The Immunoregulatory Mechanisms of Human Cytomegalovirus from Primary Infection to Reactivation
Abstract
1. Introduction
2. Primary Infection
2.1. Routes of Transmission and Susceptible Populations
2.2. Clinical Manifestations
3. Latency
3.1. Types of Latently Infected Cells
3.2. Immune Evasion
3.2.1. CMV Evasion of PRR-Mediated Antiviral Innate Immune Responses
3.2.2. CMV Evasion of T Cell- and NK Cell-Mediated Immune Surveillance
3.2.3. CMV Evades Apoptosis, Necroptosis, and Autophagy
3.2.4. CMV Inhibits Apoptosis
3.2.5. CMV Suppression of Necroptosis
3.2.6. CMV Interference with the Autophagy Pathway
4. Reactivation
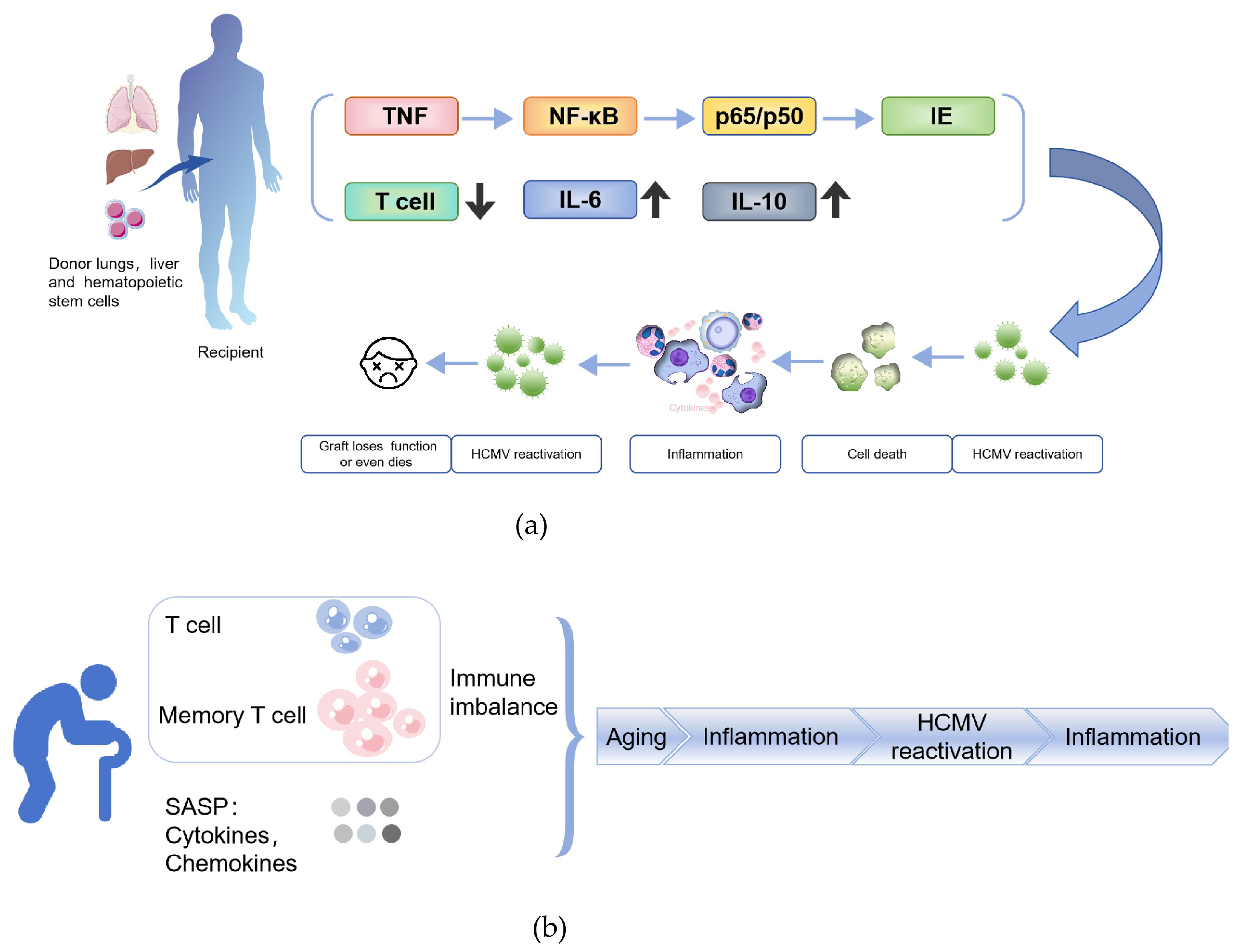
4.1. Mechanisms and Clinical Manifestations of HCMV Reactivation After Transplantation
4.2. HCMV Reactivation Driven by Immunosenescence
5. Conclusions
Funding
Conflicts of Interest
Abbreviations
| HCMV | Human cytomegalovirus |
| MHC | Major histocompatibility complex |
| Allo-HSCT | Allogeneic hematopoietic stem cell transplantation |
| PAMPs | Pathogen-associated molecular patterns |
| DAMPs | Damage-associated molecular patterns |
| CMV | Cytomegalovirus |
| IgG | Immunoglobulin G |
| AIDS | Acquired immunodeficiency syndrome |
| IE | Immediate early |
| GM-Ps | Granulocyte-macrophage progenitor cells |
| MCMV | Murine cytomegalovirus |
| LSECs | Liver sinusoidal endothelial cells |
| MIEP | Major immediate early promoter |
| PRR | Pattern recognition receptor |
| TLRs | Toll-like receptors |
| ER | Endoplasmic reticulum |
| cGAS | Cyclic GMP-AMP synthase |
| dsDNA | Double-stranded DNA |
| cGAMP | Cyclic GMP-AMP |
| IFN-I | Type I interferons |
| ERAD | ER-associated degradation |
| HLA | Human leukocyte antigen |
| TAP | Transporter associated with antigen processing |
| Ii | Invariant chain |
| MIIC | MHC class II compartment |
| NK | Natural killer |
| ULBP | UL16-binding protein |
| MICB | Major histocompatibility complex class I chain-related protein B |
| vMIA | Viral mitochondria-localized inhibitor of apoptosis |
| TNFRs | Tumor necrosis factor receptors |
| FADD | Fas-associated death domain protein |
| vICA | Viral inhibitor of caspase-8-induced apoptosis |
| RIPK1 | Receptor-interacting protein kinase 1 |
| RHIM | RIP homotypic interaction motif |
| RIPK3 | Receptor-interacting protein kinase 3 |
| MLKL | Mixed lineage kinase domain-like |
| ZBP1 | Z-DNA binding protein 1 |
| HC | Hemorrhagic cystitis |
| AML | Acute myeloid leukemia |
| CIBMTR | Center for International Blood and Marrow Transplant Research |
| IKK | Inhibitor of kappa B kinase |
| TCR | T cell receptor |
| SAMD | Senescence-associated mitochondrial dysfunction |
| ROS | Reactive oxygen species |
| DDR | DNA damage response |
| SASP | Senescence-associated secretory phenotype |
| PASC | Post-acute sequelae of SARS-CoV-2 infection |
| EBV | Epstein–Barr virus |
References
- Carmichael, A. Cytomegalovirus and the eye. Eye 2012, 26, 237–240. [Google Scholar] [CrossRef]
- Sunil-Chandra, N.P.; Jayasundara, M.; Gunathilaka, M.; Suminda, S.D.H. Human cytomegalovirus (HCMV) trends in Sri Lanka: Insights from a hospital-based seroprevalence analysis. BMC Infect. Dis. 2025, 25, 184. [Google Scholar] [CrossRef]
- Oliveira, A.D.S.; Pereira, J.G.; Nunes, G.T.; Sousa Junior, I.P.; Sarmento, D.J.S.; Lopes, J.I.F.; Amorim Filho, L.; Paula, V.S. Prevalence and investigation of Cytomegalovirus (HCMV) in blood donors from the main blood establishment in Rio de Janeiro/Brazil. Braz. J. Infect. Dis. 2025, 29, 104508. [Google Scholar] [CrossRef]
- Radoi, C.L.; Zlatian, O.; Balasoiu, M.; Dragomir, T.L.; Sorop, M.I.; Bagiu, I.C.; Boeriu, E.; Susan, M.; Sorop, B.; Oprisoni, L.A.; et al. Seroprevalence of Anti-Cytomegalovirus Antibodies in Pregnant Women from South-West Romania. Microorganisms 2024, 12, 268. [Google Scholar] [CrossRef]
- Dana Flanders, W.; Lally, C.; Dilley, A.; Diaz-Decaro, J. Estimated cytomegalovirus seroprevalence in the general population of the United States and Canada. J. Med. Virol. 2024, 96, e29525. [Google Scholar] [CrossRef]
