Antibacterial Electrophoretically Loaded Titania Nanotubes on Titanium Alloy Implants Enhance Osseointegration
Abstract
1. Introduction
2. Materials and Methods
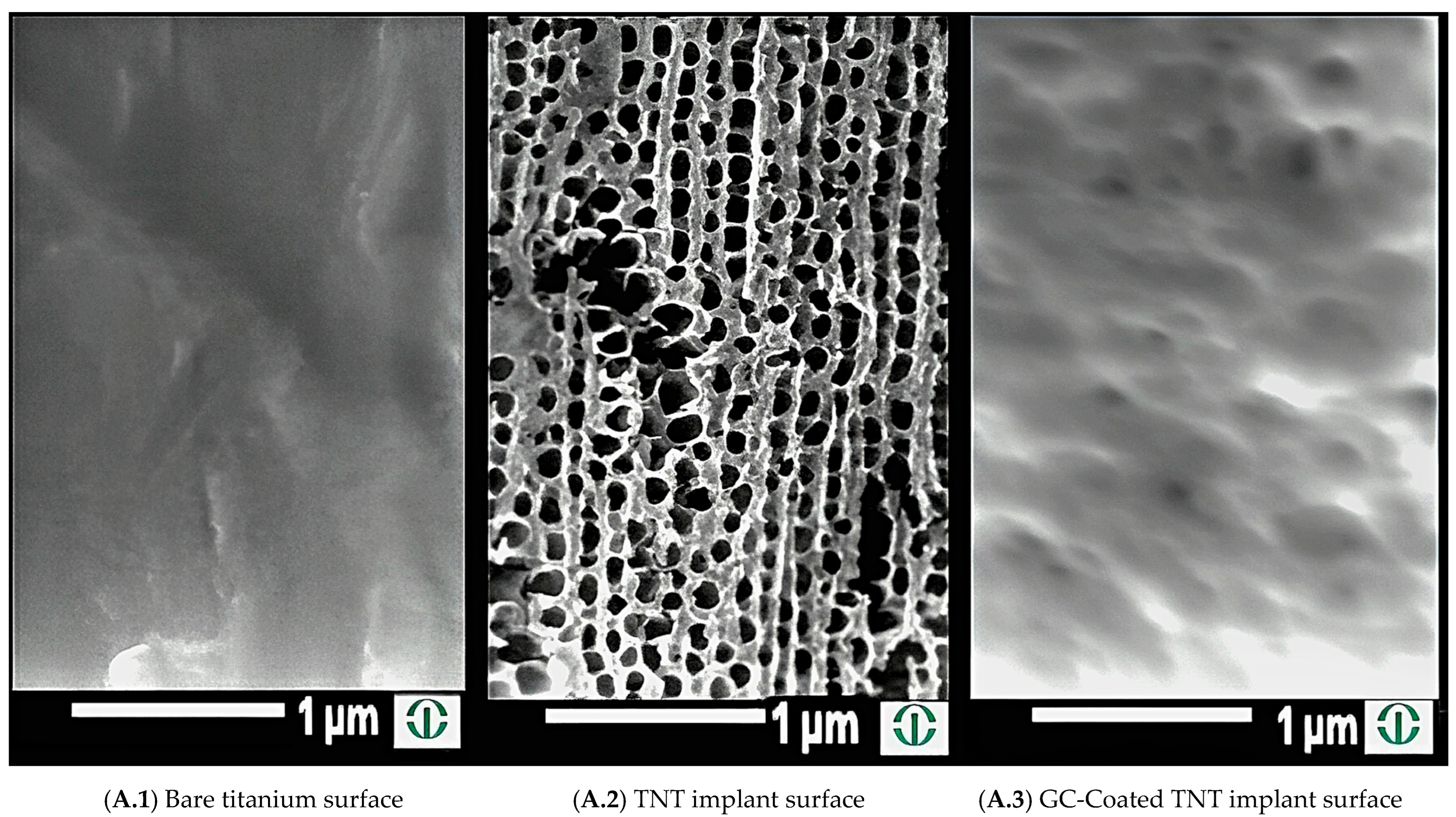
2.1. Creation of Nanotubes on Titanium Wires
2.2. Electrophoretic Disposition on Implants with TNT
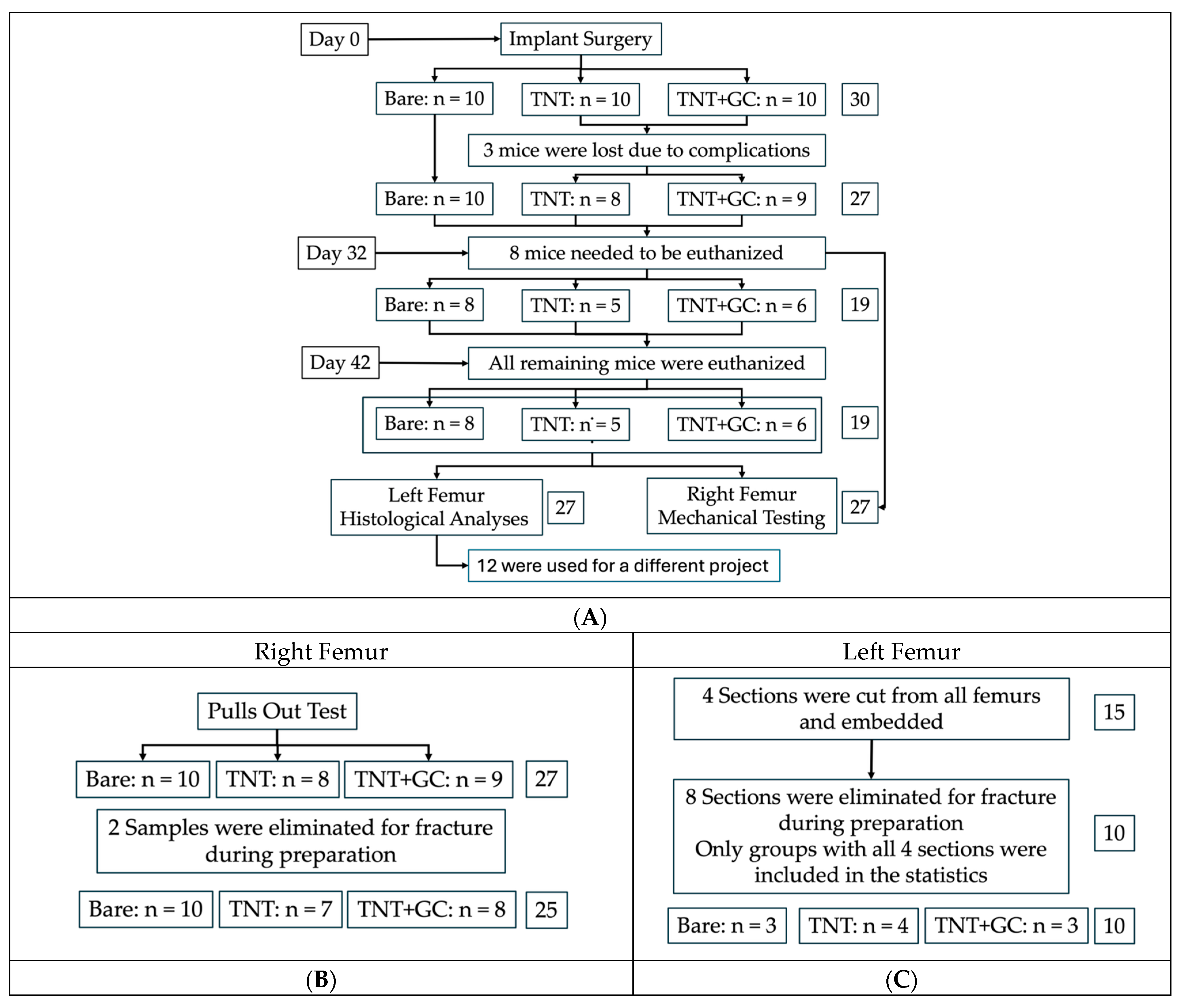
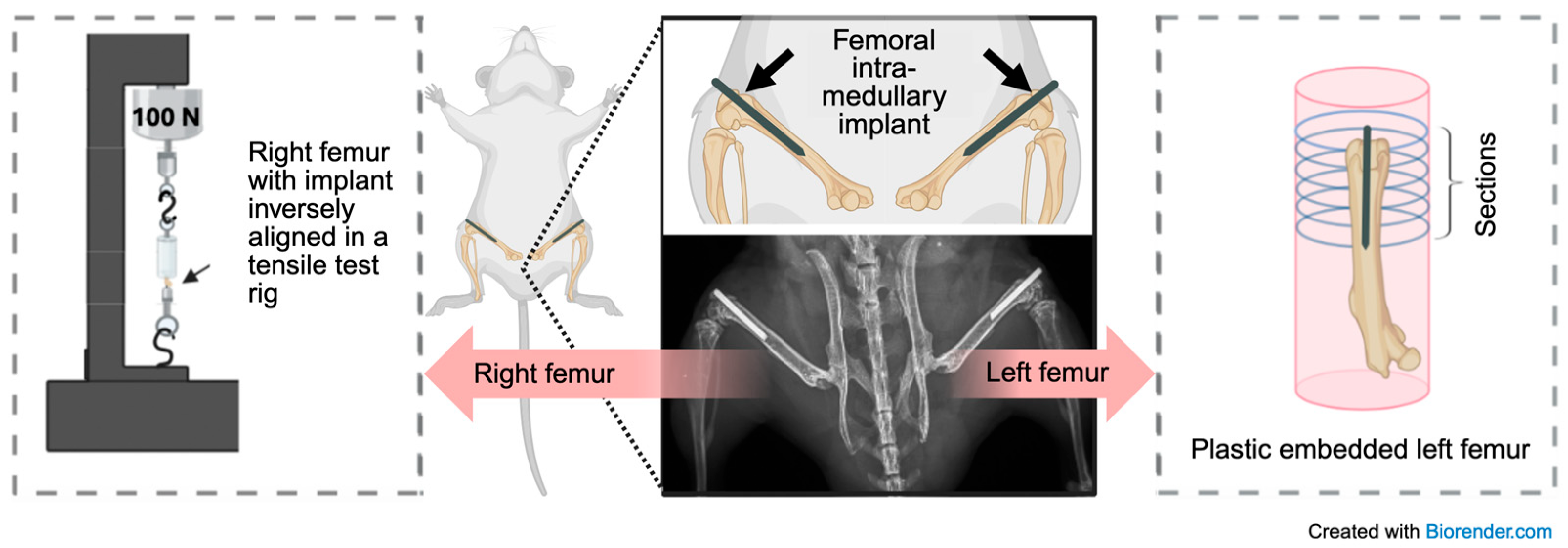
2.3. Implantation and Retrieval Protocol
2.4. Histological Analyses
2.4.1. Embedding and Sectioning
2.4.2. Point-Counting Analysis by Light Microscopy
2.4.3. Areal Analysis by SEM
2.5. Mechanical Testing
2.6. Statistical Analysis
3. Results
3.1. Histological Analysis
3.1.1. Increased Bone Share Fraction Around TNT and GC-Coated Implants Measured via Point-Counting Method
3.1.2. Increased Bone Share Fraction Around TNT and Gentamicin + Chitosan-Coated Implants Assessed via Areal Method
3.1.3. Linear Regression Control Model for Histological Analyses (BAF)
3.2. Pull-Out Analysis
3.2.1. Implants with TNTs and Implants with TNTs Coated with Gentamicin and Chitosan Resulted in Higher Maximum Fixation Strength
3.2.2. Linear Regression Control Model for Pull-Out Analysis (Fixation Strength)
3.3. Weight Results
4. Discussion
4.1. Interpretation of Results
4.2. Limitations
4.3. Conclusion and Future Directions
5. Patent
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | Alkaline phosphatase; |
| BAF | Bone area fraction = bone area/total area = BA/TA; |
| BCA | Bone contact area; |
| C | Chitosan; |
| EPD | Electrophoretic deposition; |
| GC | Gentamicin + chitosan; |
| G | Gentamicin; |
| MMA | Methyl methacrylate; |
| PJI | Periprosthetic joint infection; |
| ROI | Region of interest; |
| SEM | Standard error of the mean and scanning electron microscopy; |
| TNT | Titania nanotube. |
Appendix A
| t-Test of Coefficients (Balanced Sample) Including Control for Harvest and Method | ||
|---|---|---|
| Baseline Model | Specification Model | |
