Abstract
Urinary tract infections (UTIs) are among the most prevalent infections in the human population. Uropathogenic Escherichia coli, the primary causative agent of UTIs, may also contribute to the development of metabolic kidney stones, particularly those composed of calcium oxalate. Kidney stone disease (KSD), known as nephrolithiasis or urolithiasis, is one of the most common disorders of the urinary system. This review explores the significant clinical association between UTIs and kidney stones, focusing on the mechanisms by which UPEC may promote stone formation, including oxidative stress, inflammation, and altered citrate metabolism. It also examines the role of immune responses, particularly macrophage activity, in the progression of KSD. Recent evidence suggests that the composition of the gut microbiota and metabolic imbalances have an additional impact on stone development. In light of these findings, current prevention and treatment strategies, including microbiota-targeted therapies, probiotics, and immune modulation, are also reviewed. Understanding the complex links between UTI, immunity, and metabolism provide new insights into the pathogenesis of KSD and allows for the development of more effective treatments for this disease.
1. Introduction
Urinary tract infections (UTIs) are one of the most common bacterial infections, affecting 150 million people worldwide each year [1,2]. Evidence links UTIs to kidney stone (KS) formation, one of the most common urinary disorders, also known as nephrolithiasis or urolithiasis [3,4]. Kidney stone disease (KSD) can also be considered a systemic disease influenced by a number of interrelated factors such as gender, environmental conditions, hypertension, diabetes, dietary habits (obesity, for example, can alter urine pH and the composition of excreted substances), and genetic predisposition [4,5,6]. Stones may remain localized in the kidney or migrate through the urinary tract into the ureter. When stones remain anywhere in the urinary tract, they can obstruct the normal flow of urine, potentially causing urine to accumulate in the kidney, ureter, bladder, or urethra, which can result in renal colic [4]. Stones associated with UTIs are and prone to recurrence [7,8].
The global prevalence of KSD is estimated at 2–15% (e.g., 1–5% in Asia, 5–9% in Europe, and 13% in North America) [6,9]. In addition, it is emphasized that urolithiasis affects approximately 5–10% of the population in Western countries, with at least one episode occurring during an individual’s lifetime [10,11]. There has been a significant increase in the incidence of this urological disease over the past 30 years [7]. Although KSD has traditionally been more prevalent in men than in women, recent trends indicate a shift, with increasing incidence in women and a relative decline among men [12,13,14].
KSD remains a global health and economic concern, with an estimated 106 million new cases reported in 2021 about 67% of which affected men [15,16]. While the total number of KSD cases has increased by 26.7% since 2000, the global age-standardized incidence rate has declined by 17.5%, reflecting public health successes. Regionally, prevalence varies considerably, with the highest age-standardized incidence rates observed in Eastern Europe (up to 3560 per 100,000), while the lowest rates were reported in western sub-Saharan Africa (around 606 per 100,000). Although pediatric urolithiasis accounts for only 2–3% of all KS cases, its incidence is rising, especially in Brazil, Russia, India, China, and South Africa. India and Russia exhibit the highest pediatric burden, with peak case estimates of 16,331 and 2442, respectively, while South Africa, with only 532 cases at its peak, shows ongoing gaps in early diagnosis and treatment [4,17].
KSs are typically composed of inorganic crystalline material (such as calcium oxalate or phosphate), often embedded within an organic matrix consisting of proteins, lipids, and cellular debris. These types of mineral deposits form in the calyces or renal pelvis, either freely or attached to the renal papillae [8]. Calcium oxalate monohydrate (CaOx) stones constituted approximately 65.9% of the KSs analyzed, followed by carbapatite stones (15.6%), urate stones (12.4%), struvite stones (magnesium-ammonium phosphate; 2.7%), and brushite stones (1.7%), reflecting their relative prevalence in the patient population [3,18]. Considering the composition of the stones, it is also possible to distinguish between infectious stones (i.e., calcium stones, brushite, and urate) and non-infectious stones (i.e., carbapatite and struvite) [8]. In general, KSs can be divided into calcareous stones (calcium-containing), accounting for almost 75% of cases, and non-calcareous stones [5]. Among calcareous stones, approximately 50% are primarily composed of CaOx and about 5% consist mainly of calcium phosphate (CaP), occurring separately or in combination (45%) [5,19,20,21]. Oxalate stones are a particular problem and can constitute up to 75–85% of all urinary stones. Tubular deposition of CaOx crystals associated with the formation of KSs is often detected during biopsies of native or transplanted kidneys. In the absence of calcium deposition in the tubules and its deposition in the renal parenchyma, nephrocalcinosis develops [8]. A special type of KSs are cystine stones, which are rare in both children and adults. They arise as a result of inactivating mutations (most commonly substitution of threonine for methionine 467) in genes (e.g., in humans, the SLC3A1 gene on chromosome 2) encoding renal tubule transporters that reabsorb the amino acid cysteine. Conditions lead to elevated urinary cysteine concentrations in the urine and subsequent stone formation (Figure 1) [22,23].
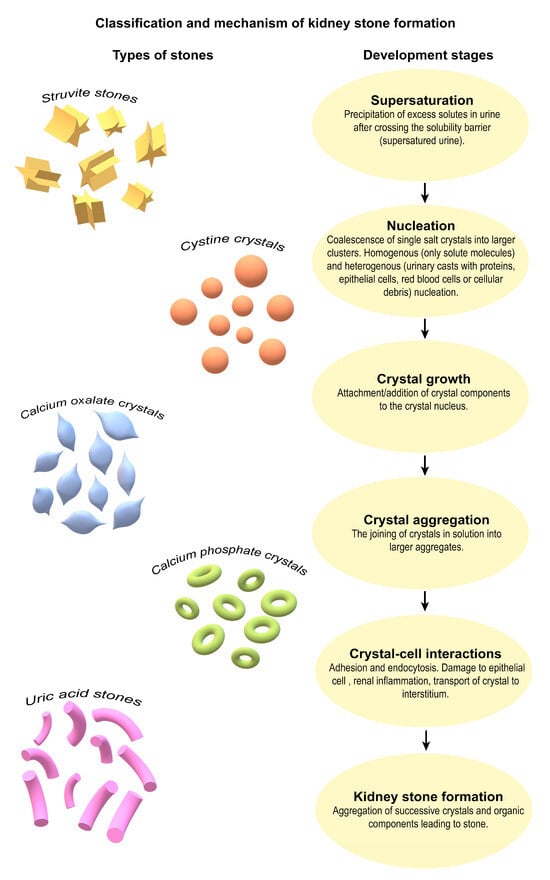
Figure 1.
Classification and shared pathophysiological steps in kidney stone formation. The diversity of KS types and the common biological pathway that underlies their formation. On the left, representative morphologies of the five major stone types (struvite, cystine, calcium oxalate, calcium phosphate, and uric acid) are shown to emphasize their compositional heterogeneity. On the right, a unified sequence of physicochemical and cellular events is presented, beginning with urine supersaturation and culminating in stone formation. This schematic highlights that, despite differences in stone composition, the mechanistic steps of crystal initiation, growth, aggregation, and epithelial interaction are shared across stone types. Understanding this convergence may be key to developing broad-spectrum preventive strategies [3,5,8,18,19,20,21,22,23].
Classification of stone-forming agents based on stone composition and clinical presentation may facilitate the determination of the risk of future symptomatic stone episodes and assist in tailoring personalized strategies for stone prevention strategies [8,24]. KSs can be highly variable and range from asymptomatic, incidentally detected stones of marginal clinical significance to painful and recurrent stones. Therefore, a potential classification system for KSs should focus mainly on categorizing stone composition and also differentiating symptomatic from asymptomatic stones, confirmed from suspected symptomatic stone episodes. Asymptomatic stones are clinically diagnosed from patient-reported symptomatic stone episodes, and symptomatic stones are diagnosed from radiographic recurrence (such as stone growth, stone disappearance or the appearance of a new stone) [24].
Epidemiological studies have shown a rising incidence of nephrolithiasis in recent years, with recurrence rates reaching up to 50% within five years of the initial stone event. Despite extensive research over the decades, effective treatment options to prevent recurrence are still rare [16,25,26]. Therefore, a deeper understanding of the various aspects of KS formation is required to develop more innovative preventive and therapeutic strategies against KSD.
2. Insight into the Mechanisms of Urolithiasis Development
The formation of KSs is fundamentally a physicochemical phenomenon initiated by the supersaturation of urine with stone-forming components such as calcium, oxalate, phosphate and uric acid. When the concentration of these solutes exceeds their solubility, crystallization becomes thermodynamically favorable. This process proceeds through a well-characterized sequence of nucleation, crystal growth, aggregation, and retention. However, it is not a purely passive process. Molecular regulators, including crystallization promoters such as calcium, urate, and oxalate, and inhibitors such as citrate, magnesium, osteopontin, and nephrocalcin modulate the kinetics of these events. Shifting the balance toward promoters fosters crystal formation and stability, while depletion or dysfunction of inhibitors predisposes individuals to stone formation [3,8].