- Álvarez-Heredia, P.; Reina-Alfonso, I.; Domínguez-Del-Castillo, J.J.; Hassouneh, F.; Gutiérrez-González, C.; Batista-Duharte, A.; Pérez, A.B.; Sarramea, F.; Jaén-Moreno, M.J.; Camacho-Rodríguez, C.; et al. Spanish HCMV Seroprevalence in the 21st Century. Viruses 2023, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Hoehl, S.; Berger, A.; Ciesek, S.; Rabenau, H.F. Thirty years of CMV seroprevalence-a longitudinal analysis in a German university hospital. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Partana, P.; Wan, W.Y.; Chow, X.Y.V.; Chan, J.K.Y.; Tan, L.K.; Tan, W.C.; Lee, P.P.; Lim, G.H.; Yang, L. Seroprevalence of cytomegalovirus among pregnant women in Singapore. Trop. Med. Health 2024, 52, 67. [Google Scholar] [CrossRef]
- Ben Shoham, A.; Schlesinger, Y.; Miskin, I.; Kalderon, Z.; Michaelson-Cohen, R.; Wiener-Well, Y. Cytomegalovirus (CMV) seroprevalence among women at childbearing age, maternal and congenital CMV infection: Policy implications of a descriptive, retrospective, community-based study. Isr. J. Health Policy Res. 2023, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Matthews, S.M.; Groves, I.J.; O’Connor, C.M. Chromatin control of human cytomegalovirus infection. mBio 2023, 14, e0032623. [Google Scholar] [CrossRef]
- Harmon, C.M.; Cooling, L.L. Current strategies and future directions for the prevention of transfusion-transmitted cytomegalovirus. Int. J. Clin. Transfus. Med. 2017, 5, 49–59. [Google Scholar] [CrossRef][Green Version]
- Sekhon, H.S.; Press, R.D.; Schmidt, W.A.; Hawley, M.; Rader, A. Identification of cytomegalovirus in a liquid-based gynecologic sample using morphology, immunohistochemistry, and DNA real-time PCR detection. Diagn. Cytopathol. 2004, 30, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Poncet, D.; Pauleau, A.L.; Szabadkai, G.; Vozza, A.; Scholz, S.R.; Le Bras, M.; Brière, J.J.; Jalil, A.; Le Moigne, R.; Brenner, C.; et al. Cytopathic effects of the cytomegalovirus-encoded apoptosis inhibitory protein vMIA. J. Cell Biol. 2006, 174, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Kenneson, A.; Cannon, M.J. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med. Virol. 2007, 17, 253–276. [Google Scholar] [CrossRef]
- Almishaal, A.A. Knowledge of cytomegalovirus infection among women in Saudi Arabia: A cross-sectional study. PLoS ONE 2022, 17, e0274863. [Google Scholar] [CrossRef]
- Wass, A.B.; Krishna, B.A.; Herring, L.E.; Gilbert, T.S.K.; Nukui, M.; Groves, I.J.; Dooley, A.L.; Kulp, K.H.; Matthews, S.M.; Rotroff, D.M.; et al. Cytomegalovirus US28 regulates cellular EphA2 to maintain viral latency. Sci. Adv. 2022, 8, eadd1168. [Google Scholar] [CrossRef]
- Gandhi, M.K.; Khanna, R. Human cytomegalovirus: Clinical aspects, immune regulation, and emerging treatments. Lancet Infect. Dis. 2004, 4, 725–738. [Google Scholar] [CrossRef]
- Singh, S.; Maheshwari, A.; Boppana, S. CMV-induced Hearing Loss. Newborn 2023, 2, 249–262. [Google Scholar] [CrossRef]
- Engman, M.L.; Malm, G.; Engstrom, L.; Petersson, K.; Karltorp, E.; Tear Fahnehjelm, K.; Uhlen, I.; Guthenberg, C.; Lewensohn-Fuchs, I. Congenital CMV infection: Prevalence in newborns and the impact on hearing deficit. Scand. J. Infect. Dis. 2008, 40, 935–942. [Google Scholar] [CrossRef]
- Ali, A.A.; Anasseri, S.; Abou-Ghaida, J.; Walker, L.; Barber, T. Cytomegalovirus Esophagitis in an Immunocompromised Patient. Cureus 2023, 15, e45634. [Google Scholar] [CrossRef]
- Liu, X.F.; Swaminathan, S.; Yan, S.; Engelmann, F.; Abbott, D.A.; VanOsdol, L.A.; Heald-Sargent, T.; Qiu, L.; Chen, Q.; Iovane, A.; et al. A novel murine model of differentiation-mediated cytomegalovirus reactivation from latently infected bone marrow haematopoietic cells. J. Gen. Virol. 2019, 100, 1680–1694. [Google Scholar] [CrossRef]
- Buehler, J.; Carpenter, E.; Zeltzer, S.; Igarashi, S.; Rak, M.; Mikell, I.; Nelson, J.A.; Goodrum, F. Host signaling and EGR1 transcriptional control of human cytomegalovirus replication and latency. PLoS Pathog. 2019, 15, e1008037. [Google Scholar] [CrossRef]
