Eighteen Years of Human Rhinovirus Surveillance in the Republic of Korea (2007–2024): Age- and Season-Specific Trends from a Single-Center Study with Public Health Implications
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Specimen Collection and Data Analysis
2.3. RNA Extraction and Real-Time Reverse Transcriptase Polymerase Chain Reaction (Real-Time RT-PCR)
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
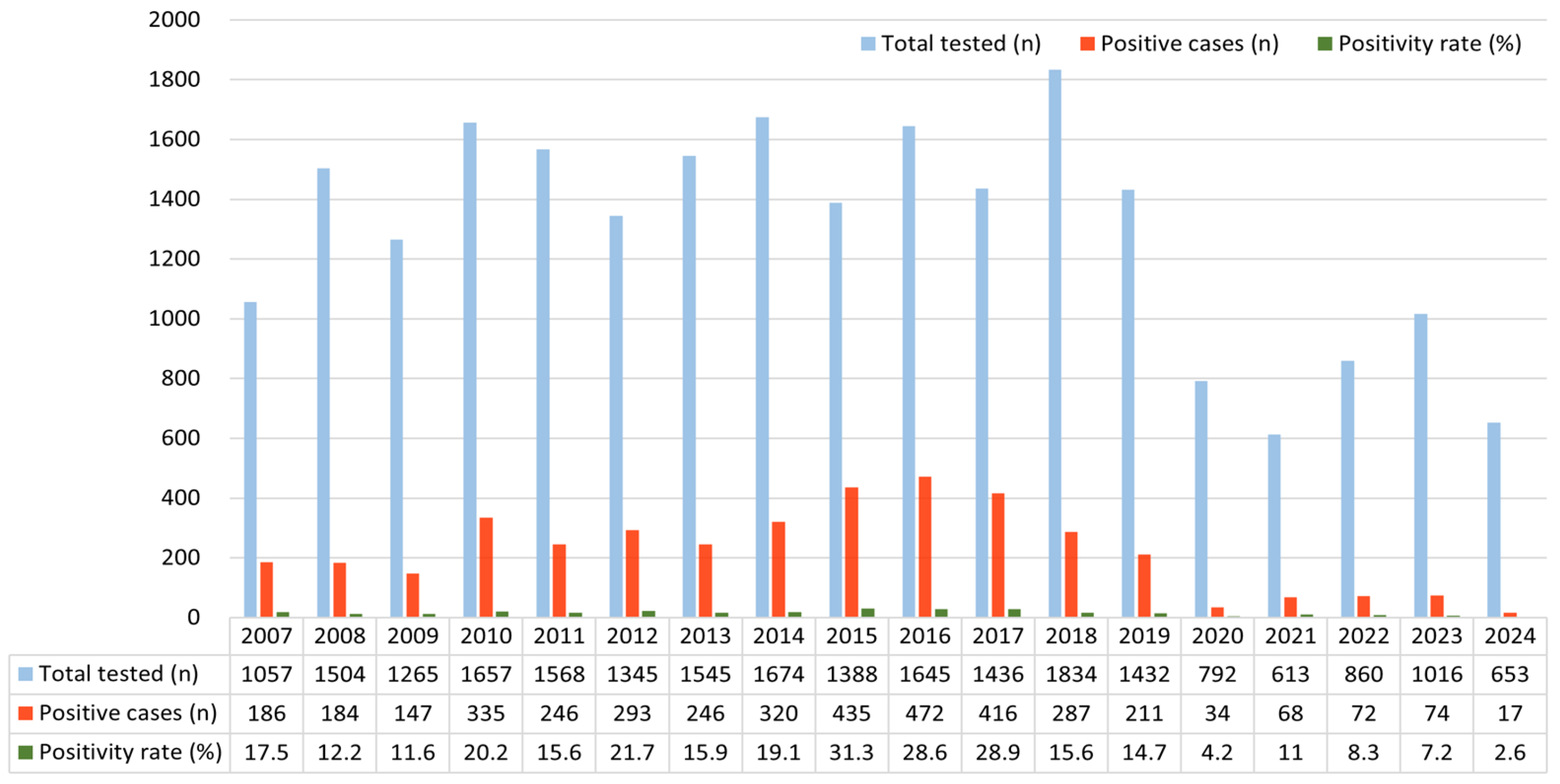
3.2. Annual HRV Positivity Trend (2007–2024)
3.3. Seasonal HRV Positivity Rate
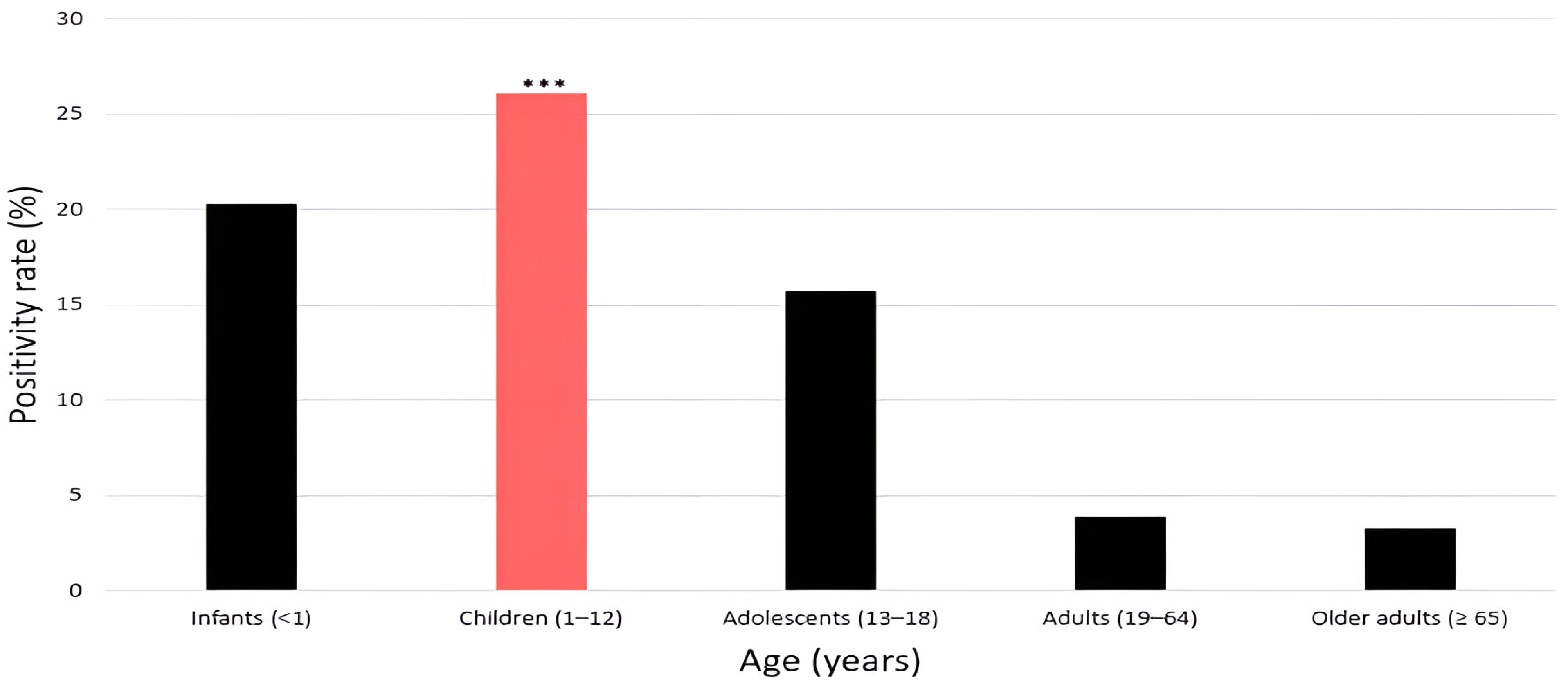
3.4. HRV Positivity Rate by Age Group
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HRV | Human rhinovirus |
| real-time RT-PCR | Real-time reverse transcriptase polymerase chain reaction |
References
- Moriyama, M.; Hugentobler, W.J.; Iwasaki, A. Seasonality of respiratory viral infections. Annu. Rev. Virol. 2020, 7, 83–101. [Google Scholar] [CrossRef]
- Esneau, C.; Duff, A.C.; Bartlett, N.W. Understanding rhinovirus circulation and impact on illness. Viruses 2022, 14, 141. [Google Scholar] [CrossRef]