| (Intercept) | 9.143 **** (1.067) | 11.855 **** (1.578) |
| TNT | 6.874 **** (1.723) | 6.884 **** (1.877) |
| TNT+GC | 10.002 **** (1.360) | 10.002 **** (1.385) |
| Harvest | 0.135 (1.999) | |
| Section 3 | −3.386 *** (1.110) | |
| Section 4 | −5.330 ** (1.992) | |
| Section 5 | −2.313 (1.903) | |
| Count | 40 | |
| R-squared | 0.439 | 0.501 |
| F Test: Linear Hypothesis Test: TNT − TNT+GC = 0 | ||
| F(1,37) = 3.851, p = 0.0573 | F(1,33) = 3.173, p = 0.0841 | |
| t-Test of Coefficients (Full Sample) Including Control for Harvest and Bone Contact Area (BCA), mm2 | ||
|---|---|---|
| Baseline Model | Specification Model | |
| (Intercept) | 0.316 *** (0.086) | 0.483 (0.889) |
| TNT | 0.563 ** (0.208) | 0.530 *** (0.201) |
| TNT+GC | 0.538 ** (0.246) | 0.524 * (0.283) |
| Harvest | −0.153 (0.268) | |
| Bone contact area (BCA) | −0.032 (0.575) | |
| Count | 25 | |
| R-squared | 0.223 | 0.172 |
| F Test: Linear Hypothesis Test: TNT+GC − TNT = 0 | ||
| F(1/22) = 0.007, p = 0.934 | F(1/20) = 0.0003, p = 0.988 | |
| t-Test of Coefficients (Balanced Sample) Including Control for Time Point of Measurement | |||||
|---|---|---|---|---|---|
| Baseline Model | Specification Model | ||||
| (Intercept) | 28.451 **** (0.525) | 27.069 **** (<0.001) | |||
| TNT | 0.242 (0.849) | 0.385 (0.901) | |||
| TNT+GC | 0.489 (0.489) | 0.599 (0.558) | |||
| As factor day 21 | 0.553 *** (0.398) | ||||
| As factor day 32 | 1.622 **** (0.432) | ||||
| As factor day 42 | 3.453 **** (0.480) | ||||
| Count | 81 | ||||
| R-squared | −0.016 | 0.338 | |||
| F Test: Linear Hypothesis Test: TNT − TNT+GC = 0 | |||||
| F(1,78) = 0.115, p = 0.736 | F(1,74) = 0.073, p = 0.7883 | ||||
References
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg.-Am. Vol. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Postler, A.; Lützner, C.; Beyer, F.; Tille, E.; Lützner, J. Analysis of total knee arthroplasty revision causes. BMC Musculoskelet. Disord. 2018, 19, 55. [Google Scholar] [CrossRef]