- Jackson, S.E.; Sedikides, G.X.; Okecha, G.; Poole, E.L.; Sinclair, J.H.; Wills, M.R. Latent Cytomegalovirus (CMV) Infection Does Not Detrimentally Alter T Cell Responses in the Healthy Old, But Increased Latent CMV Carriage Is Related to Expanded CMV-Specific T Cells. Front. Immunol. 2017, 8, 733. [Google Scholar] [CrossRef]
- Jarvis, M.A.; Nelson, J.A. Mechanisms of human cytomegalovirus persistence and latency. Front. Biosci. 2002, 7, d1575–d1582. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Pan, C.; Sheng, J.; Liang, H.; Bian, Z.; Liu, Y.; Trang, P.; Wu, J.; Liu, F.; Zhang, C.Y.; et al. Human cytomegalovirus reprogrammes haematopoietic progenitor cells into immunosuppressive monocytes to achieve latency. Nat. Microbiol. 2018, 3, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Nikitina, E.; Larionova, I.; Choinzonov, E.; Kzhyshkowska, J. Monocytes and Macrophages as Viral Targets and Reservoirs. Int. J. Mol. Sci. 2018, 19, 2821. [Google Scholar] [CrossRef] [PubMed]
- Gladkov, S.A.; Zinserling, V.A.; Shtro, A.A.; Belyaevskaya, S.V.; Zarubaev, V.V. Postmortem diagnosis of influenza during its epidemic and interepidemic periods. Arkhiv Patologii 2015, 77, 22–27. [Google Scholar] [CrossRef]
- Hoarau, J.J.; Jaffar Bandjee, M.C.; Krejbich Trotot, P.; Das, T.; Li-Pat-Yuen, G.; Dassa, B.; Denizot, M.; Guichard, E.; Ribera, A.; Henni, T.; et al. Persistent chronic inflammation and infection by Chikungunya arthritogenic alphavirus in spite of a robust host immune response. J. Immunol. 2010, 184, 5914–5927. [Google Scholar] [CrossRef]
- Henning, J.D.; Bunker, C.H.; Patrick, A.L.; Jenkins, F.J. Human herpesvirus 8 establishes a latent infection in prostates of Tobago men resulting in increased macrophage infiltration. Prostate 2016, 76, 735–743. [Google Scholar] [CrossRef]
- Abbas, W.; Tariq, M.; Iqbal, M.; Kumar, A.; Herbein, G. Eradication of HIV-1 from the macrophage reservoir: An uncertain goal? Viruses 2015, 7, 1578–1598. [Google Scholar] [CrossRef]
- Toriyabe, K.; Kitamura, A.; Ikejiri, M.; Hashizume, R.; Nakamura, M.; Teramoto, E.; Takeuchi, H.; Kondo, E.; Ikeda, T. Cytomegalovirus DNA Loads in Organs of Congenitally Infected Fetus. Viruses 2024, 16, 891. [Google Scholar] [CrossRef] [PubMed]
- Kyrrestad, I.; Larsen, A.K.; Sánchez Romano, J.; Simón-Santamaría, J.; Li, R.; Sørensen, K.K. Infection of liver sinusoidal endothelial cells with Muromegalovirus muridbeta1 involves binding to neuropilin-1 and is dynamin-dependent. Front. Cell Infect. Microbiol. 2023, 13, 1249894. [Google Scholar] [CrossRef] [PubMed]
- Reeves, M.B.; Coleman, H.; Chadderton, J.; Goddard, M.; Sissons, J.G.P.; Sinclair, J.H. Vascular endothelial and smooth muscle cells are unlikely to be major sites of latency of human cytomegalovirus in vivo. J. Gen. Virol. 2004, 85, 3337–3341. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Varghese, T.K.; Zhang, Z.; Zhao, L.C.; Thomas, G.; Hummel, M.; Abecassis, M. Renal ischemia/reperfusion injury activates the enhancer domain of the human cytomegalovirus major immediate early promoter. Am. J. Transplant. 2005, 5, 1606–1613. [Google Scholar] [CrossRef]
- Goodrum, F. Human Cytomegalovirus Latency: Approaching the Gordian Knot. Annu. Rev. Virol. 2016, 3, 333–357. [Google Scholar] [CrossRef]
- Jabłońska, A.; Jabłonowska, E.; Studzińska, M.; Kamerys, J.; Paradowska, E. Polymorphisms in the genes encoding RLR and TLR3 and CMV DNAemia in subjects coinfected with human immunodeficiency virus and cytomegalovirus. Arch. Virol. 2024, 169, 211. [Google Scholar] [CrossRef]
- Li, S.; Xie, Y.; Yu, C.; Zheng, C.; Xu, Z. The battle between host antiviral innate immunity and immune evasion by cytomegalovirus. Cell Mol. Life Sci. 2024, 81, 341. [Google Scholar] [CrossRef]
- Park, A.; Ra, E.A.; Lee, T.A.; Choi, H.J.; Lee, E.; Kang, S.; Seo, J.Y.; Lee, S.; Park, B. HCMV-encoded US7 and US8 act as antagonists of innate immunity by distinctively targeting TLR-signaling pathways. Nat. Commun. 2019, 10, 4670. [Google Scholar] [CrossRef]
