Post-COVID-19 Epidemiology of Viral Infections in Adults Hospitalized with Acute Respiratory Syndromes in Palermo, South of Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population and Clinical Samples
2.3. Microbial Pathogen Detection
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
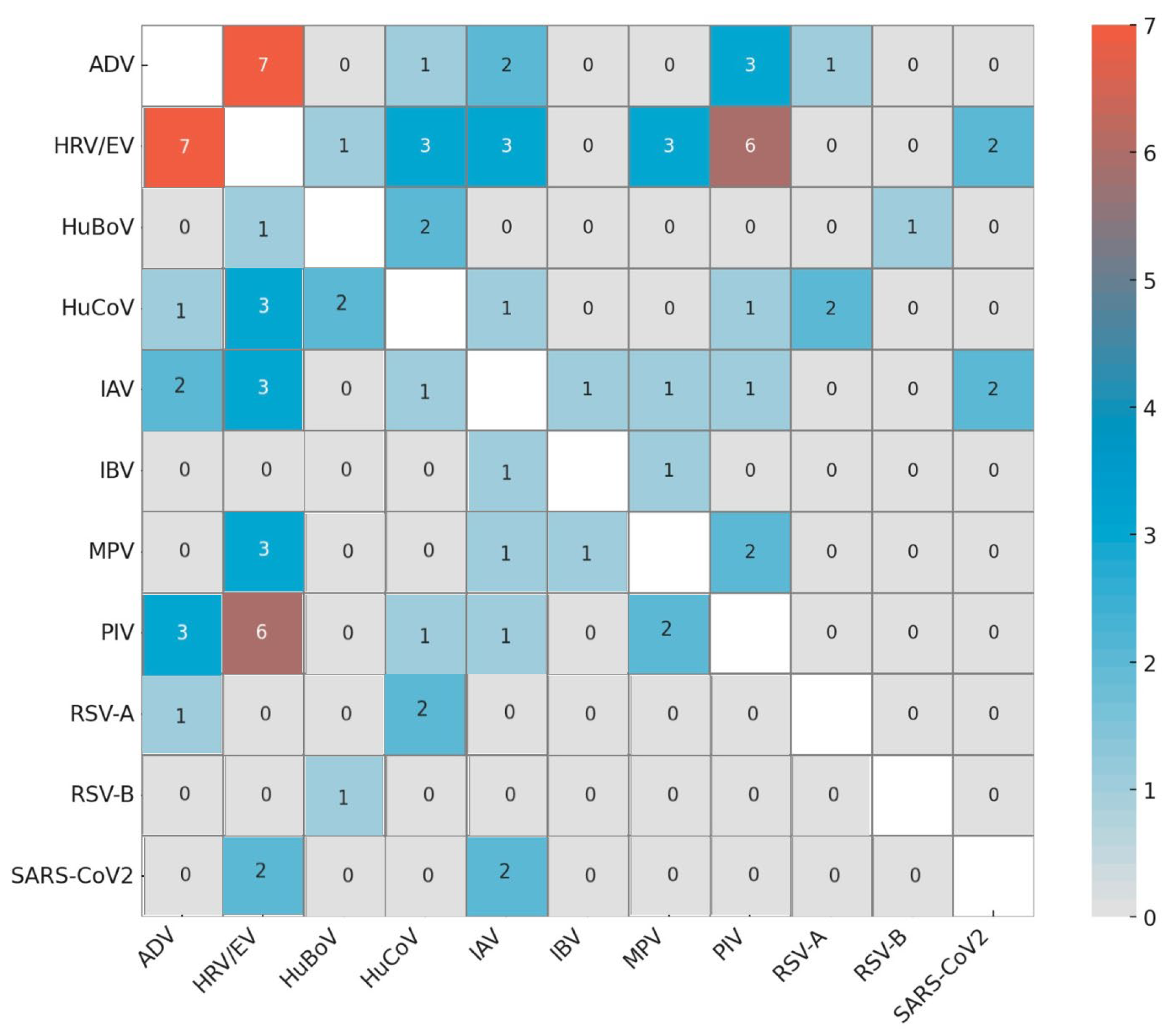
3.2. Detection of Respiratory Viruses
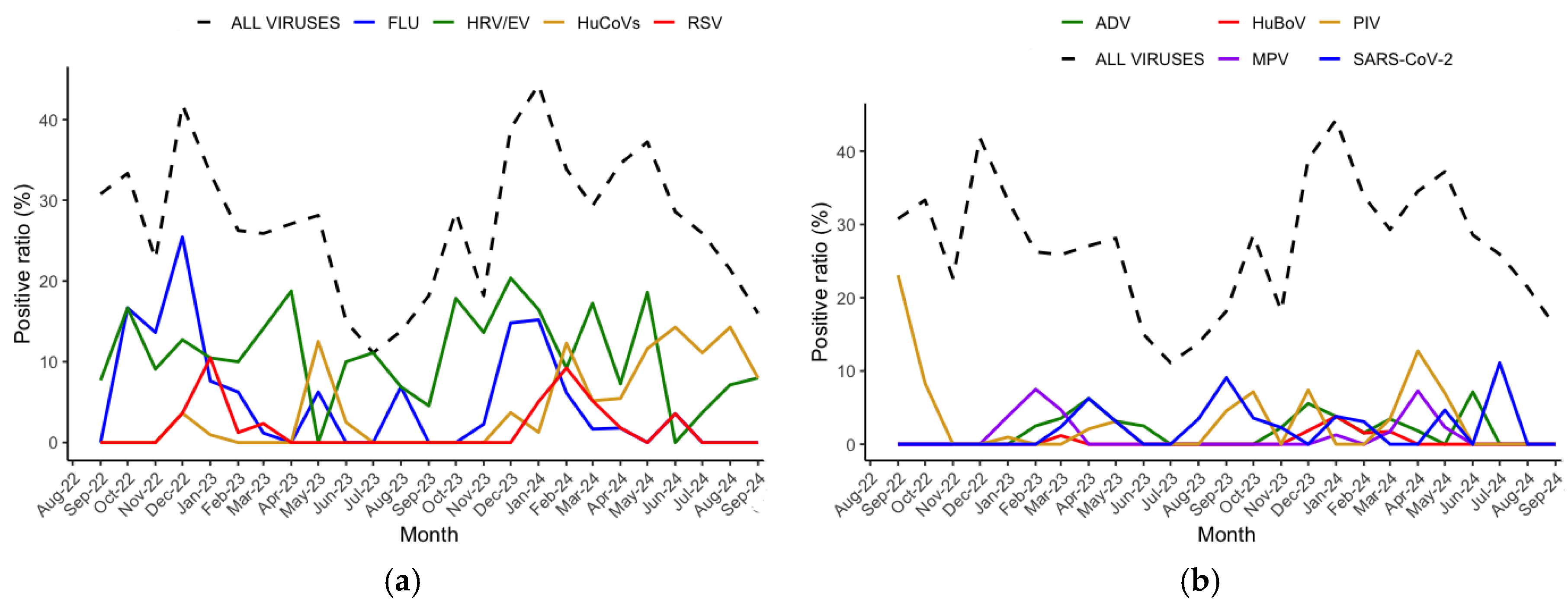
3.3. Virus Seasonality
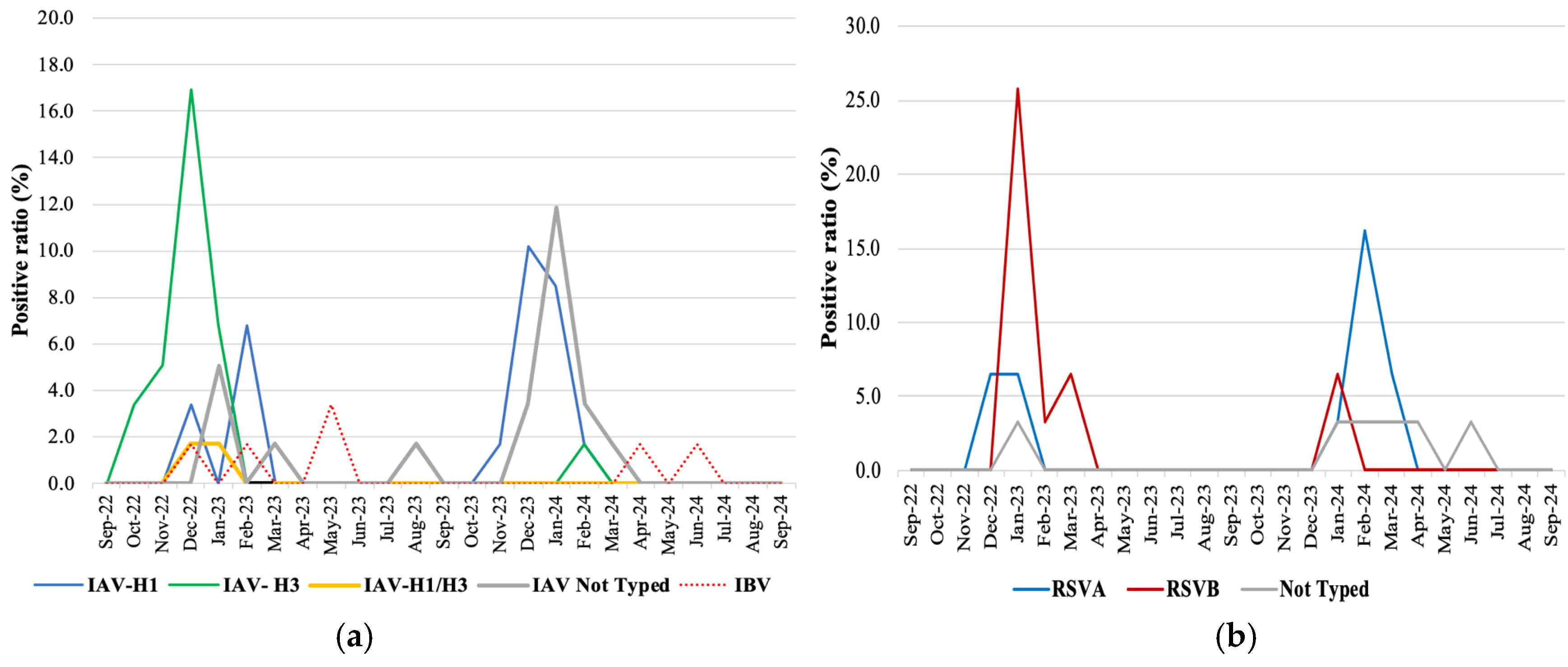
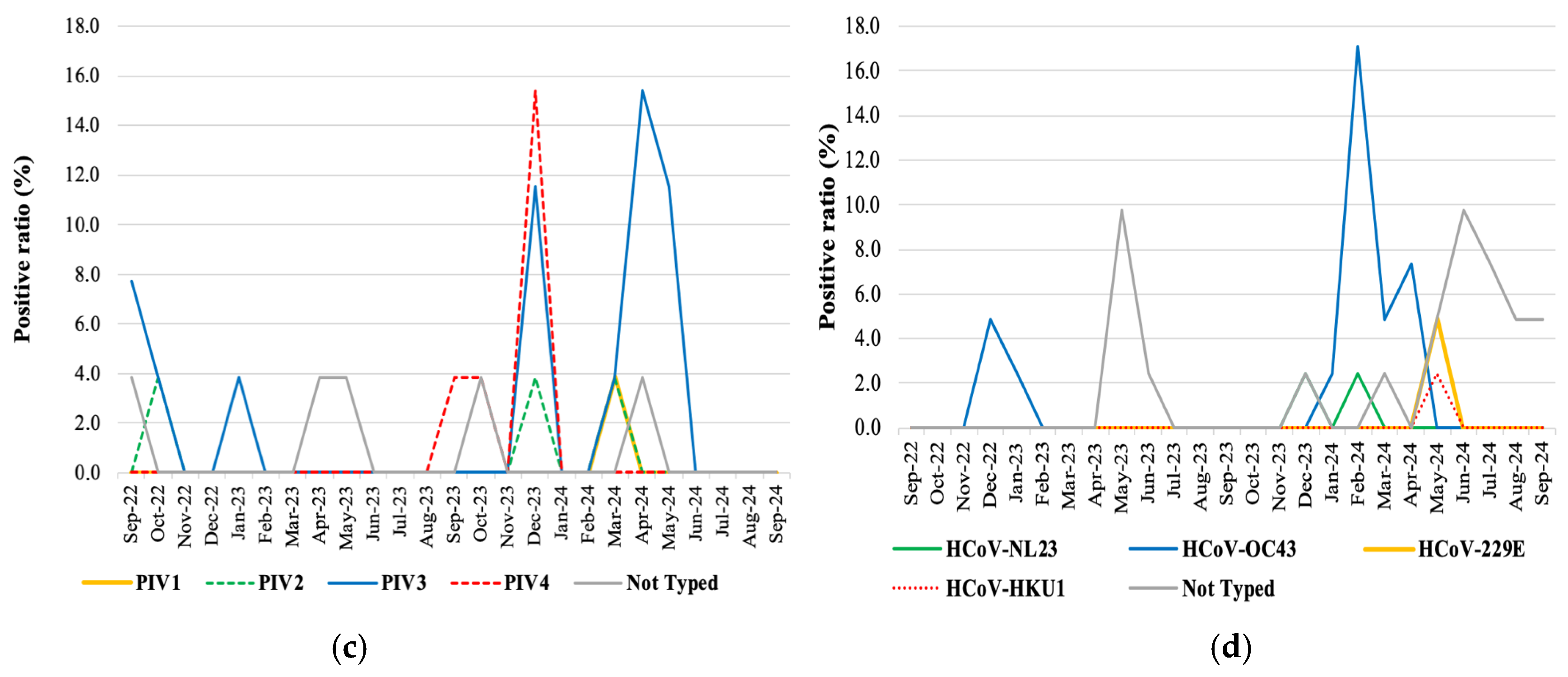
3.4. Virus Genotyping
3.5. Non-Viral Pathogen Detection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RTI | Respiratory tract infection |
| URTIs | Upper respiratory tract infections |
| LRTIs | Lower respiratory tract infections |
| COPD | Chronic obstructive pulmonary disease |
| IAV | Influenza A virus |
| IBV | Influenza B virus |
| RSV | Respiratory syncytial virus |
| PIVS | Parainfluenza viruses 1/2/3/4 |
| ADV | Adenovirus |
| SARS-CoV-2 or COVID-19 | Severe acute respiratory syndrome coronavirus 2 |
| NPIs | Non-pharmaceutical interventions |
| NPS | Nasopharyngeal swab or nasal swab |
| BAL | Bronchoalveolar lavage or sputum |
| ICU | Intensive care unit |
| non-ICU | Non-intensive care unit |
| FAPP | FilmArray Pneumonia plus |
| RP2.1 | FilmArray Respiratory Panel 2.1 |
| Ct | Threshold cycle |
| HRV/EV | Human rhinovirus/enterovirus |
| HuCoVs | Human coronaviruses (HuCoV-NL63, HuCoV-OC43, HuCoV-229E, and HuCoV-HKU1) |
| HuBoV | Human bocavirus |
| FLU | Influenza virus |
| MPV | Metapneumovirus |
| CAP | Community-acquired pneumonia |
| HAP | Hospital-acquired pneumonia |
| PCR | Polymerase Chain Reaction |
| IQR | Interquartile range |
References
- Cavallazzi, R.; Ramirez, J.A. How and when to manage respiratory infections out of hospital. Eur. Respir. Rev. 2022, 31, 220092. [Google Scholar] [CrossRef]