One of the earliest structural factors contributing to the pathogenesis of urolithiasis is the formation of Randall’s plaques of interstitial calcium phosphate deposits located beneath the urothelium of renal papillae [27]. These plaques form in the basement membrane of the thin limbs of Henle’s loop and extend into the interstitium and eventually into the urinary space. Their mineral core serves as a nidus on which CaOx crystals can anchor and overgrow. The plaques consist of a complex matrix of calcium phosphate, collagen, lipids, and vesicular structures, and are increasingly recognized as essential precursors of idiopathic calcium stone disease. Emerging evidence highlights the involvement of osteogenic transformation of interstitial fibroblasts and the regulatory role of long non-coding RNAs in the development of atherosclerotic plaques, pointing to an active, cell-mediated process rather than passive mineral deposition [27,28,29].
At the cellular level, the interaction between renal tubular epithelial cells and crystals is a key step in stone retention and growth. Adhesion of crystals triggers a cascade of proinflammatory responses characterized by oxidative stress, cytokine production and cellular damage. These events further disrupt tubular integrity, facilitating additional crystal binding and propagation [30]. Macrophages, key players in renal immunity, infiltrate these sites and exhibit functional polarization. The dynamic interaction between these macrophage phenotypes, regulated by pathways such as NLRP3 inflammasome and NF-κB, substantially influences stone pathogenesis [30,31].
Sex hormones are also involved in modifying stone risk, contributing to the observed male predominance in CaOx nephrolithiasis. Androgens enhance oxalate biosynthesis and promote tubular epithelial damage and crystal adhesion through several molecular pathways, including activation of glycolate oxidase and NADPH oxidase systems. In contrast, estrogens appear to exert protective effects by downregulating oxidative pathways and reducing the expression of crystal-binding receptors. The differential effects of sex hormones suggest the influence of hormones on both systemic metabolism and the local renal microenvironments, offering potential targets for therapeutic intervention [32,33].
The role of the microbiome in KSD has gained increasing attention, especially after the recognition of both pathogenic and protective effects of microorganisms. Recent advances in molecular techniques, including 16S rRNA gene sequencing and expanded quantitative urine culture (EQUC), have demonstrated that urine is not sterile and that the urinary tract harbors a diverse microbiome, challenging the traditional paradigm [34,35]. Distinct bacterial communities have been identified in different regions of the urinary tract, including the bladder (e.g., Lactobacillus spp. and Gardnerella vaginalis), urethra (e.g., Corynebacterium spp. and Streptococcus spp.), and renal pelvis (e.g., E. coli and Enterococcus spp.), through both molecular and enhanced culture methods [34,35,36,37]. Many of these microorganisms act as commensals that help maintain urinary tract homeostasis, while pathogenic species such as UPEC can disrupt this balance, leading to infection and potentially contributing to kidney stone formation [35,36,37]. Understanding the composition and dynamics of the urinary microbiome is essential for elucidating its role in urolithiasis and other urinary tract diseases [35,37].
Urease-producing bacteria, such as Proteus mirabilis and Klebsiella pneumoniae, catalyze the hydrolysis of urea to ammonia, increasing urinary pH and promoting the precipitation of magnesium-ammonium phosphate (struvite) stones. Meanwhile, Calcifying nanoparticles, ultra-small, calcium-binding particles are hypothesized to promote calcification through oxidative and apoptotic pathways. Although their exact biological nature remains debated, they have been detected in various types of renal stones and are believed to promote calcification through oxidative stress and apoptotic pathways [38]. On the other hand, oxalate-degrading bacteria in the gut, particularly Oxalobacter formigenes, reduce oxalate absorption and excretion in the urine, acting as a natural defense against CaOx stone formation [39]. Dysbiosis, including the depletion of such commensal species, has been linked to an increased risk of stone formation, highlighting the systemic dimension of stone pathogenesis [40].
Environmental and systemic factors provide the broader context in which KSs develop. Obesity, insulin resistance, and metabolic syndrome are consistently associated with alterations in urine composition, such as increased acidity, decreased citrate, and increased calcium or oxalate excretion. Diets high in sodium, animal protein, and fructose, combined with inadequate hydration, further increase the risk of urine supersaturation. Additionally, vitamin D deficiency, hyperparathyroidism, and hypomagnesuria disrupt mineral homeostasis and acid-base balance, promoting crystal formation. Genetic predispositions amplified by epigenetic modifications affecting the expression of calcium-sensing receptors and vitamin D metabolism add a hereditary component to the risk profile, affecting susceptibility from generation to generation [5,41].
3. Immune Mechanisms Contributing to Calcium Oxalate Crystallization and Kidney Stone Formation
KSD, particularly the formation of CaOx crystals, is a complex process in which the immune system plays a crucial role. In response to the presence of crystals in the kidneys, macrophages are recruited and accumulate at the site, serving as key regulators of both inflammation and stone development [3,30]. These phagocytic cells interact via the CD44 receptor with extracellular matrix proteins such as fibronectin (FN) and osteopontin (OPN), the expression of which is increased in renal tubular epithelial cells exposed to crystals. The interactions promote crystal aggregation and growth, highlighting the pivotal role of macrophages in the pathogenesis of kidney stones [42,43].
Macrophages can differentiate into two main phenotypes with different roles in KSs, namely pro-inflammatory M1 macrophages and anti-inflammatory M2 macrophages [43,44]. M1 macrophages are typically associated with tissue damage and can enhance crystal deposition, contributing to inflammation and CaOx stone formation. In contrast, M2 macrophages have phagocytic abilities that enable them to engulf and break down CaOx crystals, thus exerting a protective effect against stone development [45,46]. The balance between these subgroups of macrophages is crucial for controlling the progression of KSs. Beyond their phenotypic differentiation, macrophages secrete various cytokines and chemokines such as interleukin-8 (IL-8), macrophage inflammatory protein-1 (MIP-1) and monocyte chemoattractant protein-1 (MCP-1), which recruit additional immune cells including, T lymphocytes, dendritic cells, neutrophils and other macrophages to the site of crystal deposition. This cascade enhances the local immune response and may facilitate further stone growth. Furthermore, macrophages release exosomes, which are small extracellular vesicles containing proteins and signaling molecules. These vesicles modulate the activation, migration and function of immune cells (especially T lymphocytes), shaping the renal inflammatory environment [47,48].
Considering the pivotal involvement of the immune system in the formation and progression of CaOx crystals, new therapeutic strategies focusing on immune modulation have gained attention. Targeting macrophage functions and their communication via exosomes could offer a promising approach to enhance crystal removal and reduce inflammation, ultimately preventing stone formation and recurrence [46]. In addition, Randall’s plaque may play an important role in reducing inflammation mediated by M1 macrophages during CaOx crystal formation [29]. Thus, maintaining a delicate balance between pro-inflammatory M1 and anti-inflammatory M2 macrophages appears crucial in preventing the inflammatory processes that promote stone development [46].
The role of immune modulation in KS prevention is an emerging area of research, although immune therapies are not currently a standard or necessary approach. The immune system contributes to stone pathogenesis by mediating inflammation and tissue injury, which can influence crystal formation [49,50]. Experimental studies have explored potential immunomodulatory interventions aiming to reduce stone formation by controlling inflammation and oxidative stress for example, mitogen-activated macrophage polarization to the M2 phenotype, use of antioxidants like curcumin, and targeting the NLRP3 inflammasome [51,52]. However, clinical evidence supporting immune therapies for the routine prevention of KSs is still lacking. Future research is essential to assess whether targeted immune interventions could become part of comprehensive KS management [53].
In this context, immunotherapy aimed at modulating the immune system could offer an effective strategy to prevent KS recurrence in selected patients. Enhancing the activity of M2 macrophages over M1 and suppressing chronic inflammation may interrupt the cascade leading to CaOx crystal nucleation and, consequently, stone formation [3]. Nevertheless, more in-depth studies are essential to fully understand these immune mechanisms and to develop effective immunotherapies for KSD.
4. Involvement of E. coli in Calcium Oxalate Nephrolithiasis: Insights into Selected Mechanisms
The presence of E. coli in KSD adds a new dimension to our understanding of nephrolithiasis. Studies performed indicate that IS infectious stones containing ammonium, magnesium and phosphate (struvite) are closely related to UTIs [54]. Bacteria belonging to the genera Proteus and Klebsiella contribute to the development of infectious stones due to their ability to produce urease, and thus alter urine chemistry [38]. In turn, metabolic factors and disorders play an important role in the formation of metabolic CaOx stones. This is the cause of supersaturation of mineral salts in the urine, which exceeds their solubility limit and causes crystallization of stones [55]. Uropathogenic E. coli (UPEC) strains, as the predominant etiologic agent of UTIs (including recurrent cases) and despite lacking the ability to produce urease (accounting for 98.6% of cases), are also involved in the formation of CaOx stones in the kidneys by stimulating renal inflammation and increasing oxidative damage in renal tubular epithelial cells [56,57,58,59]. These processes constitute a source of substrates for the heterogeneous nucleation of CaOx crystals, strengthening their adherence to renal epithelial cells and initializing crystallization [60]. Only live and intact E. coli bacteria promote the nucleation and aggregation of CaOx crystals. In addition, E. coli were detected as the most common bacterial species in both urine and CaOx KS samples in patients treated with one-stage percutaneous nephrolithotomy. For a given patient, these strains shared common genetic features, resistance genes, virulence factors, the similar sensitivity profiles to the antimicrobial agents and the same classification in phylogenetic groups [61,62]. Similar results were obtained on the basis of urine samples collected from the catheters and stone matrices in E. coli-infected suffering from nephrolithiasis. Bacteria were found both inside the core and nucleus of the stones. Moreover, these bacteria also exhibit adaptive properties in terms of size and protein composition to survive inside the formed stones [63]. In addition, in vivo experiments confirmed that administering E. coli into the urinary tract of mice leads to renal inflammation and enhances the formation of crystal deposits [36]. This emphasizes the role of E. coli and recurrent UTIs as etiopathogenic factors responsible for the formation of KSs [64].