- Lio, C.W.; McDonald, B.; Takahashi, M.; Dhanwani, R.; Sharma, N.; Huang, J.; Pham, E.; Benedict, C.A.; Sharma, S. cGAS-STING Signaling Regulates Initial Innate Control of Cytomegalovirus Infection. J. Virol. 2016, 90, 7789–7797. [Google Scholar] [CrossRef]
- Huang, Z.F.; Zou, H.M.; Liao, B.W.; Zhang, H.Y.; Yang, Y.; Fu, Y.Z.; Wang, S.Y.; Luo, M.H.; Wang, Y.Y. Human Cytomegalovirus Protein UL31 Inhibits DNA Sensing of cGAS to Mediate Immune Evasion. Cell Host Microbe 2018, 24, 69–80.e64. [Google Scholar] [CrossRef]
- Lim, E.Y.; Jackson, S.E.; Wills, M.R. The CD4+ T Cell Response to Human Cytomegalovirus in Healthy and Immunocompromised People. Front. Cell Infect. Microbiol. 2020, 10, 202. [Google Scholar] [CrossRef]
- Sasset, L.; Petris, G.; Cesaratto, F.; Burrone, O.R. The VCP/p97 and YOD1 Proteins Have Different Substrate-dependent Activities in Endoplasmic Reticulum-associated Degradation (ERAD). J. Biol. Chem. 2015, 290, 28175–28188. [Google Scholar] [CrossRef]
- Barel, M.T.; Ressing, M.; Pizzato, N.; van Leeuwen, D.; Le Bouteiller, P.; Lenfant, F.; Wiertz, E.J. Human cytomegalovirus-encoded US2 differentially affects surface expression of MHC class I locus products and targets membrane-bound, but not soluble HLA-G1 for degradation. J. Immunol. 2003, 171, 6757–6765. [Google Scholar] [CrossRef]
- Lehner, P.J.; Karttunen, J.T.; Wilkinson, G.W.; Cresswell, P. The human cytomegalovirus US6 glycoprotein inhibits transporter associated with antigen processing-dependent peptide translocation. Proc. Natl. Acad. Sci. USA 1997, 94, 6904–6909. [Google Scholar] [CrossRef] [PubMed]
- Tomazin, R.; Boname, J.; Hegde, N.R.; Lewinsohn, D.M.; Altschuler, Y.; Jones, T.R.; Cresswell, P.; Nelson, J.A.; Riddell, S.R.; Johnson, D.C. Cytomegalovirus US2 destroys two components of the MHC class II pathway, preventing recognition by CD4+ T cells. Nat. Med. 1999, 5, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Hegde, N.R.; Tomazin, R.A.; Wisner, T.W.; Dunn, C.; Boname, J.M.; Lewinsohn, D.M.; Johnson, D.C. Inhibition of HLA-DR assembly, transport, and loading by human cytomegalovirus glycoprotein US3: A novel mechanism for evading major histocompatibility complex class II antigen presentation. J. Virol. 2002, 76, 10929–10941. [Google Scholar] [CrossRef] [PubMed]
- Odeberg, J.; Plachter, B.; Brandén, L.; Söderberg-Nauclér, C. Human cytomegalovirus protein pp65 mediates accumulation of HLA-DR in lysosomes and destruction of the HLA-DR alpha-chain. Blood 2003, 101, 4870–4877. [Google Scholar] [CrossRef]
- Miller, D.M.; Cebulla, C.M.; Rahill, B.M.; Sedmak, D.D. Cytomegalovirus and transcriptional down-regulation of major histocompatibility complex class II expression. Semin. Immunol. 2001, 13, 11–18. [Google Scholar] [CrossRef]
- Yawata, M.; Yawata, N.; Draghi, M.; Partheniou, F.; Little, A.M.; Parham, P. MHC class I-specific inhibitory receptors and their ligands structure diverse human NK-cell repertoires toward a balance of missing self-response. Blood 2008, 112, 2369–2380. [Google Scholar] [CrossRef]
- Shirane, M.; Yawata, N.; Motooka, D.; Shibata, K.; Khor, S.S.; Omae, Y.; Kaburaki, T.; Yanai, R.; Mashimo, H.; Yamana, S.; et al. Intraocular human cytomegaloviruses of ocular diseases are distinct from those of viremia and are capable of escaping from innate and adaptive immunity by exploiting HLA-E-mediated peripheral and central tolerance. Front. Immunol. 2022, 13, 1008220. [Google Scholar] [CrossRef]
- Cosman, D.; Müllberg, J.; Sutherland, C.L.; Chin, W.; Armitage, R.; Fanslow, W.; Kubin, M.; Chalupny, N.J. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity 2001, 14, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Fielding, C.A.; Aicheler, R.; Stanton, R.J.; Wang, E.C.; Han, S.; Seirafian, S.; Davies, J.; McSharry, B.P.; Weekes, M.P.; Antrobus, P.R.; et al. Two novel human cytomegalovirus NK cell evasion functions target MICA for lysosomal degradation. PLoS Pathog. 2014, 10, e1004058. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.V.; Lockridge, K.M.; Barry, P.A.; Lin, G.; Tsang, M.; Penfold, M.E.; Schall, T.J. Potent immunosuppressive activities of cytomegalovirus-encoded interleukin-10. J. Virol. 2002, 76, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Q.; Rückert, T.; Borst, E.M.; Dunst, J.; Haubner, A.; Durek, P.; Heinrich, F.; Gasparoni, G.; Babic, M.; Tomic, A.; et al. Peptide-specific recognition of human cytomegalovirus strains controls adaptive natural killer cells. Nat. Immunol. 2018, 19, 453–463. [Google Scholar] [CrossRef]
