Whole Genome Sequencing and Comparative Genomics of the Emerging Pathogen Burkholderia pseudomallei Isolated from Two Travel-Related Infections in Hungary
Abstract
1. Introduction
2. Materials and Methods
2.1. B. pseudomallei Isolates
2.2. Molecular Methods
2.3. Update on European Melioidosis Cases
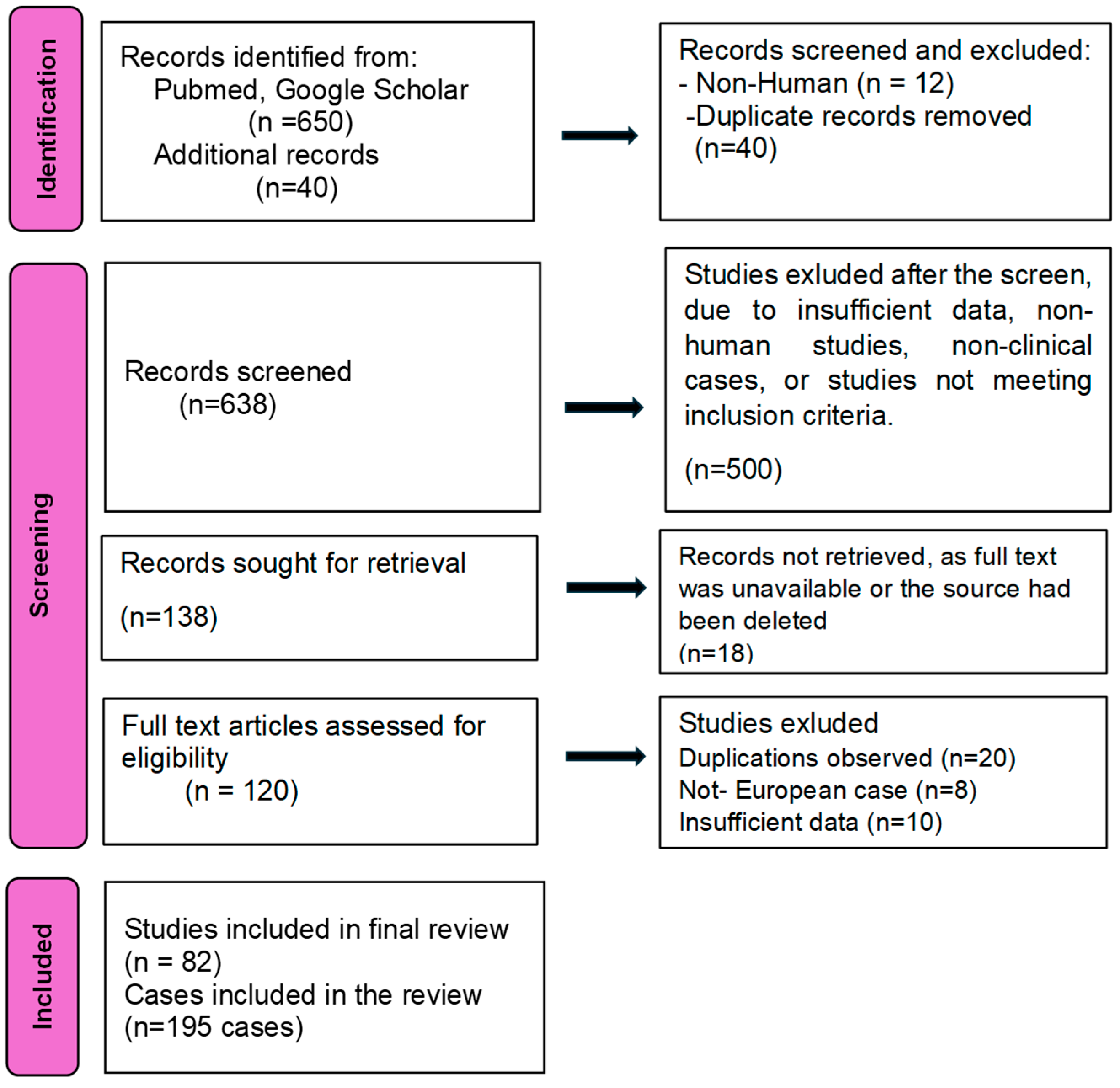
2.3.1. Search Strategy
2.3.2. Eligibility Criteria
2.3.3. Data Extraction and Analysis
Nucleotide Sequence Accession Numbers
3. Results
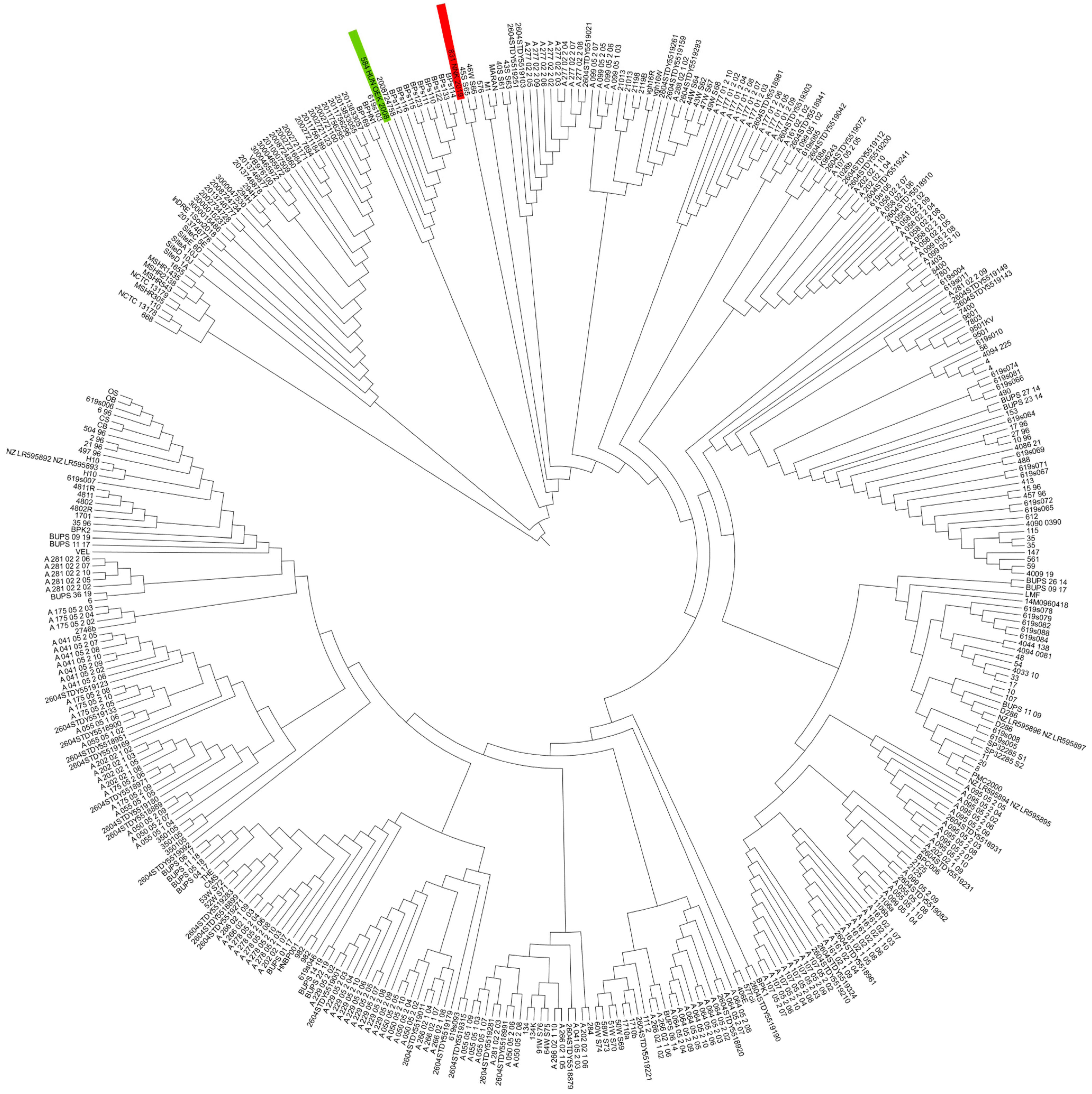
3.1. Whole-Genomic Sequencing and Comparative Genomics Results
3.2. In Silico Multilocus Sequence Typing
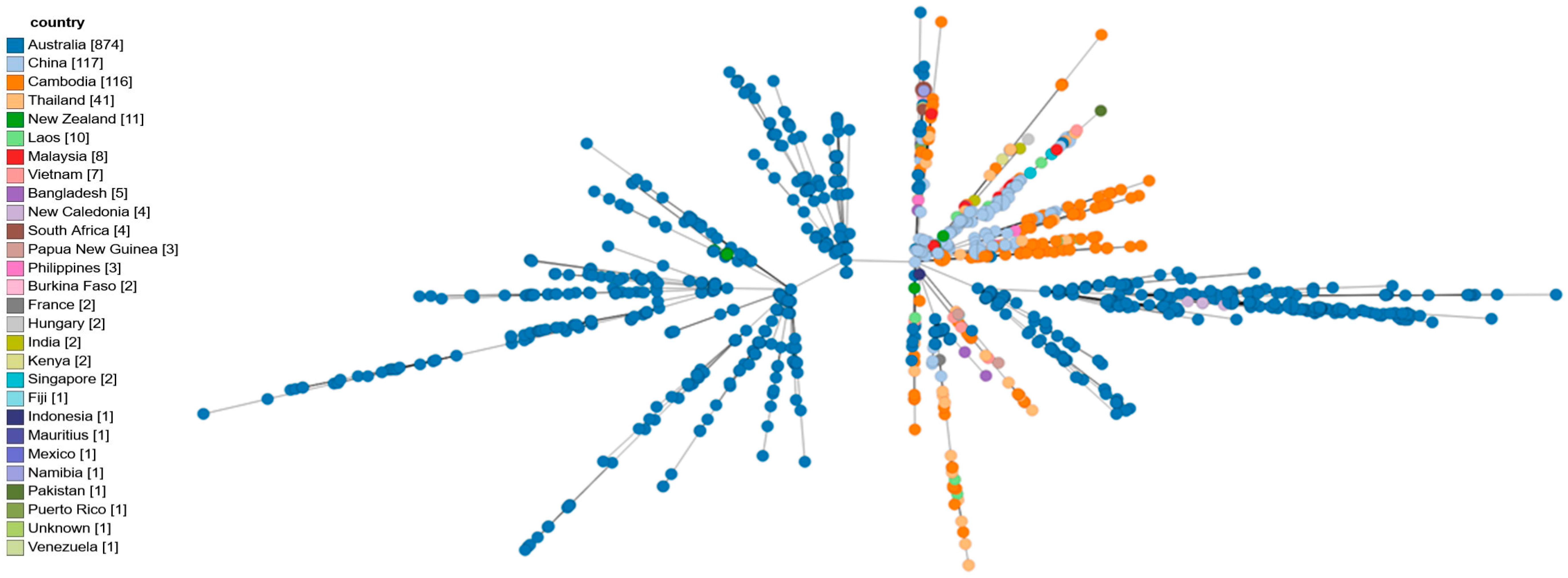
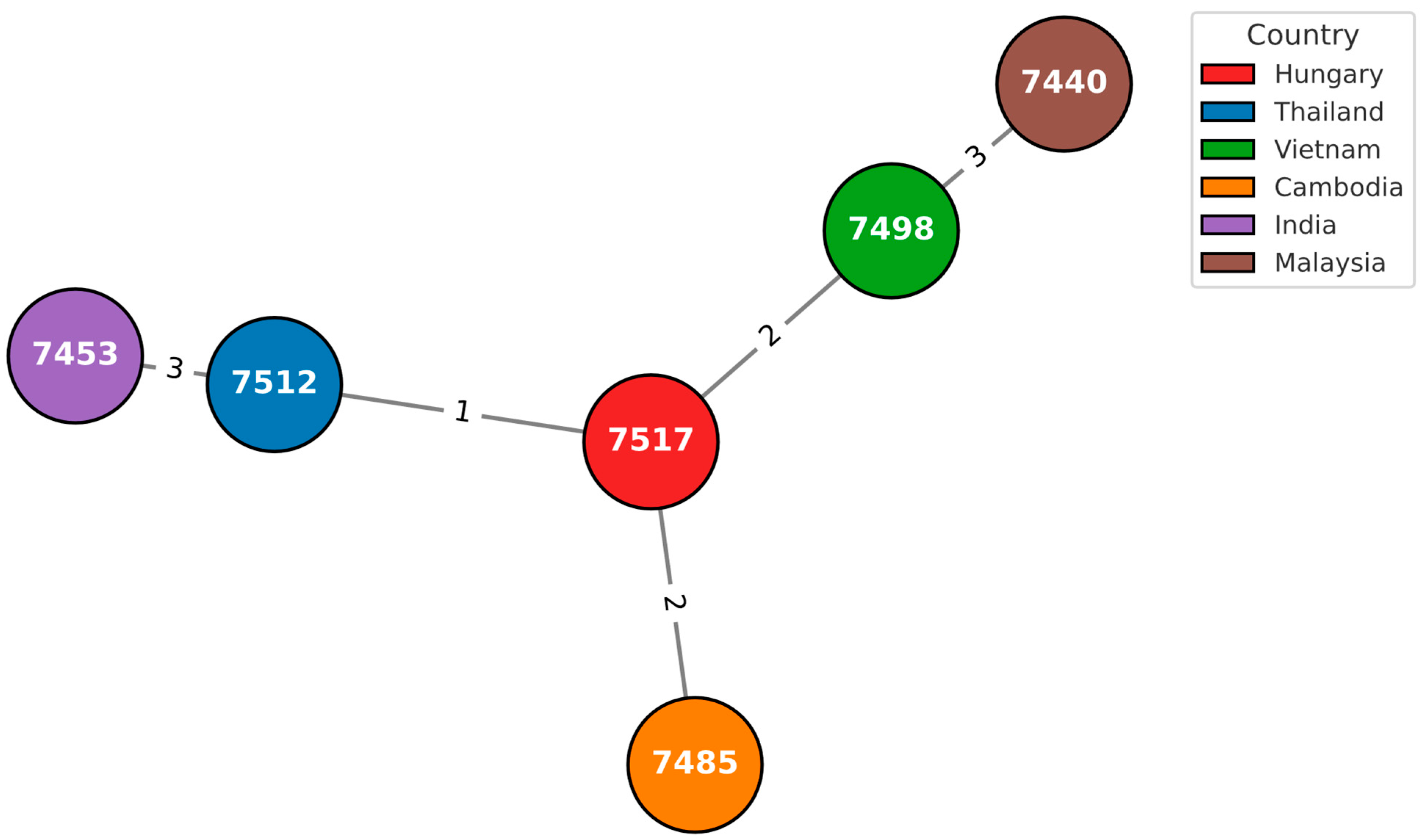
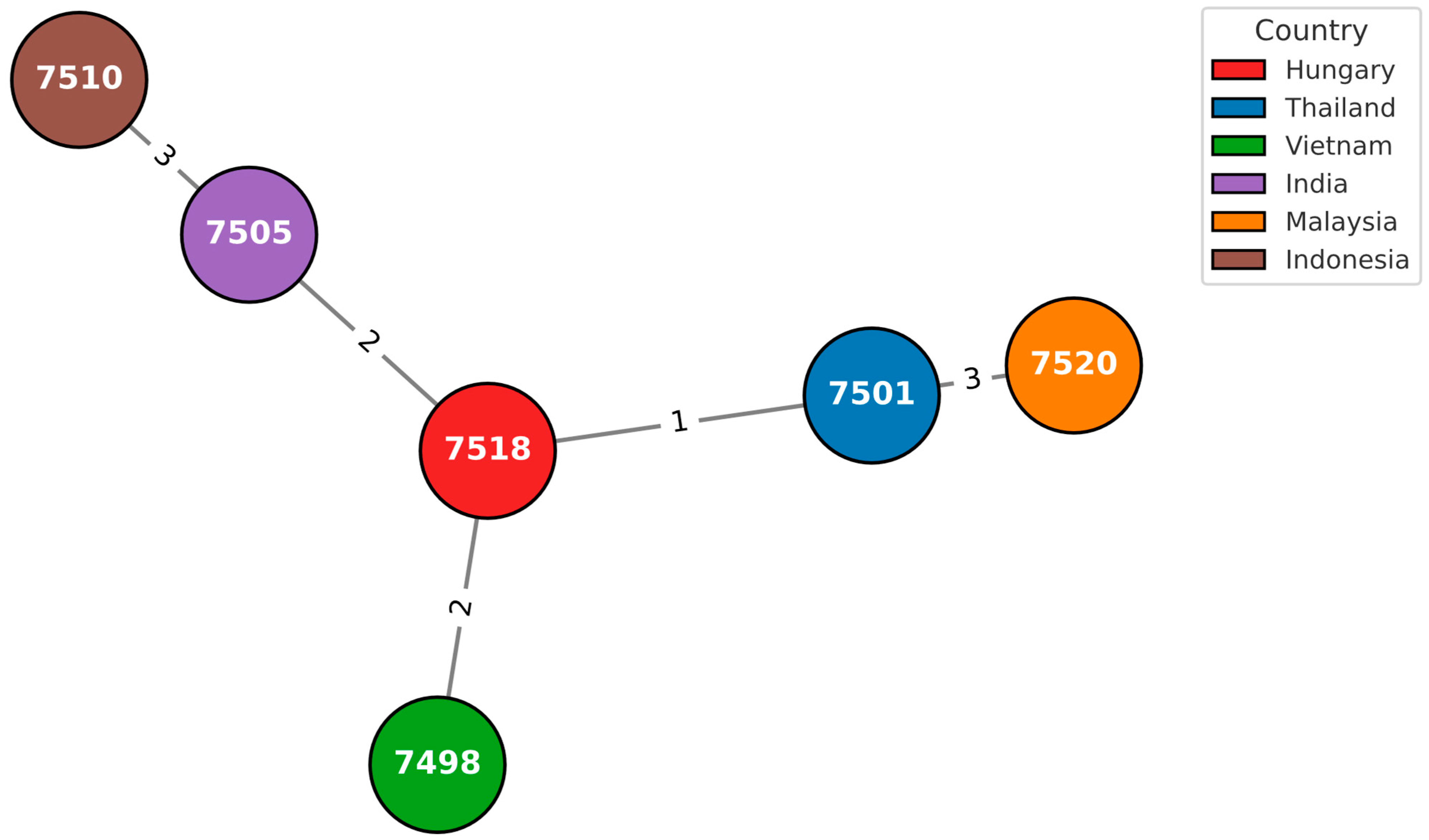
3.3. cgMLST Analysis
3.4. Antimicrobial Resistance Genes
3.5. Virulence-Associated Genes
3.6. Literature Review on Neuromelioidosis Cases in Europe
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | ATP-binding cassette transporters |