Although struvite stones are classically associated with urease-producing bacteria such as Proteus mirabilis, recent findings reveal that a small proportion of E. coli strains (approximately 1.4%) may also exhibit urease activity. Nevertheless, the majority of E. coli strains implicated in KS formation are urease-negative and primarily associated with CaOx stones [65]. These bacteria may further aggravate urinary tract injury caused by stone movement, promoting persistent colonization and inflammation, and thereby contributing to a self-perpetuating cycle of infection and lithogenesis.
Clinical investigations have consistently identified E. coli in both urine and stone matrices of patients with CaOx nephrolithiasis [62,66]. In studies involving paired isolates from urine (i.e., E. coli urine cultures [EUC]) and stone nidus (i.e., E. coli stone cultures [ESC]) in the same patients, genotypic and antimicrobial susceptibility analyses revealed a high degree of concordance. These findings indicate that E. coli bacteria in the stones likely originate from the urinary tract and are capable of surviving and adapting to the environment inside the stone. Most strains belonged to the B2 phylogenetic group, particularly sequence types ST1193 and ST131, which are well known for their high virulence and antibiotic resistance. Proteomic profiling further showed altered expression of proteins related to stress response, carbohydrate metabolism, and DNA or protein turnover, highlighting their physiological adaptation to a more hostile and nutrient-limited niche [62]. ESC strains tended to exhibit smaller colony sizes and longer cell morphology compared to their urinary counterparts [66]. Genomic comparisons of matched EUC and ESC strains revealed striking genetic similarity, including shared virulence and resistance genes and a highly conserved genomic structure, indicating a clonal relationship. Functional assays showed that both ESC and EUC strains exhibited similar, although weaker, adhesion and invasion abilities compared to the reference UPEC CFT073 (originally isolated from the blood and urine of a patient with acute pyelonephritis) [62,67]. In addition to these phenotypic and proteomic differences, experimental data have provided a wealth of information on the molecular mechanisms by which E. coli can promote stone formation. Notably, persistent infection of renal tubular epithelial cells by E. coli has been shown to enhance the adhesion of CaOx crystals to the cell surface. This effect was mediated by the upregulation and apical translocation of ezrin, a protein that connects the membrane to the cytoskeleton. Importantly, the Rho/ROCK signaling pathway has been identified as a key regulatory axis in this process. Rho-associated protein kinase (ROCK) is involved in the regulation of ezrin in its membrane-associated form, which is essential for restructuring the apical cell membrane. Phosphorylation of ezrin at threonine residue 567 by ROCK converts the protein from an inactive to an active (phosphorylated) state, enabling its relocation to the apical membrane [68]. Pharmacological inhibition of ROCK (using the selective inhibitor Y-27632 of ezrin phosphorylation) or knockdown of ezrin expression (using a small-interfering RNA, siRNA specific for this protein) significantly reduced crystal adhesion, providing a potential mechanistic link between bacterial infection and the initiation of renal calcification [69,70].
The role of E. coli in KS formation is not limited to crystal adhesion. This also includes promoting oxidative damage and inflammation in kidney tissue. Studies have identified the bacterial proteins polyphosphate kinase 1 (PPK1) and flagellin as critical mediators of KS formation. These proteins act as pathogen-associated molecular patterns (PAMPs) recognized by Toll-like receptors (TLRs), particularly TLR5 in the case of flagellin, on renal epithelial cells [71]. This interaction activates the NF-κB and p38 MAPK signaling pathways and induces oxidative stress, leading to the release of proinflammatory cytokines. As a result, tissue damage is exacerbated, and a favorable environment for crystal aggregation and stone progression is created [60,72]. Other investigations have clearly shown that E. coli strains with flagella increase the number and size of CaOx crystals. In contrast, E. coli without flagella have a smaller modulatory effect on crystal formation [61,73]. This may be due to the fact that flagella allow bacteria to rotate, which is a prerequisite for increased motility (facilitating movement toward epithelial cells), bacterial adhesion and biofilm formation. In this way, E. coli generate an environment conducive to stone formation and provide protection against the immune response [72,74].
In turn, in vitro experiments using human renal proximal tubule (HK-2) cells showed that exposure to wild-type E. coli strain CFT073 (WT-CFT073) significantly increased intracellular levels of reactive oxygen species (ROS), decreased expression of superoxide dismutase 1 (SOD1) and elevated levels of 8-hydroxy-2′-deoxyguanosine (8-OHdG), indicating oxidative DNA damage. These effects were reversed in E. coli strains deficient in PPK1 (ΔPPK1-CFT073) or flagellin (ΔFliC-CFT073), highlighting the key role of these bacterial proteins in mediating oxidative damage. Moreover, stimulation with flagellin alone induced phosphorylation of p38 and p65 proteins and increased nuclear p65 protein expression in HK-2 cells, indicating activation of the NF-κB/p38 signaling pathway. This activation was also observed after the test cells were exposed to the WT-CT073 and was dependent on PPK1 and flagellin, as mutations in these proteins attenuated the response. Additionally, in murine models, kidney inoculation with the WT-CFT073 strain led to increased CaOx deposition, enhanced oxidative damage and upregulation of proteins associated with inflammation. These effects were reduced in ΔPPK1-CFT073 and ΔFlic-CFT073 strains, confirming the involvement of these bacterial proteins in promoting kidney stone formation through oxidative stress and inflammation [60].
These findings underscore the significant role of E. coli in the pathogenesis of KS, particularly through the actions of PPK1 and flagellin in inducing oxidative stress and inflammation via the NF-κB/p38 MAPK signaling pathways [60,71]. This confirms that E. coli bacteria occupy a unique pathogenic niche. Unlike urease-producing strains, which increase urine pH through alkalization, E. coli typically maintains or even contributes to an acidic urine environment, which influences its role in the pathogenesis of KSs [75]. This dual role as both a uropathogen and a stone-promoting agent places E. coli as a critical target for the treatment of recurrent CaOx nephrolithiasis [76].
Another study conducted by Amimanan et al. (2017) [77] also emphasized the role of E. coli isolated from the urine of patients with kidney stone (i.e., E. coli-associated with urinary kidney stones, [EUK]) in promoting CaOx crystal formation. The researchers compared EUK bacterial strains with strains originating from patients who had UTIs but did not develop stones (i.e., E. coli-associated urine without stones [EUU]). They found that EUK strains showed higher expression of elongation factor Tu (EF-Tu) on the surface of outer membrane vesicles (OMVs) that significantly enhanced CaOx crystal nucleation and growth, aggregation, and adhesion (by acting as structural scaffolds that interact with crystal surfaces) compared to patients with EUU. These vesicles possess a negatively charged surface that facilitates electrostatic interactions with positively charged calcium ions, thus promoting crystal aggregation. Neutralization of EF-Tu with specific monoclonal antibodies reduced these effects, highlighting its role in stone pathogenesis. Additionally, immunofluorescence staining revealed that EF-Tu was more abundantly expressed on the surface of EUK cells in contrast to EUU strains [77]. This underscores the involvement of EF-Tu in modulating CaOx crystal formation and development. Moreover, the findings indicate that certain E. coli strains may play an active role in KS formation and represent potential targets for therapeutic intervention.
A particularly noteworthy mechanism by which E. coli affects CaOx stone formation involves the activity of citrate lyase [71,78]. Citrate, a naturally occurring compound in urine, plays an important protective role by binding to calcium ions and thereby reducing their availability for crystal nucleation and growth. In addition, citrate helps alkalize urine, further inhibiting crystallization [79]. E. coli bypasses this host defense mechanism by producing citrate lyase, an enzyme capable of breaking down citrate into oxaloacetate and acetate. This enzymatic degradation reduces the level of citrate in the urine, tipping the balance toward CaOx supersaturation and enhancing the conditions conducive to stone formation. The reduction in citrate levels changes the urine chemical profile in favor of a more lithogenic environment, making it easier for crystals facilitating crystal formation and persistence [80]. What makes this enzymatic function particularly concerning is its potential to diminish the effectiveness of standard citrate supplementation therapies used to prevent stone recurrence [81]. By metabolizing both endogenous and exogenous citrate, E. coli may not only contribute to the initiation and growth of CaOx crystals but also impair a key clinical strategy aimed at mitigating stone recurrence. This interaction suggests the need to reevaluate therapeutic strategies in patients with recurrent CaOx nephrolithiasis in the presence of E. coli colonization (Table 1) [71].