- Niederman, M.S.; Torres, A. Respiratory infections. Eur. Respir. Rev. 2022, 31, 220150. [Google Scholar] [CrossRef]
- Wang, D.Y.; Eccles, R.; Bell, J.; Chua, A.H.; Salvi, S.; Schellack, N.; Marks, P.; Wong, Y.C. Management of acute upper respiratory tract infection: The role of early intervention. Expert Rev. Respir. Med. 2021, 15, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Leung, N.H.L. Transmissibility and transmission of respiratory viruses. Nat. Rev. Microbiol. 2021, 19, 528–545. [Google Scholar] [CrossRef] [PubMed]
- Firquet, S.; Beaujard, S.; Lobert, P.-E.; Sané, F.; Caloone, D.; Izard, D.; Hober, D. Survival of Enveloped and Non-Enveloped Viruses on Inanimate Surfaces. Microbes Environ. 2015, 30, 140–144. [Google Scholar] [CrossRef]
- Principi, N.; Autore, G.; Ramundo, G.; Esposito, S. Epidemiology of Respiratory Infections during the COVID-19 Pandemic. Viruses 2023, 15, 1160. [Google Scholar] [CrossRef]
- Cilloniz, C.; Luna, C.M.; Hurtado, J.C.; Marcos, M.Á.; Torres, A. Respiratory viruses: Their importance and lessons learned from COVID-19. Eur. Respir. Rev. 2022, 31, 220051. [Google Scholar] [CrossRef]
- Price, R.H.M.; Graham, C.; Ramalingam, S. Association between viral seasonality and meteorological factors. Sci. Rep. 2019, 9, 929. [Google Scholar] [CrossRef]
- Moriyama, M.; Hugentobler, W.J.; Iwasaki, A. Seasonality of Respiratory Viral Infections. Annu. Rev. Virol. 2020, 7, 83–101. [Google Scholar] [CrossRef]
- Ke, R.; Romero-Severson, E.; Sanche, S.; Hengartner, N. Estimating the reproductive number R0 of SARS-CoV-2 in the United States and eight European countries and implications for vaccination. J. Theor. Biol. 2021, 517, 110621. [Google Scholar] [CrossRef]