- Jackson, D.J.; Gern, J.E. Rhinovirus infections and their roles in asthma: Etiology and exacerbations. J. Allergy Clin. Immunol. Pract. 2022, 10, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Erkkola, R.; Turunen, R.; Räisänen, K.; Waris, M.; Vuorinen, T.; Laine, M.; Tähtinen, P.; Gern, J.E.; Bochkov, Y.A.; Ruohola, A.; et al. Rhinovirus C is associated with severe wheezing and febrile respiratory illness in young children. Pediatr. Infect. Dis. J. 2020, 39, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Caldera, J.R.; Saleh, T.; Fuller, T.; Yang, S.; Nielsen-Saines, K. Multi-year analysis of respiratory viral dynamics reveals significance of rhinovirus in young children with severe respiratory illness. Infect. Dis. Rep. 2025, 17, 29. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Rhee, J.E.; Kang, D.; Choi, E.H.; Lee, N.J.; Woo, S.; Lee, J.; Lee, S.W.; Kim, E.J.; Yun, K.W. Epidemiology of respiratory viruses in Korean children before and after the COVID-19 pandemic: A prospective study from national surveillance system. J. Korean Med. Sci. 2024, 39, e171. [Google Scholar] [CrossRef]
- Liew, K.Y.; Koh, S.K.; Ho, H.L.; Ng, M.K.L.; Chee, H.Y.; Harith, H.H.; Israf, D.A.; Tham, C.L. Rhinovirus-induced cytokine alterations with potential implications in asthma exacerbations: A systematic review and meta-analysis. Front. Immunol. 2022, 13, 782936. [Google Scholar] [CrossRef]