Table 1.
Overview of microbial and molecular factors involved in the pathogenesis of urolithiasis [38,47,60,68,69,70,71,72,74,76,77,78,80].
Generally, these studies demonstrate the ability of E. coli to promote CaOx crystal growth and aggregation, though the precise mechanisms behind this effect remain poorly understood and require further in-depth research (Figure 2). In the future, integrated approaches that address both the microbial and metabolic dimensions of stone disease are warranted. Therapeutic strategies may include not only conventional antibiotic regimens but also targeted interventions aimed at disrupting bacterial adhesion, signaling pathways, or the host inflammatory environment. The recognition of the role of E. coli in the pathogenesis of stone disease represents a key advance in the field and encourages further research into the microbial involvement in an apparently metabolic disease.
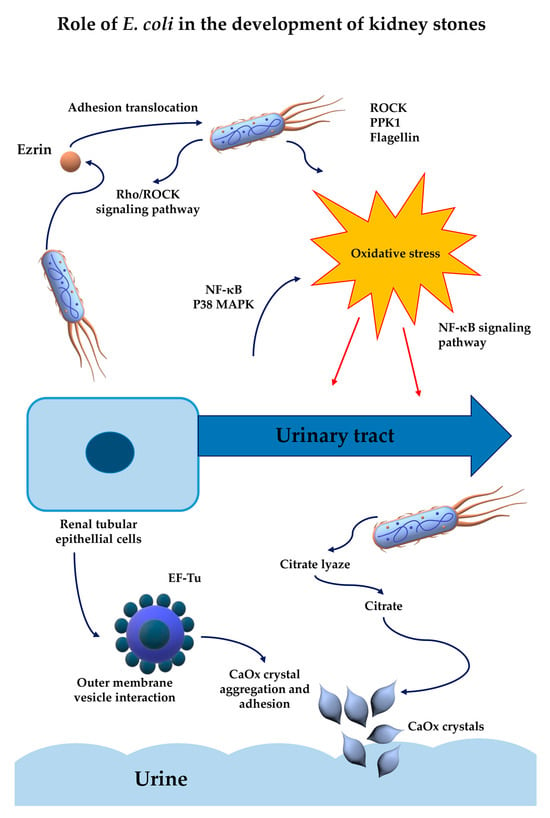
Figure 2.
Impact of E. coli on the mechanisms underlying kidney stone formation [38,60,68,69,70,72,74,77,80].
5. Potential Strategies to Prevent the Development of Kidney Stones
Prevention of nephrolithiasis, particularly CaOx stone disease, is an emerging area of research that increasingly emphasizes biological and microbiological strategies. Traditional recommendations such as increasing fluid intake, reducing dietary oxalate, or prescribing thiazide diuretics offer limited efficacy and often do not prevent recurrence, which remains a major clinical concern [14]. This has prompted a shift toward more targeted approaches that modulate oxalate metabolism at its source within the gastrointestinal tract and microbiome.
In 2020, Gupta et al. [82] highlighted the therapeutic promise of bacterial enzymes, particularly those capable of degrading oxalate, a metabolic end-product that is key to CaOx stone formation. Unlike the human host, certain gut microbes possess the enzymatic machinery to metabolize oxalate, thus reducing the pool available for urinary excretion. O. formigenes, a strictly anaerobic bacterium, utilizes oxalates as a primary carbon source, breaking them down enzymatically in the gut before they are absorbed into the systemic circulation [82,83]. Gupta’s work outlined how the use of such bacteria or their enzymes as biotherapeutic agents could significantly reduce oxalate burden, and thus serve as a primary preventive measure against nephrolithiasis [82].
This microbial perspective was further enriched by Suman et al. (2021) [84], who emphasized the role of lactic acid bacteria such as Lactobacillus and Bifidobacterium. These types of probiotics, widely used in the food and supplement industries, have demonstrated strain-specific abilities to degrade oxalate through key enzymatic pathways including oxalyl-CoA decarboxylase (oxc) and formyl-CoA transferase (frc) [84,85]. Unlike O. formigenes, which is difficult to culture and maintain in the gut, these lactic acid bacteria offer higher viability and safety profiles, making them suitable for long-term preventive use. It is worth noting that some strains have shown oxalate degradation rates of up to 100% in controlled experiments [84].Their inclusion in probiotic formulations could provide an accessible, food-based approach to reduce oxalate in urine and lower the risk of stone recurrence
Furthermore, Mani et al. (2025) [86] examined the broader role of the microbiome in the pathophysiology of KS. Considerable attention has been given to how dysbiosis, a microbial imbalance, can affect oxalate metabolism and stone development. Several commensal bacteria, including Eubacterium lentum, Enterococcus faecalis, and some strains of E. coli, were also distinguished for their potential in oxalate degradation. Researchers noted that future therapies could involve tailored microbial consortia aimed at restoring homeostasis of the gut-urinary axis. Such targeted manipulation of microbial communities may reduce intestinal oxalate absorption and improve systemic oxalate regulation, thereby offering an integrative approach to prevention [86].
Moreover, Taheri et al. (2024) [76] synthesized the results of studies on both probiotics and herbal medicine. They found convincing evidence that oral administration of probiotic strains of Oxalobacter, Lactobacillus, and Bifidobacterium can lower urinary oxalate levels and mitigate crystallization processes that initiate stone formation. Additionally, various herbal extracts (Bergenia ciliata, Pyrrosia lingua, Urtica dioica, Tribulus terrestris, Acorus calamus) administered in animal models of nephrolithiasis have been shown to reduce kidney inflammation, protect against oxidative stress and reduce the deposition of CaOx crystals. Integrating microbiota-modulating probiotics with plant-derived anti-inflammatory agents appears to represent a synergistic and promising strategy for individuals with recurrent or high-risk profiles [76].
In addition, emerging evidence underscores the key role of intestinal bacteria that produce short-chain fatty acids (SCFAs) in modulating oxalate transport, thereby influencing the risk of KS formation. Among them, Faecalibacterium prausnitzii and Roseburia hominis have attracted interest due to their indirect but significant effects on CaOx homeostasis [87,88]. Although these species do not enzymatically degrade oxalate, their metabolic byproducts particularly butyrate alter the expression of epithelial transporters by reducing SLC26A3 expression (involved in oxalate absorption) and increasing SLC26A6 expression (which promotes oxalate secretion). This bidirectional regulation reduces net oxalate absorption and decreases urinary oxalate excretion, a key factor in preventing CaOx crystallization. Microbiome analyses consistently demonstrate reduced abundance of these taxa in stone formers, and experimental studies confirm that SCFAs supplementation reduces renal crystal deposition. Despite their therapeutic potential, clinical use of F. prausnitzii and R. hominis remains limited by the challenges of cultivation, formulation, and stable colonization [89]. Nevertheless, encapsulation technologies and microbiome engineering strategies offer promising opportunities to harness their protective effects in preventing KS.
Going forward, the application of synthetic biology offers opportunities for profound innovation and change. Wan et al. (2024) [26] provided an overview of recent advances in engineering probiotics with enhanced oxalate degradation ability. By inserting genes such as oxdC, oxC, and frc into microbial hosts, the researchers have developed recombinant strains that can survive harsh intestinal conditions while expressing key oxalate-degrading enzymes. For instance, engineered Lactobacillus plantarum expressing oxdC showed significantly higher oxalate degradation in vitro and in vivo. More advanced constructs include strains equipped with transporter proteins (such as OxlT) that enhance intracellular uptake and processing of oxalate. These engineered microorganisms offer precise tools for correcting oxalate imbalance and represent a step forward in prophylactic therapy for KSD [26].
6. Approaches to Infectious Urolithiasis: Surgical Interventions, Drug Therapies and Future Directions
Effective and individualized treatment strategies are essential to manage the complexity of infectious urinary stones and remain a key goal despite the increasing attention to prevention, especially among patients with recurrent stones. Infectious stones, particularly when associated with bacterial colonization by common pathogens such as P. mirabilis and E. coli, require an individualized and comprehensive treatment approach [71]. Surgical interventions, including extracorporeal shock wave lithotripsy (ESWL), flexible urethroscopy (FURS), and percutaneous nephrolithotomy (PCNL), are widely used, although each has its own limitations. ESWL is generally avoided in infectious cases because of the risk of spreading pathogens, while PCNL, effective for larger stones, carries an increased risk of postoperative infection, especially in patients colonized with E. coli. Therefore, precise selection of preoperative antibiotics based on urine cultures is crucial. Increasing antimicrobial resistance among uropathogens requires the judicious use of antibiotics and the development of new drugs, with imipenem and amikacin remaining among the more effective options [90,91].