| AraC | Transcriptional regulator protein |
| ATP | Adenosine triphosphate |
| BapA | Bacterial adhesion protein |
| BCC | Burkholderia cepacia complex |
| BPC | Burkholderia pseudomallei complex |
| BimA | Bacterial invasion protein |
| BipB, BipC, BipD | Bip proteins are part of the type III secretion system (T3SS) |
| BopA | Bacterial outer membrane protein involved in adhesion or invasion mechanisms |
| cgMLST | Core genome multilocus sequence typing |
| CRISPRs | Clustered regularly interspaced short palindromic repeats |
| CheA, CheD, CheW, CheY | Proteins involved in chemotaxis |
| DNA | Deoxyribonucleic acid |
| ITS | Internal transcribed spacer |
| LPS | Lipopolysaccharide |
| MALDI-TOF | Matrix-assisted laser desorption/ionization time of flight |
| MLST | Multilocus sequence typing |
| NADH | Nicotinamide adenine dinucleotide (NAD) |
| OMP | Outer membrane proteins |
| PCR | Polymerase chain reaction |
| qPCR | Quantitative PCR |
| RNA | Ribonucleic acid |
| SNP | Single nucleotide polymorphism |
| T3SS | Type III secretion system |
| T3SS1 | Type 3 secretion system- cluster 1 |
| T6SS1/5 | Type 6 secretion system- cluster 1/cluster 5 |
| VF | Virulence factors |
| VFDB | Virulence factor database |
| WGS | Whole genome sequencing |
| YadA | Bacterial adhesion protein |
References
- White, N. Melioidosis. Lancet 2003, 361, 1715–1722. [Google Scholar] [CrossRef]
- Meumann, E.M.; Limmathurotsakul, D.; Dunachie, S.J.; Wiersinga, W.J.; Currie, B.J. Burkholderia pseudomallei and melioidosis. Nat. Rev. Microbiol. 2024, 22, 155–169. [Google Scholar] [CrossRef]
- Gassiep, I.; Armstrong, M.; Norton, R. Human Melioidosis. Clin. Microbiol. Rev. 2020, 33, e00006-19. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (U.S.); National Institutes of Health (U.S.). Biosafety in Microbiological and Biomedical Laboratories, 6th ed.; U.S. Department of Health and Human Services: Washington, DC, USA, 2020.
