Epidemiology, Virology, and Control of Highly Pathogenic Avian Influenza in Kazakhstan
Abstract
1. Introduction
2. Bibliographic Analysis
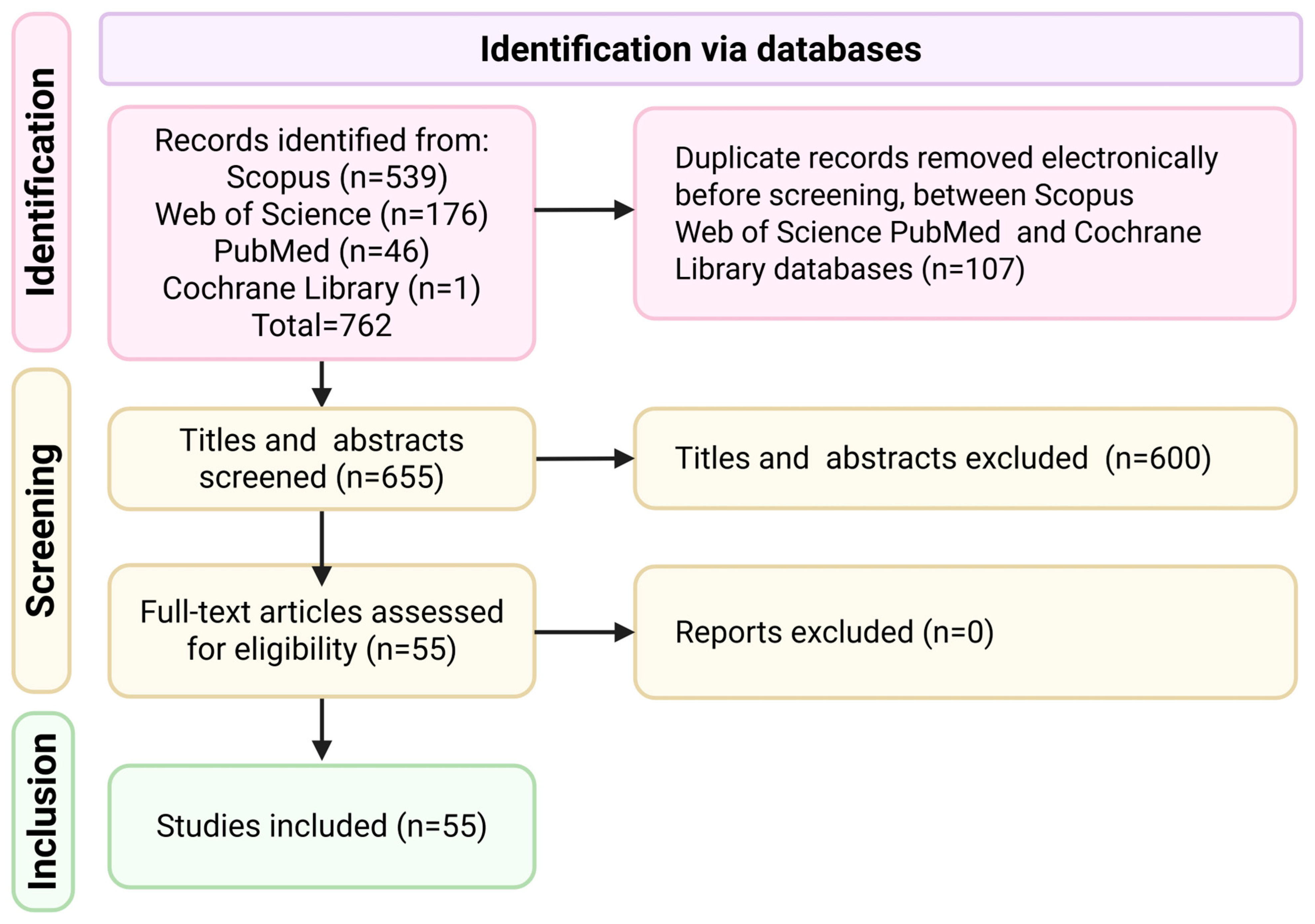
2.1. Study Selection and PRISMA Flow Diagram
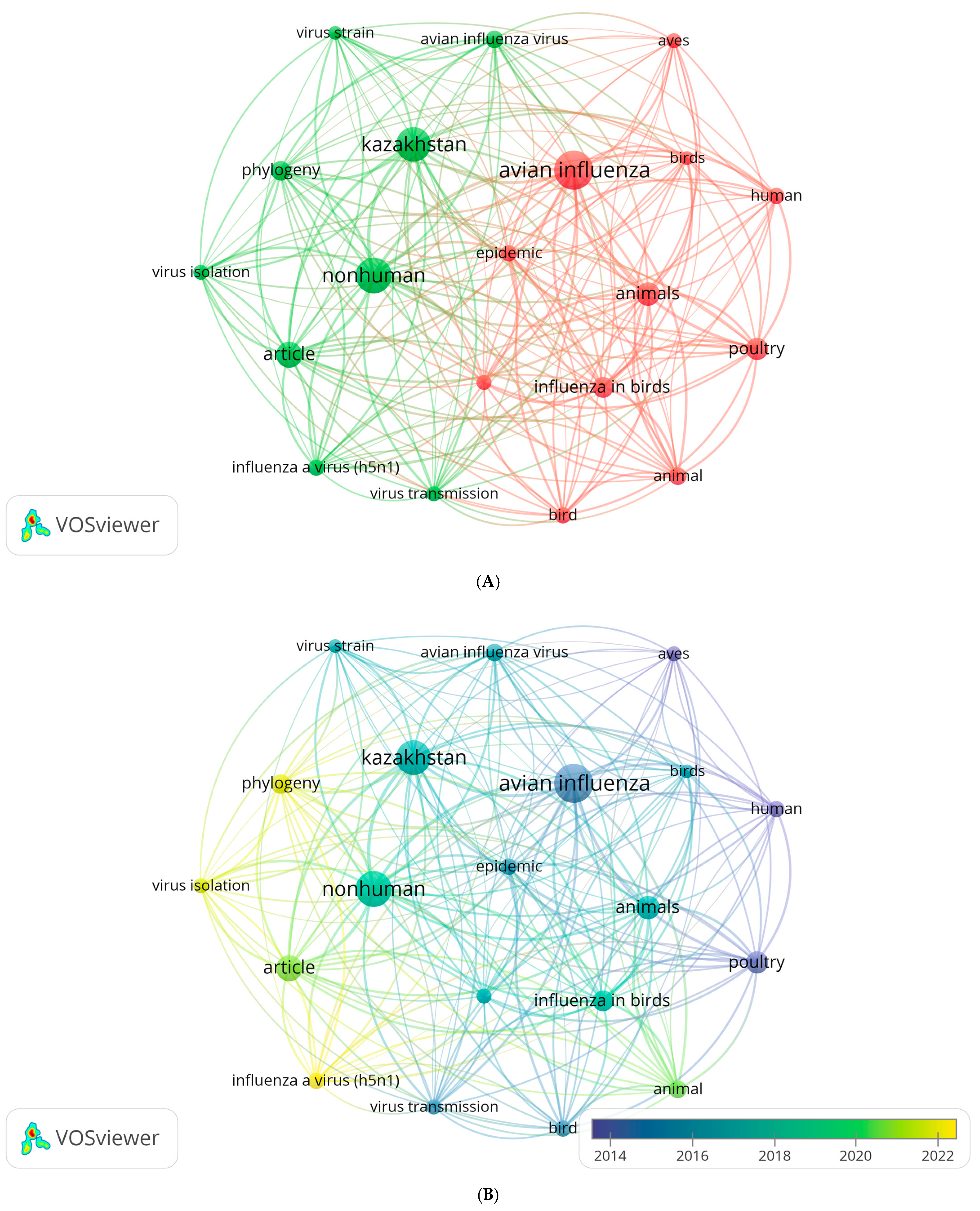
2.2. Bibliometric Analysis
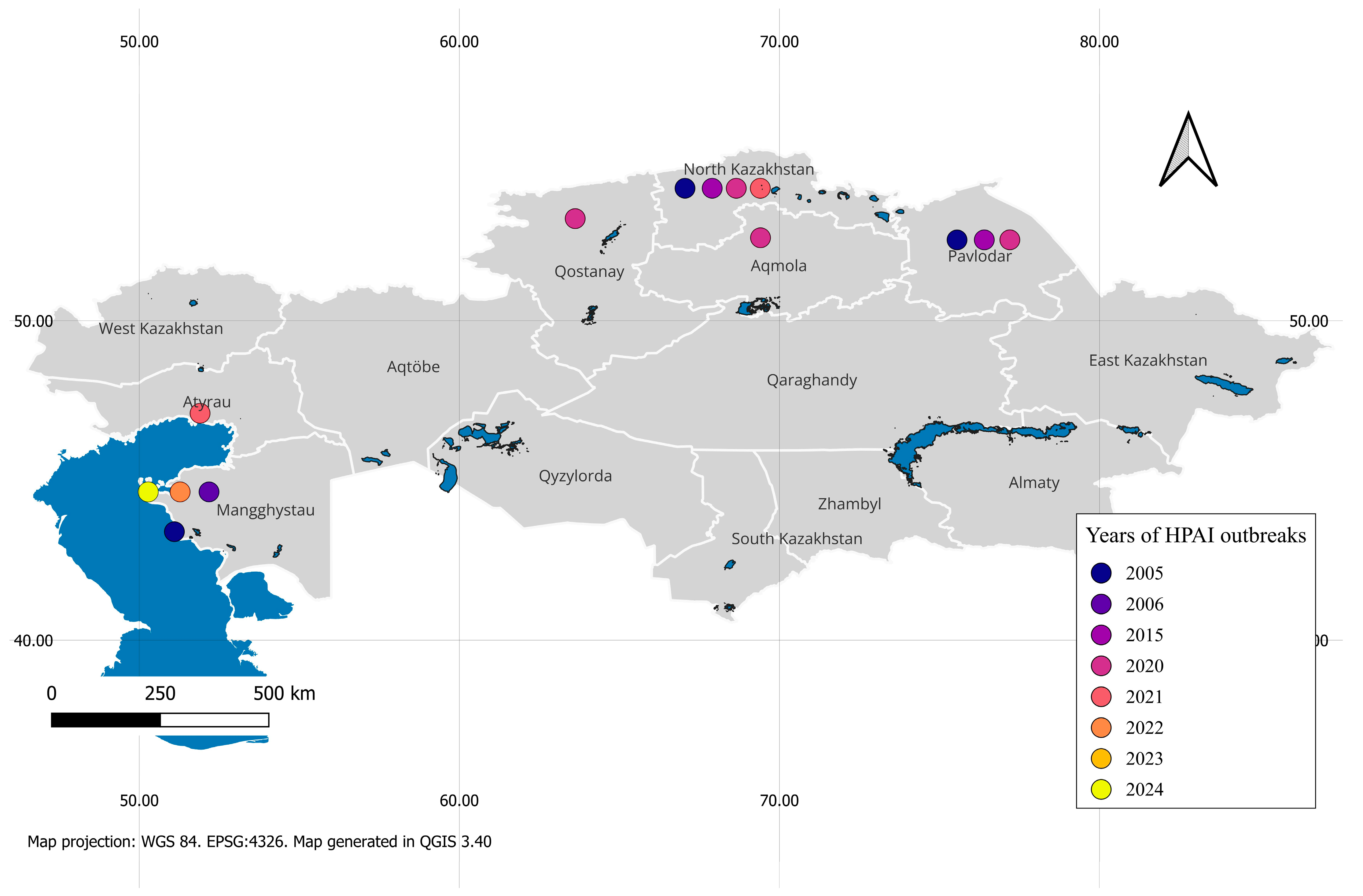
3. Epidemiology and Molecular Genetic Characterization of HPAI Viruses in Kazakhstan
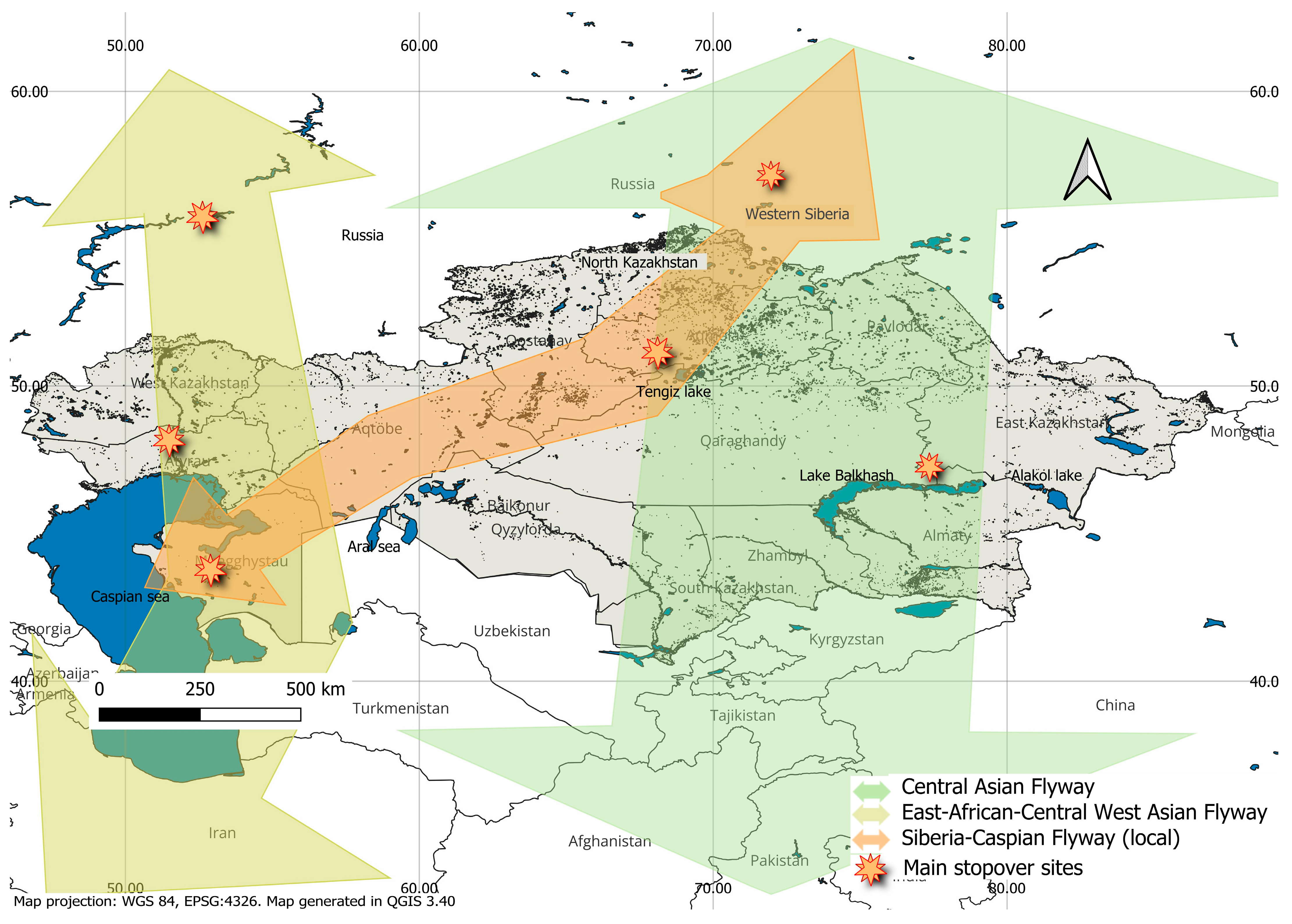
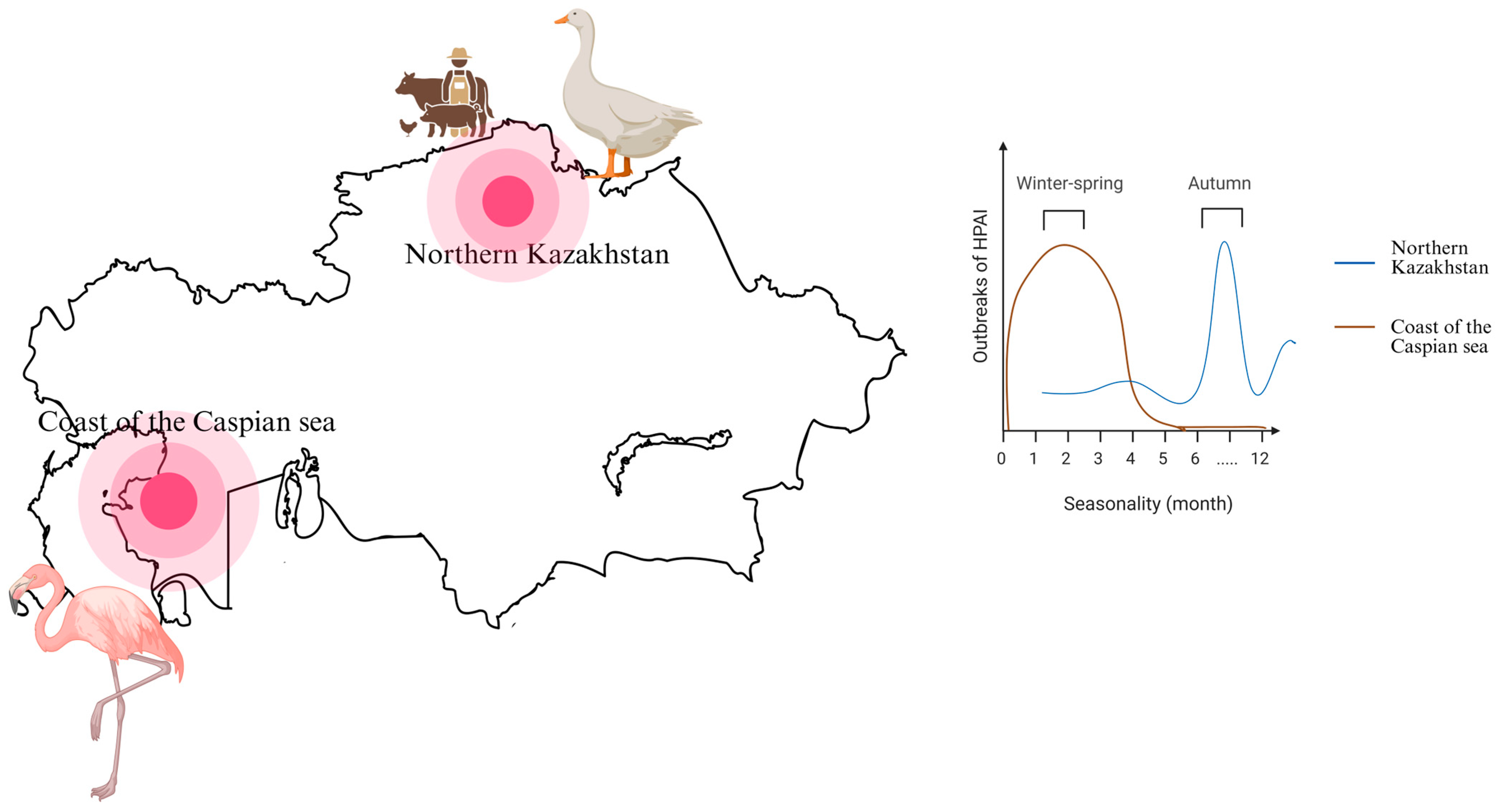
4. Risk Factors for the Spread of HPAI in Kazakhstan
5. Diagnostics of HPAI in the Field of Veterinary Medicine of the Republic of Kazakhstan
6. Vaccination of Poultry Against HPAI in Kazakhstan
7. Systematic Support for Surveillance of HPAI in Kazakhstan
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EMPRES-i+ | FAO’s Global Animal Disease Information System (Emergency Prevention System-Information System Plus) |
| FAO | Food and Agriculture Organization |
| GISRS | Global Influenza Surveillance and Response System |
| IVPI | Intravenous pathogenicity index |
| HA | Hemagglutinin |
| HPAI | Highly pathogenic avian influenza |
| LPAI | Low-pathogenic avian influenza |
| MOL-PCR | Multiplex oligonucleotide ligation–polymerase chain reaction |
| NA | Neuraminidase |
| OFFLU | The WOAH/FAO Network of Expertise on Animal Influenza |
| RT-PCR | Reverse transcription–polymerase chain reaction |
References
- Onischenko, G.G.; Netesov, S.V.; Agafonov, A.P.; Safatov, A.S.; Buryak, G.A.; Generalov, V.M.; Sergeyev, A.N.; Drozdov, I.G. Highly pathogenic avian influenza: A new pandemic threat and possibilities to resist it. Vestn. Ross. Akad. Meditsinskikh Nauk. 2006, 12, 36–42. [Google Scholar]
- World Health Organization. Cumulative Number of Confirmed Human Cases for Avian Influenza A(H5N1) Reported to WHO, 2003–2025. 2025. Available online: https://cdn.who.int/media/docs/default-source/influenza/h5n1-human-case-cumulative-table/cumulative-number-of-confirmed-human-cases-for-avian-influenza-a(h5n1)-reported-to-who--2003-20256f35bd02-b935-4063-ba3e-6b41cabfc0a9.pdf?sfvrsn=f9b20499_3 (accessed on 26 July 2025).
- Kackos, C.M.; Webby, R.J. Influenza Virus. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2023; p. B9780128225639001013. [Google Scholar] [CrossRef]
- Luczo, J.M.; Spackman, E. Epitopes in the HA and NA of H5 and H7 avian influenza viruses that are important for antigenic drift. FEMS Microbiol. Rev. 2024, 48, fuae014. [Google Scholar] [CrossRef]