- Nokhova, A.R.; Saroyan, T.A.; Solomatina, M.V.; Gutova, T.A.; Derko, A.A.; Dubovitskiy, N.A.; Murashkina, T.A.; Sharshov, K.A.; Shestopalov, A.M.; Kurskaya, O.G. Genetic diversity and epidemiology of enteroviruses and rhinoviruses in children hospitalized with acute respiratory infections in Novosibirsk, Russia (2023–2024). Viruses 2024, 16, 1924. [Google Scholar] [CrossRef]
- Yum, S.; Hong, K.; Sohn, S.; Kim, J.; Chun, B.C. Trends in viral respiratory infections during COVID-19 pandemic, South Korea. Emerg. Infect. Dis. 2021, 27, 1685–1688. [Google Scholar] [CrossRef]
- Lenglart, L.; Titomanlio, L.; Bognar, Z.; Bressan, S.; Buonsenso, D.; De, T.; Farrugia, R.; Honeyford, K.; Maconochie, I.K.; Moll, H.A.; et al. Surge of pediatric respiratory tract infections after the COVID-19 pandemic and the concept of “immune debt”. J. Pediatr. 2025, 284, 114420. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Interim Guidelines for Collecting and Handling of Clinical Specimens for COVID-19 Testing. Available online: https://www.cdc.gov/covid/hcp/clinical-care/clinical-specimen-guidelines.html?utm_source (accessed on 8 October 2025).