- Karimizadeh, Z.; Dowran, R.; Mokhtari-azad, T.; Shafiei-Jandaghi, N.-Z. The reproduction rate of severe acute respiratory syndrome coronavirus 2 different variants recently circulated in human: A narrative review. Eur. J. Med. Res. 2023, 28, 94. [Google Scholar] [CrossRef]
- Thompson, R.; Wood, J.G.; Tempia, S.; Muscatello, D.J. Global variation in early epidemic growth rates and reproduction number of seasonal influenza. Int. J. Infect. Dis. 2022, 122, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rocklöv, J. The effective reproductive number of the Omicron variant of SARS-CoV-2 is several times relative to Delta. J. Travel Med. 2022, 29, taac037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liang, H.; Xu, J.; Chen, B.; Zheng, X.; Lin, H.; Wang, W.; Yao, Y. Spatial-temporal dynamics and virus interference of respiratory viruses: Insights from multi-pathogen surveillance in China. J. Infect. 2025, 91, 106556. [Google Scholar] [CrossRef]
- Chow, E.J.; Uyeki, T.M.; Chu, H.Y. The effects of the COVID-19 pandemic on community respiratory virus activity. Nat. Rev. Microbiol. 2022, 21, 195–210. [Google Scholar] [CrossRef]
- Tang, Y.-W.; Schmitz, J.E.; Persing, D.H.; Stratton, C.W. Laboratory Diagnosis of COVID-19: Current Issues and Challenges. J. Clin. Microbiol. 2020, 58, e00512-20. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, L.; Deng, X.; Liang, R.; Su, M.; He, C.; Hu, L.; Su, Y.; Ren, J.; Yu, F.; et al. Recent advances in the detection of respiratory virus infection in humans. J. Med. Virol. 2020, 92, 408–417. [Google Scholar] [CrossRef]
- Piret, J.; Boivin, G. Viral Interference between Respiratory Viruses. Emerg. Infect. Dis. 2022, 28, 273–281. [Google Scholar] [CrossRef]