- Martín Almazán, N.; Sala, B.M.; Sandalova, T.; Sun, Y.; Resink, T.; Cichocki, F.; Söderberg-Nauclér, C.; Miller, J.S.; Achour, A.; Sarhan, D. Non-classical HLA-E restricted CMV 15-mer peptides are recognized by adaptive NK cells and induce memory responses. Front. Immunol. 2023, 14, 1230718. [Google Scholar] [CrossRef]
- Siemaszko, J.; Marzec-Przyszlak, A.; Bogunia-Kubik, K. Activating NKG2C Receptor: Functional Characteristics and Current Strategies in Clinical Applications. Arch. Immunol. Ther. Exp. 2023, 71, 9. [Google Scholar] [CrossRef]
- Mocarski, E.S. Cytomegalovirus Biology Viewed Through a Cell Death Suppression Lens. Viruses 2024, 16, 1820. [Google Scholar] [CrossRef]
- Skaletskaya, A.; Bartle, L.M.; Chittenden, T.; McCormick, A.L.; Mocarski, E.S.; Goldmacher, V.S. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proc. Natl. Acad. Sci. USA 2001, 98, 7829–7834. [Google Scholar] [CrossRef]
- Arnoult, D.; Bartle, L.M.; Skaletskaya, A.; Poncet, D.; Zamzami, N.; Park, P.U.; Sharpe, J.; Youle, R.J.; Goldmacher, V.S. Cytomegalovirus cell death suppressor vMIA blocks Bax- but not Bak-mediated apoptosis by binding and sequestering Bax at mitochondria. Proc. Natl. Acad. Sci. USA 2004, 101, 7988–7993. [Google Scholar] [CrossRef]
- Mandal, P.; Nagrani, L.N.; Hernandez, L.; McCormick, A.L.; Dillon, C.P.; Koehler, H.S.; Roback, L.; Alnemri, E.S.; Green, D.R.; Mocarski, E.S. Multiple Autonomous Cell Death Suppression Strategies Ensure Cytomegalovirus Fitness. Viruses 2021, 13, 1707. [Google Scholar] [CrossRef]
- Norris, K.L.; Youle, R.J. Cytomegalovirus proteins vMIA and m38.5 link mitochondrial morphogenesis to Bcl-2 family proteins. J. Virol. 2008, 82, 6232–6243. [Google Scholar] [CrossRef]
- Krasovec, G.; Renaud, C.; Quéinnec, É.; Sasakura, Y.; Chambon, J.P. Extrinsic apoptosis participates to tail regression during the metamorphosis of the chordate Ciona. Sci. Rep. 2024, 14, 5729. [Google Scholar] [CrossRef]
- Ebermann, L.; Ruzsics, Z.; Guzmán, C.A.; van Rooijen, N.; Casalegno-Garduño, R.; Koszinowski, U.; Čičin-Šain, L. Block of death-receptor apoptosis protects mouse cytomegalovirus from macrophages and is a determinant of virulence in immunodeficient hosts. PLoS Pathog. 2012, 8, e1003062. [Google Scholar] [CrossRef]
- Holler, N.; Zaru, R.; Micheau, O.; Thome, M.; Attinger, A.; Valitutti, S.; Bodmer, J.L.; Schneider, P.; Seed, B.; Tschopp, J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 2000, 1, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Challa, S.; Moquin, D.; Genga, R.; Ray, T.D.; Guildford, M.; Chan, F.K. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 2009, 137, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Kim, Y.S. RIPK3 in necroptosis and cancer. Mol. Cells 2025, 48, 100199. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Perez, P.; Sun, X.; Chen, K.; Fatirkhorani, R.; Mammadova, J.; Wang, Z. MLKL polymerization-induced lysosomal membrane permeabilization promotes necroptosis. Cell Death Differ. 2024, 31, 40–52. [Google Scholar] [CrossRef]
- Fletcher-Etherington, A.; Nobre, L.; Nightingale, K.; Antrobus, R.; Nichols, J.; Davison, A.J.; Stanton, R.J.; Weekes, M.P. Human cytomegalovirus protein pUL36: A dual cell death pathway inhibitor. Proc. Natl. Acad. Sci. USA 2020, 117, 18771–18779. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, W.; Zhang, Y.; Miao, J.; Bao, X.; Lu, C. MLKL as an emerging machinery for modulating organelle dynamics: Regulatory mechanisms, pathophysiological significance, and targeted therapeutics. Front. Pharmacol. 2025, 16, 1512968. [Google Scholar] [CrossRef]