- Izakovicova, P.; Borens, O.; Trampuz, A. Periprosthetic joint infection: Current concepts and outlook. EFORT Open Rev. 2019, 4, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Huotari, K.; Peltola, M.; Jämsen, E. The incidence of late prosthetic joint infections: A registry-based study of 112,708 primary hip and knee replacements. Acta Orthop. 2015, 86, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Mainardes, R.M.; Silva, L.P. Drug delivery systems: Past, present, and future. Curr. Drug Targets 2004, 5, 449–455. [Google Scholar] [CrossRef]
- Ratner, B.D.; Zhang, G. A history of biomaterials. In Biomaterials Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 21–34. [Google Scholar] [CrossRef]
- Elias, C.; Lima, J.; Valiev, R.; Meyers, M. Biomedical applications of titanium and its alloys. JOM 2008, 60, 46–49. [Google Scholar] [CrossRef]
- İzmir, M.; Ercan, B. Anodization of titanium alloys for orthopedic applications. Front. Chem. Sci. Eng. 2019, 13, 28–45. [Google Scholar] [CrossRef]
- Streicher, R.M.; Schmidt, M.; Fiorito, S. Nanosurfaces and nanostructures for artificial orthopedic implants. Nanomedicine 2007, 2, 861–874. [Google Scholar] [CrossRef]
- Dale, G.; Hamilton, J.; Dunlop, P.; Lemoine, P.; Byrne, J. Electrochemical growth of titanium oxide nanotubes: The effect of surface roughness and applied potential. J. Nanosci. Nanotechnol. 2009, 9, 4215–4219. [Google Scholar] [CrossRef]
- Minagar, S.; Berndt, C.C.; Wang, J.; Ivanova, E.; Wen, C. A review of the application of anodization for the fabrication of nanotubes on metal implant surfaces. Acta Biomater. 2012, 8, 2875–2888. [Google Scholar] [CrossRef]
- Çalışkan, N.; Bayram, C.; Erdal, E.; Karahaliloğlu, Z.; Denkbaş, E.B. Titania nanotubes with adjustable dimensions for drug reservoir sites and enhanced cell adhesion. Mater. Sci. Eng. C 2014, 35, 100–105. [Google Scholar] [CrossRef]