In addition to surgical treatment, pharmacological interventions target the biochemical environment that promotes stone formation. Urease inhibitors such as acetohydroxamic acid (AHA), have shown utility but are limited by adverse effects. New compounds, including hydroxamic acid derivatives, sulfonamides, and heterocyclic agents, are being studied for their improved efficacy and safety profiles [92,93]. Adjunctive therapies such as citrate, tartronic acid, and succinate help modulate urine chemistry, while protocatechuic acid interferes with biofilm formation by P. mirabilis. Moreover, recent metabolomic analyses suggest the potential of using specific metabolites as therapeutic targets [94]. Ultimately, the integration of antimicrobial stewardship with metabolic modulation and advanced pharmacological strategies may provide the most effective route to treating infectious nephrolithiasis and preventing recurrence.
In conclusion, an integrated understanding of the pathogenesis and treatment of KSD, from microbial modulation to surgical and pharmacological treatment represents a significant progress in both prevention and therapy [71]. Preventive strategies focused on microbiome engineering, probiotics, and dietary modification target the earliest stages of oxalate management, offering promising non-invasive options. Meanwhile, therapeutic approaches combining targeted antibiotic use, surgical precision and metabolic intervention remain essential for patients with infectious or complex stones. Further research into novel urease inhibitors, metabolic regulators and therapeutic metabolites will be essential to improve outcomes. Together, these strategies provide a comprehensive framework for reducing the incidence, severity and recurrence of KS.
7. Overview of KSD Pathogenesis and Management
KSD is a urological disorder characterized by the accumulation of minerals and acid salts that crystallize and aggregate in concentrated urine. The pathogenesis of CaOx nephrolithiasis encompasses complex interactions between microbial pathogens, host epithelial cells and the immune system [3,60]. Many factors contribute to the formation of KS. Chronic conditions such as diabetes, dyslipidemia and high blood pressure are associated with an increased risk of developing this urinary disorder [4].
Emerging evidence also implicates UPEC strains as a key contributor to KS formation, extending their pathogenic role beyond UTIs to active participation in lithogenesis. Recent studies have identified E. coli as the most frequently detected species in both urine and KSs of patients with CaOx urolithiasis [60]. Notably, the promotion of CaOx crystal nucleation and aggregation has been observed exclusively in the presence of live and intact E. coli cells [61]. Consistently, matched E. coli isolates from urine and KSs of the same patient displayed nearly identical genotypic and phenotypic profiles, including antimicrobial susceptibility, phylogenetic classification, and the presence of virulence and resistance genes [62].
In turn, mechanistic insights revealed that colonization of renal tubular epithelial cells by UPEC activates the ROCK pathway, resulting in ezrin phosphorylation and cytoskeletal changes which enhance epithelial adhesion and promote CaOx crystal retention [68]. Inhibition of this pathway reduces crystal binding, highlighting its therapeutic potential. Additionally, EF-Tu on the surface of OMVs facilitates CaOx nucleation and growth [77], while bacterial citrate lyase depletes urinary citrate, disrupting homeostasis and promoting lithogenesis [71,78].
KSs associated with E. coli present a significant clinical challenge and require well-coordinated, individualized treatment strategies [71]. The growing problem of antimicrobial resistance requires careful antibiotic selection based on microbiological data, along with continued efforts to develop novel therapeutic agents [90,91]. Surgical procedures remain fundamental in clinical practice, although their use must be carefully balanced against the risk of infection and procedural complications [71]. In turn, pharmacological interventions, including urease inhibitors and newly investigated bioactive compounds, aim to disrupt stone-promoting biochemical processes while minimizing adverse effects [92,93]. Additional therapies that modulate urine chemistry and inhibit biofilm formation, such as citrate, tartronic acid, and protocatechuic acid, further support treatment efforts [94]. Preventive strategies focused on modulation of the urinary microbiome, dietary adjustments, and the use of probiotics also show potential in reducing recurrence and improving long-term outcomes [71]. These combined approaches support a more integrated and effective framework for the management of infection-associated nephrolithiasis. While current therapeutic and preventive strategies offer promising results, further research is essential to optimize treatment efficacy, develop targeted interventions, and enhance long-term prevention of infection-related KSD.
8. Discussion
UPEC-induced nephrolithiasis involves a multifaceted pathophysiology integrating mechanisms of bacterial adhesion, enzymatic modulation of urinary inhibitors and an abnormal immune response. Targeting these interrelated pathways through antimicrobials, immune modulators and microbiome-targeted therapies may herald a paradigm shift in the prevention and treatment of CaOx kidney stones [3,71]. Although growing evidence supports the role of UPEC in lithogenesis, significant gaps remain in our understanding of the exact molecular pathways involved. These gaps limit our ability to fully characterize the pathogenic potential of UPEC and its contribution to stone development. Nevertheless, further research is needed to define the critical factors and mechanisms by which UPEC contributes to CaOx stone formation, given its consistent presence in both urine and kidney stones.
Author Contributions
Conceptualization, writing—original draft preparation, review and editing, and supervision, B.Z.-P. Writing—original draft preparation and review and editing, M.N. Contribution to writing and editing, R.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| UTIs | Urinary tract infections |
| KSs | Kidney stones |
| KSD | Kidney stone disease |
| CaOx | Calcium oxalate |
| EQUC | quantitative urine culture |
| CaP | Calcium phosphate |
| UPEC | Uropathogenic E. coli |
| IL-8 | Interleukin-8 |
| MIP-1 | Macrophage inflammatory protein-1 |
| MCP-1 | Monocyte chemoattractant protein-1 |
| EUC | E. coli urine cultures |
| ESC | E. coli stone cultures |
| ROCK | Rho-associated protein kinase |
| siRNA | Small-interfering RNA |
| PPK1 | Polyphosphate kinase 1 |
| PAMPs | Pathogen-associated molecular patterns |
| TLRs | Toll-like receptors |
| HK-2 | Human renal proximal tubule cells |
| WT-CFT073 | Wild-type E. coli strain CFT073 |
| ROS | Reactive oxygen species |
| SOD1 | Superoxide dismutase 1 |
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| EUK | E. coli-associated with urinary kidney stones |
| EUU | E. coli-associated urine without stones |
| EF-Tu | Elongation factor Tu |
| OMVs | Outer membrane vesicles |
| oxc | Oxalyl-CoA decarboxylase |
| frc | Formyl-CoA transferase |
| SCFAs | Short-chain fatty acids |
| ESWL | Extracorporeal shock wave lithotripsy |
| FURS | Flexible urethroscopy |
| PCNL | Percutaneous nephrolithotomy |