- Long, J.S.; Mistry, B.; Haslam, S.M.; Barclay, W.S. Host and viral determinants of influenza A virus species specificity. Nat. Rev. Microbiol. 2019, 17, 67–81. [Google Scholar] [CrossRef]
- Abenova, A.; Mukhanbetkaliyev, Y.Y.; Kadyrov, A.; Sytnik, I.I.; Shevtsov, A.; Korennoy, F.I.; Martin, I.I.; Perez, A.M.; Abdrakhmanov, S.K. Environmental Suitability of Kazakhstan to Highly Pathogenic Avian Influenza Using Data on Eurasian Outbreaks, 2020–2024. Viruses 2025, 17, 574. [Google Scholar] [CrossRef]
- Wu, T.; Perrings, C. The live poultry trade and the spread of highly pathogenic avian influenza: Regional differences between Europe, West Africa, and Southeast Asia. PLoS ONE 2018, 13, e0208197. [Google Scholar] [CrossRef]
- Schielzeth, H.; Eichhorn, G.; Heinicke, T.; Kamp, J.; Koshkin, M.A.; Koshkin, A.V.; Lachmann, L. Waterbird population estimates for a key staging site in Kazakhstan: A contribution to wetland conservation on the Central Asian flyway. Bird Conserv. Int. 2008, 18, 71–86. [Google Scholar] [CrossRef]
- Laleye, A.; Joannis, T.; Shittu, I.; Meseko, C.; Zamperin, G.; Milani, A.; Zecchin, B.; Fusaro, A.; Monne, I.; Abolnik, C. A two-year monitoring period of the genetic properties of clade 2.3.2.1c H5N1 viruses in Nigeria reveals the emergence and co-circulation of distinct genotypes. Infect. Genet. Evol. 2018, 57, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Iverson, S.A.; Gavrilov, A.; Katzner, T.E.; Takekawa, J.Y.; Miller, T.A.; Hagemeijer, W.; Mundkur, T.; Sivananinthaperumal, B.; Demattos, C.C.; Ahmed, L.S.; et al. Migratory movements of waterfowl in Central Asia and avian influenza emergence: Sporadic transmission of H5N1 from east to west. Ibis 2011, 153, 279–292. [Google Scholar] [CrossRef]
- Sultankulova, K.; Argimbayeva, T.; Aubakir, N.; Bopi, A.; Omarova, Z.; Melisbek, A.; Karamendin, K.; Kydyrmanov, A.; Chervyakova, O.; Kerimbayev, A.; et al. Reassortants of the Highly Pathogenic Influenza Virus A/H5N1 Causing Mass Swan Mortality in Kazakhstan from 2023 to 2024. Animals 2024, 14, 3211. [Google Scholar] [CrossRef]
- Mattila, J.; Thomas, E.; Lehtinen, P.; Vuorinen, T.; Waris, M.; Heikkinen, T. Burden of influenza during the first year of life. Influenza Respir. Viruses 2021, 15, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Goonewardene, K.B.; Chung, C.J.; Goolia, M.; Blakemore, L.; Fabian, A.; Mohamed, F.; Nfon, C.; Clavijo, A.; Dodd, K.A.; Ambagala, A. Evaluation of oral fluid as an aggregate sample for early detection of African swine fever virus using four independent pen-based experimental studies. Transbound. Emerg. Dis. 2021, 68, 2867–2877. [Google Scholar] [CrossRef]