- Pizzorno, A.; Padey, B.; Dulière, V.; Mouton, W.; Oliva, J.; Laurent, E.; Milesi, C.; Lina, B.; Traversier, A.; Julien, T.; et al. Interactions Between Severe Acute Respiratory Syndrome Coronavirus 2 Replication and Major Respiratory Viruses in Human Nasal Epithelium. J. Infect. Dis. 2022, 226, 2095–2104. [Google Scholar] [CrossRef]
- Gilbert-Girard, S.; Piret, J.; Carbonneau, J.; Hénaut, M.; Goyette, N.; Boivin, G. Viral interference between severe acute respiratory syndrome coronavirus 2 and influenza A viruses. PLoS Pathog. 2024, 20, e1012017. [Google Scholar] [CrossRef]
- Olsen, S.J.; Winn, A.K.; Budd, A.P.; Prill, M.M.; Steel, J.; Midgley, C.M.; Kniss, K.; Burns, E.; Rowe, T.; Foust, A.; et al. Changes in Influenza and Other Respiratory Virus Activity During the COVID-19 Pandemic—United States, 2020–2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1013–1019. [Google Scholar] [CrossRef]
- Dien Bard, J.; McElvania, E. Panels and Syndromic Testing in Clinical Microbiology. Clin. Lab. Med. 2020, 40, 393–420. [Google Scholar] [CrossRef] [PubMed]
- Fricke, L.M.; Glöckner, S.; Dreier, M.; Lange, B. Impact of non-pharmaceutical interventions targeted at COVID-19 pandemic on influenza burden—a systematic review. J. Infect. 2021, 82, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, P.; Bryson, A.L.; Binnicker, M.J.; Pritt, B.S.; Patel, R. Syndromic Panel-Based Testing in Clinical Microbiology. Clin. Microbiol. Rev. 2018, 31, e00024-17. [Google Scholar] [CrossRef]
- Vandendriessche, S.; Padalko, E.; Wollants, E.; Verfaillie, C.; Verhasselt, B.; Coorevits, L. Evaluation of the Seegene AllplexTM Respiratory Panel for diagnosis of acute respiratory tract infections. Acta Clin. Belg. 2019, 74, 379–385. [Google Scholar] [CrossRef]
- ECDC European Respiratory Virus Surveillance Summary. Available online: https://erviss.org (accessed on 14 March 2025).