- Lin, W.-t.; Tan, H.-l.; Duan, Z.-l.; Yue, B.; Ma, R.; He, G.; Tang, T.-t. Inhibited bacterial biofilm formation and improved osteogenic activity on gentamicin-loaded titania nanotubes with various diameters. Int. J. Nanomed. 2014, 9, 1215–1230. [Google Scholar] [CrossRef]
- Jäger, M.; Jennissen, H.; Dittrich, F.; Fischer, A.; Köhling, H.L. Antimicrobial and Osseointegration Properties of Nanostructured Titanium Orthopaedic Implants. Materials 2017, 10, 1302. [Google Scholar] [CrossRef]
- Della Fara, G.; Markovics, A.; Fischer, A.; Radice, S.; Hamilton, J.L.; Chiesa, R.; Wimmer, M.A. Electrophoretic Deposition of Gentamicin and Chitosan into Titanium Nanotubes to Target Periprosthetic Infection. J. Biomed. Mater. Res. B Appl. Biomater. 2023, 111, 1697–1704. [Google Scholar] [CrossRef]
- De Meo, D.; Cannari, F.M.; Petriello, L.; Persiani, P.; Villani, C. Gentamicin-coated tibia nail in fractures and nonunion to reduce fracture-related infections: A systematic review. Molecules 2020, 25, 5471. [Google Scholar] [CrossRef] [PubMed]
- Knaepler, H. Local application of gentamicin-containing collagen implant in the prophylaxis and treatment of surgical site infection in orthopaedic surgery. Int. J. Surg. 2012, 10, S15–S20. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Sittinger, M.; Risbud, M.V. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 2005, 26, 5983–5990. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.L.; Mohamed, M.F.; Witt, B.R.; Wimmer, M.A.; Shafikhani, S.H. Therapeutic assessment of N-formyl-methionyl-leucyl-phenylalanine (fMLP) in reducing periprosthetic joint infection. Eur. Cell. Mater. 2021, 42, 122–138. [Google Scholar] [CrossRef]
- Hamilton, J.L.; Gianotti, S.; Fischer, J.; Della Fara, G.; Impergre, A.; De Vecchi, F.; AbuAlia, M.; Fischer, A.; Markovics, A.; Wimmer, M.A. Electrophoretic Deposition of Gentamicin Into Titania Nanotubes Prevents Evidence of Infection in a Mouse Model of Periprosthetic Joint Infection. J. Orthop. Res. 2025, 43, 671–681. [Google Scholar] [CrossRef]