- O’Connor, R.E.; Romanov, M.N.; Kiazim, L.G.; Barrett, P.M.; Farré, M.; Damas, J.; Ferguson-Smith, M.; Valenzuela, N.; Larkin, D.M.; Griffin, D.K. Reconstruction of the diapsid ancestral genome permits chromosome evolution tracing in avian and non-avian dinosaurs. Nat. Commun. 2018, 9, 1883. Available online: https://www.nature.com/articles/s41467-018-04267-9 (accessed on 26 July 2025). [CrossRef] [PubMed]
- Zikibayeva, K.; Svanbayev, A.; Akhmetsadykov, N.; Kudaibergenova, K.; Akhmetsadykova, S.; Nurolda, E.; Kydyrmanov, A. Epidemiological investigation of poultry infectious in Kazakhstan (2021–2024). Front. Vet. Sci. 2025, 11, 1520606. [Google Scholar] [CrossRef] [PubMed]
- Perfumo, C.J.; Pereda, A.; Jongkaewwattana, A.; Chen, Z.; Perez, D.R.; Ma, J. Editorial: Emerging Swine Viruses. Front. Vet. Sci. 2020, 7, 132. [Google Scholar] [CrossRef] [PubMed]
- Kydyrmanov, A.; Karamendin, K.; Kassymbekov, Y.; Daulbayeva, K.; Sabyrzhan, T.; Khan, Y.; Nuralibekov, S.; Baikara, B.; Fereidouni, S. Mass Mortality in Terns and Gulls Associated with Highly Pathogenic Avian Influenza Viruses in Caspian Sea, Kazakhstan. Viruses 2024, 16, 1661. [Google Scholar] [CrossRef]
- Mamadaliyev, S.; Koshemetov, Z.; Matveyeva, V.; Kydyrbayev, Z.; Zaitsev, V.; Khairullin, B.; Mambetaliyev, M.; Sandybayev, N.; Nurabayev, S.; Azhibayev, A.; et al. Avian influenza virus H5N1 subtype A diagnosed in sick and dead wild and domestic birds in Pavlodar oblast, Republic of Kazakhstan. Afr. J. Agric. Res. 2007, 2, 360–365. [Google Scholar]
- Issabek, A.; Burashev, Y.; Chervyakova, O.; Orynbayev, M.; Kydyrbayev, Z.; Kassenov, M.; Zakarya, K.; Sultankulova, K. Complete genome sequence of the highly pathogenic strain A/domestic Goose/Pavlodar/1/05 (H5N1) of the avian influenza virus, isolated in Kazakhstan in 2005. Microbiol. Resour. Announc. 2020, 9, e00109-20. [Google Scholar] [CrossRef]
- Samorek-Salamonowicz, E.; Czekaj, H.; Kozdruń, W. Ptasia grypa—Aspekty epidemiologiczne. Med. Weter. 2006, 62, 488–492. [Google Scholar]
- Parry, J. Best defence against avian flu is to fight the virus in Asia. Bull. World Health Organ. 2005, 83, 887–889. [Google Scholar]
- H5N1 avian influenza virus expands its range. Vet. Rec. 2005, 157, 242. [CrossRef]
- Li, M.; Liu, H.; Bi, Y.; Sun, J.; Wong, G.; Liu, D.; Li, L.; Liu, J.; Chen, Q.; Wang, H.; et al. Highly Pathogenic Avian Influenza A(H5N8) Virus in Wild Migratory Birds, Qinghai Lake, China. Emerg. Infect. Dis. 2017, 23, 637–641. [Google Scholar] [CrossRef]
