Canine Leishmaniasis in Europe over the Last Decade: A Review of Geographic Trends and Epidemiological Data
Abstract
1. Introduction
2. Materials and Methods
- original studies published between 2015 and 2024;
- studies conducted in Europe (specifically, in European countries or territories);
- explicit reporting of Leishmania spp. prevalence in dogs;
- specification of the employed diagnostic method and the type of sample;
- identification of Leishmania species involved, where possible.
3. Results
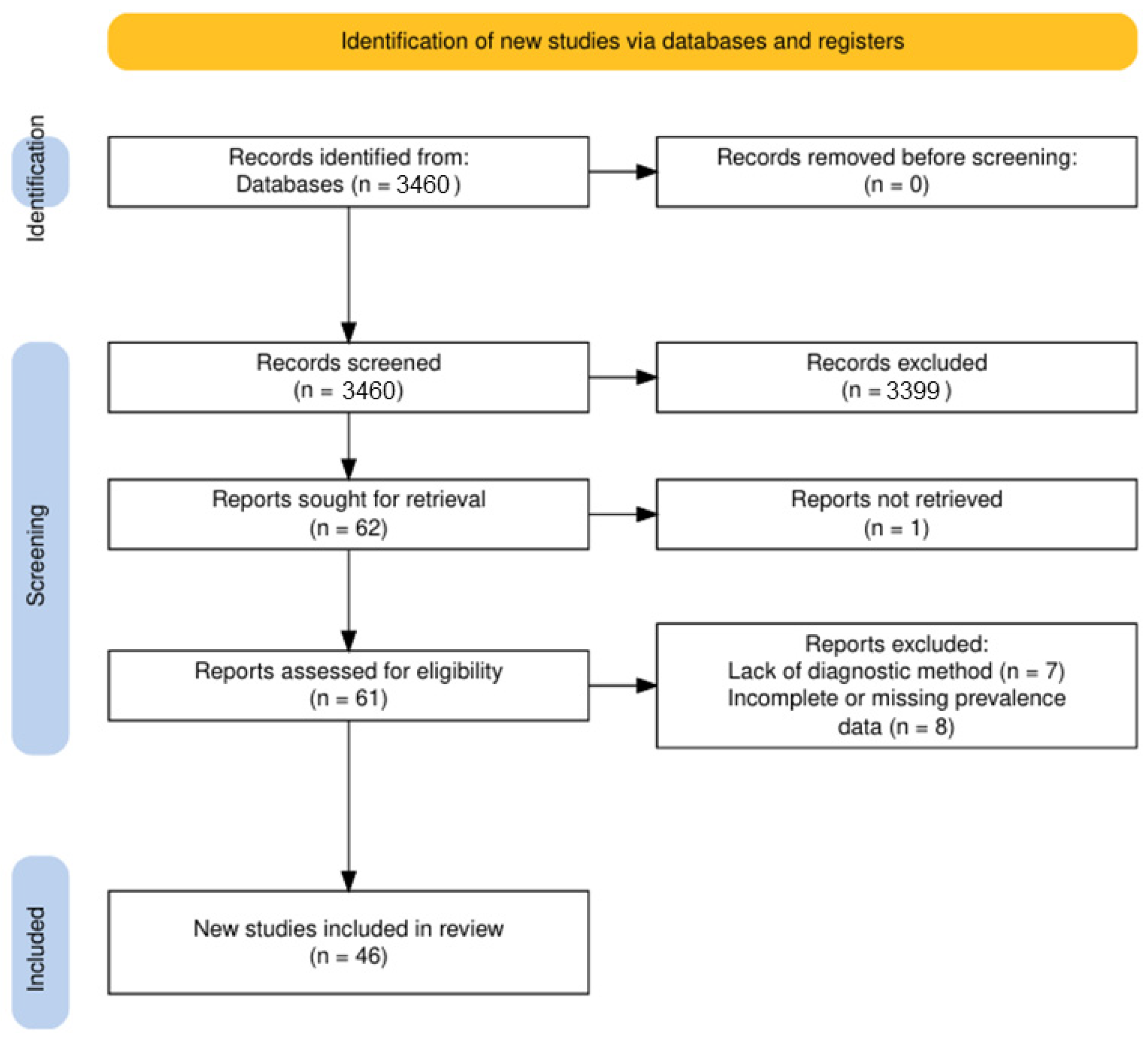
3.1. Study Selection Process
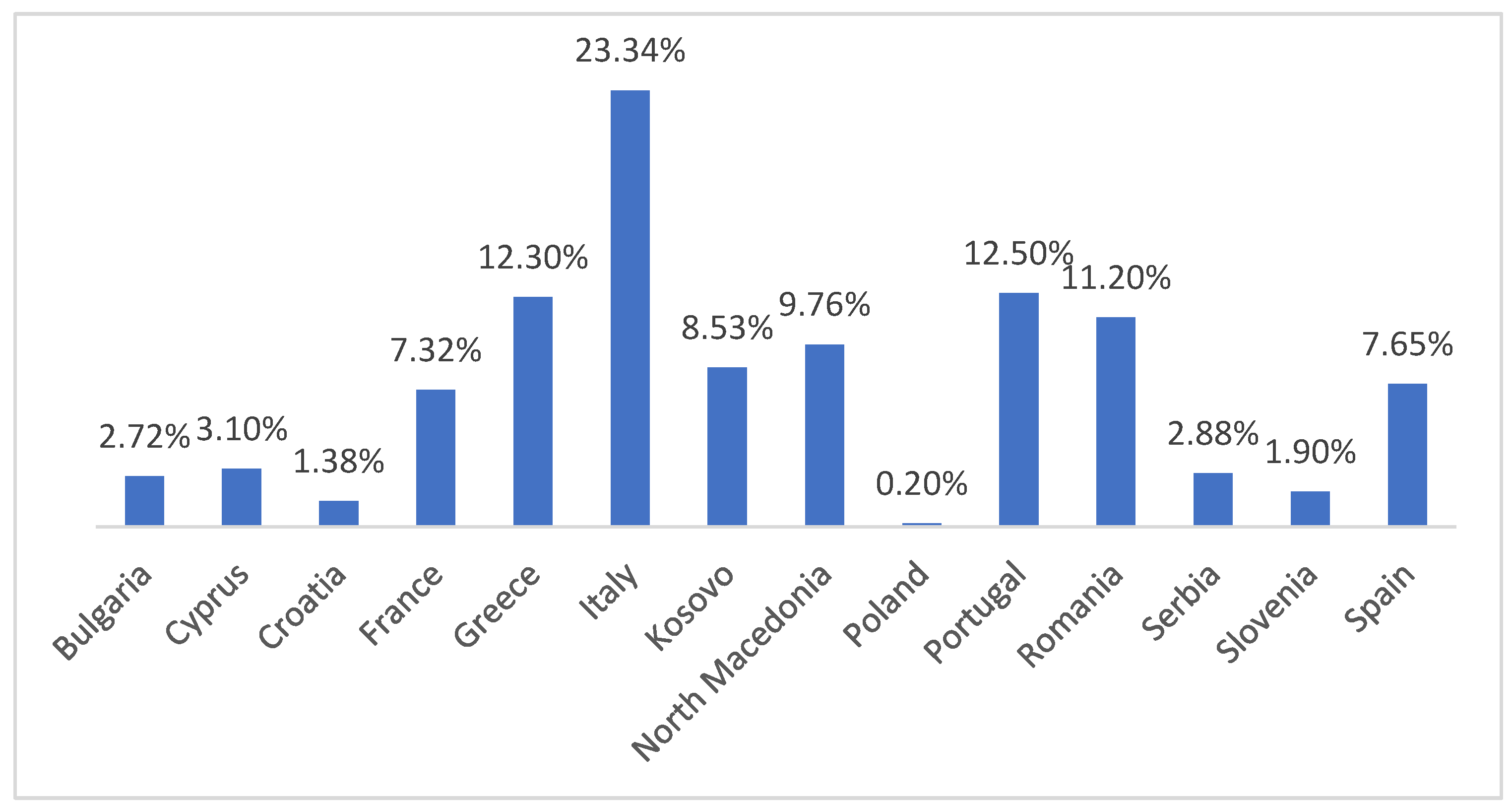
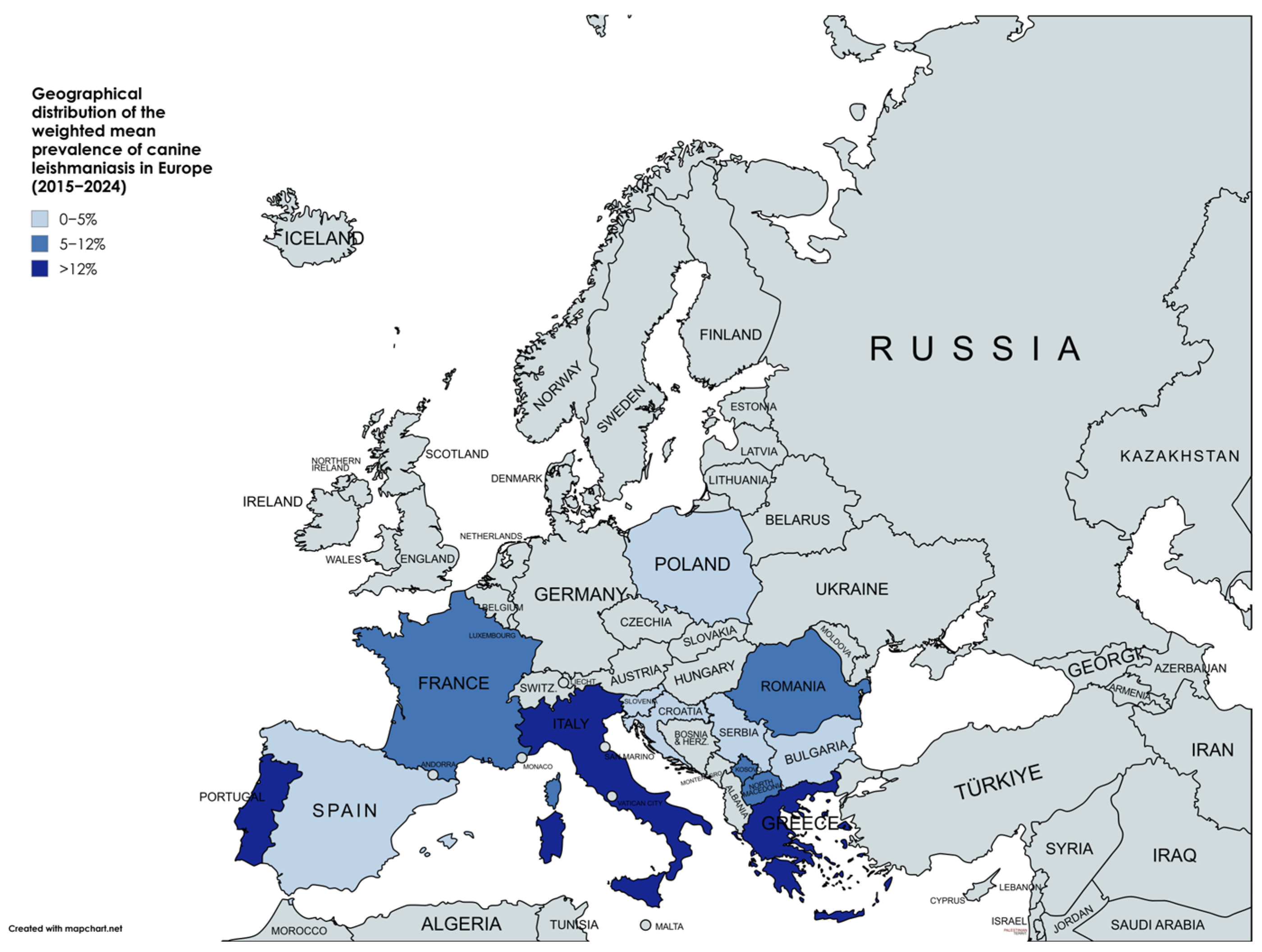
3.2. Geographical Distribution and Prevalence
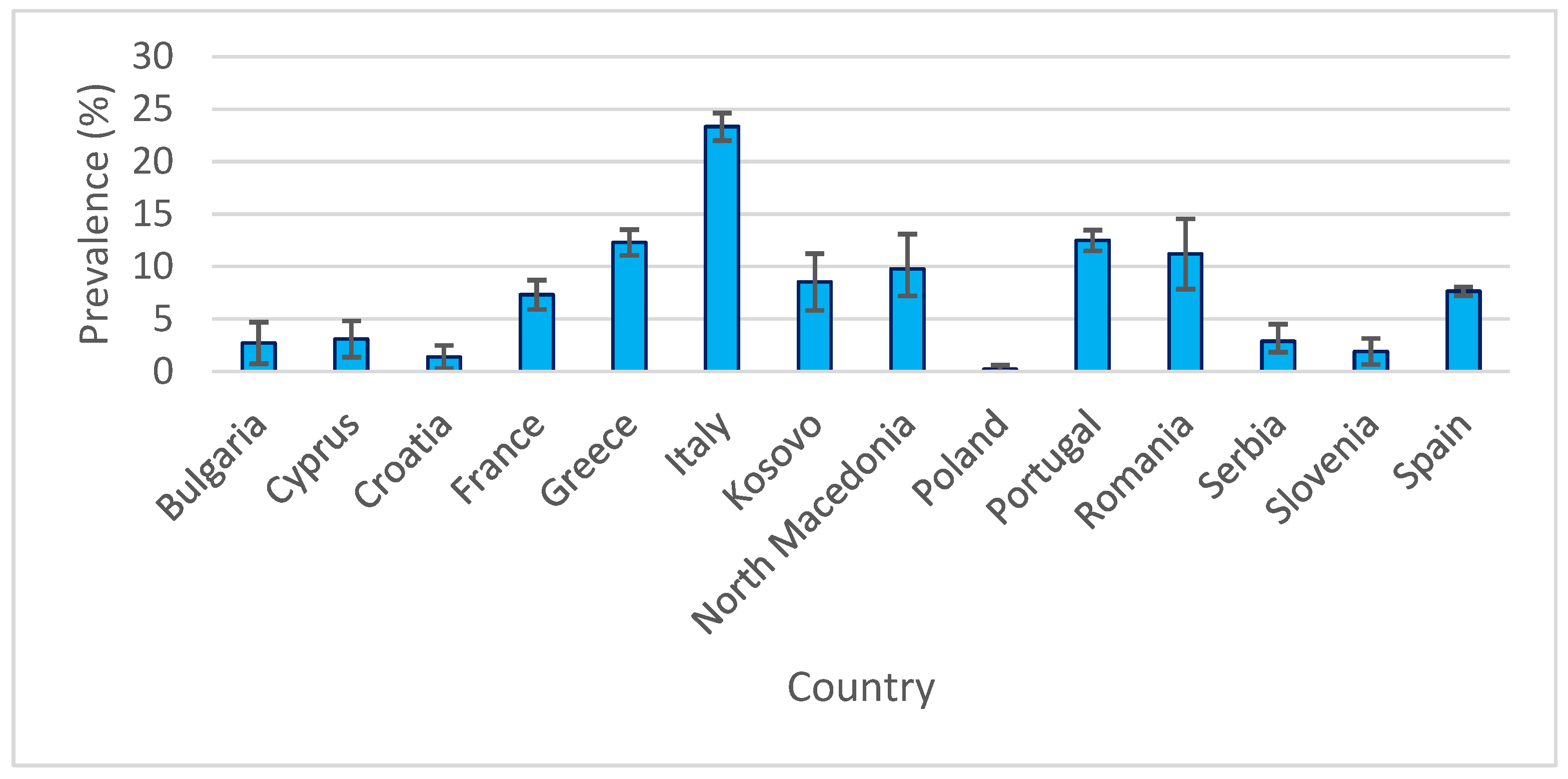
3.3. Weighted Means Prevalence
3.4. Confidence Intervals
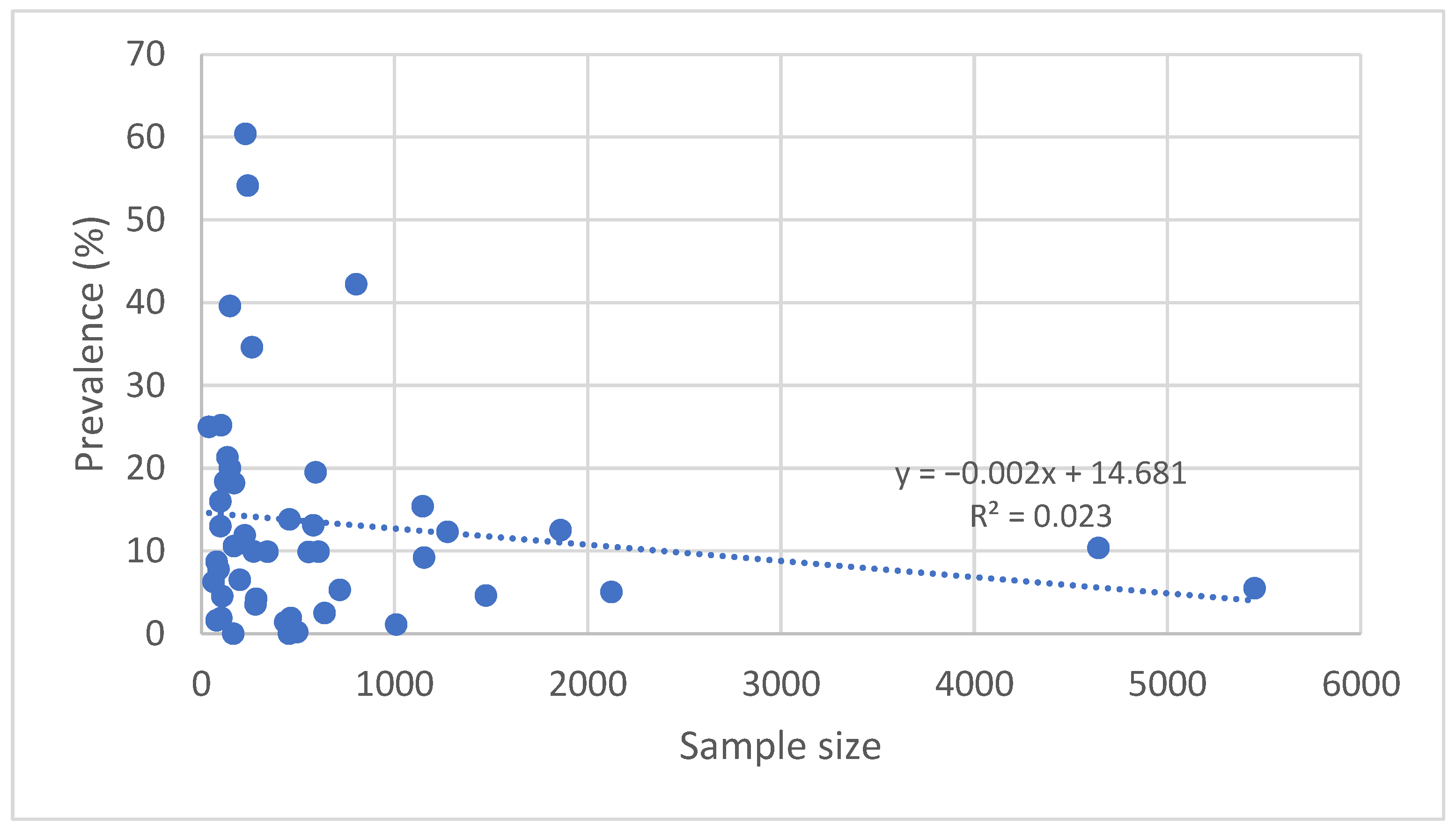
3.5. Relationship Between the Size of the Cohort and Prevalence
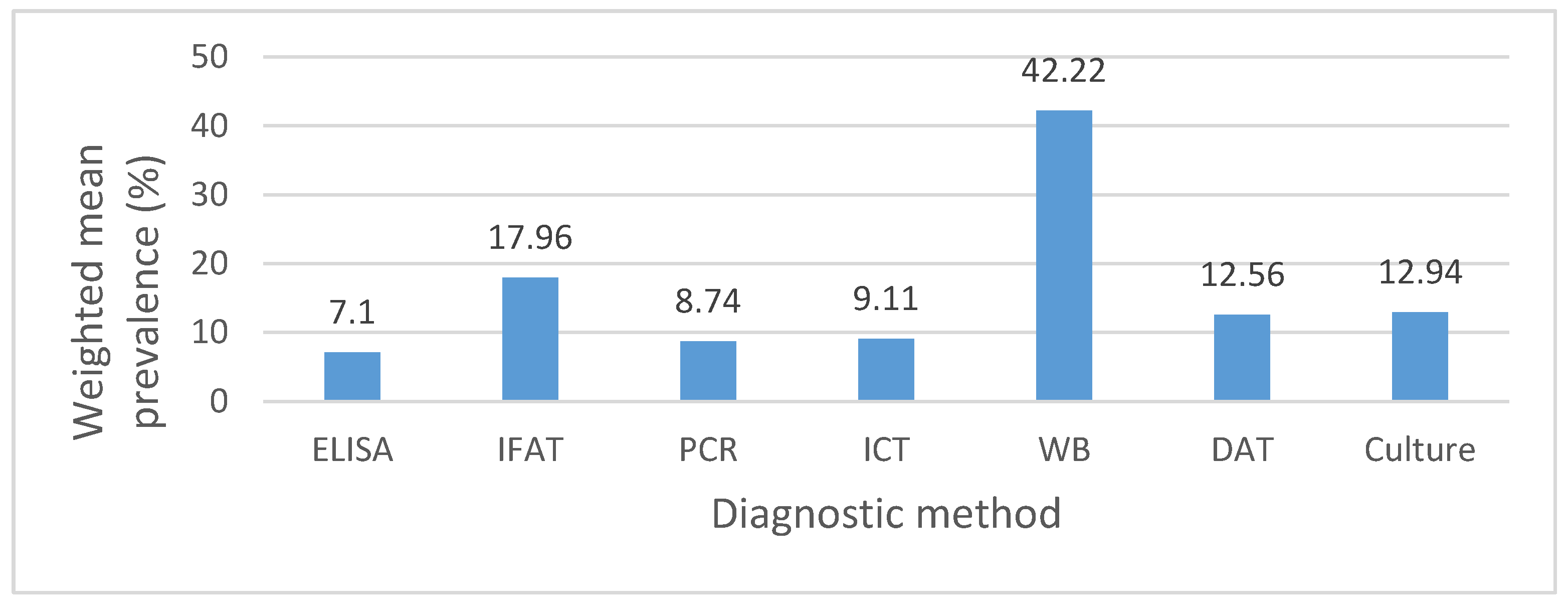
3.6. The Influence of Diagnostic Methods on Prevalence Rate Calculations
4. Discussion