- Kim, J.K.; Jeon, J.S.; Kim, J.W.; Rheem, I. Epidemiology of respiratory viral infection using multiplex RT-PCR in Cheonan, Korea (2006–2010). J. Microbiol. Biotechnol. 2013, 23, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Rollinger, J.M.; Schmidtke, M. The human rhinovirus: Human-pathological impact, mechanisms of antirhinoviral agents, and strategies for their discovery. Med. Res. Rev. 2011, 31, 42–92. [Google Scholar] [CrossRef]
- Xu, B.; Wang, J.; Li, Z.; Xu, C.; Liao, Y.; Hu, M.; Yang, J.; Lai, S.; Wang, L.; Yang, W. Seasonal association between viral causes of hospitalised acute lower respiratory infections and meteorological factors in China: A retrospective study. Lancet Planet. Health 2021, 5, e154–e163. [Google Scholar] [CrossRef]
- An, T.J.; Lee, J.; Shin, M.; Rhee, C.K. Seasonality of common respiratory viruses: Analysis of nationwide time-series data. Respirology 2024, 29, 985–993. [Google Scholar] [CrossRef]
- Park, M.; Choi, W.S.; Cowling, B.J. Shifts in influenza and respiratory syncytial virus infection patterns in Korea after the COVID-19 pandemic resulting from immunity debt: Retrospective observational study. JMIR Public Health Surveill. 2025, 11, e68058. [Google Scholar] [CrossRef]
- Han, J.S.; Jang, S.H.; Jeon, J.-S.; Kim, J.K. Long-term trends in respiratory syncytial virus A infections (2007–2024) in Korea. Diseases 2025, 13, 147. [Google Scholar] [CrossRef]
- Shek, L.P.C.; Lee, B.W. Epidemiology and seasonality of respiratory tract virus infections in the tropics. Paediatr. Respir. Rev. 2003, 4, 105–111. [Google Scholar] [CrossRef]
- Contes, K.M.; Liu, B.M. Epidemiology, clinical significance, and diagnosis of respiratory viruses and their co-infections in the post-COVID era. Pathogens 2025, 14, 262. [Google Scholar] [CrossRef]
- Zeng, Z.; Guan, W.; Liu, Y.; Lin, Z.; Liang, W.; Liang, J.; Chen, B.; Wu, T.; Wang, Y.; Yang, C.; et al. Different circulation pattern of multiple respiratory viruses in southern China during the COVID-19 pandemic. Front. Microbiol. 2021, 12, 801946. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, M.; Udagawa, T.; Kato, T.; Tanaka, I.; Yamamoto, R.; Sakaguchi, H.; Sekikawa, Y. Observational study on the clinical reality of community-acquired respiratory virus infections in adults and older individuals. Pathogens 2024, 13, 983. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, D.; Kitano, T.; Furumori, M.; Suzuki, S.; Shintani, Y.; Suzuki, Y.; Nakano, A.; Nakano, R.; Nishiyama, A.; Yoshida, S.; et al. Epidemiology of respiratory tract infections using multiplex PCR in a Japanese acute care hospital during the COVID19 pandemic. Heliyon 2023, 9, e14424. [Google Scholar] [CrossRef] [PubMed]
- Gikandi, A.; Hallet, J.; Koerkamp, B.G.; Clark, C.J.; Lillemoe, K.D.; Narayan, R.R.; Mamon, H.J.; Zenati, M.A.; Wasif, N.; Safran, D.G.; et al. Distinguishing clinical from statistical significances in contemporary comparative effectiveness research. Ann. Surg. 2024, 279, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Giardina, F.A.M.; Piralla, A.; Ferrari, G.; Zavaglio, F.; Cassaniti, I.; Baldanti, F. Molecular epidemiology of rhinovirus/enterovirus and their role on cause severe and prolonged infection in hospitalized patients. Microorganisms 2022, 10, 755. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Salgado, E.; Briseno-Ramírez, J.; Vega-Cornejo, G.; Damian-Negrete, R.; Rosales-Chavez, G.; De Arcos-Jiménez, J.C. Seasonal Shifts in Influenza, Respiratory Syncytial Virus, and Other Respiratory Viruses After the COVID-19 Pandemic: An Eight-Year Retrospective Study in Jalisco, Mexico. Viruses 2024, 16, 1892. [Google Scholar] [CrossRef]
- Morelli, T.; Freeman, A.; Staples, K.J.; Wilkinson, T.M.A. Hidden in plain sight: The impact of human rhinovirus infection in adults. Respir. Res. 2025, 26, 120. [Google Scholar] [CrossRef]
- Almeida, T.; Guimarães, J.T.; Rebelo, S. Epidemiological changes in respiratory viral infections in children: The influence of the COVID-19 pandemic. Viruses 2023, 15, 1880. [Google Scholar] [CrossRef]
- He, Y.; Liu, W.J.; Jia, N.; Richardson, S.; Huang, C. Viral respiratory infections in a rapidly changing climate: The need to prepare for the next pandemic. eBioMedicine 2023, 93, 104593. [Google Scholar] [CrossRef]
- Thomas, E.; Mattila, J.M.; Lehtinen, P.; Vuorinen, T.; Waris, M.; Heikkinen, T. Burden of respiratory syncytial virus infection during the first year of life. J. Infect. Dis. 2021, 223, 811–817. [Google Scholar] [CrossRef]
- Fahim, M.; AbdElGawad, B.; Hassan, H.; Naguib, A.; Ahmed, E.; Afifi, S.; Abu ElSood, H.; Mohsen, A. Epidemiology and outcome of influenza-associated infections among hospitalized patients with acute respiratory infections, Egypt national surveillance system, 2016–2019. Influenza Other Respir. Viruses 2021, 15, 599–607. [Google Scholar] [CrossRef]
- Palmenberg, A.C.; Gern, J.E. Classification and evolution of human rhinoviruses. In Rhinoviruses Protocol; Humana Press: New York, NY, USA, 2014; pp. 1–10. [Google Scholar]
- Ratnayake, R.; Checchi, F.; Jarvis, C.I.; Edmunds, W.J.; Finger, F. Inference is bliss: Simulation for power estimation for an observational study of a cholera outbreak intervention. PLoS Negl. Trop. Dis. 2022, 16, e0010163. [Google Scholar] [CrossRef]
- McIntyre, C.L.; Knowles, N.J.; Simmonds, P. Proposals for the classification of human rhinovirus species A, B and C into genotypically assigned types. J. Gen. Virol. 2013, 94, 1791–1806. [Google Scholar] [CrossRef]
- Kim, H.; Kim, K.; Kim, D.W.; Jung, H.D.; Min Cheong, H.; Kim, K.H.; Soo Kim, D.; Kim, Y.J. Identification of recombinant human rhinovirus A and C in circulating strains from upper and lower respiratory infections. PLoS ONE 2013, 8, e68081. [Google Scholar] [CrossRef] [PubMed]
- He, J.; He, X.; He, N.; Wang, P.; Gao, O.; Sheng, J.; Tan, J. Epidemiological trends and pathogen analysis of pediatric acute respiratory infections in Hanzhong Hospital, China: Insights from 2023 to 2024. Front. Public Health 2025, 13, 1557076. [Google Scholar] [CrossRef] [PubMed]
- Khor, C.S.; Sam, I.C.; Hooi, P.S.; Quek, K.F.; Chan, Y.F. Epidemiology and seasonality of respiratory viral infections in hospitalized children in Kuala Lumpur, Malaysia: A retrospective study of 27 years. BMC Pediatr. 2012, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Burda, B.U.; Chambers, A.R.; Johnson, J.C. Appraisal of guidelines developed by the World Health Organization. Public Health 2014, 128, 444–474. [Google Scholar] [CrossRef]