- Chervyakova, O.; Strochkov, V.; Sultankulova, K.; Sandybayev, N.; Zaitsev, V.L.; Mamadaliyev, S. Molecular and genetic analysis of NS gene from high pathogenic strains of the avian influenza (H5N1) virus isolated in Kazakhstan. Gene 2011, 476, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Karamendin, K.; Kydyrmanov, A.; Kasymbekov, Y.; Daulbayeva, K.; Khan, E.; Seidalina, A.; Sayatov, M. A Highly Pathogenic H5N1 Influenza A Virus Isolated from a Flamingo on the Caspian Sea Shore. Microbiol. Resour. Announc. 2020, 9, e00508-20. [Google Scholar] [CrossRef] [PubMed]
- Baikara, B.; Seidallina, A.; Baimakhanova, B.; Kasymbekov, Y.; Sabyrzhan, T.; Daulbaeva, K.; Nuralibekov, S.; Khan, Y.; Karamendin, K.; Sultanov, A.; et al. Genome Sequence of Highly Pathogenic Avian Influenza Virus A/Chicken/North Kazakhstan/184/2020 (H5N8). Microbiol. Resour. Announc. 2023, 12, e0115122. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, I.; Gadzhiev, A.; Sharshov, K.; Ohlopkova, O.; Stolbunova, K.; Fadeev, A.; Dubovitskiy, N.; Glushchenko, A.; Irza, V.; Perkovsky, M.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus–Induced Mass Death of Wild Birds, Caspian Sea, Russia, 2022. Emerg. Infect. Dis. 2023, 29, 2528–2532. [Google Scholar] [CrossRef]
- Marchenko, V.Y.; Goncharova, N.I.; Gavrilova, E.V.; Maksyutov, R.A.; Ryzhikov, A.B. Overview of the Epizootiological Situation on Highly Pathogenic Avian Influenza in Russia in 2020. Probl. Osob. Opasnykh Infektsii 2021, 2, 33–40. [Google Scholar] [CrossRef]
- World Organization for Animal Health. Highly Pathogenic Avian Influenza (HPAI) Report N° 15: September 11 to October 1, 2020; World Animal Health Information and Analysis Department: Paris, France, 2020; p. 2. Available online: https://www.woah.org (accessed on 9 August 2025).
- World Organization for Animal Health. Highly Pathogenic Avian Influenza (HPAI) Report N° 16: October 2 to October 22, 2020; World Animal Health Information and Analysis Department: Paris, France, 2020; p. 2. Available online: https://www.woah.org/app/uploads/2022/03/16-hpai-asof22102020.pdf (accessed on 9 August 2025).
- World Organization for Animal Health. Highly Pathogenic Avian Influenza (HPAI) Report N° 18: November 13 to December 3, 2020; World Animal Health Information and Analysis Department: Paris, France, 2020; p. 2. Available online: https://www.woah.org/app/uploads/2022/03/18-hpai-asof03122020.pdf (accessed on 9 August 2025).