4.1. Geographical Variation in Prevalence
4.2. Impact of Diagnostic Methods
4.3. Relationship Between Sample Size and Prevalence
4.4. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akhoundi, M.; Kuhls, K.; Cannet, A.; Votýpka, J.; Marty, P.; Delaunay, P.; Sereno, D. A historical overview of the classification, evolution, and dispersion of Leishmania parasites and sandflies. PLoS Neglected Trop. Dis. 2016, 10, e0004770. [Google Scholar] [CrossRef]
- Cosma, C.; Maia, C.; Khan, N.; Infantino, M.; Del Riccio, M. Leishmaniasis in humans and animals: A One Health approach for surveillance, prevention and control in a changing world. Trop. Med. Infect. Dis. 2024, 9, 258. [Google Scholar] [CrossRef]
- Zavitsanou, A.; Koutis, C.; Babatsikou, F. Leishmaniasis: An overlooked public health concern. Health Sci. J. 2008, 2, 196–205. [Google Scholar]
- Dărăbuș, G.; Morariu, S.; Oprescu, I.; Mederle, N.; Ilie, M.; Imre, M. Parazitologie și Boli Parazitare; Mirton, Ed.; Mirton: Timișoara, Romania, 2022. [Google Scholar]
- Gramiccia, M.; Gradoni, L. The current status of zoonetic leishmaniasis and approaches to disease control. Int. J. Parasitol. 2005, 35, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Ayele, A.; Zewdu, S. Review on canine leishmaniasis, etiology, clinical sign, pathogenesis, treatment and control methods. Glob. Vet. 2016, 17, 343–352. [Google Scholar]
- Maroli, M.; Feliciangeli, M.D.; Bichaud, L.; Charrel, R.N.; Gradoni, L. Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Med. Vet. Entomol. 2012, 27, 123–147. [Google Scholar] [CrossRef] [PubMed]
- Ready, P.D. Biology of phlebotomine sand flies as vectors of disease agents. Annu. Rev. Entomol. 2013, 58, 227–250. [Google Scholar] [CrossRef]
- Serafim, T.D.; Coutinho-Abreu, I.V.; Dey, R.; Kissinger, R.; Valenzuela, J.G.; Oliveira, F.; Kamhawi, S. Leishmaniasis: The act of transmission. Trends Parasitol. 2021, 37, 976–987. [Google Scholar] [CrossRef]
- Vilas-Boas, D.F.; Nakasone, E.K.N.; Gonçalves, A.A.M.; Lair, D.F.; Oliveira, D.S.d.; Pereira, D.F.S.; Silva, G.G.; Conrado, I.D.S.S.; Resende, L.A.; Zaldívar, M.F.; et al. Global Distribution of Canine Visceral Leishmaniasis and the Role of the Dog in the Epidemiology of the Disease. Pathogens 2024, 13, 455. [Google Scholar] [CrossRef]
- Hide, M.; Bucheton, B.; Kamhavi, S.; Bras-Gonçavales, R.; Sundar, S.; Lemesre, J.L.; Banuls, A.L. Understanding Human Leishmaniasis. The Need for an Integrated Approach. In Encyclopedia of Infectious Diseases: Modern Methodologies; John Wiley & Sons: Hoboken, NJ, USA, 2007; Chapter 6; pp. 87–123. [Google Scholar]
- Alemayehu, B.; Alemayehu, M. Leishmaniasis: A review on parasite, vector and reservoir host. Health Sci. J. 2017, 11, 519. [Google Scholar] [CrossRef]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M.; WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- Pennisi, M.G. Leishmaniosis of companion animals in Europe: An update. Vet. Parasitol. 2015, 208, 35–47. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Leishmaniasis. Available online: https://www.who.int/news-room/fact-sheets/detail/Leishmaniasis (accessed on 25 June 2025).
