifaceted roles of HPs in fungal biology, highlighting class II HPs as potential targets for novel strategies in disease mitigation of
S. sclerotiorum.
2. Materials and Methods
2.1. Fungal Strains and Culture Conditions
The wild-type (WT) strain S. sclerotiorum 1980 and all the knockout (KO) mutant strains generated in this background were cultured on potato dextrose agar (PDA, Shanghai Bio-way technology, Shanghai, China) at room temperature. Strains were maintained either on PDA slants at 4 °C or preserved as sclerotia. Selection and purification of KO mutants were conducted on PDA with hygromycin B (50 μg/mL, MilliporeSigma, Oakville, ON, Canada) to verify successful transformant isolation.
Carrot medium was prepared from sliced, autoclaved carrots and used to cultivate large sclerotia for apothecia production. Mycelia from PDA plates were inoculated onto the carrot medium and incubated at room temperature for 3–4 weeks until sclerotia matured.
2.2. Apothecium Induction
For apothecium formation, large sclerotia harvested from carrot medium were gently cleaned to remove adhering mycelia and carrot residues. After air-drying at room temperature, the sclerotia were immersed in 33% (v/v) bleach solution (Clorox Disinfecting Bleach, The Clorox Company, Oakland, CA, USA) for about 6 min for surface sterilization, followed by three rinses using sterile water to remove the remaining bleach. The sterilized sclerotia were then placed onto pre-autoclaved sands in containers covered with aluminum foil and incubated at 4 °C for approximately 4 weeks. After cold and dark treatment, the foil was replaced with alcohol-sterilized plastic wrap, and the containers were transferred to a plant growth chamber set at 16 °C with a 16 h light/8 h dark photoperiod. Apothecium development was typically observed at approximately 3–5 weeks.
2.3. Target Genes Knockout (KO)
The split-marker strategy was employed to construct
SsHP1,
SsHP2, and
SsHP3 gene replacement cassettes and generate KO mutants, following the procedure as previously described [
31]. Briefly, the gene replacement fragment, containing the flanking sequences of the target gene surrounding the hygromycin-resistance gene (
HYG), was introduced into WT
S. sclerotiorum protoplasts. Transformants showing hygromycin B resistances were selected and were further purified through sequential tip mycelia transfers followed by protoplast purification. This process was repeated until PCR analysis with the 5F/6R primer pair confirmed the absence of WT band, verifying successful gene deletion. All the primers used in this study are listed in
Supplemental Table S1.
The double mutant was generated using a previously described mycelial fusion method [
32]. Mycelial disks of
Sshp2 and
Sshp3 single mutants were positioned in direct contact, face-to-face, and co-inoculated onto carrot medium to facilitate fusion and the development of large sclerotia. These large sclerotia were subsequently induced to produce apothecia, and single colonies derived from the resulting ascospores were screened on PDA containing hygromycin B. Putative double KO mutants were verified by PCR, where no WT-specific bands were detected using the 5F/6R primer pairs for both genes.