- Baikara, B.; Karamendin, K.; Kassymbekov, Y.; Daulbayeva, K.; Sabyrzhan, T.; Nuralibekov, S.; Khan, Y.; Sandybayev, N.; Fereidouni, S.; Kydyrmanov, A. Genetic Characterization of Kazakhstan Isolates: Avian Influenza H9N2 Viruses Demonstrate Their Potential to Infect Mammals. Viruses 2025, 17, 685. [Google Scholar] [CrossRef]
- Adlhoch, C.; Fusaro, A.; Kuiken, T.; Niqueux, É.; Staubach, C.; Terregino, C.; Muñoz Guajardo, I.; Baldinelli, F.; Authority, E.F.S.; Prevention, E.C.; et al. Avian influenza overview May–August 2020. EFSA J. 2020, 18, e06270. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control and European Union Reference Laboratory for Avian Influenza; Adlhoch, C.; Fusaro, A.; Kuiken, T.; Niqueux, E.; Staubach, C.; Terregino, C.; Guajardo, I.M.; Baldinelli, F. Avian influenza overview February–May 2020. EFS2 2020, 18, e06194. [Google Scholar] [CrossRef]
- World Organisation for Animal Health (OIE). Saudi Arabia—Highly Pathogenic Avian Influenza (Poultry)—Immediate Notification; OIE: Paris, France, 2020; p. 3. Available online: https://wahis.woah.org/#/in-review/3118?reportId=16866&fromPage=event-dashboard-url (accessed on 16 August 2025).
- Zhu, Y.; Cong, Y.; Sun, Y.; Han, J.; Gai, L.; Yang, T.; Liu, C.; Zhao, L.; Cong, Y. Isolation and Identification of Novel Highly Pathogenic Avian Influenza Virus (H5N8) Subclade 2.3.4.4b from Geese in Northeastern China. Appl. Environ. Microbiol. 2023, 89, e01572-22. [Google Scholar] [CrossRef]
- Ali, A.A.H.; Mansour, S.; Hefeny, S.M.; Sultan, S. Molecular characterization of avian influenza viruses (H5N2, H5N8, H5Nx and H9N2) isolated from chickens and ducks in the South of Egypt 2020–2021. J. Adv. Vet. Res. 2024, 14, 356–361. [Google Scholar]
- Tabynov, K.; Strochkov, V.; Sandybayev, N.; Karibayev, T.; Berdikulov, M.; Yelchibayeva, L.; Zharmambet, K.; Kuanyshbek, A.; Zhumadilova, Z.; Tabynov, K. Detection and genomic characterization of an avian influenza virus A/mute swan/Mangystau/1-S24R-2/2024 (H5N1; clade 2.3.4.4b) strain isolated from the lung of a dead swan in Kazakhstan. Microbiol. Resour. Announc. 2024, 13, e00260-24. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, M.O.B.; Rashid, P.M.A.; Rahim, Z.H.; Marouf, A.S.; Saeed, S.S. Molecular characterization and genetic analysis of highly pathogenic H5N1 clade 2.3.4.4b in seagulls from Dukan Lake, Iraq. Virus Genes 2025, 61, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Yang, J.; Jiao, W.; Li, X.; Iqbal, M.; Liao, M.; Dai, M. Clade 2.3.4.4b highly pathogenic avian influenza H5N1 viruses: Knowns, unknowns, and challenges. J. Virol. 2025, 99, e00424-25. [Google Scholar] [CrossRef]
- Tabynov, K.; Sansyzbay, A.; Sandybayev, N.; Mambetaliyev, M. The pathogenicity of swan derived H5N1 virus in birds and mammals and its gene analysis. Virol. J. 2014, 11, 207. [Google Scholar] [CrossRef]
- Bopi, A.K.; Omarova, Z.D.; Rystayeva, R.A.; Tulendibayev, A.B.; Argimbayeva, T.U.; Alibekova, D.A.; Aubakir, N.A.; Ermekbay, T.T.; Serikbay, A.A.; Orynbayev, M.B.; et al. Monitoring of Highly Pathogenic Avian Influenza in Kazakhstan. Bopi 2022, 24–30. [Google Scholar] [CrossRef]
- World Organisation for Animal Health (OIE). Highly pathogenic avian influenza in Kazakhstan: Follow-up report No. 1. Dis. Inf. 2005, 18, 258. [Google Scholar]
- OIE. Disease Information; OIE: Paris, France, 2006; Volume 19, p. 142. Available online: https://web.oie.int/downld/infos_san_archives/eng/2006/en_060309v19n10.pdf (accessed on 26 July 2025).
- FAO. Global Animal Disease Intelligence Report; Report No.: 1/2015; FAO: Rome, Italy, 2015; p. 16. [Google Scholar]
- Amirgazin, A.; Shevtsov, A.; Karibayev, T.; Berdikulov, M.; Kozhakhmetova, T.; Syzdykova, L.; Ramankulov, Y.; Shustov, A. Highly pathogenic avian influenza virus of the A/H5N8 subtype, clade 2.3.4.4b, caused outbreaks in Kazakhstan in 2020. PeerJ 2022, 10, e13038. [Google Scholar] [CrossRef]
- Burashev, E.D.; Orynbayev, M.B.; Sultankulova, K.T.; Omarova, Z.D.; Tulendibayev, A.B.; Argimbayeva, T.U.; Aubakir, H.A.; Ermekbai, T.T. Monitoring the spread of avian influenza virus in Kazakhstan. Agrar. Nauka 2024, 41–44. [Google Scholar] [CrossRef]
- World Organization for Animal Health. Highly Pathogenic Avian Influenza (HPAI)—Situation report 8 December 2023 to 5 January 2024; World Organization for Animal Health: Paris, France, 2024. [Google Scholar]
- Lee, J.-Y.; Nam, H.-K.; Park, J.-Y.; Kang, S.-G.; Batbayar, N.; Kim, D.-W.; Hwang, J.-W.; Tsend, O.; Natsagdorj, T.; Nergui, J.; et al. Migration routes and differences in migration strategies of Whooper Swans between spring and autumn. Avian Res. 2023, 14, 100113. [Google Scholar] [CrossRef]
- Li, S.; Meng, W.; Liu, D.; Yang, Q.; Chen, L.; Dai, Q.; Ma, T.; Gao, R.; Ru, W.; Li, Y.; et al. Migratory Whooper Swans Cygnus cygnus Transmit H5N1 Virus Between China and Mongolia: Combination Evidence from Satellite Tracking and Phylogenetics Analysis. Sci. Rep. 2018, 8, 7049. [Google Scholar] [CrossRef] [PubMed]
- Petherbridge, G.; Gadzhiev, A.A.; Shestopalov, A.M.; Alekseev, A.Y.; Sharshov, K.A.; Daudova, M.G. An early warning system for highly pathogenic viruses borne by waterbird species and related dynamics of climate change in the Caspian Sea region: Outlines of a concept. South Russ. Ecol. Dev. 2022, 17, 233–263. [Google Scholar] [CrossRef]
- Kydyrmanov, A.; Sayatov, M.; Karamendin, K.; Zhumatov, K.; Asanova, S.; Daulbayeva, K.; Starick, E.; Fereidouni, S. Monitoring of influenza A viruses in wild bird populations in Kazakhstan in 2002–2009. Arch. Virol. 2017, 162, 147–155. [Google Scholar] [CrossRef]
- Zeng, J.; Du, F.; Xiao, L.; Sun, H.; Lu, L.; Lei, W.; Zheng, J.; Wang, L.; Shu, S.; Li, Y.; et al. Spatiotemporal genotype replacement of H5N8 avian influenza viruses contributed to H5N1 emergence in 2021/2022 panzootic. J. Virol. 2024, 98, e01401-23. [Google Scholar] [CrossRef] [PubMed]
- Burashev, Y.; Strochkov, V.; Sultankulova, K.; Orynbayev, M.; Kassenov, M.; Kozhabergenov, N.; Shorayeva, K.; Sadikaliyeva, S.; Issabek, A.; Almezhanova, M.; et al. Near-complete genome sequence of an H5N1 avian influenza virus strain isolated from a Swan in southwest Kazakhstan in 2006. Microbiol. Resour. Announc. 2020, 9, e00016-20. [Google Scholar] [CrossRef]
- Jindal, M.; Stone, H.; Lim, S.; MacIntyre, C.R. A Geospatial Perspective Toward the Role of Wild Bird Migrations and Global Poultry Trade in the Spread of Highly Pathogenic Avian Influenza H5N1. GeoHealth 2025, 9, e2024GH001296. [Google Scholar] [CrossRef]
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992, 56, 152–179. [Google Scholar] [CrossRef]
- Kim, D.-H.; Lee, D.-Y.; Seo, Y.; Song, C.-S.; Lee, D.-H. Immediate PB2-E627K amino acid substitution after single infection of highly pathogenic avian influenza H5N1 clade 2.3.4.4b in mice. Virol. J. 2025, 22, 183. [Google Scholar] [CrossRef]
- Taft, A.S.; Ozawa, M.; Fitch, A.; Depasse, J.V.; Halfmann, P.J.; Hill-Batorski, L.; Hatta, M.; Friedrich, T.C.; Lopes, T.J.S.; Maher, E.A.; et al. Identification of mammalian-adapting mutations in the polymerase complex of an avian H5N1 influenza virus. Nat. Commun. 2015, 6, 7491. [Google Scholar] [CrossRef]
- New Zealand Institute for Public Health and Forensic Science. Overseas Emerging Respiratory Virus Intelligence; Institute for Public Health and Forensic Science: Auckland, New Zealand, 2025. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Global Avian Influenza Viruses with Zoonotic Potential Situation Update. 2025. Available online: https://www.fao.org/animal-health/situation-updates/global-aiv-with-zoonotic-potential/en (accessed on 6 August 2025).