- Alvar, J.; Canavate, C.; Molina, R.; Moreno, J.; Nieto, J. Canine leishmaniasis. Adv. Parasitol. 2004, 57, 1–88. [Google Scholar]
- Kaszak, I.; Planellas, M.; Dworecka-Kaszak, B. Canine leishmaniosis—An emerging disease. Ann. Parasitol. 2015, 61, 69–76. [Google Scholar]
- Roberio, R.R.; Marques Michalick, M.S.; da Silva, M.E.; Dos Santos, C.C.P.; Frezard, F.J.G.; da Silva, S.M. Canine Leishmaniasis: An Overview of the Current Status and Strategies for Control. BioMed Res. Int. 2018, 2018, 3296893. [Google Scholar] [CrossRef]
- Paltinieri, S.; Gardoni, L.; Roura, X.; Zatelli, A.; Zini, E. Laboratory tests for diagnosing and monitoring canine leishmaniasis. Vet. Clin. Pathol. 2016, 45, 552–578. [Google Scholar] [CrossRef]
- Morales-Yuste, M.; Martín-Sánchez, J.; Corpas-Lopez, V. Canine Leishmaniasis: Update on Epidemiology, Diagnosis, Treatment, and Prevention. Vet. Sci. 2022, 9, 387. [Google Scholar] [CrossRef] [PubMed]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef] [PubMed]
- Stoimenov, G.M.; Tchakarova, S. Detection of Leishmania infantum antibodies in stray dogs from nonendemic areas in Bulgaria. Vector-Borne Zoonotic Dis. 2024, 24, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Pantchev, N.; Schnyder, M.; Vrhovec, M.G.; Schaper, R.; Tsachev, I. Current Surveys of the Seroprevalence of Borrelia burgdorferi, Ehrlichia canis, Anaplasma phagocytophilum, Leishmania infantum, Babesia canis, Angiostrongylus vasorum and Dirofilaria immitis in Dogs in Bulgaria. Parasitol. Res. 2015, 114 (Suppl. S1), 117–130. [Google Scholar] [CrossRef]
- Beyhan, Y.E.; Çelebi, B.; Ergene, O.; Mungan, M. Seroprevalance of Leishmaniasis in Dogs from Hatay and Burdur Provinces of Turkey and Northern Cyprus. Turk. J. Parasitol. 2016, 40, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Çanakçı, T.; Kurtdede, A.; Paşa, S.; Töz Özensoy, S.; Özbel, Y. Seroprevalence of Canine Leishmaniasis in Northern Cyprus. Turk. J. Parasitol. 2016, 40, 117–120. [Google Scholar] [CrossRef]
- Mrljak, V.; Kuleš, J.; Mihaljević, Ž.; Torti, M.; Gotić, J.; Crnogaj, M.; Živičnjak, T.; Mayer, I.; Šmit, I.; Bhide, M.; et al. Prevalence and Geographic Distribution of Vector-Borne Pathogens in Apparently Healthy Dogs in Croatia. Vector-Borne Zoonotic Dis. 2017, 17, 398–408. [Google Scholar] [CrossRef]
- Laidoudi, Y.; Sevestre, J.; Bedjaoui, S.; Watier-Grillot, S.; Davoust, B. Serosurvey of canine leishmaniasis in five departments near an identified human clinical case in Marseille (France). One Health 2024, 19, 100855. [Google Scholar] [CrossRef]
- Hide, M.; Michel, G.; Legueult, K.; Pin, R.; Leonard, S.; Simon, L.; Bañuls, A.L.; Delaunay, P.; Marty, P.; Pomares, C. Asymptomatic Leishmania infantum infection in dogs and dog owners in an endemic area in southeast France. Parasite 2024, 31, 16. [Google Scholar] [CrossRef]
- Kostopoulou, D.; Gizzarelli, M.; Ligda, P.; Foglia Manzillo, V.; Saratsi, K.; Montagnaro, S.; Schunack, B.; Boegel, A.; Pollmeier, M.; Oliva, G.; et al. Mapping the canine vector-borne disease risk in a Mediterranean area. Parasites Vectors 2020, 13, 282. [Google Scholar] [CrossRef] [PubMed]
- Ntais, P.; Christodoulou, V.; Dokianakis, E.; Antoniou, M. Leishmania infantum and Dirofilaria immitis coinfection in dogs in Greece. Parasitol. Open 2016, 2, e17. [Google Scholar] [CrossRef]
- Hofmann, M.; Hodžić, A.; Pouliou, N.; Joachim, A. Vector-borne pathogens affecting shelter dogs in eastern Crete, Greece. Parasitol. Res. 2019, 118, 1661–1666. [Google Scholar] [CrossRef]
- Diakou, A.; Di Cesare, A.; Morelli, S.; Colombo, M.; Halos, L.; Simonato, G.; Tamvakis, A.; Beugnet, F.; Paoletti, B.; Traversa, D. Endoparasites and vector-borne pathogens in dogs from Greek islands: Pathogen distribution and zoonotic implications. PLoS Neglected Trop. Dis. 2019, 13, e0007003. [Google Scholar] [CrossRef] [PubMed]
- Morosetti, G.; Toson, M.; Trevisiol, K.; Idrizi, I.; Natale, A.; Lucchese, L.; Michelutti, A.; Ceschi, P.; Lorenzi, G.; Piffer, C.; et al. Canine leishmaniosis in the Italian northeastern Alps: A survey to assess serological prevalence in dogs and distribution of phlebotomine sand flies in the Autonomous Province of Bolzano—South Tyrol, Italy. Vet. Parasitol. Reg. Stud. Rep. 2020, 21, 100432. [Google Scholar] [CrossRef]
- Foglia Manzillo, V.; Gizzarelli, M.; Vitale, F.; Montagnaro, S.; Torina, A.; Sotera, S.; Oliva, G. Serological and entomological survey of canine leishmaniasis in Lampedusa island, Italy. BMC Vet. Res. 2018, 14, 286. [Google Scholar] [CrossRef]
- Tamponi, C.; Scarpa, F.; Carta, S.; Knoll, S.; Sanna, D.; Gai, C.; Pipia, A.P.; Dessì, G.; Casu, M.; Varcasia, A.; et al. Seroprevalence and risk factors associated with Leishmania infantum in dogs in Sardinia (Italy), an endemic island for leishmaniasis. Parasitol. Res. 2021, 120, 289–300. [Google Scholar] [CrossRef]
- Mendoza-Roldan, J.A.; Latrofa, M.S.; Iatta, R.R.S.; Manoj, R.; Panarese, R.; Annoscia, G.; Pombi, M.; Zatelli, A.; Beugnet, F.; Otranto, D. Detection of Leishmania tarentolae in lizards, sand flies and dogs in southern Italy, where Leishmania infantum is endemic: Hindrances and opportunities. Parasites Vectors 2021, 14, 461. [Google Scholar] [CrossRef] [PubMed]
- Vitale, F.; Bruno, F.; Migliazzo, A.; Galante, A.; Vullo, A.; Graziano, R.; D’Avola, S.; Caputo, V.; Castelli, G. Cross-sectional survey of canine leishmaniasis in Pantelleria island in Sicily. Vet. Ital. 2020, 56, 103–107. [Google Scholar]