- Su, E.; Justin, D.; Pratt, C.; Sarin, V.; Nguyen, V.; Oh, S.; Jin, S. Effects of titanium nanotubes on the osseointegration, cell differentiation, mineralisation and antibacterial properties of orthopaedic implant surfaces. Bone Jt. J. 2018, 100, 9–16. [Google Scholar] [CrossRef]
- ISO 5832-3:2021; Implants for Surgery—Metallic Materials; Part 3: Wrought Titanium 6-Aluminum 4-Vanadium Alloy. International Organization for Standardization: Geneva, Switzerland, 2021.
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Bernthal, N.M.; Stavrakis, A.I.; Billi, F.; Cho, J.S.; Kremen, T.J.; Simon, S.I.; Cheung, A.L.; Finerman, G.A.; Lieberman, J.R.; Adams, J.S.; et al. A Mouse Model of Post-Arthroplasty Staphylococcus aureus Joint Infection to Evaluate In Vivo the Efficacy of Antimicrobial Implant Coatings. PLoS ONE 2010, 5, e12580. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.M.; Moran, M.M.; Meagher, M.J.; Ross, R.D.; Mashiatulla, M.; Virdi, A.S.; Sumner, D.R. Early changes in serum osteocalcin and body weight are predictive of implant fixation in a rat model of implant loosening. J. Orthop. Res. 2020, 38, 1216–1227. [Google Scholar] [CrossRef]
- Brörmann, K.; Barel, I.; Urbakh, M.; Bennewitz, R. Friction on a microstructured elastomer surface. Tribol. Lett. 2013, 50, 3–15. [Google Scholar] [CrossRef]
- Wang, N.; Li, H.; Lü, W.; Li, J.; Wang, J.; Zhang, Z.; Liu, Y. Effects of TiO2 nanotubes with different diameters on gene expression and osseointegration of implants in minipigs. Biomaterials 2011, 32, 6900–6911. [Google Scholar] [CrossRef]
- Wang, J.; Meng, F.; Song, W.; Jin, J.; Ma, Q.; Fei, D.; Fang, L.; Chen, L.; Wang, Q.; Zhang, Y. Nanostructured titanium regulates osseointegration via influencing macrophage polarization in the osteogenic environment. Int. J. Nanomed. 2018, 10, 4029–4043. [Google Scholar] [CrossRef]