- Phillips, E.D.; Garcia, E.C. Garcia Burkholderia pseudomallei. Trends Microbiol. 2023, 32, 105–106. [Google Scholar] [CrossRef] [PubMed]
- Benoit, T.J.; Blaney, D.D.; Doker, T.J.; Gee, J.E.; Elrod, M.G.; Rolim, D.B.; Inglis, T.J.J.; Hoffmaster, A.R.; Bower, W.A.; Walke, H.T. A Review of Melioidosis Cases in the Americas. Am. J. Trop. Med. Hyg. 2015, 93, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Birnie, E.; James, A.; Peters, F.; Olajumoke, M.; Traore, T.; Bertherat, E.; Trinh, T.T.; Naidoo, D.; Steinmetz, I.; Wiersinga, W.J.; et al. Melioidosis in Africa: Time to Raise Awareness and Build Capacity for Its Detection, Diagnosis, and Treatment. Am. J. Trop. Med. Hyg. 2022, 106, 394–397. [Google Scholar] [CrossRef]
- Norman, F.F.; Blair, B.M.; Chamorro-Tojeiro, S.; González-Sanz, M.; Chen, L.H. The Evolving Global Epidemiology of Human Melioidosis: A Narrative Review. Pathogens 2024, 13, 926. [Google Scholar] [CrossRef]
- Limmathurotsakul, D.; Peacock, S.J. Melioidosis: A clinical overview. Br. Med. Bull. 2011, 99, 125–139. [Google Scholar] [CrossRef]
- Gee, J.E.; Bower, W.A.; Kunkel, A.; Petras, J.; Gettings, J.; Bye, M.; Firestone, M.; Elrod, M.G.; Liu, L.; Blaney, D.D.; et al. Multistate Outbreak of Melioidosis Associated with Imported Aromatherapy Spray. N. Engl. J. Med. 2022, 386, 861–868. [Google Scholar] [CrossRef]
- Pumpuang, A.; Chantratita, N.; Wikraiphat, C.; Saiprom, N.; Day, N.P.; Peacock, S.J.; Wuthiekanun, V. Survival of Burkholderia pseudomallei in distilled water for 16 years. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 598–600. [Google Scholar] [CrossRef]
- Wuthiekanun, V.; Amornchai, P.; Langla, S.; White, N.J.; Day, N.P.J.; Limmathurotsakul, D. Survival of Burkholderia pseudomallei and Pathogenic Leptospira in Cola, Beer, Energy Drinks, and Sports Drinks. Am. J. Trop. Med. Hyg. 2020, 103, 249–252. [Google Scholar] [CrossRef]
- Shams, A.M.; Rose, L.J.; Hodges, L.; Arduino, M.J. Survival of Burkholderia pseudomallei on Environmental Surfaces. Appl. Environ. Microbiol. 2007, 73, 8001–8004. [Google Scholar] [CrossRef]
- O’Rourke, A.; Lee, M.D.; Nierman, W.C.; Everroad, R.C.; Dupont, C.L. Genomic and phenotypic characterization of Burkholderia isolates from the potable water system of the International Space Station. PLoS ONE 2020, 15, e0227152. [Google Scholar] [CrossRef] [PubMed]
- Sarli, D.A.; Sánchez, L.A.; Delgado, O.D. Burkholderia gladioli MB39 an Antarctic Strain as a Biocontrol Agent. Curr. Microbiol. 2021, 78, 2332–2344. [Google Scholar] [CrossRef] [PubMed]
- Savelkoel, J.; Dance, D.A.B.; Currie, B.J.; Limmathurotsakul, D.; Wiersinga, W.J. A call to action: Time to recognise melioidosis as a neglected tropical disease. Lancet Infect. Dis. 2022, 22, e176–e182. [Google Scholar] [CrossRef]
- Larsen, J.C.; Johnson, N.H. Pathogenesis of Burkholderia pseudomallei and Burkholderia mallei. Mil. Med. 2009, 174, 647–651. [Google Scholar] [CrossRef]
- Birnie, E.; Biemond, J.J.; Wiersinga, W.J. Drivers of melioidosis endemicity: Epidemiological transition, zoonosis, and climate change. Curr. Opin. Infect. Dis. 2022, 35, 196–204. [Google Scholar] [CrossRef]
- Abbink, F.C.; Orendi, J.M.; de Beaufort, A.J. Mother-to-Child Transmission of Burkholderia pseudomallei. N. Engl. J. Med. 2001, 344, 1171–1172. [Google Scholar] [CrossRef]
- Chang, C.Y.; Lau, N.L.J.; Currie, B.J.; Podin, Y. Disseminated melioidosis in early pregnancy—An unproven cause of foetal loss. BMC Infect. Dis. 2020, 20, 201. [Google Scholar] [CrossRef]
- Currie, B.J.; Ward, L.; Cheng, A.C. The Epidemiology and Clinical Spectrum of Melioidosis: 540 Cases from the 20 Year Darwin Prospective Study. PLOS Neglected Trop. Dis. 2010, 4, e900. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, O.; Seng, S.; Fitzgerald, D.A. Paediatric melioidosis. Paediatr. Respir. Rev. 2023, 50, 31–37. [Google Scholar] [CrossRef]
- Nemhauser, J.; LaRocque, R.; Alvarado-Ramy, F.; Angelo, K.; Ericsson, C.; Gertz, A.; Kozarsky, P.; Ostroff, S.; Ryan, E.; Shlim, D.; et al. Copyright Page. In CDC Yellow Book; Oxford University Press: New York, NY, USA, 2024. [Google Scholar]
- Cheng, A.C.; Currie, B.J. Melioidosis: Epidemiology, Pathophysiology, and Management. Clin. Microbiol. Rev. 2005, 18, 383–416. [Google Scholar] [CrossRef]
- Ngauy, V.; Lemeshev, Y.; Sadkowski, L.; Crawford, G. Cutaneous Melioidosis in a Man Who Was Taken as a Prisoner of War by the Japanese during World War II. J. Clin. Microbiol. 2005, 43, 970–972. [Google Scholar] [CrossRef] [PubMed]
- Zueter, A.; Yean, C.Y.; Abumarzouq, M.; Rahman, Z.A.; Deris, Z.Z.; Harun, A. The epidemiology and clinical spectrum of me-lioidosis in a teaching hospital in a North-Eastern state of Malaysia: A fifteen-year review. BMC Infect. Dis. 2016, 16, 333. [Google Scholar] [CrossRef]
- Virk, H.S.; Mukhopadhyay, C.; Wiersinga, W.J. Melioidosis: A Neglected Cause of Community-Acquired Pneumonia. Semin. Respir. Crit. Care Med. 2020, 41, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Deuble, M.; Aquilina, C.; Norton, R. Neurologic Melioidosis. Am. J. Trop. Med. Hyg. 2013, 89, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, N.G.; Flüh, G.; Zange, S.; Aytulun, A.; Turowski, B.; Hartung, H.-P.; Meuth, S.G.; Gliem, M. Case report: First case of neuromelioidosis in Europe: CNS infection caused by Burkholderia pseudomallei. Front. Neurol. 2022, 13, 899396. [Google Scholar] [CrossRef]