- Otranto, D.; Napoli, E.; Latrofa, M.S.; Annoscia, G.; Tarallo, V.D.; Greco, G.; Lorusso, E.; Gulotta, L.; Falsone, L.; Basano, F.S.; et al. Feline and canine leishmaniosis and other vector-borne diseases in the Aeolian Islands: Pathogen and vector circulation in a confined environment. Vet. Parasitol. 2017, 236, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Sauda, F.; Malandrucco, L.; Macrì, G.; Scarpulla, M.; De Liberato, C.; Terracciano, G.; Fichi, G.; Berrilli, F.; Perrucci, S. Leishmania infantum, Dirofilaria spp. and other endoparasite infections in kennel dogs in central Italy. Parasite 2018, 25, 2. [Google Scholar] [CrossRef] [PubMed]
- Iatta, R.; Mendoza-Roldan, J.A.; Latrofa, M.S.; Cascio, A.; Brianti, E.; Pombi, M.; Gabrielli, S.; Otranto, D. Leishmania tarentolae and Leishmania infantum in humans, dogs and cats in the Pelagie archipelago, southern Italy. PLoS Negl. Trop. Dis. 2021, 15, e0009817. [Google Scholar] [CrossRef]
- Ferroglio, E.; Battisti, E.; Zanet, S.; Bolla, C.; Concialdi, E.; Trisciuoglio, A.; Khalili, S.; Biglino, A. Epidemiological evaluation of Leishmania infantum zoonotic transmission risk in the recently established endemic area of Northwestern Italy. Zoonoses Public Health 2018, 65, 675–682. [Google Scholar] [CrossRef]
- Xhekaj, B.; Stefanovska, J.; Sherifi, K.; Rexhepi, A.; Bizhga, B.; Rashikj, L.; Nikolovski, M.; Kniha, E.; Cvetkovikj, A. Seroprevalence of canine leishmaniosis in asymptomatic dogs in Kosovo. Parasitol. Res. 2023, 122, 607–614. [Google Scholar] [CrossRef]
- Xhekaj, B.; Alishani, M.; Rexhepi, A.; Jakupi, X.; Sherifi, K. Serological and clinical survey of Canine Leishmaniasis in Southwestern Region of Kosovo. Vet. Ital. 2020, 56, 47–50. [Google Scholar]
- Mamuti, D.; Murati, E.; Seferi, N.; Elezi, B.; Ademi, Z.; Idrizi, H. Detection of Leishmaniasis Disease in Stray Dogs in the Tetovo Region and Breeds with Higher Susceptibility. J. Agric. Sustain. Rural. Dev. 2023, 1, 51–56. [Google Scholar]
- Petrov, E.A.; Novakov, T.; Gjorgjievska, S.; Celeska, I. Haematology and serum biochemistry parameters as indicators of the most common canine vector borne diseases in RN Macedonia. In Tradition and Modernity in Veterinary Medicine; Faculty of Veterinary Medicine, University of Forestry: Sofia, Bulgaria, 2023; Volume 8, pp. 3–10. [Google Scholar]
- Tasić-Otašević, S.; Savić, S.; Jurhar-Pavlova, M.; Stefanovska, J.; Stalević, M.; Ignjatović, A.; Ranđelović, M.; Gajić, B.; Cvetkovikj, A.; Gabrielli, S. Molecular Survey of Dirofilaria and Leishmania Species in Dogs from Central Balkan. Animals 2022, 12, 911. [Google Scholar] [CrossRef]
- Adaszek, Ł.; Staniec, M.; Dokuzeylül, B.; Pisarek, M.; Skrzypczak, M.; Żółkiewski, P.; Rutkowska-Szulczyk, M.; Deneka, Ł.; Or, M.E.; Winiarczyk, S. Vector-borne diseases imported to Poland between 2021 and 2023. J. Vet. Res. 2024, 68, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Afonso, P.; Coelho, A.C.; Quintas, H.; Cardoso, L. Leishmania Seroprevalence in Dogs: Comparing Shelter and Domestic Communities. Animals 2023, 13, 2352. [Google Scholar] [CrossRef]
- Almeida, M.; Maia, C.; Cristóvão, J.M.; Morgado, C.; Barbosa, I.; Ibars, R.F.; Campino, L.; Gonçalves, L.; Cortes, S. Seroprevalence and Risk Factors Associated with Leishmania Infection in Dogs from Portugal. Microorganisms 2022, 10, 2262. [Google Scholar] [CrossRef]
- Maia, C.; Almeida, B.; Coimbra, M.; Fernandes, M.C.; Cristóvão, J.M.; Ramos, C.; Martins, Â.; Martinho, F.; Silva, P.; Neves, N.; et al. Bacterial and protozoal agents of canine vector-borne diseases in the blood of domestic and stray dogs from southern Portugal. Parasites Vectors 2015, 8, 138. [Google Scholar] [CrossRef]
- Maia, C.; Coimbra, M.; Ramos, C.; Cristóvão, J.M.; Cardoso, L.; Campino, L. Serological investigation of Leishmania infantum, Dirofilaria immitis and Angiostrongylus vasorum in dogs from southern Portugal. Parasites Vectors 2015, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Alho, A.M.; Pita, J.; Amaro, A.; Amaro, F.; Schnyder, M.; Grimm, F.; Custódio, A.C.; Cardoso, L.; Deplazes, P.; de Carvalho, L.M. Seroprevalence of vector-borne pathogens and molecular detection of Borrelia afzelii in military dogs from Portugal. Parasites Vectors 2016, 9, 225. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.; Alwassouf, S.; Cristóvão, J.M.; Ayhan, N.; Pereira, A.; Charrel, R.N.; Campino, L. Serological association between Leishmania infantum and sand fly fever Sicilian (but not Toscana) virus in sheltered dogs from southern Portugal. Parasites Vectors 2017, 10, 92. [Google Scholar] [CrossRef]
- Maia, C.; Altet, L.; Serrano, L.; Cristóvão, J.M.; Tabar, M.D.; Francino, O.; Cardoso, L.; Campino, L.; Roura, X. Molecular detection of Leishmania infantum, filariae and Wolbachia spp. in dogs from southern Portugal. Parasites Vectors 2016, 9, 170. [Google Scholar] [CrossRef]
- Dumitrache, M.O.; Nachum-Biala, Y.; Gilad, M.; Mircean, V.; Cazan, C.D.; Mihalca, A.D.; Baneth, G. The quest for canine leishmaniasis in Romania: The presence of an autochthonous focus with subclinical infections in an area where disease occurred. Parasites Vectors 2016, 9, 297. [Google Scholar] [CrossRef]
- Cazan, C.D.; Ionică, A.M.; Matei, I.A.; D’Amico, G.; Muñoz, C.; Berriatua, E.; Dumitrache, M.O. Detection of Leishmania infantum DNA and antibodies against Anaplasma spp., Borrelia burgdorferi s.l. and Ehrlichia canis in a dog kennel in South-Central Romania. Acta Vet. Scand. 2020, 62, 42. [Google Scholar] [CrossRef]
- Cîmpan, A.A.; Diakou, A.; Papadopoulos, E.; Miron, L.D. Serological study of exposure to Leishmania in dogs living in shelters, in south-east Romania. Rev. Romana Med. Vet. 2019, 29, 54–58. [Google Scholar]
- Savic, S.; Vidic, B.; Grgic, Z.; Petrovic, T.; Potkonjak, A.; Cupina, A.; Vaselek, S. Dirofilariosis and Leishmaniasis in the Northern Region of Serbia. In An Overview of Tropical Diseases; IntechOpen: Rijeka, Croatia, 2015; Chapter 6; p. 107. [Google Scholar]