2.4. S. sclerotiorum Growth Rate and Colony Morphology
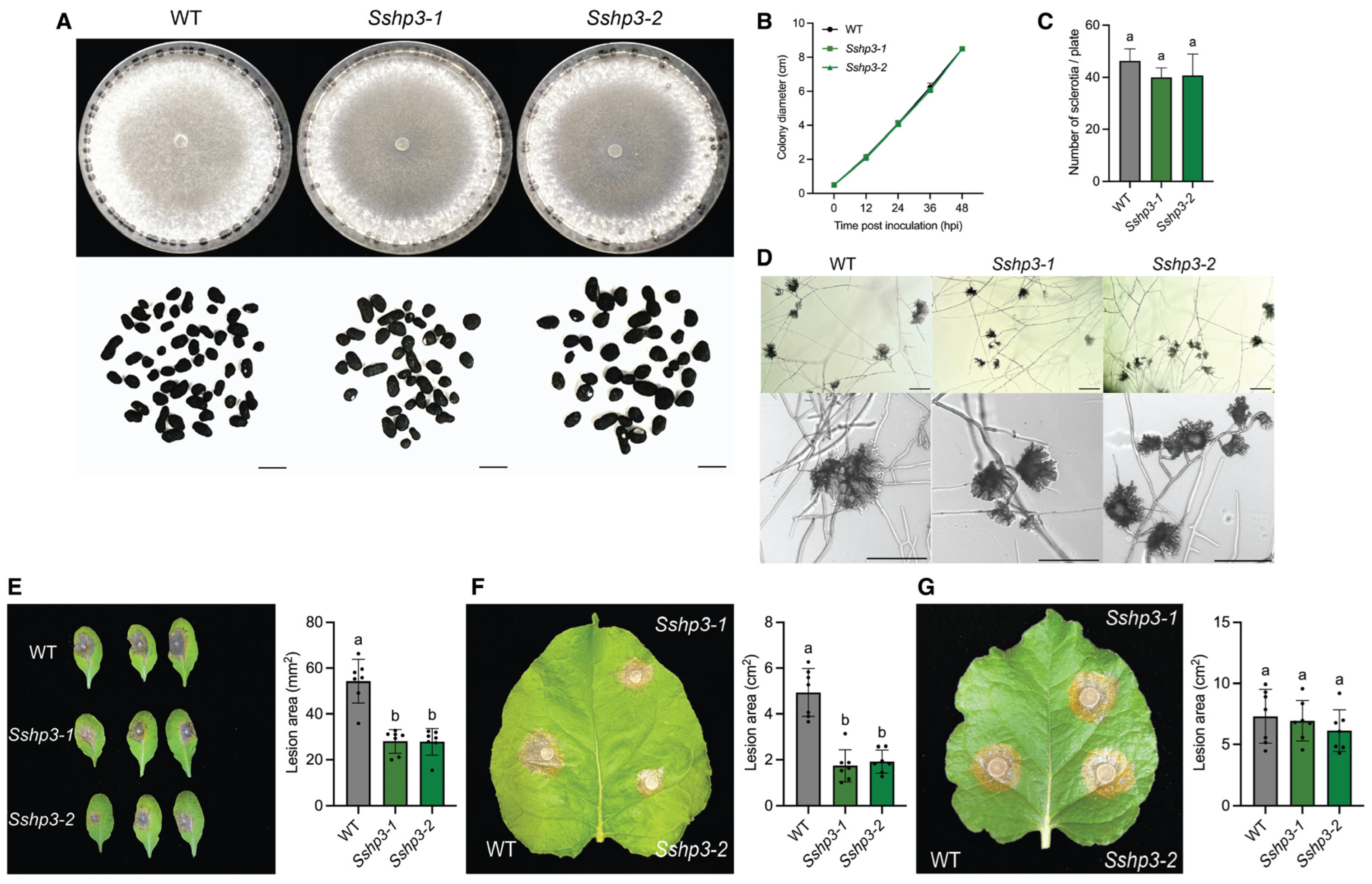
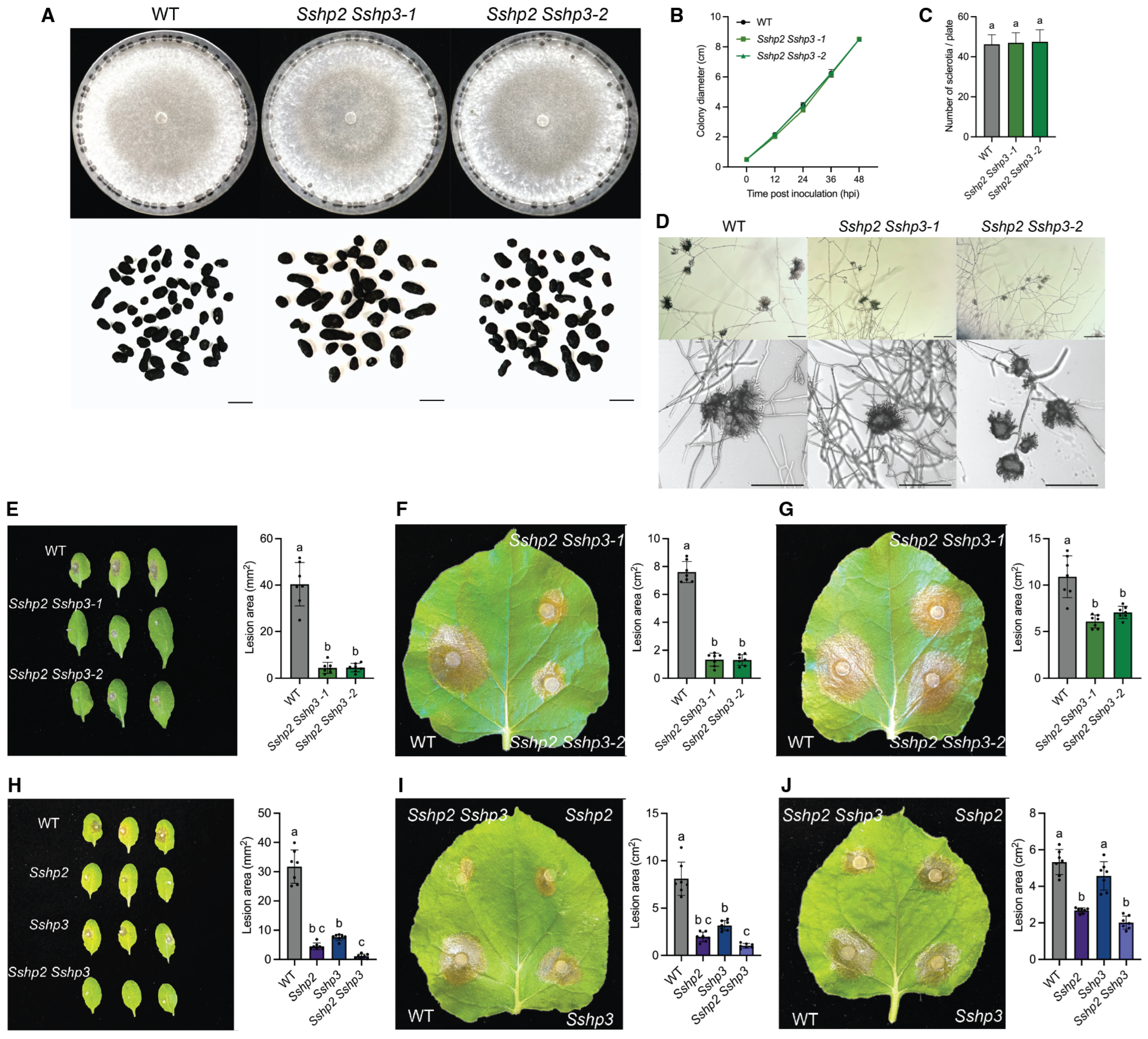
To evaluate growth rate and assess colony morphology, 5 mm mycelial agar disks were excised from the margins of 2-day-old WT and mutant colonies using a sterile pipette tip and placed at the center of fresh 85 mm PDA plates, which were then incubated at room temperature. For each genotype, three biological replicates were performed. Colony diameter was measured every 12 h until the plates were fully colonized. Colony morphology and sclerotia formation were documented by imaging the plates 7 days post-inoculation. The number of sclerotia was evaluated on two-week-old PDA plates, also with three biological replicates, and the sclerotia were subsequently collected for imaging. The same WT strain served as the control as the plates were grown at the same time. For clarity and consistency, identical WT images are presented across relevant figures for different mutants.
2.5. Plant Infection Assay
Fresh mycelial plugs (1 mm or 5 mm in diameter) taken from the colony margins of 2-day-old cultures were inoculated on detached leaves of approximately 4-week-old A. thaliana (Col-0) or N. benthamiana, with or without prior mechanical wounding. The inoculated leaves were placed on moistened paper towels inside trays covered with lids to ensure consistent humidity. The trays were then incubated in a growth chamber at 23 °C under a 16 h light/8 h dark condition. Lesion sizes were quantified using ImageJ software (1.53v).
2.6. Compound Appressoria Observation
Fresh mycelial plugs (5 mm in diameter) taken from the colony margins of 2-day-old cultures were placed onto glass slides and incubated on moistened paper towels in Petri dishes at room temperature for 2 days. Compound appressoria formation was examined using a ZEISS light microscope, and images were captured at 100× or 200× magnification. Scale bars are included in each image and described in the corresponding figure legend. The same WT strain served as the control as the experiments were carried out at the same time. For clarity and consistency, identical WT images are presented across relevant figures for different mutants.