- Andersen, E.W.; Mackay, M.T.; Ryan, M.M. Ryan Neurologic Melioidosis: Case Report of a Rare Cause of Acute Flaccid Paralysis. J. Pediatr. 2016, 170, 319–321. [Google Scholar] [CrossRef]
- Saravu, K.; Kadavigere, R.; Shastry, A.B.; Pai, R.; Mukhopadhyay, C. Neurologic melioidosis presented as encephalomyelitis and subdural collection in two male labourers in India. J. Infect. Dev. Ctries. 2015, 9, 1289–1293. [Google Scholar] [CrossRef]
- Karunanayake, P. Melioidosis: Clinical aspects. Clin. Med. 2022, 22, 6–8. [Google Scholar] [CrossRef]
- Guo, R.F.; Wong, F.L.; Perez, M.L. Splenic abscesses in a returning traveler. Infect. Dis. Rep. 2015, 7, 10–12. [Google Scholar] [CrossRef]
- Raj, S.; Sistla, S.; Sadanandan, D.M.; Kadhiravan, T.; Rameesh, B.M.S.; Amalnath, D. Clinical Profile and Predictors of Mortality among Patients with Melioidosis. J. Glob. Infect. Dis. 2023, 15, 72–78. [Google Scholar] [CrossRef]
- Le Tohic, S.; Montana, M.; Koch, L.; Curti, C.; Vanelle, P. A review of melioidosis cases imported into Europe. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1395–1408. [Google Scholar] [CrossRef]
- Limmathurotsakul, D.; Wongratanacheewin, S.; Teerawattanasook, N.; Wongsuvan, G.; Chaisuksant, S.; Chetchotisakd, P.; Chaowagul, W.; Day, N.P.; Peacock, S.J. Increasing Incidence of Human Melioidosis in Northeast Thailand. Am. J. Trop. Med. Hyg. 2010, 82, 1113–1117. [Google Scholar] [CrossRef]
- Schweizer, H.P. Mechanisms of Antibiotic Resistance in Burkholderia pseudomallei: Implications for Treatment of Melioidosis. Futur. Microbiol. 2012, 7, 1389–1399. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Virk, H.S.; Torres, A.G.; Currie, B.J.; Peacock, S.J.; Dance, D.A.B.; Limmathurotsakul, D. Melioidosis. Nat. Rev. Dis. Primers 2018, 4, 17107. [Google Scholar] [CrossRef] [PubMed]
- Lipsitz, R.; Garges, S.; Aurigemma, R.; Baccam, P.; Blaney, D.D.; Cheng, A.C.; Currie, B.J.; Dance, D.; Gee, J.E.; Larsen, J.; et al. Workshop on Treatment of and Postexposure Prophylaxis for Burkholderia pseudomallei and B. mallei Infection. Emerg. Infect. Dis. 2010, 18, e2. [Google Scholar] [CrossRef] [PubMed]
- Galeas-Pena, M.; Morici, L.A. Vaccine Development Against Melioidosis. In Vaccines for Neglected Pathogens: Strategies, Achievements and Challenges; Springer International Publishing: Cham, Switzerland, 2023; pp. 329–344. [Google Scholar]
- Lichtenegger, S.; Trinh, T.T.; Assig, K.; Prior, K.; Harmsen, D.; Pesl, J.; Zauner, A.; Lipp, M.; Que, T.A.; Mutsam, B.; et al. Development and Validation of a Burkholderia pseudomallei Core Genome Multilocus Sequence Typing Scheme to Facilitate Molecular Surveillance. J. Clin. Microbiol. 2021, 59, e0009321. [Google Scholar] [CrossRef]
- Pakdeerat, S.; Boonklang, P.; Angchagun, K.; Chomkatekaew, C.; Apichaidejudom, N.; Dokket, Y.; Faosap, A.; Wongsuwan, G.; Wuthiekanun, V.; Aramrueung, P.; et al. Benchmarking CRISPR-BP34 for point-of-care melioidosis detection in low-income and middle-income countries: A molecular diagnostics study. Lancet Microbe 2024, 5, e379–e389. [Google Scholar] [CrossRef] [PubMed]
- Holden, M.T.G.; Titball, R.W.; Peacock, S.J.; Cerdeño-Tárraga, A.M.; Atkins, T.; Crossman, L.C.; Pitt, T.; Churcher, C.; Mungall, K.; Bentley, S.D.; et al. Genomic plasticity of the causative agent of melioidosis, Burkholderia pseudomallei. Proc. Natl. Acad. Sci. USA 2004, 101, 14240–14245. [Google Scholar] [CrossRef]
- Bzdyl, N.M.; Moran, C.L.; Bendo, J.; Sarkar-Tyson, M. Pathogenicity and virulence of Burkholderia pseudomallei. Virulence 2022, 13, 1945–1965. [Google Scholar] [CrossRef]
- Sarovich, D.S.; Price, E.P.; Webb, J.R.; Ward, L.M.; Voutsinos, M.Y.; Tuanyok, A.; Mayo, M.; Kaestli, M.; Currie, B.J. Variable virulence factors in Burkholderia pseudomallei (Melioidosis) associated with human disease. PLoS ONE 2014, 9, e91682. [Google Scholar] [CrossRef]
- Stevens, M.P.; Stevens, J.M.; Jeng, R.L.; Taylor, L.A.; Wood, M.W.; Hawes, P.; Monaghan, P.; Welch, M.D.; Galyov, E.E. Identification of a bacterial factor required for actin-based motility of Burkholderia pseudomallei. Mol. Microbiol. 2005, 56, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Tuanyok, A.; Stone, J.K.; Mayo, M.; Kaestli, M.; Gruendike, J.; Georgia, S.; Warrington, S.; Mullins, T.; Allender, C.J.; Wagner, D.M.; et al. The Genetic and Molecular Basis of O-Antigenic Diversity in Burkholderia pseudomallei Lipopolysaccharide. PLOS Neglected Trop. Dis. 2012, 6, e1453. [Google Scholar] [CrossRef] [PubMed]
- Sarkar-Tyson, M.; Thwaite, J.E.; Harding, S.V.; Smither, S.J.; Oyston, P.C.F.; Atkins, T.P.; Titball, R.W. Polysaccharides and virulence of Burkholderia pseudomallei. J. Med. Microbiol. 2007, 56, 1005–1010. [Google Scholar] [CrossRef]
- Dance, D.A. Melioidosis: The tip of the iceberg? Clin. Microbiol. Rev. 1991, 4, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Siddique, M.H.; Samad, M.A.; Memoon, A.; Naqvi, S.Z.H.; Rehman, F.U.; Kalim, F.; Ali, A.; Qureshi, M.A.; Khawar, W. Burkholderia (Mallei and Pseudomallei) Related Zoonosis Drastic Zoonotic and Biological Warfare Potential. Int. J. Agric. Biosci. 2023, 4, 82–99. [Google Scholar] [CrossRef]