- RespiVirNet. Epidemiological Reports 2022–23 and 2023–24. Available online: https://respivirnet.iss.it/default.aspx?ReturnUrl=%2f (accessed on 14 March 2025).
- Zhang, D.; He, Z.; Xu, L.; Zhu, X.; Wu, J.; Wen, W.; Zheng, Y.; Deng, Y.; Chen, J.; Hu, Y.; et al. Epidemiology characteristics of respiratory viruses found in children and adults with respiratory tract infections in southern China. Int. J. Infect. Dis. 2014, 25, 159–164. [Google Scholar] [CrossRef]
- De Francesco, M.A.; Pollara, C.; Gargiulo, F.; Giacomelli, M.; Caruso, A. Circulation of Respiratory Viruses in Hospitalized Adults before and during the COVID-19 Pandemic in Brescia, Italy: A Retrospective Study. Int. J. Environ. Res. Public Health 2021, 18, 9525. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Zhang, Y.-F.; Xu, Q.; Qiu, Y.; Lu, Q.-B.; Wang, T.; Zhang, X.-A.; Lin, S.-H.; Lv, C.-L.; Jiang, B.-G.; et al. Infection and co-infection patterns of community-acquired pneumonia in patients of different ages in China from 2009 to 2020: A national surveillance study. Lancet Microbe 2023, 4, e330–e339. [Google Scholar] [CrossRef]
- Jones, R.P.; Ponomarenko, A. COVID-19-Related Age Profiles for SARS-CoV-2 Variants in England and Wales and States of the USA (2020 to 2022): Impact on All-Cause Mortality. Infect. Dis. Rep. 2023, 15, 600–634. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Severe Acute Respiratory Infections Treatment Centre. In Practical Manual to Set Up and Manage a SARI Treatment Centre and a SARI Screening Facility in Health Care Facilities; World Health Organization: Geneva, Switzerland, 2020; Available online: https://reliefweb.int/report/world/severe-acute-respiratory-infections-treatment-centre-practical-manual-set-and-manage (accessed on 25 March 2024).
- Schüz, M.L.; Dallmeyer, L.; Fragkou, P.C.; Omony, J.; Krumbein, H.; Hünerbein, B.L.; Skevaki, C. Global prevalence of respiratory virus infections in adults and adolescents during the COVID-19 pandemic: A systematic review and meta-analysis. Int. J. Infect. Dis. 2023, 137, 16–24. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Z.; Yan, Y.; Shi, Y.; Huang, J.; Du, H.; Du, Q.; Li, Y.; Lin, Y.; Liu, D.; et al. Prevalence of respiratory viruses among hospitalized children with lower respiratory tract infections during the COVID-19 pandemic in Wuhan, China. Int. J. Infect. Dis. 2024, 139, 6–12. [Google Scholar] [CrossRef]
- Sumitomo, T.; Kawabata, S. Respiratory tract barrier dysfunction in viral-bacterial co-infection cases. Jpn. Dent. Sci. Rev. 2024, 60, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Barberi, C.; Castelnuovo, E.; Dipasquale, A.; Mrakic Sposta, F.; Vatteroni, G.; Canziani, L.M.; Alloisio, M.; Ciccarelli, M.; Selmi, C.; Ferraroli, G.M. Bronchoalveolar lavage in suspected COVID-19 cases with a negative nasopharyngeal swab: A retrospective cross-sectional study in a high-impact Northern Italy area. Intern. Emerg. Med. 2021, 16, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- Bouzid, D.; Hingrat, Q.L.; Salipante, F.; Ferré, V.M.; Chevallier, T.; Tubiana, S.; Lucet, J.C.; Choquet, C.; Yazdanpanah, Y.; Timsit, J.F.; et al. Agreement of respiratory viruses’ detection between nasopharyngeal swab and bronchoalveolar lavage in adults admitted for pneumonia: A retrospective study. Clin. Microbiol. Infect. 2023, 29, 942.e1–942.e6. [Google Scholar] [CrossRef] [PubMed]