- Kotnik, T.; Moreno, J.; Šoba, B.; Krt, B.; Skvarč, M.; Vergles Rataj, A.; Gorišek Bajc, M.; Ravnik Verbič, U. Canine Leishmaniasis Prevalence in the Slovenian Dog Population. J. Vet. Res. 2021, 65, 161–167. [Google Scholar] [CrossRef]
- Cabezón, O.; Martínez-Orellana, P.; Ribas, M.P.; Baptista, C.J.; Gassó, D.; Velarde, R.; Aguilar, X.F.; Solano-Gallego, L. Leishmania Infection in Wild Lagomorphs and Domestic Dogs in North-East Spain. Animals 2024, 14, 1080. [Google Scholar] [CrossRef]
- Topla, V.; Peña i Subirà, R.N. Canine Leishmaniasis: A Comparative Study of Real-Time and Conventional PCR Protocols in Lleida Province, Spain. Ph.D. Thesis, Universitat de Lleida. Escola Tècnica Superior d’Enginyeria Agroalimentària i Forestal i de Veterinària, Lleida, Spain, 2023. [Google Scholar]
- Montoya-Alonso, J.A.; Morchón, R.; Costa-Rodríguez, N.; Matos, J.I.; Falcón-Cordón, Y.; Carretón, E. Current Distribution of Selected Vector-Borne Diseases in Dogs in Spain. Front. Vet. Sci. 2020, 7, 564429. [Google Scholar] [CrossRef] [PubMed]
- Miró, G.; Müller, A.; Montoya, A.; Checa, R.; Marino, V.; Marino, E.; Fuster, F.; Escacena, C.; Descalzo, M.A.; Gálvez, R. Epidemiological role of dogs since the human leishmaniosis outbreak in Madid. Parasites Vectors 2017, 10, 209. [Google Scholar] [CrossRef] [PubMed]
- Pérez Pérez, P.; Rodríguez-Escolar, I.; Carretón, E.; Sánchez Agudo, J.Á.; Lorenzo-Morales, J.; Montoya-Alonso, J.A.; Morchón, R. Serological Survey of Canine Vector-Borne Infections in North-Center Spain. Front. Vet. Sci. 2021, 8, 784331. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Regañón, D.; Roura, X.; Suárez, M.L.; León, M.; Sainz, Á. Serological evaluation of selected vector-borne pathogens in owned dogs from northern Spain based on a multicenter study using a commercial test. Parasites Vectors 2020, 13, 301. [Google Scholar] [CrossRef]
- Baxarias, M.; Jornet-Rius, O.; Donato, G.; Mateu, C.; Alcover, M.M.; Pennisi, M.G.; Solano-Gallego, L. Signalment, Immunological and Parasitological Status and Clinicopathological Findings of Leishmania-Seropositive Apparently Healthy Dogs. Animals 2023, 13, 1649. [Google Scholar] [CrossRef]
- Velez, R.; Ballart, C.; Domenech, E.; Abras, A.; Fernández-Arévalo, A.; Gómez, S.A.; Tebar, S.; Muñoz, C.; Cairó, J.; Gállego, M. Seroprevalence of canine Leishmania infantum infection in the Mediterranean region and identification of risk factors: The example of North-Eastern and Pyrenean areas of Spain. Prev. Vet. Med. 2018, 162, 67–75. [Google Scholar] [CrossRef] [PubMed]
- MapChart. Available online: https://www.mapchart.net/europe.html (accessed on 30 June 2025).
- Ferroglio, E.; Maroli, M.; Gastaldo, S.; Mignone, W.; Rossi, L. Canine leishmaniasis, Italy. Emerg. Infect. Dis. 2005, 11, 1618–1620. [Google Scholar] [CrossRef]
- Ntais, P.; Sifaki-Pistola, D.; Christodoulou, V.; Messaritakis, I.; Pratlong, F.; Poulapos, G.; Antoniou, M. Leishmaniases in Greece. Am. J. Trop. Med. Hyg. 2013, 89, 906–915. [Google Scholar] [CrossRef]
- Ivović, V.; Patakakis, M.; Tselentis, Y.; Chaniotis, B. Faunistic study of sandflies in Greece. Med. Vet. Entomol. 2007, 21, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Deplazes, P.; Grimm, F.; Papaprodromou, M.; Cavaliero, T.; Gramiccia, M.; Christofi, G.; Christofi, N.; Economides, P.; Eckert, J. Canine leishmaniosis in Cyprus due to Leishmania infantum MON 1. Acta Trop. 1998, 71, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Töz, S.O.; Ertabaklar, H.; Göçmen, B.; Demir, S.; Karakuş, M.; Arserim, S.K.; Balcıoğlu, I.C.; Canakçı, T.; Ozbel, Y. An epidemiological study on canine leishmaniasis (CanL) and sand flies in Northern Cyprus. Turk. J. Parasitol. 2013, 37, 107–112. [Google Scholar]
- Cazan, C.D.; Păstrav, I.R.; Ionică, A.M.; Oguz, G.; Erisoz Kasap, O.; Dvorak, V.; Halada, P.; Dumitrache, M.O.; Volf, P.; Alten, B.; et al. Updates on the distribution and diversity of sand flies (Diptera: Psychodidae) in Romania. Parasites Vectors 2019, 12, 247. [Google Scholar] [CrossRef]
- Cazan, C.D.; Sándor, A.D.; Erisoz Kasap, O.; Alten, B.; Mihalca, A.D. Sand fly fauna of South-Eastern Romania, with the description of Phlebotomus (Transphlebotomus) simonahalepae n. sp. (Diptera: Psychodidae). Parasites Vectors 2021, 14, 448. [Google Scholar] [CrossRef]
- Didkowska, A.; Martín-Santander, V.; Wojciechowska, M.; Olech, W.; Anusz, K.; Fernández, A.; Davies, J.E.; Ruíz de Arcaute, M.; Lacasta, D.; Villanueva-Saz, S.; et al. Serologic Evidence of Exposure to Leishmania infantum in Captive and Free-Ranging European Bison (Bison bonasus) in Poland, 2017–23. J. Wildl. Dis. 2025, 61, 253–257. [Google Scholar] [CrossRef]
- Šiško-Kraljević, K.; Jerončić, A.; Mohar, B.; Punda-Polić, V. Asymptomatic Leishmania infantum infections in humans living in endemic and non-endemic areas of Croatia, 2007 to 2009. Eurosurveillance 2013, 18, 20533. [Google Scholar] [CrossRef]
- Vaselek, S.; Oguz, G.; Ayhan, N.; Ozbel, Y.; Kadriaj, P.; Ćupina, A.I.; Velo, E.; Muja, N.; Baymak, D.; Alishani, M.; et al. Sandfly surveillance and investigation of Leishmania spp. DNA in sandflies in Kosovo. Med. Vet. Entomol. 2020, 34, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Xhekaj, B.; Hoxha, I.; Platzgummer, K.; Kniha, E.; Walochnik, J.; Sherifi, K.; Rexhepi, A.; Behluli, B.; Dvořák, V.; Fuehrer, H.P.; et al. First Detection and Molecular Analysis of Leishmania infantum DNA in Sand Flies of Kosovo. Pathogens 2023, 12, 1190. [Google Scholar] [CrossRef]
- Ready, P.D. Leishmaniasis emergence in Europe. Eurosurveillance 2010, 15, 19505. [Google Scholar] [CrossRef] [PubMed]
- Medlock, J.M.; Hansford, K.M.; Van Bortel, W.; Zeller, H.; Alten, B. A summary of the evidence for the change in European distribution of phlebotomine sand flies (Diptera: Psychodidae) of public health importance. J. Vector Ecol. 2014, 39, 72–77. [Google Scholar] [CrossRef]