- Chewapreecha, C.; Holden, M.T.G.; Vehkala, M.; Välimäki, N.; Yang, Z.; Harris, S.R.; Mather, A.E.; Tuanyok, A.; De Smet, B.; Le Hello, S.; et al. Global and regional dissemination and evolution of Burkholderia pseudomallei. Nat. Microbiol. 2017, 2, 16263. [Google Scholar] [CrossRef]
- Li, S.; Xu, L.; Jiao, Y.; Li, S.; Yang, Y.; Lan, F.; Chen, S.; Man, C.; Du, L.; Chen, Q.; et al. Risk Assessment of Global Animal Melioidosis Under Current and Future Climate Scenarios. Animals 2025, 15, 455. [Google Scholar] [CrossRef]
- Lasch, P.; Nattermann, H.; Erhard, M.; Stämmler, M.; Grunow, R.; Bannert, N.; Appel, B.; Naumann, D. MALDI-TOF Mass Spectrometry Compatible Inactivation Method for Highly Pathogenic Microbial Cells and Spores. Anal. Chem. 2008, 80, 2026–2034. [Google Scholar] [CrossRef]
- Novak, R.T.; Glass, M.B.; Gee, J.E.; Gal, D.; Mayo, M.J.; Currie, B.J.; Wilkins, P.P. Development and Evaluation of a Real-Time PCR Assay Targeting the Type III Secretion System of Burkholderia pseudomallei. J. Clin. Microbiol. 2006, 44, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Leggett, R.M.; Ramirez-Gonzalez, R.H.; Clavijo, B.J.; Waite, D.; Davey, R.P. Sequencing quality assessment tools to enable da-ta-driven informatics for high throughput genomics. Front. Genet. 2013, 4, 288. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Schwengers, O.; Jelonek, L.; Dieckmann, M.A.; Beyvers, S.; Blom, J.; Goesmann, A. Bakta: Rapid and standardized annotation of bacterial genomes via alignment-free sequence identification. Microb. Genom. 2021, 7, 000685. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef]
- Couvin, D.; Bernheim, A.; Toffano-Nioche, C.; Touchon, M.; Michalik, J.; Néron, B.; Rocha, E.P.C.; Vergnaud, G.; Gautheret, D.; Pourcel, C. CRISPRCasFinder, an update of CRISRFinder, includes a portable version, enhanced performance and inte-grates search for Cas proteins. Nucleic Acids Res. 2018, 46, W246–W251. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zheng, D.; Liu, B.; Yang, J.; Jin, Q. VFDB 2016: Hierarchical and refined dataset for big data analysis—10 years on. Nucleic Acids Res. 2016, 44, D694–D697. [Google Scholar] [CrossRef]
- Florensa, A.F.; Kaas, R.S.; Clausen, P.T.L.C.; Aytan-Aktug, D.; Aarestrup, F.M. ResFinder—An open online resource for identification of antimicrobial resistance genes in next-generation sequencing data and prediction of phenotypes from geno-types. Microb. Genom. 2022, 8, 000748. [Google Scholar] [CrossRef]
- Jünemann, S.; Sedlazeck, F.J.; Prior, K.; Albersmeier, A.; John, U.; Kalinowski, J.; Mellmann, A.; Goesmann, A.; von Haeseler, A.; Stoye, J.; et al. Updating benchtop sequencing performance comparison. Nat. Biotechnol. 2013, 31, 294–296. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL): An online tool for phylogenetic tree display and annotation. Bioinformatics 2007, 23, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Alikhan, N.-F.; Sergeant, M.J.; Luhmann, N.; Vaz, C.; Francisco, A.P.; Carriço, J.A.; Achtman, M. GrapeTree: Visualization of core genomic relationships among 100,000 bacterial pathogens. Genome Res. 2018, 28, 1395–1404. [Google Scholar] [CrossRef]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef]
- Birnie, E.; Savelkoel, J.; Reubsaet, F.; Roelofs, J.J.; Soetekouw, R.; Kolkman, S.; Cremers, A.L.; Grobusch, M.P.; Notermans, D.W.; Wiersinga, W.J.; et al. Melioidosis in travelers: An analysis of Dutch melioidosis registry data 1985–2018. Travel Med. Infect. Dis. 2019, 32, 101461. [Google Scholar] [CrossRef] [PubMed]
- Saïdani, N.; Griffiths, K.; Million, M.; Gautret, P.; Dubourg, G.; Parola, P.; Brouqui, P.; Lagier, J.-C. Melioidosis as a travel-associated infection: Case report and review of the literature. Travel Med. Infect. Dis. 2015, 13, 367–381. [Google Scholar] [CrossRef]
- Hinchcliffe, A.; Hylton, C.; Brown, K.; Mortimer, K.; Gurusinghe, D. An unusually sore head: Melioidosis case report. Clin. Microbiol. Infect. 2025, 31, 1400–1401. [Google Scholar] [CrossRef]
- O’COnnor, C.; Kenna, D.; Walsh, A.; Zamarreño, D.V.; Dance, D. Imported melioidosis in the United Kingdom: Increasing in-cidence but continued under-reporting. Clin. Infect. Pract. 2020, 7–8, 100051. [Google Scholar] [CrossRef]
- Hesstvedt, L.; Reikvam, D.H.; Dunlop, O. Neurological melioidosis in Norway presenting with a cerebral abscess. IDCases 2015, 2, 16–18. [Google Scholar] [CrossRef]
- Lübbert, C.; Wendt, S.; Sander, C.; Beeskow, A.; Becker-Rux, D. Intracranial hypertension and vasculitic infarction in a patient with severe cerebral melioidosis. Lancet Infect. Dis. 2018, 18, 1160. [Google Scholar] [CrossRef]
- Corell, A.; Yilmaz, A.; Almotairi, F.S.; Farahmand, D. Intracranial Manifestation of Melioidosis: A Case Report and Long-Term Follow-Up. Cureus 2020, 12, e12367. [Google Scholar] [CrossRef] [PubMed]
- Demas, A.; Labbé, F.; Vandendriessche, A.; Langlois, V. Focal pachymeningitis in a returning traveler: Don’t forget melioidosis. IDCases 2023, 33, e01834. [Google Scholar] [CrossRef] [PubMed]
- Limmathurotsakul, D.; Golding, N.; Dance, D.A.B.; Messina, J.P.; Pigott, D.M.; Moyes, C.L.; Rolim, D.B.; Bertherat, E.; Day, N.P.J.; Peacock, S.J.; et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat. Microbiol. 2016, 1, 15008. [Google Scholar] [CrossRef]
- Mahidol Oxford Tropical Medicine Research Unit Raising Awareness of Melioidosis. Available online: https://www.melioidosis.info/infobox.aspx?pageID=101 (accessed on 9 September 2025).