- Bjursten, L.M.; Rasmusson, L.; Oh, S.; Smith, G.C.; Brammer, K.S.; Jin, S. Titanium dioxide nanotubes enhance bone bonding in vivo. J. Biomed. Mater. Res. Part A 2010, 92, 1218–1224. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, J.; Scheideler, L.; Reichl, R.; Geis-Gerstorfer, J. Effects of topography and composition of titanium surface oxides on osteoblast responses. Biomaterials 2004, 25, 4087–4103. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Medina, P.; Sundaram, P.; Diffoot-Carlo, N. Titanium oxide: A bioactive factor in osteoblast differentiation. Int. J. Dent. 2015, 2015, 357653. [Google Scholar] [CrossRef] [PubMed]
- Mathews, S.; Gupta, P.; Bhonde, R.; Totey, S. Chitosan enhances mineralization during osteoblast differentiation of human bone marrow-derived mesenchymal stem cells, by upregulating the associated genes. Cell Prolif. 2011, 44, 537–549. [Google Scholar] [CrossRef]
- Lahiji, A.; Sohrabi, A.; Hungerford, D.S.; Frondoza, C.G. Chitosan supports the expression of extracellular matrix proteins in human osteoblasts and chondrocytes. J. Biomed. Mater. Res. 2000, 51, 586–595. [Google Scholar] [CrossRef]
- Arpornmaeklong, P.; Suwatwirote, N.; Pripatnanont, P.; Oungbho, K. Growth and differentiation of mouse osteoblasts on chitosan–collagen sponges. Int. J. Oral Maxillofac. Surg. 2007, 36, 328–337. [Google Scholar] [CrossRef]
- Fakhry, A.; Schneider, G.B.; Zaharias, R.; Şenel, S. Chitosan supports the initial attachment and spreading of osteoblasts preferentially over fibroblasts. Biomaterials 2004, 25, 2075–2079. [Google Scholar] [CrossRef] [PubMed]