| AHA | Acetohydroxamic acid |
References
- Foxman, B. Urinary Tract Infection Syndromes: Occurrence, Recurrence, Bacteriology, Risk Factors, and Disease Burden. Infect. Dis. Clin. N. Am. 2014, 28, 1–13. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Marra, M.; Zummo, S.; Biondo, C. Urinary Tract Infections: The Current Scenario and Future Prospects. Pathogens 2023, 12, 623. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Y.; Zhang, J.; Deng, Q.; Liang, H. Recent Advances on the Mechanisms of Kidney Stone Formation (Review). Int. J. Mol. Med. 2021, 48, 149. [Google Scholar] [CrossRef]
- Vilapurathu, J.K.; Carlmana, C.A.; Francisa, D.; Varghesea, S. A Comprehensive Investigation of the Prevalence and Risk Factors Associated with Renal Calculi in Southern India: A Prospective Study. Urol. Sci. 2025, 36, 20–24. [Google Scholar] [CrossRef]
- D’Ambrosio, V.; Ferraro, P.M.; Lombardi, G.; Friso, S.; Gambaro, G. Unravelling the Complex Relationship between Diet and Nephrolithiasis: The Role of Nutrigenomics and Nutrigenetics. Nutrients 2022, 14, 4961. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yoo, D.M.; Bang, W.J.; Choi, H.G. Obesity Is Positively Associated and Alcohol Intake Is Negatively Associated with Nephrolithiasis. Nutrients 2022, 14, 4122. [Google Scholar] [CrossRef]
- Wigner, P.; Grębowski, R.; Bijak, M.; Szemraj, J.; Saluk-Bijak, J. The Molecular Aspect of Nephrolithiasis Development. Cells 2021, 10, 1926. [Google Scholar] [CrossRef]
- Tamborino, F.; Cicchetti, R.; Mascitti, M.; Litterio, G.; Orsini, A.; Ferretti, S.; Basconi, M.; De Palma, A.; Ferro, M.; Marchioni, M.; et al. Pathophysiology and Main Molecular Mechanisms of Urinary Stone Formation and Recurrence. Int. J. Mol. Sci. 2024, 25, 3075. [Google Scholar] [CrossRef]
- Sui, W.; Hancock, J.; Asplin, J.R.; Gould, E.R.; Hsi, R.S. Nephrolithiasis and Elevated Urinary Ammonium: A Matched Comparative Study. Urology 2020, 144, 77–82. [Google Scholar] [CrossRef]
- Stern, J.M.; Moazami, S.; Qiu, Y.; Kurland, I.; Chen, Z.; Agalliu, I.; Burk, R.; Davies, K.P. Evidence for a Distinct Gut Microbiome in Kidney Stone Formers Compared to Non-Stone Formers. Urolithiasis 2016, 44, 399–407. [Google Scholar] [CrossRef]
- Zhao, E.; Zhang, W.; Geng, B.; You, B.; Wang, W.; Li, X. Intestinal Dysbacteriosis Leads to Kidney Stone Disease. Mol. Med. Rep. 2020, 23, 180. [Google Scholar] [CrossRef]
- Cicerello, E.; Ciaccia, M.; Cova, G.D.; Mangano, M.S. The New Patterns of Nephrolithiasis: What Has Been Changing in the Last Millennium? Arch. Ital. Urol. E Androl. 2021, 93, 195–199. [Google Scholar] [CrossRef]
- Cicerello, E.; Mangano, M.S.; Cova, G.; Ciaccia, M. Changing in Gender Prevalence of Nephrolithiasis. Urol. J. 2021, 88, 90–93. [Google Scholar] [CrossRef]
- Peerapen, P.; Thongboonkerd, V. Protein Network Analysis and Functional Enrichment via Computational Biotechnology Unravel Molecular and Pathogenic Mechanisms of Kidney Stone Disease. Biomed. J. 2023, 46, 100577. [Google Scholar] [CrossRef]
- Awedew, A.F.; Han, H.; Berice, B.N.; Dodge, M.; Schneider, R.D.; Abbasi-Kangevari, M.; Al-Aly, Z.; Almidani, O.; Alvand, S.; Arabloo, J.; et al. The Global, Regional, and National Burden of Urolithiasis in 204 Countries and Territories, 2000–2021: A Systematic Analysis for the Global Burden of Disease Study 2021. eClinicalMedicine 2024, 78, 102924. [Google Scholar] [CrossRef]
- Nazarian, R.; Lin, N.; Thaker, S.; Yang, R.; Wong, G.C.L.; Scotland, K.B. What Causes Calcium Oxalate Kidney Stones to Form? An Update on Recent Advances. Uro 2025, 5, 6. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, Q.; Zhou, Q.; Xu, N.; Zheng, D.; Pan, Q.; Wang, X.; Xu, R. Trend Analysis of Pediatric Urolithiasis Prevalence from 1990 to 2021 in the BRICS. Front. Pediatr. 2025, 13, 1551046. [Google Scholar] [CrossRef]
- Alelign, T.; Petros, B. Kidney Stone Disease: An Update on Current Concepts. Adv. Urol. 2018, 2018, 3068365. [Google Scholar] [CrossRef]
- Aggarwal, K.P.; Narula, S.; Kakkar, M.; Tandon, C. Nephrolithiasis: Molecular Mechanism of Renal Stone Formation and the Critical Role Played by Modulators. BioMed Res. Int. 2013, 2013, 292953. [Google Scholar] [CrossRef]
- Tavasoli, S.; Taheri, M. Vitamin D and Calcium Kidney Stones: A Review and a Proposal. Int. Urol. Nephrol. 2019, 51, 101–111. [Google Scholar] [CrossRef]
- Ye, Z.; Zeng, G.; Yang, H.; Li, J.; Tang, K.; Wang, G.; Wang, S.; Yu, Y.; Wang, Y.; Zhang, T.; et al. The Status and Characteristics of Urinary Stone Composition in China. BJU Int. 2020, 125, 801–809. [Google Scholar] [CrossRef]
- Sakhaee, K. Pathogenesis and Medical Management of Cystinuria. Semin. Nephrol. 1996, 16, 435–447. [Google Scholar] [PubMed]
- Strologo, L.D.; Pras, E.; Pontesilli, C.; Beccia, E.; Ricci-Barbini, V.; De Sanctis, L.; Ponzone, A.; Gallucci, M.; Bisceglia, L.; Zelante, L.; et al. Comparison between SLC3A1 and SLC7A9 Cystinuria Patients and Carriers: A Need for a New Classification. J. Am. Soc. Nephrol. 2002, 13, 2547–2553. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Krambeck, A.E.; Rule, A.D. Determining the True Burden of Kidney Stone Disease. Nat. Rev. Nephrol. 2020, 16, 736–746. [Google Scholar] [CrossRef]
- Geraghty, R.; Wood, K.; Sayer, J.A. Calcium Oxalate Crystal Deposition in the Kidney: Identification, Causes and Consequences. Urolithiasis 2020, 48, 377–384. [Google Scholar] [CrossRef]
- Wan, W.; Wu, W.; Amier, Y.; Li, X.; Yang, J.; Huang, Y.; Xun, Y.; Yu, X. Engineered Microorganisms: A New Direction in Kidney Stone Prevention and Treatment. Synth. Syst. Biotechnol. 2024, 9, 294–303. [Google Scholar] [CrossRef]
- Evan, A.P.; Worcester, E.M.; Coe, F.L.; Williams, J.; Lingeman, J.E. Mechanisms of Human Kidney Stone Formation. Urolithiasis 2015, 43, 19–32. [Google Scholar] [CrossRef]
- Chung, H.-J. The Role of Randall Plaques on Kidney Stone Formation. Transl. Androl. Urol. 2014, 3, 251–254. [Google Scholar] [CrossRef]
- Khan, S.R.; Canales, B.K.; Dominguez-Gutierrez, P.R. Randall’s Plaque and Calcium Oxalate Stone Formation: Role for Immunity and Inflammation. Nat. Rev. Nephrol. 2021, 17, 417–433. [Google Scholar] [CrossRef]
- Taguchi, K.; Okada, A.; Unno, R.; Hamamoto, S.; Yasui, T. Macrophage Function in Calcium Oxalate Kidney Stone Formation: A Systematic Review of Literature. Front. Immunol. 2021, 12, 673690. [Google Scholar] [CrossRef]
- Anders, H.-J.; Suarez-Alvarez, B.; Grigorescu, M.; Foresto-Neto, O.; Steiger, S.; Desai, J.; Marschner, J.A.; Honarpisheh, M.; Shi, C.; Jordan, J.; et al. The Macrophage Phenotype and Inflammasome Component NLRP3 Contributes to Nephrocalcinosis-Related Chronic Kidney Disease Independent from IL-1–Mediated Tissue Injury. Kidney Int. 2018, 93, 656–669. [Google Scholar] [CrossRef]
- Liang, L.; Li, L.; Tian, J.; Lee, S.O.; Dang, Q.; Huang, C.-K.; Yeh, S.; Erturk, E.; Bushinsky, D.; Chang, L.S.; et al. Androgen Receptor Enhances Kidney Stone-CaOx Crystal Formation via Modulation of Oxalate Biosynthesis & Oxidative Stress. Mol. Endocrinol. 2014, 28, 1291–1303. [Google Scholar] [CrossRef]
- Fuster, D.G.; Morard, G.A.; Schneider, L.; Mattmann, C.; Lüthi, D.; Vogt, B.; Dhayat, N.A. Association of Urinary Sex Steroid Hormones with Urinary Calcium, Oxalate and Citrate Excretion in Kidney Stone Formers. Nephrol. Dial. Transplant. 2022, 37, 335–348. [Google Scholar] [CrossRef]