- DiEuliis, D.; Imperiale, M.J.; Berger, K.M. Biosecurity Assessments for Emerging Transdisciplinary Biotechnologies: Revisiting Biodefense in an Age of Synthetic Biology. Appl. Biosaf. 2024, 29, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Singh, S.; Kumar, T.P.; Malla, S.; Sethi, A.; Boodman, C.; Broucke, S.V.D.; Vlieghe, E.; Bottieau, E.; Grobusch, M.P.; et al. Tunnel sign on magnetic resonance imaging in neuromelioidosis: A systematic literature review. New Microbes New Infect. 2025, 68, 101639. [Google Scholar] [CrossRef]
| Sample ID | Travel History | Incubation Period (Calculated from Arrival Home) | Specimen Type | Symptoms | Therapy | Antibiotic Therapy | Patient Status | Culture and Identification System | |
|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 584_OEK_2008 | India | 21 days | Blood | Weakness, fever, headache, facial neuralgia, sepsis | ICU supportive care + antibiotic therapy | Empiric therapy: Rocephin, Herpesin; after the diagnosis, meropenem 3 × 1 g/day and Amikacin 1 g/day for 18 days; | Recovered with long term neurological manifestations | Blood agar, chocolate agar followed by Biolog System |
| CNS infection; CSF protein 1.69; Cell count 343; glucose 2.8; CRP elevated; | |||||||||
| Case 2 | 831_NNK_2019 | Thailand | 14 days | Abscess | Respiratory symptoms followed by neck pain, swollen lymph nodes | Ultrasound showed a neck abscess, which was surgically removed + antibiotic therapy | Amoxicillin Clavulanate followed the standard dosage | Fully recovered | Blood agar, chocolate agar followed by MALDI-TOF MS |
| Isolate_1 (Signed 584_2008_OEK) | Isolate_2 (Signed 831_2019_NNK) | |
|---|---|---|
| Genome size | 7.2 Mb | 7.13 Mb |
| GC content | 67.9% | 68.1% |
| CDS | 5877 | 5857 |
| Gene codes | 7394 | 7460 |
| Virulence-associated genes (annotated) | 299 | 300 |
| tRNAs | 60 | 83 |
| rRNAs | 4 | 2 |
| tmRNA | 1 | 1 |
| ncRNA | 30 | 32 |
| ncRNA regions | 19 | 19 |
| sORF | 7 | 7 |
| oriC | 2 | 2 |
| oriV | 0 | 0 |
| oriT | 0 | 0 |
| Gap | 4 | 0 |
| CRISPR-Cas systems | 0 | 0 |
| Strain ID | MLST | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ace | gltB | gmhD | lepA | lipA | nark | ndh | ST | Isolate ID | Genomic ID | |
| 584_2008_OEK | 1 | 12 | 6 | 4 | 1 | 8 | 87 | 1643 | 5254 | 7517 |
| 831_2019_NNK | 1 | 12 | 6 | 1 | 1 | 2 | 1 | 1051 | 6554 | 7518 |
| pubMLST ID | Isolate | Aliases | Country | Travel History | Year | Source |
|---|---|---|---|---|---|---|
| 3230 | BCC23 | BPCC 24 | Australia | na | 1999 | Environmental |
| 3231 | BCC24 | BPCC 25 | Australia | na | 1999 | Environmental |
| 3232 | BCC32 | BPCC 33 | Australia | na | 2000 | Environmental |
| 5004 | Ma154 | India | no | 2016 | Human | |
| 5331 | H 04-2015 | Vietnam | no | 2015 | Human | |
| 5335 | QB 03 | Vietnam | no | 2015 | Human | |
| 5512 | Ma-35 | India | no | 2012 | Human | |
| 7518 | 831_2019_NNK | Hungary | Thailand | 2019 | Human |
| Country | Years | Number of Cases | Patient Characteristics | Source Country/Region | Clinical Presentation |
|---|---|---|---|---|---|
| United Kingdom | 1988–2025 | 69 | Male (n = 49), Female (n = 19); not specified (n = 1) | Thailand (n = 18); India (n = 3); Nigeria (n = 2); Banghladesh (n = 5); Cambogia (n = 2); Caribbean (n = 2); Ghana (n = 2); Malaysia (n = 2); Nigeria (n = 2); Borneo (n = 1); Singapore (n = 1); Vietnam (n = 1); China (n = 1); Shanghai (n = 1); Palau (n = 1); Pakistan (n = 1); Multiple country (n = 6), not specified (n = 6); Asia (n = 22) | Respiratory infection (n = 31), abscess (n = 13), sepsis (n = 17); genito-urinary infection (n = 6), gastrintestinal infection (n = 3), central nervous system infection (n = 2), cutaneus/soft tissue infection (n = 2);sore head (n = 1); not specified (n = 15) |
| The Netherlands | 1990–2018 | 39 | Male (n = 29), female (n = 10) | Thailand (n = 21), Brazil (n = 3); Vietnam (n = 3), Indonesia (n = 4); Gambia (n = 1); Sri Lanka (n = 1); Nepal (n = 1); Myanmar (n = 1); Malaysia (n = 1); Australia (n = 3), Panama (n = 1); Cambodia (n = 1); Australia (n = 1); not specified (n = 5), | Sepsis (n = 10), abscess (n = 10), respiratory infection (n = 20); genito-urinary infection (n = 6); central nervous system (n = 1), otitis externa (n = 1); mycotic aneurysm (n = 1) |
| Finland | 1995–2014 | 4 | Male (n = 3), female (n = 1) | Thailand (n = 4) | Cutaneous/soft tissues infection (n = 3), genito-urinary infection (n = 1) |
| Belgium | 2001–2012 | 4 | Male (n = 2), female (n = 2) | Bangladesh (n = 1); Vietnam (n = 1); Thailand (n = 1); Madagascar (n = 1) | Respiratory infection (n = 1), cutaneus/soft tissue infection (n = 1); genito-urinary infection (n = 1); lymphadenopathy (n = 1) |
| Denmark | 1982–2024 | 9 | Male (n = 6); Female (n = 1) | Kenya (n = 1); Thailand (n = 4); Laos (n = 1); Vietnam (n = 1); Cambodia (n = 1); Southeast Asia (n = 1) | Sepsis (n = 1); cutaneus/soft tissue infection (n = 1); genito-urinary infection (n = 1); abscess (n = 1); respiratory infection (n = 4) |
| Spain | 2009, 2011, 2023–2024 | 5 | Male (n = 4); Female (n = 1) | Gambia (n = 1); Guinea Bissau (n = 1); Senegal (n = 1); Madagascar (n = 1); West-Africa (n = 1); Colombia (n = 1); Thailand (n = 1) | Sepsis (n = 2); cutaneus/soft tissue infection (n = 1); abscess (n = 1); osteomyelitis (n = 1) |
| France | 1995–2024 | 21 | Male (n = 18), Female (n = 3) | Africa (n = 1); Thailand (n = 6); Cambodia (n = 4); Cameron (n = 1); Madagascar (n = 1); Guadalupe (n = 1), Vietnam (n = 3); Indonesia (n = 1); Phillippines (n = 1); not specified (n = 1) | Respiratory infection (n = 11); cervical lymphadenitis (n = 2); abdominal infection (n = 2); mycotic aneurysm (n = 3); saccular aneurysm (n = 1); central nervous system infection (n = 1); osteomyelytis (n = 1) |