- Gao, F.; Wang, Q.; Qiu, C.; Luo, J.; Li, X. Pandemic preparedness of effective vaccines for the outbreak of newly H5N1 highly pathogenic avian influenza virus. Virol. Sin. 2024, 39, 981–985. [Google Scholar] [CrossRef]
- Krammer, F.; Hermann, E.; Rasmussen, A.L. Highly pathogenic avian influenza H5N1: History, current situation, and outlook. J. Virol. 2025, 99, e02209-24. [Google Scholar] [CrossRef] [PubMed]
- Blagodatski, A.; Trutneva, K.; Glazova, O.; Mityaeva, O.; Shevkova, L.; Kegeles, E.; Onyanov, N.; Fede, K.; Maznina, A.; Khavina, E.; et al. Avian Influenza in Wild Birds and Poultry: Dissemination Pathways, Monitoring Methods, and Virus Ecology. Pathogens 2021, 10, 630. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhou, S.; Dong, L.; Van Boeckel, T.P.; Cui, Y.; Newman, S.H.; Takekawa, J.Y.; Prosser, D.J.; Xiao, X.; Wu, Y.; et al. Avian influenza H5N1 viral and bird migration networks in Asia. Proc. Natl. Acad. Sci. USA 2015, 112, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Van Der Kolk, J.H. Role for migratory domestic poultry and/or wild birds in the global spread of avian influenza? Vet. Q. 2019, 39, 161–167. [Google Scholar] [CrossRef]
- World Organisation for Animal Health (WOAH). Self-Declaration of Freedom from Infection with Highly Pathogenic Avian Influenza Viruses (HPAI) in Poultry by the Republicof Kazakhstan; WOAH: Paris, France, 2025; Available online: https://www.woah.org/app/uploads/2025/05/2025-05-kazakhstan-hpai-selfd.pdf (accessed on 20 September 2025).
- Gilbert, M.; Xiao, X.; Domenech, J.; Lubroth, J.; Martin, V.; Slingenbergh, J. Anatidae migration in the western Palearctic and spread of highly pathogenic avian influenza H5N1 virus. Emerg. Infect. Dis. 2006, 12, 1650–1656. [Google Scholar] [CrossRef]
- Gubin, B.M. Birds of the deserts of Kazakhstan. In Part 1: Birds of Mangyshlak, Ustyurt and Buzachi Peninsula; Kolor Master: Almaty, Kazakhstan, 2015. [Google Scholar]
- Si, Y.; Skidmore, A.K.; Wang, T.; De Boer, W.F.; Debba, P.; Toxopeus, A.G.; Li, L.; Prins, H.H.T. Spatio-temporal dynamics of global H5N1 outbreaks match bird migration patterns. Geospat. Health 2009, 4, 65. [Google Scholar] [CrossRef]
- An, Q.; Li, Y.; Sun, Z.; Gao, X.; Wang, H. Spatial and Temporal Characteristic Analysis and Risk Assessment of Global Highly Pathogenic Avian Influenza H5N8 Subtype. Transbound. Emerg. Dis. 2024, 2024, 5571668. [Google Scholar] [CrossRef]
- Chechet, O.; Korniienko, L.; Ukhovskyi, V.; Dovgal, O.; Bilyk, S.; Tsarenko, T. Potential Role of Intensive Bird Growing During Outbreaks of Viral Zoonosis in Ukraine, Russian Federation, Kazakhstan and Belarus (on the Model Viruses Highly Pathogenic Influenza and Newcastle Diseases): Systematic Review. J. Pure Appl. Microbiol. 2022, 16, 2363–2400. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhong, D.; Han, Y.; Zhou, Y.; Zhou, H. Knowledge, attitudes, and practices regarding avian influenza among poultry farmers near migratory bird habitats in Guidong County, China. Front. Public Health 2025, 13, 1618292. [Google Scholar] [CrossRef]
- Sultankulova, K.; Dzhekebekov, K.; Orynbayev, M.; Burashev, Y.; Melisbek, A.M.; Barmak, S.; Kozhabergenov, N.; Issabek, A.; Chervyakova, O.; Namet, A.M.; et al. Evidence for flock transmission of individual subtypes and strains of avian influenza viruses: A monitoring study of wild birds in Kazakhstan. Virus Res. 2022, 320, 198898. [Google Scholar] [CrossRef]
- Dhingra, M.S.; Artois, J.; Dellicour, S.; Lemey, P.; Dauphin, G.; Von Dobschuetz, S.; Van Boeckel, T.P.; Castellan, D.M.; Morzaria, S.; Gilbert, M. Geographical and Historical Patterns in the Emergences of Novel Highly Pathogenic Avian Influenza (HPAI) H5 and H7 Viruses in Poultry. Front. Vet. Sci. 2018, 5, 84. [Google Scholar] [CrossRef]
- Barman, S.; Turner, J.C.M.; Hasan, M.K.; Akhtar, S.; Jeevan, T.; Franks, J.; Walker, D.; Mukherjee, N.; Seiler, P.; Kercher, L.; et al. Reassortment of newly emergent clade 2.3.4.4b A(H5N1) highly pathogenic avian influenza A viruses in Bangladesh. Emerg. Microbes Infect. 2025, 14, 2432351. [Google Scholar] [CrossRef]
- Sims, L.D. Avian influenza: Past, present and future. Rev. Sci. Tech. OIE 2024, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Edwards, K.M.; Wille, M.; Wei, X.; Wong, S.-S.; Zanin, M.; El-Shesheny, R.; Ducatez, M.; Poon, L.L.M.; Kayali, G.; et al. The episodic resurgence of highly pathogenic avian influenza H5 virus. Nature 2023, 622, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Karamendin, K.; Kydyrmanov, A.; Zhumatov, K.; Asanova, S.; Ishmukhametova, N.; Sayatov, M. Phylogenetic analysis of avian influenza viruses of H11 subtype isolated in Kazakhstan. Virus Genes 2011, 43, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Murashkina, T.; Sharshov, K.; Gadzhiev, A.; Petherbridge, G.; Derko, A.; Sobolev, I.; Dubovitskiy, N.; Loginova, A.; Kurskaya, O.; Kasianov, N.; et al. Avian Influenza Virus and Avian Paramyxoviruses in Wild Waterfowl of the Western Coast of the Caspian Sea (2017–2020). Viruses 2024, 16, 598. [Google Scholar] [CrossRef]
- Rafique, S.; Rashid, F.; Mushtaq, S.; Ali, A.; Li, M.; Luo, S.; Xie, L.; Xie, Z. Global review of the H5N8 avian influenza virus subtype. Front. Microbiol. 2023, 14, 1200681. [Google Scholar] [CrossRef]
- Niu, Q.; Jiang, Z.; Wang, L.; Ji, X.; Baele, G.; Qin, Y.; Lin, L.; Lai, A.; Chen, Y.; Veit, M.; et al. Prevention and control of avian influenza virus: Recent advances in diagnostic technologies and surveillance strategies. Nat. Commun. 2025, 16, 3558. [Google Scholar] [CrossRef]
- Charostad, J.; Rezaei Zadeh Rukerd, M.; Mahmoudvand, S.; Bashash, D.; Hashemi, S.M.A.; Nakhaie, M.; Zandi, K. A comprehensive review of highly pathogenic avian influenza (HPAI) H5N1: An imminent threat at doorstep. Travel Med. Infect. Dis. 2023, 55, 102638. [Google Scholar] [CrossRef]
- Dawson, E.D.; Moore, C.L.; Dankbar, D.M.; Mehlmann, M.; Townsend, M.B.; Smagala, J.A.; Smith, C.B.; Cox, N.J.; Kuchta, R.D.; Rowlen, K.L. Identification of A/H5N1 influenza viruses using a single gene diagnostic microarray. Anal. Chem. 2007, 79, 378–384. [Google Scholar] [CrossRef]