- Siddiqui, H.; Nederbragt, A.J.; Lagesen, K.; Jeansson, S.L.; Jakobsen, K.S. Assessing Diversity of the Female Urine Microbiota by High Throughput Sequencing of 16S rDNA Amplicons. BMC Microbiol. 2011, 11, 244. [Google Scholar] [CrossRef]
- Mehta, M.; Goldfarb, D.S.; Nazzal, L. The Role of the Microbiome in Kidney Stone Formation. Int. J. Surg. 2016, 36, 607–612. [Google Scholar] [CrossRef]
- Neugent, M.L.; Hulyalkar, N.V.; Nguyen, V.H.; Zimmern, P.E.; De Nisco, N.J. Advances in Understanding the Human Urinary Microbiome and Its Potential Role in Urinary Tract Infection. mBio 2020, 11, 10–1128. [Google Scholar] [CrossRef]
- Lu, J.; Tao, K. Urinary Microbiota in Patients with Kidney Stones: A Systematic Review and Meta-Analysis. Microb. Pathog. 2025, 205, 107641. [Google Scholar] [CrossRef]
- Espinosa-Ortiz, E.J.; Eisner, B.H.; Lange, D.; Gerlach, R. Current Insights into the Mechanisms and Management of Infection Stones. Nat. Rev. Urol. 2019, 16, 35–53. [Google Scholar] [CrossRef]
- Trinchieri, A. Urinary Calculi and Infection. Urol. J. 2014, 81, 93–98. [Google Scholar] [CrossRef]
- Falony, G. Beyond Oxalobacter: The Gut Microbiota and Kidney Stone Formation. Gut 2018, 67, 2078–2079. [Google Scholar] [CrossRef]
- Rodgers, A.L. Physicochemical Mechanisms of Stone Formation. Urolithiasis 2017, 45, 27–32. [Google Scholar] [CrossRef]
- Okada, A.; Yasui, T.; Fujii, Y.; Niimi, K.; Hamamoto, S.; Hirose, M.; Kojima, Y.; Itoh, Y.; Tozawa, K.; Hayashi, Y.; et al. Renal Macrophage Migration and Crystal Phagocytosis via Inflammatory-Related Gene Expression during Kidney Stone Formation and Elimination in Mice: Detection by Association Analysis of Stone-Related Gene Expression and Microstructural Observation. J. Bone Miner. Res. 2010, 25, 2701–2711. [Google Scholar] [CrossRef]
- Nikolic-Paterson, D.J.; Wang, S.; Lan, H.Y. Macrophages Promote Renal Fibrosis through Direct and Indirect Mechanisms. Kidney Int. Suppl. 2014, 4, 34–38. [Google Scholar] [CrossRef]
- Taguchi, K.; Okada, A.; Hamamoto, S.; Unno, R.; Moritoki, Y.; Ando, R.; Mizuno, K.; Tozawa, K.; Kohri, K.; Yasui, T. M1/M2-Macrophage Phenotypes Regulate Renal Calcium Oxalate Crystal Development. Sci. Rep. 2016, 6, 35167. [Google Scholar] [CrossRef]
- Kusmartsev, S.; Dominguez-Gutierrez, P.R.; Canales, B.K.; Bird, V.G.; Vieweg, J.; Khan, S.R. Calcium Oxalate Stone Fragment and Crystal Phagocytosis by Human Macrophages. J. Urol. 2016, 195, 1143–1151. [Google Scholar] [CrossRef]
- Dominguez-Gutierrez, P.R.; Kusmartsev, S.; Canales, B.K.; Khan, S.R. Calcium Oxalate Differentiates Human Monocytes into Inflammatory M1 Macrophages. Front. Immunol. 2018, 9, 1863. [Google Scholar] [CrossRef]
- Singhto, N.; Kanlaya, R.; Nilnumkhum, A.; Thongboonkerd, V. Roles of Macrophage Exosomes in Immune Response to Calcium Oxalate Monohydrate Crystals. Front. Immunol. 2018, 9, 316. [Google Scholar] [CrossRef]
- Singhto, N.; Thongboonkerd, V. Exosomes Derived from Calcium Oxalate-Exposed Macrophages Enhance IL-8 Production from Renal Cells, Neutrophil Migration and Crystal Invasion through Extracellular Matrix. J. Proteom. 2018, 185, 64–76. [Google Scholar] [CrossRef]
- Speer, T.; Dimmeler, S.; Schunk, S.J.; Fliser, D.; Ridker, P.M. Targeting Innate Immunity-Driven Inflammation in CKD and Cardiovascular Disease. Nat. Rev. Nephrol. 2022, 18, 762–778. [Google Scholar] [CrossRef]
- Sakhaee, K. Exploring the Role of Inflammation toward the Pathogenesis of Calcium Nephrolithiasis. Clin. J. Am. Soc. Nephrol. 2022, 17, 338–339. [Google Scholar] [CrossRef]
- Paniri, A.; Akhavan-Niaki, H. Emerging Role of IL-6 and NLRP3 Inflammasome as Potential Therapeutic Targets to Combat COVID-19: Role of lncRNAs in Cytokine Storm Modulation. Life Sci. 2020, 257, 118114. [Google Scholar] [CrossRef]
- Ding, T.; Zhao, T.; Li, Y.; Liu, Z.; Ding, J.; Ji, B.; Wang, Y.; Guo, Z. Vitexin Exerts Protective Effects against Calcium Oxalate Crystal-Induced Kidney Pyroptosis In Vivo and In Vitro. Phytomedicine 2021, 86, 153562. [Google Scholar] [CrossRef]
- Yao, H.; Yu, D.; He, Q.; Ning, X. Causal Associations of Inflammatory Cytokines and Urinary Stones: A Two-Sample Mendelian Randomization Study. Transl. Androl. Urol. 2025, 14, 258–265. [Google Scholar] [CrossRef]
- Bichler, K.-H.; Eipper, E.; Naber, K.; Braun, V.; Zimmermann, R.; Lahme, S. Urinary Infection Stones. Int. J. Antimicrob. Agents 2002, 19, 488–498. [Google Scholar] [CrossRef]
- He, Z.; Jing, Z.; Jing-Cun, Z.; Chuan-Yi, H.; Fei, G. Compositional Analysis of Various Layers of Upper Urinary Tract Stones by Infrared Spectroscopy. Exp. Ther. Med. 2017, 14, 3165–3169. [Google Scholar] [CrossRef]
- Hirose, M.; Yasui, T.; Okada, A.; Hamamoto, S.; Shimizu, H.; Itoh, Y.; Tozawa, K.; Kohri, K. Renal Tubular Epithelial Cell Injury and Oxidative Stress Induce Calcium Oxalate Crystal Formation in Mouse Kidney. Int. J. Urol. 2010, 17, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R. Reactive Oxygen Species as the Molecular Modulators of Calcium Oxalate Kidney Stone Formation: Evidence from Clinical and Experimental Investigations. J. Urol. 2013, 189, 803–811. [Google Scholar] [CrossRef]
- Djojodimedjo, T.; Soebadi, D.M. Soetjipto Escherichia Coli Infection Induces Mucosal Damage and Expression of Proteins Promoting Urinary Stone Formation. Urolithiasis 2013, 41, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Jiang, C.; Liang, X.; Zhong, F.; Huang, J.; Lin, Y.; Zhao, Z.; Duan, X.; Zeng, G.; Wu, W. Early and Rapid Prediction of Postoperative Infections Following Percutaneous Nephrolithotomy in Patients with Complex Kidney Stones. BJU Int. 2019, 123, 1041–1047. [Google Scholar] [CrossRef]
- An, L.; Wu, W.; Li, S.; Lai, Y.; Chen, D.; He, Z.; Chang, Z.; Xu, P.; Huang, Y.; Lei, M.; et al. Escherichia Coli Aggravates Calcium Oxalate Stone Formation via PPK1/Flagellin-Mediated Renal Oxidative Injury and Inflammation. Oxidative Med. Cell. Longev. 2021, 2021, 9949697. [Google Scholar] [CrossRef]
- Chutipongtanate, S.; Sutthimethakorn, S.; Chiangjong, W.; Thongboonkerd, V. Bacteria Can Promote Calcium Oxalate Crystal Growth and Aggregation. JBIC J. Biol. Inorg. Chem. 2013, 18, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.; Wu, W.; Chen, D.; Lai, Y.; Tiselius, H.-G.; Jiang, C.; Huang, J.; Duan, X.; Choong, S.; Liang, Y.; et al. The Characteristic and Relationship of Escherichia Coli Isolated from Urine and Stones in Patients with Calcium Oxalate Stones. Urolithiasis 2021, 49, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Tavichakorntrakool, R.; Prasongwattana, V.; Sungkeeree, S.; Saisud, P.; Sribenjalux, P.; Pimratana, C.; Bovornpadungkitti, S.; Sriboonlue, P.; Thongboonkerd, V. Extensive Characterizations of Bacteria Isolated from Catheterized Urine and Stone Matrices in Patients with Nephrolithiasis. Nephrol. Dial. Transplant. 2012, 27, 4125–4130. [Google Scholar] [CrossRef] [PubMed]
- Bauza, J.L.; Pieras, E.C.; Grases, F.; Tubau, V.; Guimerà, J.; Sabaté, X.A.; Pizà, P. Urinary Tract Infection’s Etiopathogenic Role in Nephrolithiasis Formation. Med. Hypotheses 2018, 118, 34–35. [Google Scholar] [CrossRef]
- Gault, M.H.; Longerich, L.L.; Crane, G.; Cooper, R.; Dow, D.; Best, L.; Stockall, E.; Brown, W. Bacteriology of Urinary Tract Stones. J. Urol. 1995, 153, 1164–1170. [Google Scholar] [CrossRef]
- Tavichakorntrakool, R.; Boonsiri, P.; Prasongwatana, V.; Lulitanond, A.; Wongkham, C.; Thongboonkerd, V. Differential Colony Size, Cell Length, and Cellular Proteome of Escherichia Coli Isolated from Urine vs. Stone Nidus of Kidney Stone Patients. Clin. Chim. Acta 2017, 466, 112–119. [Google Scholar] [CrossRef]