- Adamson, C.S.; Nevels, M.M. Bright and Early: Inhibiting Human Cytomegalovirus by Targeting Major Immediate-Early Gene Expression or Protein Function. Viruses 2020, 12, 110. [Google Scholar] [CrossRef]
- Knuth, A.K.; Rösler, S.; Schenk, B.; Kowald, L.; van Wijk, S.J.L.; Fulda, S. Interferons Transcriptionally Up-Regulate MLKL Expression in Cancer Cells. Neoplasia 2019, 21, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Heusel, A.T.; Rapp, S.; Stamminger, T.; Scherer, M. IE1 of Human Cytomegalovirus Inhibits Necroptotic Cell Death via Direct and Indirect Modulation of the Necrosome Complex. Viruses 2024, 16, 290. [Google Scholar] [CrossRef] [PubMed]
- Upton, J.W.; Kaiser, W.J.; Mocarski, E.S. DAI/ZBP1/DLM-1 Complexes with RIP3 to Mediate Virus-Induced Programmed Necrosis that Is Targeted by Murine Cytomegalovirus vIRA. Cell Host Microbe 2019, 26, 564. [Google Scholar] [CrossRef] [PubMed]
- Nogusa, S.; Thapa, R.J.; Dillon, C.P.; Liedmann, S.; Oguin, T.H., 3rd; Ingram, J.P.; Rodriguez, D.A.; Kosoff, R.; Sharma, S.; Sturm, O.; et al. RIPK3 Activates Parallel Pathways of MLKL-Driven Necroptosis and FADD-Mediated Apoptosis to Protect against Influenza A Virus. Cell Host Microbe 2016, 20, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.L.; Shanmugam, N.; Strange, M.; O’Carroll, A.; Brown, J.W.; Sierecki, E.; Gambin, Y.; Steain, M.; Sunde, M. Viral M45 and necroptosis-associated proteins form heteromeric amyloid assemblies. EMBO Rep. 2019, 20, e46518. [Google Scholar] [CrossRef]
- Chaumorcel, M.; Lussignol, M.; Mouna, L.; Cavignac, Y.; Fahie, K.; Cotte-Laffitte, J.; Geballe, A.; Brune, W.; Beau, I.; Codogno, P.; et al. The human cytomegalovirus protein TRS1 inhibits autophagy via its interaction with Beclin 1. J. Virol. 2012, 86, 2571–2584. [Google Scholar] [CrossRef]
- Krishna, B.A.; Wass, A.B.; Dooley, A.L.; O’Connor, C.M. CMV-encoded GPCR pUL33 activates CREB and facilitates its recruitment to the MIE locus for efficient viral reactivation. J. Cell Sci. 2021, 134, jcs254268. [Google Scholar] [CrossRef]
- Hill, J.A.; Mayer, B.T.; Xie, H.; Leisenring, W.M.; Huang, M.L.; Stevens-Ayers, T.; Milano, F.; Delaney, C.; Sorror, M.L.; Sandmaier, B.M.; et al. The cumulative burden of double-stranded DNA virus detection after allogeneic HCT is associated with increased mortality. Blood 2017, 129, 2316–2325. [Google Scholar] [CrossRef]
- Einsele, H.; Ljungman, P.; Boeckh, M. How I treat CMV reactivation after allogeneic hematopoietic stem cell transplantation. Blood 2020, 135, 1619–1629. [Google Scholar] [CrossRef]
- George, B.; Pati, N.; Gilroy, N.; Ratnamohan, M.; Huang, G.; Kerridge, I.; Hertzberg, M.; Gottlieb, D.; Bradstock, K. Pre-transplant cytomegalovirus (CMV) serostatus remains the most important determinant of CMV reactivation after allogeneic hematopoietic stem cell transplantation in the era of surveillance and preemptive therapy. Transpl. Infect. Dis. 2010, 12, 322–329. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, J.; He, Q.; Zhang, H.; Wen, P.; Wen, P.; Ge, J.; Yang, Y.; Zhang, T.; Wang, R. Impact of varied immunosuppressive agents and post-transplant diabetes mellitus on prognosis among diverse transplant recipients (experimental studies). Int. J. Surg. 2024, 110, 2007–2024. [Google Scholar] [CrossRef]
- Forte, E.; Swaminathan, S.; Schroeder, M.W.; Kim, J.Y.; Terhune, S.S.; Hummel, M. Tumor Necrosis Factor Alpha Induces Reactivation of Human Cytomegalovirus Independently of Myeloid Cell Differentiation following Posttranscriptional Establishment of Latency. mBio 2018, 9, e01560-18. [Google Scholar] [CrossRef]
- Yong, M.K.; Cameron, P.U.; Slavin, M.; Morrissey, C.O.; Bergin, K.; Spencer, A.; Ritchie, D.; Cheng, A.C.; Samri, A.; Carcelain, G.; et al. Identifying Cytomegalovirus Complications Using the Quantiferon-CMV Assay After Allogeneic Hematopoietic Stem Cell Transplantation. J. Infect. Dis. 2017, 215, 1684–1694. [Google Scholar] [CrossRef]