2.7. Surface Hydrophobicity Assay
All S. sclerotiorum WT and HP mutant strains were cultured on PDA media for 10 days before experimentation. 10 μL of sterile distilled water, 0.2%SDS, and 2%SDS were placed on the surface of the plate culture. The results were observed and documented at different time points.
2.8. Oxalic Acid (OA) Accumulation Assay
Agar disks (5 mm in diameter) containing mycelia from WT and different mutant strains were excised from the edges of colonies using a sterile pipette tip and placed at the center of 85 mm Petri dishes containing PDA medium supplemented with bromophenol blue (which appears violet at pH > 4.6 and yellow when pH < 3.0). Plates were incubated at room temperature and color changes were documented. Each assay included three technical replicates.
2.9. Cell Wall Integrity and Stress Treatment Assay
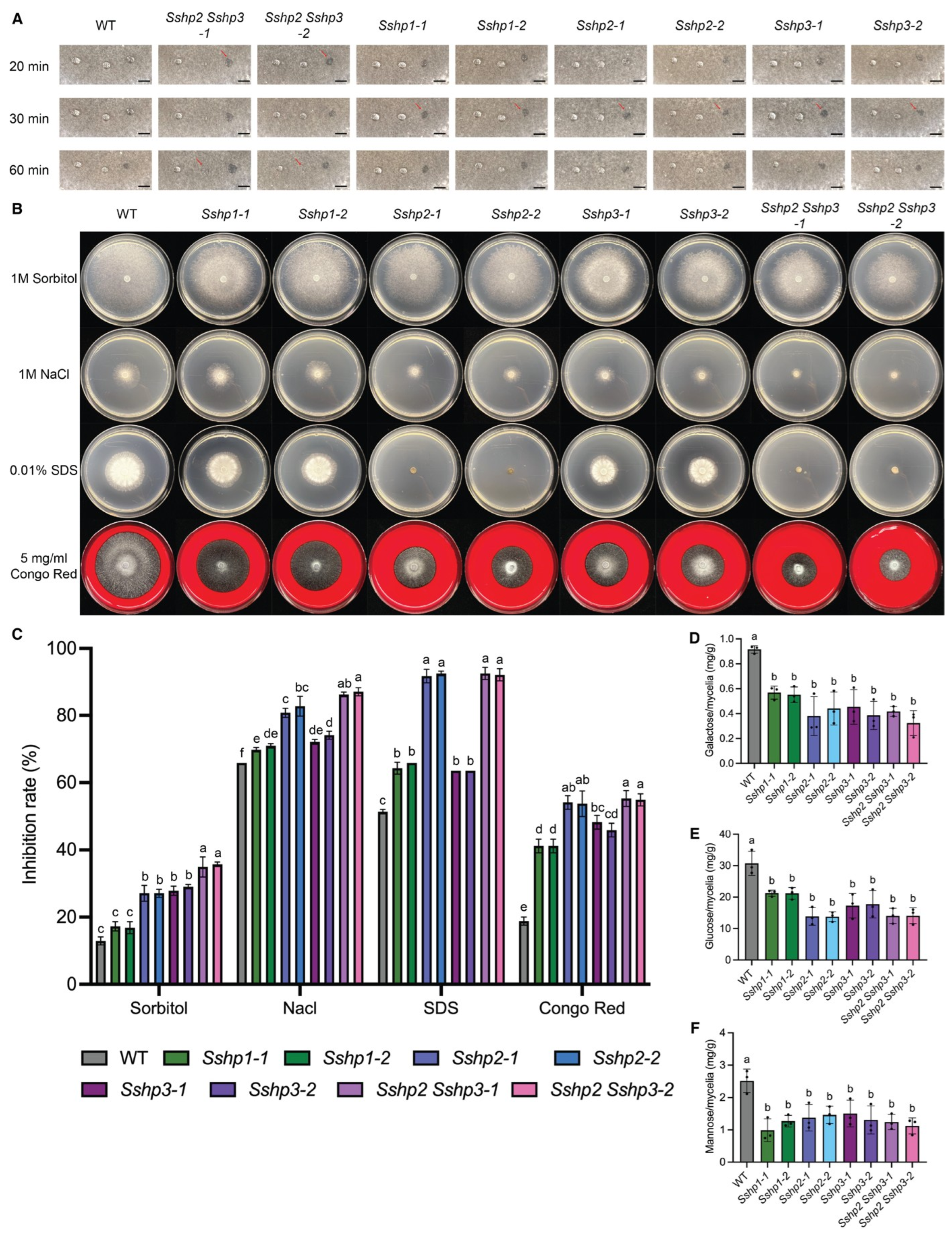
Mycelial agar disks (5 mm in diameter) from the actively growing margins of WT and mutant colonies were obtained using a sterile pipette tip and placed at the center of 85 mm Petri dishes containing PDA medium supplemented with various stress agents: 5 mg/mL Congo Red (CR, Sigma-Aldrich, St. Louis, MO, USA), 0.01% Sodium Dodecyl Sulfate (SDS, BIO-RAD, Hercules, CA, USA), and osmotic stressors 1 M NaCl (BioShop, Burlington, ON, Canada)and 1 M Sorbitol (Thermo Fisher Scientific, Waltham, MA, USA). Plates were incubated at room temperature, and colony diameters were recorded after 48 h of growth under each stress condition. Colony morphology was also documented. Additionally, the inhibition rate (%) was determined using the formula: 100 × [(colony diameter on PDA) − (colony diameter under stress)]/(colony diameter on PDA). Each assay included three technical replicates.
2.10. Cell Wall Carbohydrate Composition Determination
Cell wall extraction was performed following a previously described procedure with some modifications [
33]. Colonies of WT and HP mutant lines were cultured on PDA plates with cellophane for 2 days to obtain fresh mycelia. About 0.02 g mycelial tissue was harvested and disrupted in 500 μL of 10 mM Tris-Cl (pH8) using three glass beads and the TissueLyser III (QIAGEN, Hilden, Germany) for four cycles of 20 s each, with 20 s cooling intervals on ice. The resulting cell suspension was collected, and the glass beads were thoroughly rinsed with cold Tris buffer three times to recover residual material. The combined supernatant and wash fragments were centrifuged at 3800×