- Sultankulova, K.; Kozhabergenov, N.; Strochkov, V.; Burashev, Y.; Shorayeva, K.; Chervyakova, O.; Rametov, N.; Sandybayev, N.; Sansyzbay, A.; Orynbayev, M. New oligonucleotide microarray for rapid diagnosis of avian viral diseases. Virol. J. 2017, 14, 69. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.; Sims, L.D.; Stegeman, A.; Swayne, D.E.; Harder, T.; Pavade, G.; Awada, L.; Torres, G.; Abolnik, C.; Delgado, M. Strategic challenges in the global control of high pathogenicity avian influenza. Rev. Sci. Tech. OIE 2024, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zeng, X.; Cui, P.; Yan, C.; Chen, H. Alarming situation of emerging H5 and H7 avian influenza and effective control strategies. Emerg. Microbes Infect. 2023, 12, 2155072. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, N.C.; Abolnik, C.; Baldinelli, F.; Brown, I.; Cameron, A.; De Wit, S.; Dhingra, M.; Espeisse, O.; Guérin, J.-L.; Harder, T.; et al. Vaccination and surveillance for high pathogenicity avian influenza in poultry—Current situation and perspectives. Biologicals 2025, 91, 101840. [Google Scholar] [CrossRef]
- Bilal, M.; Samoon, F.A.; Fahad, M.; Shafiq, M.S.; Iftikhar, M.A.; Ahmad, Y.; Fatima, N.; Mahmood, S.; Cheema, I.A.; Abdullah, O.M. Strategies for Improving Immunity and Production in Broilers: Impact of Vaccination on Poultry Health. Haya Saudi J. Life Sci. 2025, 10, 94–103. [Google Scholar] [CrossRef]
- Astemirov, B.; Mamadaliyev, S.; Perfiliyeva, Y.; Kopochenya, M.A. Comparative Assessment of Seroconversion in Poultry Vaccinated with Two Avian Influenza Vaccines. Am. J. Anim. Vet. Sci. 2022, 17, 211–218. [Google Scholar] [CrossRef]
- EFSA Panel on Animal Health and Animal Welfare (AHAW), European Union Reference Laboratory for Avian Influenza; Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Gortázar, C.; et al. Vaccination of poultry against highly pathogenic avian influenza—Part 1. Available vaccines and vaccination strategies. EFSA J. 2023, 21, e08271. [Google Scholar] [CrossRef]
- Tabynov, K.; Kuanyshbek, A.; Zharmambet, K.; Yelchibayeva, L.; Karibayev, T.; Berdikulov, M.; Zhumadilova, Z.; Tabynov, K. Evaluation of commercial vaccines for efficacy and transmission control against the emergent H5N8 (clade 2.3.4.4b) avian influenza virus in Kazakhstan. Virology 2025, 610, 110601. [Google Scholar] [CrossRef]
- Sansyzbay, A.R.; Erofeeva, M.K.; Khairullin, B.M.; Sandybayev, N.T.; Kydyrbayev, Z.K.; Mamadaliyev, S.M.; Kassenov, M.M.; Sergeeva, M.V.; Romanova, J.R.; Krivitskaya, V.Z.; et al. An inactivated, adjuvanted whole virion clade 2.2 H5N1 (A/Chicken/Astana/6/05) influenza vaccine is safe and immunogenic in a single dose in humans. Clin. Vaccine Immunol. 2013, 20, 1314–1319. [Google Scholar] [CrossRef]
- Myrzakhmetova, B.S.; Zhapparova, G.A.; Tlenchiyeva, T.M.; Bissenbayeva, K.B.; Tussipova, A.A.; Zhugunissov, K.D.; Kutumbetov, L.B. Assessment of biological risks in infectious diseases to ensure biological safety. Eurasian J. Appl. Biotechnol. 2024, 4, 52–59. [Google Scholar] [CrossRef]
- Awada, L.; Vrancken, B.; Thézé, J.; Ducrot, C.; Tizzani, P.; Dellicour, S.; Fusaro, A.; Chalvet-Monfray, K. Quantifying Time-Dependent Predictors for the International Spatial Spread of Highly Pathogenic Avian Influenza H5NX: Focus on Trade and Surveillance Efforts. Transbound. Emerg. Dis. 2025, 2025, 2020766. [Google Scholar] [CrossRef]
- FAO. Preparing for Highly Pathogenic Avian Influenza; FAO Animal Production and Health Manual; Revised; FAO: Rome, Italy, 2006; Volume 3, Available online: https://www.fao.org/4/i0808e/i0808e00.htm (accessed on 11 August 2025).
- One Health High-Level Expert Panel (OHHLEP); Adisasmito, W.B.; Almuhairi, S.; Behravesh, C.B.; Bilivogui, P.; Bukachi, S.A.; Casas, N.; Cediel Becerra, N.; Charron, D.F.; Chaudhary, A.; et al. One Health: A new definition for a sustainable and healthy future. PLoS Pathog. 2022, 18, e1010537. [Google Scholar]
- Lambertucci, S.A.; Santangeli, A.; Plaza, P.I. The threat of avian influenza H5N1 looms over global biodiversity. Nat. Rev. Biodivers. 2025, 1, 7–9. [Google Scholar] [CrossRef]
- Kuiken, T.; Vanstreels, R.E.T.; Banyard, A.; Begeman, L.; Breed, A.C.; Dewar, M.; Fijn, R.; Serafini, P.P.; Uhart, M.; Wille, M. Emergence, spread, and impact of high-pathogenicity avian influenza H5 in wild birds and mammals of South America and Antarctica. Conserv. Biol. 2025, e70052. [Google Scholar] [CrossRef]
- Mackenzie, J.S.; Jeggo, M. The One Health Approach—Why Is It So Important? Trop. Med. Infect. Dis. 2019, 4, 88. [Google Scholar] [CrossRef]
- Sikkema, R.S.; Koopmans, M.P.G. Preparing for Emerging Zoonotic Viruses. In Encyclopedia of Virology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 256–266. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780128145159001508 (accessed on 11 August 2025).
- FAO. Fifth Report on the Global Programme for the Prevention and Control of HPAI (January 2011–January 2012); FAO: Rome, Italy, 2013. [Google Scholar]
- Lewis, N.S.; Banyard, A.C.; Whittard, E.; Karibayev, T.; Al Kafagi, T.; Chvala, I.; Byrne, A.; Meruyert, S.; King, J.; Harder, T.; et al. Emergence and spread of novel H5N8, H5N5 and H5N1 clade 2.3.4.4 highly pathogenic avian influenza in 2020. Emerg. Microbes Infect. 2021, 10, 148–151. [Google Scholar] [CrossRef]
- Spatayev, Y.; Belot, G. Systems Thinking Methodology Application to One Health Enabling Factors Analysis: Case Study from Kazakhstan. One Health Cases 2023, 2023, ohcs20230003. [Google Scholar] [CrossRef]
- WHO. Building One Health Preparedness Capacities: Implementation of the National Bridging Workshop Roadmap in Kazakhstan. 2023. Available online: https://www.who.int/news-room/feature-stories/detail/building-one-health-preparedness-capacities (accessed on 27 August 2025).
- WHO. Adaptation Guide on the One Health Approach for the WHO European Region. 2024. Available online: https://www.who.int/europe/publications/m/item/adaptation-guide-on-the-one-health-approach-for-the-who-european-region (accessed on 27 August 2025).