- Hummel, M.; Zhang, Z.; Yan, S.; DePlaen, I.; Golia, P.; Varghese, T.; Thomas, G.; Abecassis, M.I. Allogeneic transplantation induces expression of cytomegalovirus immediate-early genes in vivo: A model for reactivation from latency. J. Virol. 2001, 75, 4814–4822. [Google Scholar] [CrossRef]
- Liu, X.F.; Jie, C.; Zhang, Z.; Yan, S.; Wang, J.J.; Wang, X.; Kurian, S.; Salomon, D.R.; Abecassis, M.; Hummel, M. Transplant-induced reactivation of murine cytomegalovirus immediate early gene expression is associated with recruitment of NF-κB and AP-1 to the major immediate early promoter. J. Gen. Virol. 2016, 97, 941–954. [Google Scholar] [CrossRef]
- Lambe, G.; Mansukhani, D.; Khodaiji, S.; Shetty, A.; Rodrigues, C.; Kapadia, F. Immune Modulation and Cytomegalovirus Reactivation in Sepsis-induced Immunosuppression: A Pilot Study. Indian J. Crit. Care Med. 2022, 26, 53–61. [Google Scholar] [CrossRef]
- Zhang, L.; Khadka, B.; Wu, J.; Feng, Y.; Long, B.; Xiao, R.; Liu, J. CMV infection is a risk factor for hemorrhagic cystitis after hematopoietic stem cell transplantation. Ann. Hematol. 2023, 102, 1193–1201. [Google Scholar] [CrossRef]
- Li, S.; Xiao, Y.; Jia, M. Prior cytomegalovirus reactivation may lead to worse bacterial bloodstream infection outcomes in HSCT patients. Transpl. Immunol. 2024, 84, 102038. [Google Scholar] [CrossRef]
- Wu, X.; Ma, X.; Song, T.; Liu, J.; Sun, Y.; Wu, D. The indirect effects of CMV reactivation on patients following allogeneic hematopoietic stem cell transplantation: An evidence mapping. Ann. Hematol. 2024, 103, 917–933. [Google Scholar] [CrossRef]
- Green, M.L.; Leisenring, W.M.; Xie, H.; Walter, R.B.; Mielcarek, M.; Sandmaier, B.M.; Riddell, S.R.; Boeckh, M. CMV reactivation after allogeneic HCT and relapse risk: Evidence for early protection in acute myeloid leukemia. Blood 2013, 122, 1316–1324. [Google Scholar] [CrossRef]
- Teira, P.; Battiwalla, M.; Ramanathan, M.; Barrett, A.J.; Ahn, K.W.; Chen, M.; Green, J.S.; Saad, A.; Antin, J.H.; Savani, B.N.; et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: A CIBMTR analysis. Blood 2016, 127, 2427–2438. [Google Scholar] [CrossRef]
- Lum, J.; Koval, C. The changing landscape of infections in the lung transplant recipient. Curr. Opin. Pulm. Med. 2024, 30, 382–390. [Google Scholar] [CrossRef]
- Almaghrabi, R.S.; Omrani, A.S.; Memish, Z.A. Cytomegalovirus infection in lung transplant recipients. Expert. Rev. Respir. Med. 2017, 11, 377–383. [Google Scholar] [CrossRef]
- Snyder, L.D.; Finlen-Copeland, C.A.; Turbyfill, W.J.; Howell, D.; Willner, D.A.; Palmer, S.M. Cytomegalovirus pneumonitis is a risk for bronchiolitis obliterans syndrome in lung transplantation. Am. J. Respir. Crit. Care Med. 2010, 181, 1391–1396. [Google Scholar] [CrossRef]
- Jothimani, D.; Venugopal, R.; Vij, M.; Rela, M. Post liver transplant recurrent and de novo viral infections. Best Pract. Res. Clin. Gastroenterol. 2020, 46–47, 101689. [Google Scholar] [CrossRef]
- Puliti Reigada, C.H.; de Ataide, E.C.; de Almeida Prado Mattosinho, T.; Boin, I. Hepatic Artery Thrombosis After Liver Transplantation: Five-Year Experience at the State University of Campinas. Transplant. Proc. 2017, 49, 867–870. [Google Scholar] [CrossRef]
- Newton, K.; Strasser, A.; Kayagaki, N.; Dixit, V.M. Cell death. Cell 2024, 187, 235–256. [Google Scholar] [CrossRef]
- Karunakaran, D.; Nguyen, M.A.; Geoffrion, M.; Vreeken, D.; Lister, Z.; Cheng, H.S.; Otte, N.; Essebier, P.; Wyatt, H.; Kandiah, J.W.; et al. RIPK1 Expression Associates With Inflammation in Early Atherosclerosis in Humans and Can Be Therapeutically Silenced to Reduce NF-κB Activation and Atherogenesis in Mice. Circulation 2021, 143, 163–177. [Google Scholar] [CrossRef]
- Aiello, A.; Farzaneh, F.; Candore, G.; Caruso, C.; Davinelli, S.; Gambino, C.M.; Ligotti, M.E.; Zareian, N.; Accardi, G. Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Front. Immunol. 2019, 10, 2247. [Google Scholar] [CrossRef]
- Quan, X.Q.; Ruan, L.; Zhou, H.R.; Gao, W.L.; Zhang, Q.; Zhang, C.T. Age-related changes in peripheral T-cell subpopulations in elderly individuals: An observational study. Open Life Sci. 2023, 18, 20220557. [Google Scholar] [CrossRef]