g for 5 min. The resulting pellet, representing the cell wall fraction, was subsequently washed three additional times with cold deionized water.
The cell wall pellet was suspended in 200 μL of 2N-trifluoroacetic acid (TFA, Sigma-Aldrich, St. Louis, MO, USA) and hydrolyzed in sealed tubes at 121 °C for 1 h. following hydrolysis, TFA was evaporated under nitrogen gas, and the resulting dry samples were resuspended in 1 mL of MilliQ water. The samples were then centrifuged at the maximum speed for 10 min, and the supernatant was collected for quantification of monomeric sugars.
Monosaccharide concentrations were determined using high-performance anion-exchange liquid chromatography (HPAELC) on a Dionex ICS-5000 system equipped with Dionex CarboPac PA1 analytical column (Thermo Fisher Scientific, Waltham, MA, USA) and corresponding guard column. Detection was performed with a pulsed amperometric detector containing a gold working electrode. The mobile phase was delivered at a flow rate of 0.8 mL/min, using Nanopure water as eluent A (0–35 min), followed by 0.2 M NaOH as eluent B for column washing (35–45 min), and re-equilibration with Nanopure water for 15 min. Individual sugars were identified and quantified by comparison with external calibration standards prepared in serial dilutions.
2.11. Bleach Immersion and Ultraviolet (UV) Irradiation Assays on Sclerotia
Bleach and UV resistance assays were conducted based on a previously described method with slight modifications [
31]. For chemical treatment, sclerotia from two-week-old cultures of the WT and HP mutants were immersed in 15% bleach (Cloromax, The Clorox Company, Okland, CA, USA) for 30, 60, or 90 min. For physical stress, sclerotia were exposed to UV irradiation at an energy dose of 9000 mJ/cm
2 using a TL-2000 Ultraviolet Translinker (Fisherscientific, Hampton, NH, USA) for 10, 20, or 30 min. For each treatment and strain, 45 sclerotia were collected from multiple independent culture plates to avoid biological variation. After treatment, sclerotia were transferred onto three independent PDA plates and incubated at room temperature for 2 days to assess germination. Germination rate was calculated as the proportion of sclerotia that germinated out of the total number of sclerotia treated. Two independent experiments were conducted with similar results.