- Advancing the Implementation of the One Health Approach in the WHO European Region. 2024. Available online: https://www.who.int/europe/news/item/29-10-2024-advancing-the-implementation-of-the-one-health-approach-in-the-who-european-region (accessed on 27 August 2025).
- Yamaji, R.; Saad, M.D.; Davis, C.T.; Swayne, D.E.; Wang, D.; Wong, F.Y.K.; McCauley, J.W.; Peiris, J.S.M.; Webby, R.J.; Fouchier, R.A.M.; et al. Pandemic potential of highly pathogenic avian influenza clade 2.3.4.4 A(H5) viruses. Rev. Med. Virol. 2020, 30, e2099. [Google Scholar] [CrossRef]
| № | Analyzed Strain | Clade | Phylogeographic Inference | Sources |
|---|---|---|---|---|
| 1 | A virus/domestic goose/Pavlodar/1/05 (H5N1) | 2.2 | Qinghai–Siberian lineage | [19] |
| 2 | A/swan/Mangystau/3/2006 (H5N1) | EA-nonGsGD | Probably a reassortant strain; Russian–Far East–Japan | [41] |
| 3 | A/flamingo/Mangistau/6570/2015 (H5N1) | 2.3.2.1c | Asia–Middle East–Eastern Europe–West Africa lineage | [25] |
| 4 | A/chicken/ Akmola/62/21 (H5N8) | 2.3.4.4b | Europe–Central Asia–Middle East | [42] |
| 5 | A/chicken/North Kazakhstan/184/2020 (H5N8) | 2.3.4.4b | Middle East–West Africa lineage | [26] |
| 6 | A/Caspian tern/Atyrau/9184/2022(H5N1) | 2.3.4.4b | Russian–Caspian Sea | [17] |
| 7 | A/Mute swan/Mangystau/9809/2023 (H5N1) | 2.3.4.4b | Reassortant strain; Siberia–Egypt–North Africa | [11] |
| 8 | A/Cygnus cygnus/Karakol lake/01/2024 (H5N1) | 2.3.4.4b | Russia–Caspian Sea | [11,38] |
| 9 | A/Mute swan/Karakol Lake/02/2024 (H5N1) | 2.3.4.4b | The virus recombined with viruses from ducks in Russia | [11,38] |
| 10 | A/mute swan/Mangystau/1-S24R-2/2024 (H5N1) | 2.3.4.4b | Egypt–Black Sea–Caspian route | [38] |
| Year | Season of Year, Month in Kazakhstan | HPAI Subtypes | Region | Host Category | Species | Sources | |
|---|---|---|---|---|---|---|---|
| H5N1 | H5N8 | ||||||
| 2005 | Late summer–early autumn | + | - | North Kazakhstan region | Poultry | Geese | [18,43] |
| Wild birds | Whooper swans | ||||||
| 2006 | Spring: March | + | - | Coast of the Caspian Sea | Wild birds | Swans | [41,44] |
| 2015 | Spring: May | + | - | Coast of the Caspian Sea | Wild birds | Flamingoes | [9,25,45], |
| 2020 | Autumn: September–November | - | + | Coast of the Caspian Sea North Kazakhstan region | Poultry | Chickens, ducks, and geese | [36,46,47] |
| 2021 | Autumn: October | - | + | Qostanay region, North Kazakhstan region, East Kazakhstan region, and Aqmola | Poultry | Chickens, geese, and turkeys | [42] |
| 2022 | Summer: June–July | + | - | Coast of the Caspian Sea | Wild birds | Terns and gulls | [17] |
| 2023–2024 | Winter: December–January | + | - | Coast of the Caspian Sea | Wild birds | Whooper swans and mute swans | [11,38,48] |
| № | Virus Strain, Bird/Place/ Year of the Flu Outbreak | Characteristic Features | Source |
|---|---|---|---|
| 1 | A/domestic goose/Pavlodar/1/05 (H5N1) (GS/1/05)—domestic geese, northern Kazakhstan, 2005 | The presence of a polybasic proteolytic cleavage site of HA; HA, NA, and NS1—increased tropism to mammals, resistance to interferons, and tumor necrosis factor | [19] |
| 2 | A/swan/Mangystau/3/2006 (H5N1)—dead swan, southeastern coast of the Caspian Sea, 2006 | HA—lacks a polybasic cleavage site; however, according to the WOAH classification *, the strain was considered highly pathogenic, as its intravenous pathogenicity index (IVPI) (N) was 2.34 | [54] |
| 3 | A/flamingo/Mangistau/6570/2015 (H5N1)—flamingo, Caspian Sea, 2015 | The presence of a polybasic proteolytic cleavage site of HA, the PQRERRRKR*GLF motif, and the HPAI marker. | [25] |
| 4 | A/chicken/North Kazakhstan/184/2020 (H5N8)—chicken, Northern Kazakhstan 2020 | The presence of a polybasic proteolytic cleavage site of HA, the KRRKR/GLF motif (identical to European strains in 2016–2017), and the HPAI marker | [26] |
| 5 | A/chicken/ Akmola/62/21 (H5N8) | PLREKRRKR/G cleavage site-marker HPAIV | [42] |
| A/wild goose/ Qostanay/83/21 (H5N8) | |||
| A/domestic goose/Akmola/65/21 (H5N8) | |||
| A/chicken/ North Kazakhstan/97/21 (H5N8) | |||
| 6 | A/Caspian tern/Atyrau/9184/2022(H5N1)—terns, north-eastern coast of the Caspian Sea in 2022 | The presence of an HA proteolytic cleavage site (PLREKRRKR*GLF); the isolate was classified as HPAIV | [17] |
| 7 | A/Mute swan/Mangystau/9809/2023(H5N1)—mute swan, Mangystau 2023 | The presence of a polybasic proteolytic cleavage site of the HA—PLREKRRRKR/G marker of HPAIV | [11] |
| 8 | A/Cygnus cygnus/Karakol lake/01/2024(H5N1)—whooper Swan Lake Karakol 2024 | [11] | |
| 9 | A/Mute swan/Karakol lake/02/2024(H5N1)—mute swan of Lake Karakol 2024 | [11] | |
| 10 | A/mute swan/Mangystau/1-S24R-2/2024 (H5N1; clade 2.3.4.4b)—mute swan, lake Karakol 2024 | The presence of the HA cleavage site—PLREKRRKRGLF marker HPAIV | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekshin, Z.; Temirbekova, A.; Nurbekova, Z.; Amirkhanova, N.; Satenova, A.; Askarov, A.; Zakarya, K.; Abduraimov, Y.; Rsaliyev, A. Epidemiology, Virology, and Control of Highly Pathogenic Avian Influenza in Kazakhstan. Pathogens 2025, 14, 1084. https://doi.org/10.3390/pathogens14111084
Bekshin Z, Temirbekova A, Nurbekova Z, Amirkhanova N, Satenova A, Askarov A, Zakarya K, Abduraimov Y, Rsaliyev A. Epidemiology, Virology, and Control of Highly Pathogenic Avian Influenza in Kazakhstan. Pathogens. 2025; 14(11):1084. https://doi.org/10.3390/pathogens14111084
Chicago/Turabian StyleBekshin, Zhandarbek, Aliya Temirbekova, Zhadyrassyn Nurbekova, Nurgul Amirkhanova, Akbota Satenova, Albert Askarov, Kunsulu Zakarya, Yergali Abduraimov, and Aralbek Rsaliyev. 2025. "Epidemiology, Virology, and Control of Highly Pathogenic Avian Influenza in Kazakhstan" Pathogens 14, no. 11: 1084. https://doi.org/10.3390/pathogens14111084
APA StyleBekshin, Z., Temirbekova, A., Nurbekova, Z., Amirkhanova, N., Satenova, A., Askarov, A., Zakarya, K., Abduraimov, Y., & Rsaliyev, A. (2025). Epidemiology, Virology, and Control of Highly Pathogenic Avian Influenza in Kazakhstan. Pathogens, 14(11), 1084. https://doi.org/10.3390/pathogens14111084