- Maia, C.; Campino, L. Methods for diagnosis of canine leishmaniasis and immune response to infection. Vet. Parasitol. 2008, 158, 274–287. [Google Scholar] [CrossRef]
- Smyrli, A.; Dumitrache, M.O.; Kalmar, Z.; Cozma-Petruţ, A.; Naic, A.T.; Daskalakis, M.; Aleantinou, V.; Panopoulos, A.; Christoforaki, S.; Restivakis, I.; et al. Canine leishmaniasis in Crete, Greece: Epidemiology, diagnosis and control of the disease. Sci. Parasitol. 2019, 20, 12–18. [Google Scholar]
- Naing, L.; Winn, T.; Rusli, B.N. Practical issues in calculating the sample size for prevalence studies. Arch. Orofac. Sci. 2006, 1, 9–14. [Google Scholar]
- Thrusfield, M. Veterinary Epidemiology; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
| No. | Country | Sample | Diag. Method | Species | No. of Samples | Positive Samples | Prevalence | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1. | Bulgaria | Serum | ELISA | L. infantum | 90 | 7 | 7.77% | [22] |
| Serum | Elisa | No. sp. | 167 | 0 | 0% | [23] | ||
| 2. | Cyprus | Serum | IFAT | L. infantum | 105 | 2 | 1.9% | [24] |
| Serum | IFAT | L. infantum | 281 | 10 | 3.55% | [25] | ||
| 3. | Croatia | Serum | IFAT | L. infantum | 435 | 6 | 1.38% | [26] |
| 4. | France | Serum | ELISA | L. infantum | 718 | 37 | 5.3% | [27] |
| Blood | PCR | 23 | 3.2% | |||||
| Serum | ELISA | L. infantum | 607 | 60 | 9.9% | [28] | ||
| 5. | Greece | Serum | ELISA | Leishmania spp. | 1154 | 106 | 9.2% | [29] |
| Blood | Culture | Leishmania spp. | 1275 | 165 | 12.94% | [30] | ||
| Serum | ELISA | L. infantum | 103 | 26 | 25.2% | [31] | ||
| Blood | PCR | L. infantum | 200 | 13 | 6.5% | [32] | ||
| 6. | Italy | Serum | IFAT | L. infantum | 457 | 63 | 13.8% | [33] |
| Serum | IFAT | L. infantum | 242 | 130 | 54.13% | [34] | ||
| Serum | IFAT | L. infantum | 1147 | 176 | 15.4% | [35] | ||
| Serum | IFAT | L. infantum, L. tarentolae | 100 | 16 | 16% | [36] | ||
| Lymph nodes | PCR | L. infantum | 136 | 29 | 21.32% | [37] | ||
| Serum | IFAT | L. infantum | 263 | 91 | 34.6% | [38] | ||
| Conj. swab | PCR | 32 | 12.2% | |||||
| Blood | 24 | 9.1% | ||||||
| Serum | IFAT | L. infantum | 639 | 16 | 2.5% | [39] | ||
| Serum | IFAT | Leishmania spp. | 149 | 59 | 39.6% | [40] | ||
| Blood | PCR | 5 | 2.98% | |||||
| Serum | WB | L. infantum | 803 | 339 | 42.22% | [41] | ||
| 7. | Kosovo | Serum | ELISA | L. infantum | 285 | 12 | 4.21% | [42] |
| IFAT | 10 | 3.51% | ||||||
| Serum | ELISA | L. infantum | 125 | 23 | 18.4% | [43] | ||
| 8. | North Macedonia | Blood | ICT | L. infantum | 272 | 27 | 9.92% | [44] |
| ICT | L. infantum | 40 | 10 | 25% | [45] | |||
| PCR | L. infantum | 80 | 2 | 1.6% | [46] | |||
| 9. | Poland | Blood | PCR | L. infantum | 497 | 1 | 0.2% | [47] |
| 10. | Portugal | Serum | DAT | Leishmania spp. | 343 | 34 | 9.9% | [48] |
| Serum | DAT | L. infantum | 1860 | 233 | 12.5% | [49] | ||
| Blood | PCR | L. infantum | 1010 | 11 | 1.1% | [50] | ||
| Blood | DAT | L. infantum | 170 | 31 | 18.2% | [51] | ||
| Serum | ELISA | L. infantum | 100 | 13 | 13% | [52] | ||
| Serum | ELISA | Leishmania spp. | 581 | 76 | 13.1% | [53] | ||
| Blood | PCR | L. infantum | 230 | 139 | 60.4% | [54] | ||
| 11. | Romania | Serum | ELISA | L. infantum | 80 | 3 | 3.7% | [55] |
| Conj. swab | PCR | Leishmania spp. | 7 | 8.7% | ||||
| Blood | 1 | 1.2% | ||||||
| Blood, conj. swab | PCR | L. infantum | 149 | 30 | 20.1% | [56] | ||
| Serum | ICT | - | 0 | 0% | ||||
| Serum | ELISA | Leishmania spp. | 110 | 5 | 4.54% | [57] | ||
| 12. | Serbia | Blood | PCR | - | 455 | 0 | 0% | [46] |
| Serum | ELISA | L. infantum | 170 | 18 | 10.59% | [58] | ||
| 13. | Slovenia | Serum | ELISA | Leishmania spp. | 465 | 9 | 1.9% | [59] |
| Conj. swab, blood | PCR | 0 | 0% | |||||
| 14. | Spain | Serum | ELISA | Leishmania spp. | 226 | 27 | 11.9% | [60] |
| Blood | PCR | L. infantum | 65 | 4 | 6.25% | [61] | ||
| Serum | ICT | L. infantum | 4643 | 481 | 10.36% | [62] | ||
| Serum | IFAT | L. infantum | 2123 | 107 | 5.05% | [63] | ||
| Blood | ICT | L. infantum | 1474 | 68 | 4.61% | [64] | ||
| Serum | ELISA | L. infantum | 556 | 55 | 9.88% | [65] | ||
| Serum | ELISA | L. infantum | 5451 | 300 | 5.5% | [66] | ||
| Serum | ELISA | L. infantum | 593 | 116 | 19.5% | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plesko, A.; Florea, T.; Imre, M.; Hoffman, D.; Dreghiciu, I.C.; Pocinoc, A.; Morariu, F.; Marin, A.-M.; Mederle, N.; Darabus, G.; et al. Canine Leishmaniasis in Europe over the Last Decade: A Review of Geographic Trends and Epidemiological Data. Pathogens 2025, 14, 1082. https://doi.org/10.3390/pathogens14111082
Plesko A, Florea T, Imre M, Hoffman D, Dreghiciu IC, Pocinoc A, Morariu F, Marin A-M, Mederle N, Darabus G, et al. Canine Leishmaniasis in Europe over the Last Decade: A Review of Geographic Trends and Epidemiological Data. Pathogens. 2025; 14(11):1082. https://doi.org/10.3390/pathogens14111082
Chicago/Turabian StylePlesko, Anamaria, Tiana Florea, Mirela Imre, Diana Hoffman, Ioan Cristian Dreghiciu, Alexandra Pocinoc, Florica Morariu, Ana-Maria Marin, Narcisa Mederle, Gheorghe Darabus, and et al. 2025. "Canine Leishmaniasis in Europe over the Last Decade: A Review of Geographic Trends and Epidemiological Data" Pathogens 14, no. 11: 1082. https://doi.org/10.3390/pathogens14111082
APA StylePlesko, A., Florea, T., Imre, M., Hoffman, D., Dreghiciu, I. C., Pocinoc, A., Morariu, F., Marin, A.-M., Mederle, N., Darabus, G., Oprescu, I., Ilie, M. S., & Morariu, S. (2025). Canine Leishmaniasis in Europe over the Last Decade: A Review of Geographic Trends and Epidemiological Data. Pathogens, 14(11), 1082. https://doi.org/10.3390/pathogens14111082