2.12. Ascospores Quantification and Asci Observation
Each mature apothecium of WT and mutant strains was collected into an individual 1.5 mL Eppendorf tube containing 1 mL of sterile distilled water. Samples were vortexed for 3 min to release ascospores, and the resulting suspensions were used for ascospore quantification. For microscopic observation of asci, small sections of apothecia were sliced and gently squashed on glass slides and examined using a ZEISS AXIO Imager M2 microscope (Jena, Germany) with 200× and 630× magnification. Scale bars are included in each image and described in the corresponding figure legend.
2.13. Statistical Analysis
Statistical analyses were performed using GraphPad Prism 10 software (GraphPad Software Inc., San Diego, CA, USA:
http://www.graphpad.com/, accessed on 11 July 2023). Differences among means were evaluated by one-way ANOVA with Tukey’s post hoc test. Statistically significant differences were indicated by distinct letters.
4. Discussion
HPs are essential in the life cycle of filamentous fungi. They cover the aerial hyphae and spores to enable hydrophobicity, allowing them to grow into air or disperse into new environment during differentiation or dissemination [
34]. HPs also play important roles in host–pathogen interactions. Although HPs have been extensively studied in many fungal species, their roles in
S. sclerotiorum remain poorly understood. In this study, we identified and functionally characterized three
HP genes in
S. sclerotiorum: the sole class I HP,
SsHP1, and two class II HPs,
SsHP2 and
SsHP3. Several features support their classification as members of the HP family: they encode small proteins of approximately 100 amino acids, contain an N-terminal signal peptide indicative of secretion, and display the conserved spacing of eight cysteine residues. In addition to sequence similarity with HPs from other fungi, the
S. sclerotiorum HPs share known functional properties, including conferring surface hydrophobicity and contributing to protection against environmental stress.
Using homologous recombination, we generated single deletion mutants of all HP genes in S. sclerotiorum and analyzed their roles in growth, development, and plant infection. SsHP1 was found to primarily regulate sclerotia development, while being dispensable for vegetative growth and virulence. In contrast, SsHP2 and SsHP3 play broader roles in fungal biology, being essential for proper compound appressoria function and full virulence. In addition, by employing a mycelial fusion strategy, double mutants lacking both class II HP genes were successfully generated, enabling an assessment of functional redundancy that might be masked in single mutants. The Sshp2 Sshp3 double mutants exhibited more severe phenotypes, including defective appressoria formation, markedly decreased virulence, compromised cell wall integrity, and reduced sclerotia stress tolerance. These results indicate the functional redundancy and overlapping contributions of SsHP2 and SsHP3 to host penetration, colonization, and fungal fitness, highlighting their multifaceted roles in S. sclerotiorum biology.
Our study in
S. sclerotiorum revealed that both class I and class II HPs contribute to surface hydrophobicity, while also performing distinct functions: SsHP1 primarily regulates sclerotia development, whereas SsHP2 and SsHP3 are more critical to appressoria formation and virulence. These findings are consistent with previous observations in
M. oryzae but also diverge in specific aspects. In
M. oryzae, both class I and II HPs are required for surface hydrophobicity, appressoria development and virulence; the class I HP contributes to rodlet layer formation and surface sensing, whereas the class II HP plays a more central role in plant colonization [
25,
26]. Taken together, these results point to both conserved and divergent roles of HPs across fungal species.