- Vanderwall, E.R.; Barrow, K.A.; Rich, L.M.; Read, D.F.; Trapnell, C.; Okoloko, O.; Ziegler, S.F.; Hallstrand, T.S.; White, M.P.; Debley, J.S. Airway epithelial interferon response to SARS-CoV-2 is inferior to rhinovirus and heterologous rhinovirus infection suppresses SARS-CoV-2 replication. Sci. Rep. 2022, 12, 6972. [Google Scholar] [CrossRef]
- Dee, K.; Goldfarb, D.M.; Haney, J.; Amat, J.A.R.; Herder, V.; Stewart, M.; Szemiel, A.M.; Baguelin, M.; Murcia, P.R. Human Rhinovirus Infection Blocks Severe Acute Respiratory Syndrome Coronavirus 2 Replication Within the Respiratory Epithelium: Implications for COVID-19 Epidemiology. J. Infect. Dis. 2021, 224, 31–38. [Google Scholar] [CrossRef]
- Mosscrop, L.G.; Williams, T.C.; Tregoning, J.S. Respiratory syncytial virus after the SARS-CoV-2 pandemic—what next? Nat. Rev. Immunol. 2022, 22, 589–590. [Google Scholar] [CrossRef]
- Watanabe, Y.; Kamioka, Y.; Seki, M. Rhinovirus-Infected Patients in the COVID-19 Pandemic Period. Infect. Drug Resist. 2021, 14, 609–611. [Google Scholar] [CrossRef]
- Park, K.Y.; Seo, S.; Han, J.; Park, J.Y. Respiratory virus surveillance in Canada during the COVID-19 pandemic: An epidemiological analysis of the effectiveness of pandemic-related public health measures in reducing seasonal respiratory viruses test positivity. PLoS ONE 2021, 16, e0253451. [Google Scholar] [CrossRef]
- De Maio, F.; Fiori, B.; Bianco, D.M.; Sanguinetti, M.; Sali, M. Respiratory viruses in the pre and post-pandemic periods in an Italian tertiary hospital. Immun. Inflamm. Dis. 2023, 11, e909. [Google Scholar] [CrossRef]
- Esneau, C.; Duff, A.C.; Bartlett, N.W. Understanding Rhinovirus Circulation and Impact on Illness. Viruses 2022, 14, 141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, T.; Zou, M.; Wang, L.; Sai, L. The epidemiological features of respiratory tract infection using the multiplex panels detection during COVID-19 pandemic in Shandong province, China. Sci. Rep. 2023, 13, 6319. [Google Scholar] [CrossRef] [PubMed]
- Sclavi, L.; Bertelli, M.; Messali, S.; Caruso, A.; Caccuri, F. Epidemiology and molecular characterization of respiratory viruses at the end of COVID-19 pandemic in Lombardy, Northern Italy. New Microbiol. 2024, 47, 80–87. [Google Scholar]
- Eden, J.-S.; Sikazwe, C.; Xie, R.; Deng, Y.-M.; Sullivan, S.G.; Michie, A.; Levy, A.; Cutmore, E.; Blyth, C.C.; Britton, P.N.; et al. Off-season RSV epidemics in Australia after easing of COVID-19 restrictions. Nat. Commun. 2022, 13, 2884. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Influenza Surveillance and Response System (GISRS). Available online: https://www.who.int/initiatives/global-influenza-surveillance-and-response-system (accessed on 14 March 2025).
- Istituto Superiore di Sanità National. Integrated Epidemiological Surveillance of Respiratory Viruses 2022/23 and 2023/24 Season, Italy. Available online: https://www.epicentro.iss.it/influenza/respivirnet (accessed on 14 March 2025).