- Welch, R.A.; Burland, V.; Plunkett, G.; Redford, P.; Roesch, P.; Rasko, D.; Buckles, E.L.; Liou, S.-R.; Boutin, A.; Hackett, J.; et al. Extensive Mosaic Structure Revealed by the Complete Genome Sequence of Uropathogenic Escherichia Coli. Proc. Natl. Acad. Sci. USA 2002, 99, 17020–17024. [Google Scholar] [CrossRef]
- Hébert, M.; Potin, S.; Sebbagh, M.; Bertoglio, J.; Bréard, J.; Hamelin, J. Rho-ROCK-Dependent Ezrin-Radixin-Moesin Phosphorylation Regulates Fas-Mediated Apoptosis in Jurkat Cells. J. Immunol. 2008, 181, 5963–5973. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, D.; Guo, Z.; Zhao, J.; Wu, B.; Deng, H.; Zhou, T.; Xiang, H.; Gao, F.; Yu, X.; et al. Rho Kinase Phosphorylation Promotes Ezrin-Mediated Metastasis in Hepatocellular Carcinoma. Cancer Res. 2011, 71, 1721–1729. [Google Scholar] [CrossRef]
- Kanlaya, R.; Thongboonkerd, V. Persistent Escherichia Coli Infection in Renal Tubular Cells Enhances Calcium Oxalate Crystal–Cell Adhesion by Inducing Ezrin Translocation to Apical Membranes via Rho/ROCK Pathway. Cell. Mol. Life Sci. 2022, 79, 381. [Google Scholar] [CrossRef]
- Li, H.; Xue, X.; Meng, G.; He, C.; Tong, L.; Lai, Y. The Roles of Bacteria on Urolithiasis Progression and Associated Compounds. Biochem. Pharmacol. 2025, 237, 116958. [Google Scholar] [CrossRef]
- Kanlaya, R.; Naruepantawart, O.; Thongboonkerd, V. Flagellum Is Responsible for Promoting Effects of Viable Escherichia Coli on Calcium Oxalate Crystallization, Crystal Growth, and Crystal Aggregation. Front. Microbiol. 2019, 10, 2507. [Google Scholar] [CrossRef]
- Bayer, M.E.; Sloyer, J.L. The Electrophoretic Mobility of Gram-Negative and Gram-Positive Bacteria: An Electrokinetic Analysis. J. Gen. Microbiol. 1990, 136, 867–874. [Google Scholar] [CrossRef]
- Ballén, V.; Cepas, V.; Ratia, C.; Gabasa, Y.; Soto, S.M. Clinical Escherichia Coli: From Biofilm Formation to New Antibiofilm Strategies. Microorganisms 2022, 10, 1103. [Google Scholar] [CrossRef]
- Lai, H.-C.; Chang, S.-N.; Lin, H.-C.; Hsu, Y.-L.; Wei, H.-M.; Kuo, C.-C.; Hwang, K.-P.; Chiang, H.-Y. Association between Urine pH and Common Uropathogens in Children with Urinary Tract Infections. J. Microbiol. Immunol. Infect. 2021, 54, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Taheri, H.; Feizabadi, M.M.; Keikha, R.; Afkari, R. Therapeutic Effects of Probiotics and Herbal Medications on Oxalate Nephrolithiasis: A Mini Systematic Review. Iran. J. Microbiol. 2024, 16, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Amimanan, P.; Tavichakorntrakool, R.; Fong-ngern, K.; Sribenjalux, P.; Lulitanond, A.; Prasongwatana, V.; Wongkham, C.; Boonsiri, P.; Umka Welbat, J.; Thongboonkerd, V. Elongation Factor Tu on Escherichia Coli Isolated from Urine of Kidney Stone Patients Promotes Calcium Oxalate Crystal Growth and Aggregation. Sci. Rep. 2017, 7, 2953. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-C.; Huang, W.-H.; Lo, S.-C.; Huang, E.; Chiang, E.-P.I.; Huang, C.-C.; Yang, Y.-T. Conversion of Escherichia Coli into Mixotrophic CO2 Assimilation with Malate and Hydrogen Based on Recombinant Expression of 2-Oxoglutarate:Ferredoxin Oxidoreductase Using Adaptive Laboratory Evolution. Microorganisms 2023, 11, 253. [Google Scholar] [CrossRef]
- Ruiz-Agudo, E.; Burgos-Cara, A.; Ruiz-Agudo, C.; Ibañez-Velasco, A.; Cölfen, H.; Rodriguez-Navarro, C. A Non-Classical View on Calcium Oxalate Precipitation and the Role of Citrate. Nat. Commun. 2017, 8, 768. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.; Birowo, P.; Widyahening, I.S.; Rasyid, N. Effect of Citrus-Based Products on Urine Profile: A Systematic Review and Meta-Analysis. F1000Research 2017, 6, 220. [Google Scholar] [CrossRef]
- Edin-Liljegren, A.; Hedelin, H.H.; Grenabo, L.; Pettersson, S. Impact of Escherichia Coli on Urine Citrate and Urease-Induced Crystallization. Scanning Microsc. 1995, 9, 901–905. [Google Scholar]
- Gupta, S.; Kanwar, S.S. Therapeutic Applications of Microbial Enzymes in the Management of Kidney Stone Diseases. In Microbial Enzymes: Roles and Applications in Industries; Arora, N.K., Mishra, J., Mishra, V., Eds.; Microorganisms for Sustainability; Springer: Singapore, 2020; Volume 11, pp. 319–329. ISBN 978-981-15-1709-9. [Google Scholar]
- Daniel, S.L.; Moradi, L.; Paiste, H.; Wood, K.D.; Assimos, D.G.; Holmes, R.P.; Nazzal, L.; Hatch, M.; Knight, J. Forty Years of Oxalobacter Formigenes, a Gutsy Oxalate-Degrading Specialist. Appl. Environ. Microbiol. 2021, 87, e00544-21. [Google Scholar] [CrossRef] [PubMed]
- Suman, D. Oxalate Degrading Lactic Acid Bacteria in Prevention and Management of Kidney Stone. Open Access J. Complement. Altern. Med. 2021, 3, 420–425. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Zhao, Y.-Y.; Pahl, M.V. Altered Intestinal Microbial Flora and Impaired Epithelial Barrier Structure and Function in CKD: The Nature, Mechanisms, Consequences and Potential Treatment. Nephrol. Dial. Transplant. 2016, 31, 737–746. [Google Scholar] [CrossRef]
- Mani, R.R.; Ranganathan, V.; Panneerselvam, J.; Begam, S.; Chinnappan, S.; Anbalagan, M. Therapeutic Applications of Oxalate-Degrading Bacteria in Kidney Stone Prevention. Nat. Prod. J. 2025, 15, e22103155352643. [Google Scholar] [CrossRef]
- Suryavanshi, M.V.; Bhute, S.S.; Gune, R.P.; Shouche, Y.S. Functional Eubacteria Species along with Trans-Domain Gut Inhabitants Favour Dysgenic Diversity in Oxalate Stone Disease. Sci. Rep. 2018, 8, 16598. [Google Scholar] [CrossRef] [PubMed]
- Denburg, M.R.; Koepsell, K.; Lee, J.-J.; Gerber, J.; Bittinger, K.; Tasian, G.E. Perturbations of the Gut Microbiome and Metabolome in Children with Calcium Oxalate Kidney Stone Disease. J. Am. Soc. Nephrol. 2020, 31, 1358–1369. [Google Scholar] [CrossRef]
- Noonin, C.; Thongboonkerd, V. Beneficial Roles of Gastrointestinal and Urinary Microbiomes in Kidney Stone Prevention via Their Oxalate-Degrading Ability and Beyond. Microbiol. Res. 2024, 282, 127663. [Google Scholar] [CrossRef]
- He, M.; Dong, Y.; Cai, W.; Cai, J.; Xie, Y.; Yu, M.; Li, C.; Wen, L. Recent Advances in the Treatment of Renal Stones Using Flexible Ureteroscopys. Int. J. Surg. 2024, 110, 4320–4328. [Google Scholar] [CrossRef] [PubMed]
- Papatsoris, A.; Alba, A.B.; Galán Llopis, J.A.; Musafer, M.A.; Alameedee, M.; Ather, H.; Caballero-Romeu, J.P.; Costa-Bauzá, A.; Dellis, A.; El Howairis, M.; et al. Management of Urinary Stones: State of the Art and Future Perspectives by Experts in Stone Disease. Arch. Ital. Urol. E Androl. 2024, 96, 12703. [Google Scholar] [CrossRef]
- Yang, X.; Koohi-Moghadam, M.; Wang, R.; Chang, Y.-Y.; Woo, P.C.Y.; Wang, J.; Li, H.; Sun, H. Metallochaperone UreG Serves as a New Target for Design of Urease Inhibitor: A Novel Strategy for Development of Antimicrobials. PLoS Biol. 2018, 16, e2003887. [Google Scholar] [CrossRef] [PubMed]
- Milo, S.; Heylen, R.A.; Glancy, J.; Williams, G.T.; Patenall, B.L.; Hathaway, H.J.; Thet, N.T.; Allinson, S.L.; Laabei, M.; Jenkins, A.T.A. A Small-Molecular Inhibitor against Proteus Mirabilis Urease to Treat Catheter-Associated Urinary Tract Infections. Sci. Rep. 2021, 11, 3726. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Gao, C.; Lu, J.; Liao, S.; Gong, G. Key Biological Processes and Essential Genes for Proteus Mirabilis Biofilm Development Inhibition by Protocatechuic Acid. Int. J. Food Microbiol. 2024, 412, 110570. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).