| Germany | 1996–2024 | 14 | Male (n = 11); Female (n = 3) | South East Asia (n = 1); China (n = 1); Taiwan (n = 7); Maldives (n = 1); Sri Lanka (n = 1); Cambodia (n = 1); Thailand (n = 2), Vietnam (n = 1); Indonesia (n = 1); Mexico (n = 1); Dominican Republic (n = 1); USA (n = 1); Costa Rica (n = 1); No significant travel history (n = 1) | Respiratory infection (7); central nervous system infection (n = 1); abscess (n = 1); pericardial effusion (n = 1), urosepsis (n = 1); abdominal mycotic aortic aneurysm (n = 1); wound infection (n = 1) |
| Sweden | Not specified | 5 | Male (n = 4); Female (n = 1) | Thailand (n = 4); Malaysia (n = 1) | Respiratory infection (n = 1); central nervous system infection (n = 1); otitis externa (n = 1); abscess (n = 2); cutaneus/soft tissue (n = 1) |
| Austria | 2014; 2020 | 2 | Male (n = 2) | Thailand (n = 2) | Sepsis (n = 1); lymphadenitis (n = 1) |
| Hungary | 2008–2019 | 2 | Male (n = 1); Female (n = 1) | India, Thailand | Central nervous system infection (n = 1); abscess (n = 1) |
| Portugal | 2011; 2025 | 3 | Male (n = 1); Female (n = 1); Not specified (n = 1) | Thailand (n = 1), Brazil (n = 1), Not specified (n = 1) | Erythema nodosum (n = 1); sepsis (n = 1); gluteal abscess (n = 1); not specified (n = 1) |
| Slovenia | 2007 | 1 | Male (n = 1) | Asia (Brunei) | Osteomyelitis of parietal bone |
| Italy | 1997, 2002, 2014, | 3 | Male (n = 2), female (n = 1) | Thailand (n = 2); Singapure (n = 1) | Respiratory infection (n = 3), multiple abscess (n = 1) |
| Norway | 2011, 2014 | 3 | Male (n = 3) | Sri Lanka (n = 1); Thailand (n = 1); Cambodia (n = 1) | Bacteraemia and splenic and prostatic abscesses (n = 2); neurological (n = 1) |
| Switzerland | 2008–2012 | 4 | Male (n = 4) | Thailand (n = 3), Caribbean, Martinique Island (n = 1) | Respiratory infection (n = 1); abscess (n = 1); systemic inflammatory response syndrome (n = 1); cutaneous/soft tissues (n = 1) |
| Iceland | Not specified | 4 | Male (n = 4) | Thailand (n = 3); Southeast Asia (n = 1); not specified (n = 1) | Respiratory infection (n = 2); necrotizing granulomatous inflammation (n = 1); pleural abscess (n = 1); osteomyelitis (n = 1) |
| Country | Year | Travel Anamnesis | Clinical Picture | Reference |
|---|---|---|---|---|
| The Netherlands | 1993 | Not specified | Meningoencephalitis | [67] |
| Hungary | 2008 | India | Meningoencephalitis | in this study |
| Norway | 2014 | Cambogia | Cerebral abscess | [71] |
| The Netherlands | 2015 | Thailand | Brain abscesses | [67] |
| Germany | 2018 | Thailand | Cerebral melioidosis | [72] |
| Sweden | 2021 | Thailand | Intracranial melioidosis | [73] |
| Germany | 2022 | Unknown, several countries, possibility of autochthonous infection | Neuromelioidosis | [29] |
| France | 2023 | Thailand | Pneumonia, Meningitis | [74] |
| United Kingdom | 2024 | Sri Lanka | Neuromelioidosis | [35,69] |
| Feature | Isolate 584_2008_OEK | Isolate 831_2019_NNK | Implications |
|---|---|---|---|
| Clinical manifestation | Neuromelioidosis (meningoencephalitis) | Neck abscess | Bm variant of bimA, associated with neurological disease |
| MLST/sequence type (ST) | ST 1640 (novel) | ST 1051 | ST 1051 shows overlap with Australian strains |
| bimA variant | Bm | Bp | Bm variant linked to neurological complications |
| YLF gene cluster | Present | Present | Enhances adhesion and biofilm formation; common in Asian strains |
| BTFC cluster | Absent | Absent | Characteristic of Australian isolates; affects motility/chemotaxis |
| ITS type | Type C (622 bp) | Type C (622 bp) | Commonly observed in Asian isolates |
| fhaB3 | Present | Present | Adhesion and tissue invasion; contributes to virulence |
| Capsule polysaccharide operon (wcb) | Present, no key mutations | Present, no key mutations | Important for immune evasion |
| Toxin A (toxA) | Present | Present | Disrupts host cell cytoskeleton |
| T3SS | Present | Present | Type III secretion system; facilitates host cell manipulation |
| β-lactamase genes | blaOXA-57, blaPenI, amrAB | blaOXA-59 | Contributes to antibiotic resistance (aminoglycosides, β-lactams) |
| Geographical link/phylogeny | First neuromelioidosis case in Europe | Linked to Asian strains via YLF cluster | Highlights travel-related risk and global strain diversity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henczkó, J.; Tóth, Á.; Knausz, M.; Gartner, B.; Reményi, Á.; Bíró, E.; Létay, E.; Rókusz, L.; Tóth, S.; Pályi, B.; et al. Whole Genome Sequencing and Comparative Genomics of the Emerging Pathogen Burkholderia pseudomallei Isolated from Two Travel-Related Infections in Hungary. Pathogens 2025, 14, 1108. https://doi.org/10.3390/pathogens14111108
Henczkó J, Tóth Á, Knausz M, Gartner B, Reményi Á, Bíró E, Létay E, Rókusz L, Tóth S, Pályi B, et al. Whole Genome Sequencing and Comparative Genomics of the Emerging Pathogen Burkholderia pseudomallei Isolated from Two Travel-Related Infections in Hungary. Pathogens. 2025; 14(11):1108. https://doi.org/10.3390/pathogens14111108
Chicago/Turabian StyleHenczkó, Judit, Ákos Tóth, Márta Knausz, Béla Gartner, Ákos Reményi, Edit Bíró, Erzsébet Létay, László Rókusz, Szilárd Tóth, Bernadett Pályi, and et al. 2025. "Whole Genome Sequencing and Comparative Genomics of the Emerging Pathogen Burkholderia pseudomallei Isolated from Two Travel-Related Infections in Hungary" Pathogens 14, no. 11: 1108. https://doi.org/10.3390/pathogens14111108
APA StyleHenczkó, J., Tóth, Á., Knausz, M., Gartner, B., Reményi, Á., Bíró, E., Létay, E., Rókusz, L., Tóth, S., Pályi, B., Mag, T., Erdősi, T., Deézsi-Magyar, N., Molnár, Z., & Kis, Z. (2025). Whole Genome Sequencing and Comparative Genomics of the Emerging Pathogen Burkholderia pseudomallei Isolated from Two Travel-Related Infections in Hungary. Pathogens, 14(11), 1108. https://doi.org/10.3390/pathogens14111108