- Han, J.S.; Jang, S.H.; Jeon, J.-S.; Lee, K.B.; Kim, J.K. Epidemiological shifts in respiratory virus infections among older adults (≥65 years) before and after the COVID-19 pandemic: An 18-year retrospective study in the Republic of Korea. Microorganisms 2025, 13, 2301. [Google Scholar] [CrossRef]

| Age Group | Male (n) | Female (n) | Total (n) | Positivity Rate (%) |
|---|---|---|---|---|
| Infants (<1 year) | 2704 | 1839 | 4543 (19.5%) | 20.3 |
| Children (1–12 years) | 5992 | 4545 | 10,537 (45.3%) | 26.1 |
| Adolescents (13–18 years) | 336 | 241 | 577 (2.5%) | 15.7 |
| Adults (19–64 years) | 1893 | 1042 | 2935 (12.6%) | 3.9 |
| Older adults (≥65 years) | 3036 | 1656 | 4692 (20.2%) | 3.3 |
| Total | 13,961 | 9323 | 23,284 (100%) |
| Month | Total Tested (n) | Positive Case (n) | Positivity Rate (%) | p-Value |
|---|---|---|---|---|
| 1 (January) | 2118 | 148 | 6.9 | <0.001 |
| 2 (February) | 1813 | 150 | 8.2 | |
| 3 (March) | 1912 | 321 | 16.7 | |
| 4 (April) | 2180 | 447 | 20.5 | |
| 5 (May) | 2299 | 478 | 20.7 | |
| 6 (June) | 1736 | 369 | 21.2 | |
| 7 (July) | 1535 | 376 | 24.4 | |
| 8 (August) | 1539 | 295 | 19.1 | |
| 9 (September) | 1496 | 361 | 24.1 | |
| 10 (October) | 1794 | 412 | 22.9 | |
| 11 (November) | 2317 | 387 | 16.7 | |
| 12 (December) | 2545 | 299 | 11.7 |
| Age Group | Total Tested (n) | Positive Case (n) | Negative Case (n) | Positivity Rate (%) | p-Value |
|---|---|---|---|---|---|
| Infants (<1 year) | 4543 | 924 | 3619 | 20.3 | <0.001 |
| Children (1–12 years) | 10,537 | 2752 | 7785 | 26.1 | |
| Adolescents (13–18 years) | 577 | 91 | 486 | 15.7 | |
| Adults (19–64 years) | 2935 | 117 | 2818 | 3.9 | |
| Older adults (≥65 years) | 4692 | 159 | 4533 | 3.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.J.; Han, J.S.; Jang, S.H.; Jeon, J.-S.; Kim, J.K. Eighteen Years of Human Rhinovirus Surveillance in the Republic of Korea (2007–2024): Age- and Season-Specific Trends from a Single-Center Study with Public Health Implications. Pathogens 2025, 14, 1098. https://doi.org/10.3390/pathogens14111098
Kim YJ, Han JS, Jang SH, Jeon J-S, Kim JK. Eighteen Years of Human Rhinovirus Surveillance in the Republic of Korea (2007–2024): Age- and Season-Specific Trends from a Single-Center Study with Public Health Implications. Pathogens. 2025; 14(11):1098. https://doi.org/10.3390/pathogens14111098
Chicago/Turabian StyleKim, Yu Jeong, Jeong Su Han, Sung Hun Jang, Jae-Sik Jeon, and Jae Kyung Kim. 2025. "Eighteen Years of Human Rhinovirus Surveillance in the Republic of Korea (2007–2024): Age- and Season-Specific Trends from a Single-Center Study with Public Health Implications" Pathogens 14, no. 11: 1098. https://doi.org/10.3390/pathogens14111098
APA StyleKim, Y. J., Han, J. S., Jang, S. H., Jeon, J.-S., & Kim, J. K. (2025). Eighteen Years of Human Rhinovirus Surveillance in the Republic of Korea (2007–2024): Age- and Season-Specific Trends from a Single-Center Study with Public Health Implications. Pathogens, 14(11), 1098. https://doi.org/10.3390/pathogens14111098