- Maida, C.M.; Mazzucco, W.; Priano, W.; Palermo, R.; Graziano, G.; Costantino, C.; Russo, A.; Andolina, G.; Restivo, I.; Giangreco, V.; et al. Detection of influenza virus in urban wastewater during the season 2022/2023 in Sicily, Italy. Front. Public Health 2024, 12, 1383536. [Google Scholar] [CrossRef]
- Maggi, S.; Veronese, N.; Burgio, M.; Cammarata, G.; Ciuppa, M.E.; Ciriminna, S.; Di Gennaro, F.; Smith, L.; Trott, M.; Dominguez, L.J.; et al. Rate of Hospitalizations and Mortality of Respiratory Syncytial Virus Infection Compared to Influenza in Older People: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 2092. [Google Scholar] [CrossRef]
- Redlberger-Fritz, M.; Springer, D.N.; Aberle, S.W.; Camp, J.V.; Aberle, J.H. Respiratory syncytial virus surge in 2022 caused by lineages already present before the COVID-19 pandemic. J. Med. Virol. 2023, 95, e28830. [Google Scholar] [CrossRef]
- Pierangeli, A.; Midulla, F.; Piralla, A.; Ferrari, G.; Nenna, R.; Pitrolo, A.M.G.; Licari, A.; Marseglia, G.L.; Abruzzese, D.; Pellegrinelli, L.; et al. Sequence analysis of respiratory syncytial virus cases reveals a novel subgroup -B strain circulating in north-central Italy after pandemic restrictions. J. Clin. Virol. 2024, 173, 105681. [Google Scholar] [CrossRef]
- Hatter, L.; Eathorne, A.; Hills, T.; Bruce, P.; Beasley, R. Respiratory syncytial virus: Paying the immunity debt with interest. Lancet Child Adolesc. Health 2021, 5, e44–e45. [Google Scholar] [CrossRef]
- Pius-Sadowska, E.; Kulig, P.; Niedźwiedź, A.; Baumert, B.; Rogińska, D.; Łuczkowska, K.; Sobuś, A.; Parczewski, M.; Kawa, M.; Paczkowska, E.; et al. The micro-RNA expression profile predicts the severity of SARS-CoV-2 infection. Sci. Rep. 2025, 15, 17139. [Google Scholar] [CrossRef]
- Kesheh, M.M.; Hosseini, P.; Soltani, S.; Zandi, M. An overview on the seven pathogenic human coronaviruses. Rev. Med. Virol. 2022, 32, e2282. [Google Scholar] [CrossRef]
- Russell, E.; Ison, M.G. Parainfluenza Virus in the Hospitalized Adult. Clin. Infect. Dis. 2017, 65, 1570–1576. [Google Scholar] [CrossRef]
- Voiriot, G.; Visseaux, B.; Cohen, J.; Nguyen, L.B.L.; Neuville, M.; Morbieu, C.; Burdet, C.; Radjou, A.; Lescure, F.-X.; Smonig, R.; et al. Viral-bacterial coinfection affects the presentation and alters the prognosis of severe community-acquired pneumonia. Crit. Care 2016, 20, 375. [Google Scholar] [CrossRef]
- Di Maio, V.C.; Scutari, R.; Forqué, L.; Colagrossi, L.; Coltella, L.; Ranno, S.; Linardos, G.; Gentile, L.; Galeno, E.; Vittucci, A.C.; et al. Presence and Significance of Multiple Respiratory Viral Infections in Children Admitted to a Tertiary Pediatric Hospital in Italy. Viruses 2024, 16, 750. [Google Scholar] [CrossRef]
- Chan, M.; Koo, S.H.; Jiang, B.; Lim, P.Q.; Tan, T.Y. Comparison of the Biofire FilmArray Respiratory Panel, Seegene AnyplexII RV16, and Argene for the detection of respiratory viruses. J. Clin. Vir. 2018, 106, 13–17. [Google Scholar] [CrossRef]
- Kiselev, D.; Matsvay, A.; Abramov, I.; Dedkov, V.; Shipulin, G.; Khafizov, K. Current Trends in Diagnostics of Viral Infections of Unknown Etiology. Viruses 2020, 12, 211. [Google Scholar] [CrossRef] [PubMed]
| Variable | Number of Patients (%) | Virus-Positive Patients (%) | p-Value |
|---|---|---|---|
| Male | 611 (56.5) | 173 (54.1) | 0.297 |
| Age in years, median | 71 | 69 | |
| Age ranges (2022–2024) | |||
| 18–30 yrs | 46 (4.3) | 13 (28.3) | 0.014 a |
| 31–40 yrs | 41 (3.8) | 19 (46.3) | |
| 41–50 yrs | 73 (6.8) | 27 (37.0) | |
| 51–60 yrs 61–70 yrs 71–80 yrs 81–90 yrs >91 yrs | 151 (14.0) | 46 (30.5) | |
| 219 (20.3) | 71 (32.3) | ||
| 336 (31.1) | 75 (22.1) | ||
| 194 (17.0) | 62 (31.8) | ||
| 21 (1.9) | 7 (33.3) | ||
| Admission ward | |||
| ICU | 242 | 49 (20.2) | 0.0003 a |
| Non-ICU | 839 | 271 (32.3) | |
| Virus detection rate | 320 (29.6) | 0.701 | |
| Single virus detection | 285 (26.4) | ||
| Multiple virus detection | 35 (3.2) | ||