- Philp, A.M.; Raja, S.; Philp, A.; Ede, M.P.N.; Jones, S.W. The effect of vancomycin and gentamicin antibiotics on human osteoblast proliferation, metabolic function, and bone mineralization. Spine 2017, 42, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Shaygani, H.; Seifi, S.; Shamloo, A.; Golizadeh, M.; Rahnamaee, S.Y.; Alishiri, M.; Ebrahimi, S. Novel bilayer coating on gentamicin-loaded titanium nanotube for orthopedic implants applications. Int. J. Pharm. 2023, 636, 122764. [Google Scholar] [CrossRef] [PubMed]
- Kagiwada, H.; Fukuchi, T.; Machida, H.; Yamashita, K.; Ohgushi, H. Effect of gentamicin on growth and differentiation of human mesenchymal stem cells. J. Toxicol. Pathol. 2008, 21, 61–67. [Google Scholar] [CrossRef]
- Semiatin, S.L.; Knisley, S.L.; Fagin, P.N.; Barker, D.R.; Zhang, F.J.M. Microstructure evolution during alpha-beta heat treatment of Ti-6Al-4V. Metall. Mater. Trans. A 2003, 34, 2377–2386. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, J.; Hall, D.J.; Moran, M.M.; Markovics, A.; Pennekamp, P.H.; Hamilton, J.L.; Wimmer, M.A. Antibacterial Electrophoretically Loaded Titania Nanotubes on Titanium Alloy Implants Enhance Osseointegration. Pathogens 2025, 14, 1072. https://doi.org/10.3390/pathogens14111072
Fischer J, Hall DJ, Moran MM, Markovics A, Pennekamp PH, Hamilton JL, Wimmer MA. Antibacterial Electrophoretically Loaded Titania Nanotubes on Titanium Alloy Implants Enhance Osseointegration. Pathogens. 2025; 14(11):1072. https://doi.org/10.3390/pathogens14111072
Chicago/Turabian StyleFischer, Julia, Deborah J. Hall, Meghan M. Moran, Adrienn Markovics, Peter H. Pennekamp, John L. Hamilton, and Markus A. Wimmer. 2025. "Antibacterial Electrophoretically Loaded Titania Nanotubes on Titanium Alloy Implants Enhance Osseointegration" Pathogens 14, no. 11: 1072. https://doi.org/10.3390/pathogens14111072
APA StyleFischer, J., Hall, D. J., Moran, M. M., Markovics, A., Pennekamp, P. H., Hamilton, J. L., & Wimmer, M. A. (2025). Antibacterial Electrophoretically Loaded Titania Nanotubes on Titanium Alloy Implants Enhance Osseointegration. Pathogens, 14(11), 1072. https://doi.org/10.3390/pathogens14111072