- Koch, S.; Larbi, A.; Derhovanessian, E.; Ozcelik, D.; Naumova, E.; Pawelec, G. Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immun. Ageing 2008, 5, 6. [Google Scholar] [CrossRef]
- Tu, W.; Rao, S. Mechanisms Underlying T Cell Immunosenescence: Aging and Cytomegalovirus Infection. Front. Microbiol. 2016, 7, 2111. [Google Scholar] [CrossRef]
- Sylwester, A.W.; Mitchell, B.L.; Edgar, J.B.; Taormina, C.; Pelte, C.; Ruchti, F.; Sleath, P.R.; Grabstein, K.H.; Hosken, N.A.; Kern, F.; et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 2005, 202, 673–685. [Google Scholar] [CrossRef]
- Korolchuk, V.I.; Miwa, S.; Carroll, B.; von Zglinicki, T. Mitochondria in Cell Senescence: Is Mitophagy the Weakest Link? eBioMedicine 2017, 21, 7–13. [Google Scholar] [CrossRef]
- Saavedra, D.; Añé-Kourí, A.L.; Barzilai, N.; Caruso, C.; Cho, K.H.; Fontana, L.; Franceschi, C.; Frasca, D.; Ledón, N.; Niedernhofer, L.J.; et al. Aging and chronic inflammation: Highlights from a multidisciplinary workshop. Immun. Ageing 2023, 20, 25. [Google Scholar] [CrossRef]
- Wiech, M.; Chroscicki, P.; Swatler, J.; Stepnik, D.; De Biasi, S.; Hampel, M.; Brewinska-Olchowik, M.; Maliszewska, A.; Sklinda, K.; Durlik, M.; et al. Remodeling of T Cell Dynamics During Long COVID Is Dependent on Severity of SARS-CoV-2 Infection. Front. Immunol. 2022, 13, 886431. [Google Scholar] [CrossRef]
- Schultheiß, C.; Willscher, E.; Paschold, L.; Gottschick, C.; Klee, B.; Henkes, S.S.; Bosurgi, L.; Dutzmann, J.; Sedding, D.; Frese, T.; et al. The IL-1β, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep. Med. 2022, 3, 100663. [Google Scholar] [CrossRef]
- Navarro-Bielsa, A.; Gracia-Cazaña, T.; Aldea-Manrique, B.; Abadías-Granado, I.; Ballano, A.; Bernad, I.; Gilaberte, Y. COVID-19 infection and vaccines: Potential triggers of Herpesviridae reactivation. An. Bras. Dermatol. 2023, 98, 347–354. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Nikolich-Žugich, J.; Čicin-Šain, L.; Collins-McMillen, D.; Jackson, S.; Oxenius, A.; Sinclair, J.; Snyder, C.; Wills, M.; Lemmermann, N. Advances in cytomegalovirus (CMV) biology and its relationship to health, diseases, and aging. Geroscience 2020, 42, 495–504. [Google Scholar] [CrossRef]
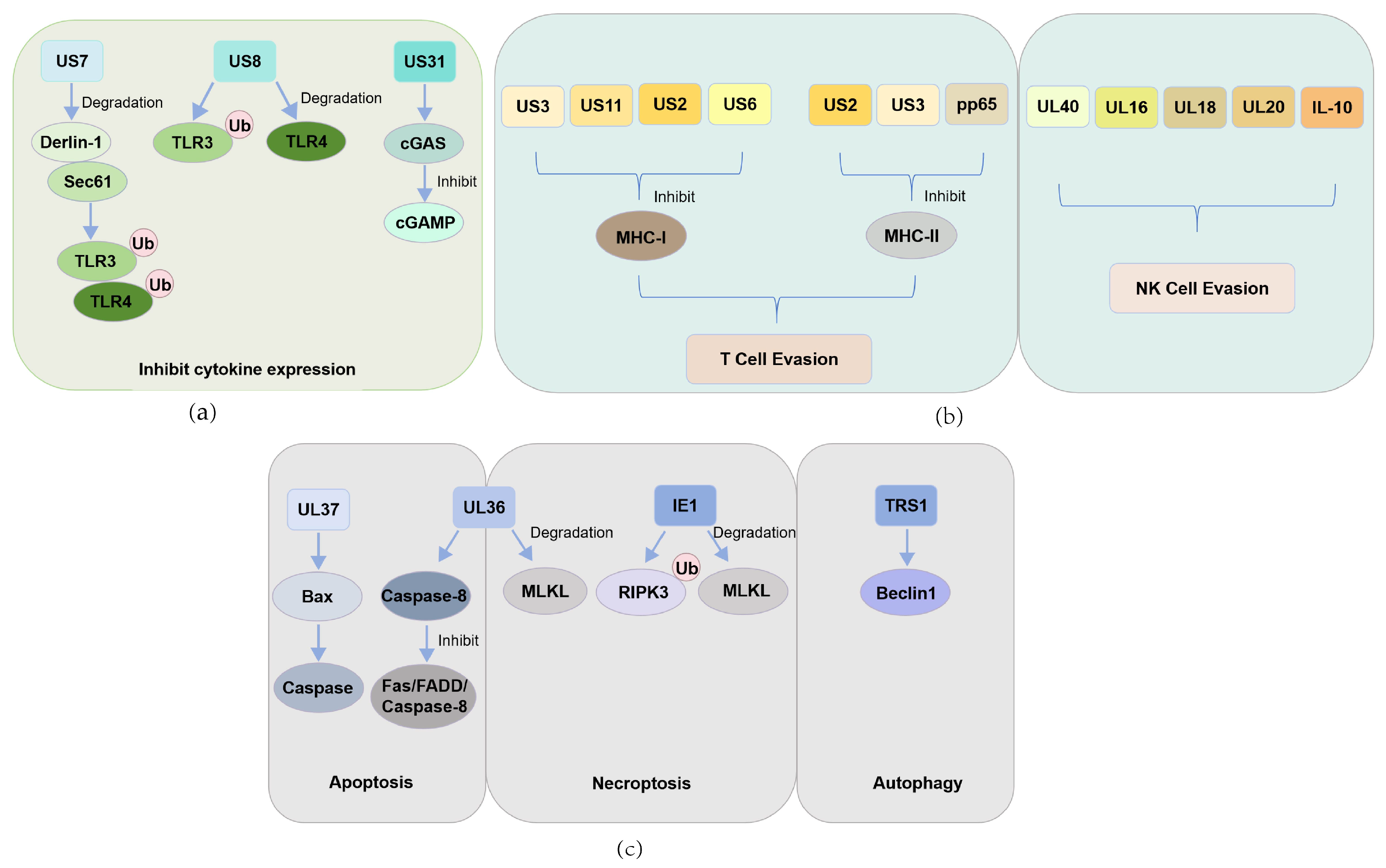
| Antiviral Pathway | Virus Type | CMV-Encoded Regulatory Proteins | Target Host Proteins | Reference |
|---|---|---|---|---|
| PRR recognition | HCMV | US7 | TLR3, TLR4 | [38] |
| US8 | TLR3, TLR4 | [38] | ||
| UL31 | cGAS | [40] | ||
| T cell | HCMV | US3 | MHC-I, MHC-II | [42,46] |
| US11 | MHC-I | [42] | ||
| US2 | MHC-I, MHC-II | [43,45] | ||
| US6 | MHC-I | [44] | ||
| pp65 | MHC-II | [47] | ||
| NK cell | HCMV | UL40 | HLA-E | [50] |
| UL16 | NKG2D | [51] | ||
| UL18 | MICA | [52] | ||
| UL20 | MICA | [52] | ||
| IL-10 | HLA-G | [53] | ||
| Apoptosis | HCMV | UL36 | Caspase-8 | [58] |
| UL37 | BAX | [59] | ||
| MCMV | M36 | Caspase-8 | [63] | |
| M38.5 | BAX | [61] | ||
| Necroptosis | HCMV | UL36 | MLKL | [68] |
| IE1 | RIPK3 | [70] | ||
| IE1 | MLKL | [72] | ||
| MCMV | M45 | RIPK3 | [75] | |
| Autophagy | HCMV | TRS1 | Beclin1 | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Liu, C.; Zhang, T. The Immunoregulatory Mechanisms of Human Cytomegalovirus from Primary Infection to Reactivation. Pathogens 2025, 14, 998. https://doi.org/10.3390/pathogens14100998
Liu X, Liu C, Zhang T. The Immunoregulatory Mechanisms of Human Cytomegalovirus from Primary Infection to Reactivation. Pathogens. 2025; 14(10):998. https://doi.org/10.3390/pathogens14100998
Chicago/Turabian StyleLiu, Xiaodan, Chang Liu, and Ting Zhang. 2025. "The Immunoregulatory Mechanisms of Human Cytomegalovirus from Primary Infection to Reactivation" Pathogens 14, no. 10: 998. https://doi.org/10.3390/pathogens14100998
APA StyleLiu, X., Liu, C., & Zhang, T. (2025). The Immunoregulatory Mechanisms of Human Cytomegalovirus from Primary Infection to Reactivation. Pathogens, 14(10), 998. https://doi.org/10.3390/pathogens14100998