| Total | 1081 | 320 |
| Identified Virus | ICU (n = 242) | NON-ICU (n = 839) | p-Value ICU vs. NON-ICU | Total (n = 1081) | BAL (n = 318) | NPS (n = 792) | p-Value BAL vs. NPS | Total (n = 1110) |
|---|---|---|---|---|---|---|---|---|
| HRV/EV | 18 (7.4) | 110 (13.1) | 0.017 a | 128 (11.8) | 30 (9.4) | 98 (12.4) | 0.178 | 128 (11.5) |
| IAV | 8 (3.3) | 51 (6.1) | 0.108 | 59 (5.4) | 13 (4.1) | 46 (5.8) | 0.301 | 59 (5.3) |
| IBV | 1 (0.4) | 5 (0.6) | 1.0 | 6 (0.6) | 1 (0.3) | 5 (0.6) | 0.680 | 6 (0.5) |
| SARS-CoV-2 | 1 (0.4) | 20 (2.4) | 0.061 | 21 (1.9) | 3 (0.9) | 18 (2.3) | 0.221 | 21 (1.9) |
| RSV | 2 (0.8) | 29 (3.4) | 0.028 a | 31 (2.9) | 6 (1.9) | 25 (3.1) | 0.315 | 31 (2.8) |
| MPV | 3 (1.2) | 18 (2.1) | 0.596 | 21 (1.9) | 6 (1.9) | 15 (1.9) | 1.00 | 21 (1.9) |
| ADV | 1 (0.4) | 22 (2.6) | 0.040 a | 23 (2.1) | 2 (0.6) | 21 (2.6) | 0.035 a | 23 (2.1) |
| PIV | 3 (1.2) | 23 (2.7) | 0.236 | 26 (2.4) | 5 (1.6) | 21 (2.6) | 0.389 | 26 (2.3) |
| HuCoVs | 15 (6.2) | 26 (3.1) | 0.035 a | 41 (3.8) | 22 (6.9) | 19 (2.4) | 0.0006 a | 41 (3.7) |
| HuBoV b | 0 | 7 (0.9) | 0.360 | 7 (0.9) | 0 | 7 (1.0) | 0.202 | 7 (0.9) |
| Season | Viral Infections (%) | Non-Viral Infections (%) | Mixed Infections (%) | No Pathogens Detected (%) | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2022–2023 | 42 (10.5) | 156 (39.0) | 61 (15.3) | 141 (35.2) | 400 | |||||
| 2023–2024 | 48 (11.1) | 166 (38.2) | 76 (17.5) | 144 (33.2) | 434 | |||||
| Age ranges | M | F | M | F | M | F | M | F | M | F |
| 18–30 yrs | 2 (8.7) | 1 (9.1) | 14 (60.9) | 3 (27.3) | 2 (8.7) | 4 (36.4) | 5 (21.7) | 3 (27.3) | 23 | 11 |
| 31–40 yrs | 3 (15.8) | 4 (28.6) | 8 (42.1) | 2 (14.3) | 2 (10.5) | 5 (35.7) | 6 (31.6) | 3 (21.4) | 19 | 14 |
| 41–50 yrs | 3 (9.7) | 4 (19.0) | 9 (29.0) | 11 (52.4) | 11 (35.5) | 1 (4.8) | 8 (25.8) | 5 (23.8) | 31 | 21 |
| 51–60 yrs | 8 (14.0) | 7 (11.5) | 19 (33.3) | 24 (39.3) | 7 (12.3) | 13 (21.3) | 23 (40.4) | 17 (27.9) | 57 | 61 |
| 61–70 yrs | 17 (17.0) | 13 (18.1) | 39 (39.0) | 22 (30.6) | 16 (16.0) | 10 (13.9) | 29 (29.0) | 27 (37.5) | 100 | 72 |
| 71–80 yrs | 20 (12.5) | 12 (10.3) | 71 (44.4) | 51 (43.6) | 12 (7.5) | 14 (12.0) | 57 (35.6) | 40 (34.2) | 160 | 117 |
| 81–90 yrs | 11 (14.7) | 8 (13.1) | 28 (37.3) | 16 (26.2) | 8 (10.7) | 7 (11.5) | 28 (37.3) | 30 (49.2) | 75 | 61 |
| >91 yrs | 2 (20.0) | 0 (0.0) | 4 (40.0) | 1 (50.0) | 0 (0.0) | 1 (50.0) | 4 (40.0) | 0 (0.0) | 10 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizzo, M.; Bonura, F.; Cacioppo, F.; Palazzotto, E.; Filizzolo, C.; Russo, S.; Pistoia, D.; Capra, G.; Ferraro, D.; Giammanco, G.M.; et al. Post-COVID-19 Epidemiology of Viral Infections in Adults Hospitalized with Acute Respiratory Syndromes in Palermo, South of Italy. Pathogens 2025, 14, 997. https://doi.org/10.3390/pathogens14100997
Pizzo M, Bonura F, Cacioppo F, Palazzotto E, Filizzolo C, Russo S, Pistoia D, Capra G, Ferraro D, Giammanco GM, et al. Post-COVID-19 Epidemiology of Viral Infections in Adults Hospitalized with Acute Respiratory Syndromes in Palermo, South of Italy. Pathogens. 2025; 14(10):997. https://doi.org/10.3390/pathogens14100997
Chicago/Turabian StylePizzo, Mariangela, Floriana Bonura, Federica Cacioppo, Emilia Palazzotto, Chiara Filizzolo, Sharon Russo, Daniela Pistoia, Giuseppina Capra, Donatella Ferraro, Giovanni M. Giammanco, and et al. 2025. "Post-COVID-19 Epidemiology of Viral Infections in Adults Hospitalized with Acute Respiratory Syndromes in Palermo, South of Italy" Pathogens 14, no. 10: 997. https://doi.org/10.3390/pathogens14100997
APA StylePizzo, M., Bonura, F., Cacioppo, F., Palazzotto, E., Filizzolo, C., Russo, S., Pistoia, D., Capra, G., Ferraro, D., Giammanco, G. M., & De Grazia, S. (2025). Post-COVID-19 Epidemiology of Viral Infections in Adults Hospitalized with Acute Respiratory Syndromes in Palermo, South of Italy. Pathogens, 14(10), 997. https://doi.org/10.3390/pathogens14100997