The orthologs of SsHP1, SsHP2, and SsHP3 have also been characterized in the closely related pathogenic species
B. cinerea. However, our results reveal striking differences in HP function between these two closely related fungi. In
B. cinerea, HPs are largely dispensable for surface hydrophobicity and virulence but play critical roles in sexual development, particularly apothecia formation [
13,
30]. In contrast, all
S. sclerotiorum HP mutants formed normal apothecia and asci, but released significantly fewer ascospores than the WT. This indicates that HPs are not required for the morphogenesis of sexual structures in
S. sclerotiorum but are crucial for the biophysical process of ascospore dispersal. Given their ability to modify surface hydrophobicity and influence biophysical properties, HPs may facilitate ascus dehiscence or reduce adhesion forces that otherwise restrict spore discharge. They may also contribute to the mechanical tension of ascus or spore cell walls, promoting efficient spore dispersal. This reveals a previously unrecognized role for HPs in fungal spore dissemination. The contrasting outcomes in
S. sclerotiorum and
B. cinerea likely reflect differences in both ecological specialization and reproductive strategies.
S. sclerotiorum relies on long-living sclerotia for survival and infect hosts through compound appressoria, while
B. cinerea depends heavily on massive number of conidia for dispersal and reproduction. It is also possible that the
HP expression pattern differ between the two species, with
B. cinerea HPs being predominantly expressed during sexual development, while
S. sclerotiorum HPs might be more tightly associated with infection-related processes [
13]. This divergence highlights the evolutionary flexibility of HP function. Even within closely related fungi, HPs have been co-opted to fulfill distinct roles in development, reproduction, and virulence.
In this study, we also revealed novel roles of
S. sclerotiorum HPs in maintaining cell wall integrity and composition, with the double HP mutants exhibiting the most severe cell wall-related defects. To our knowledge, this represents the first demonstration of HPs influencing cell wall organization in a plant pathogenic fungus. A robust cell wall is essential not only for appressoria-mediated host penetration but also for resisting against host-derived stresses. Comparable observations have been reported in the Basidiomycete
S. commune, where deletion of the major HP
SC3 led to pronounced alterations in cell wall composition, including increased secretion of soluble β-glucan mucilage and a reduction in chitin-linked glucans [
21]. Moreover, in the insect pathogenic fungus
Beauveria bassiana, loss of
HP genes down-regulated cell wall integrity pathway genes and impaired plant roots colonization by the insect [
35]. In
S. sclerotiorum, SsHP1 appears to function as a “surface organizer”, primarily affecting mannose and consequently influencing the assembly of mannoproteins in the outer cell wall layer. In contrast, Class II HPs act as “global cell wall stabilizer”, as class II HP mutants exhibited marked reductions in all major sugars, including glucose, galactose and mannose. These alterations in cell wall composition align with observed phenotypes: class I HP mainly support survival-related traits with limited role in infection, whereas class II HPs serve major roles in maintaining cell wall integrity and regulating infection-related development. The mechanisms by which HPs affect cell wall integrity remain to be elucidated. One possibility is direct structural interaction with cell wall components. Given their amphipathic properties, HPs may cross-link with glucans or mannans, influencing cell wall stability and organization. Alternatively, HPs may act indirectly through signaling pathways, potentially interacting with the cell wall integrity (CWI) MAPK cascade, which monitors and responses to cell wall stress.
In summary, our findings broaden the understanding of HPs by linking them to sclerotia development, cell wall integrity, stress tolerance and virulence in S. sclerotiorum. The discovery that HPs contribute to cell wall integrity, virulence and ascospore dispersal in S. sclerotiorum has important implications for understand host–pathogen interactions. HPs may act directly by interacting with cell wall polymers or indirectly by modulating cell wall integrity signaling pathways. Moreover, their unique biophysical properties that alter surface hydrophobicity likely facilitate ascospore dispersal. However, the precise mechanisms underlying these effects remain unclear and require further investigation. In addition, comparative analyses of HP gene expression patterns in S. sclerotiorum and other species could help elucidate potential functional diversification among HPs across species. Given their multifunctional roles in both development and virulence, HPs could be promising targets for novel disease control strategies. Approaches such as host-induced gene silencing could provide innovative avenues for sustainable S. sclerotiorum disease management. Nevertheless, several practical limitations should be considered. For example, functional redundancy among HP members may reduce the effectiveness of single-gene targeting, and the intrinsic stability of HPs allows them to form persistent protein layers that are difficult to disrupt. Therefore, effective control may require multiple targeting or integrated management approaches to overcome these challenges and achieve durable disease suppression.