Prevalence and Risk Mapping of Intestinal Parasites in the “Hungry Valleys” Region of Slovakia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
Primary Data Collection
2.3. Secondary Data Collection
2.4. Data Analysis
Parasitological Analysis
2.5. Statistical Analysis
2.6. Spatial Analysis
3. Results
4. Discussion
4.1. Prevalence of Intestinal Parasites
4.2. Risk Varies According to the Sub-Population
4.3. Determinants of Intestinal Parasite Transmission Risk
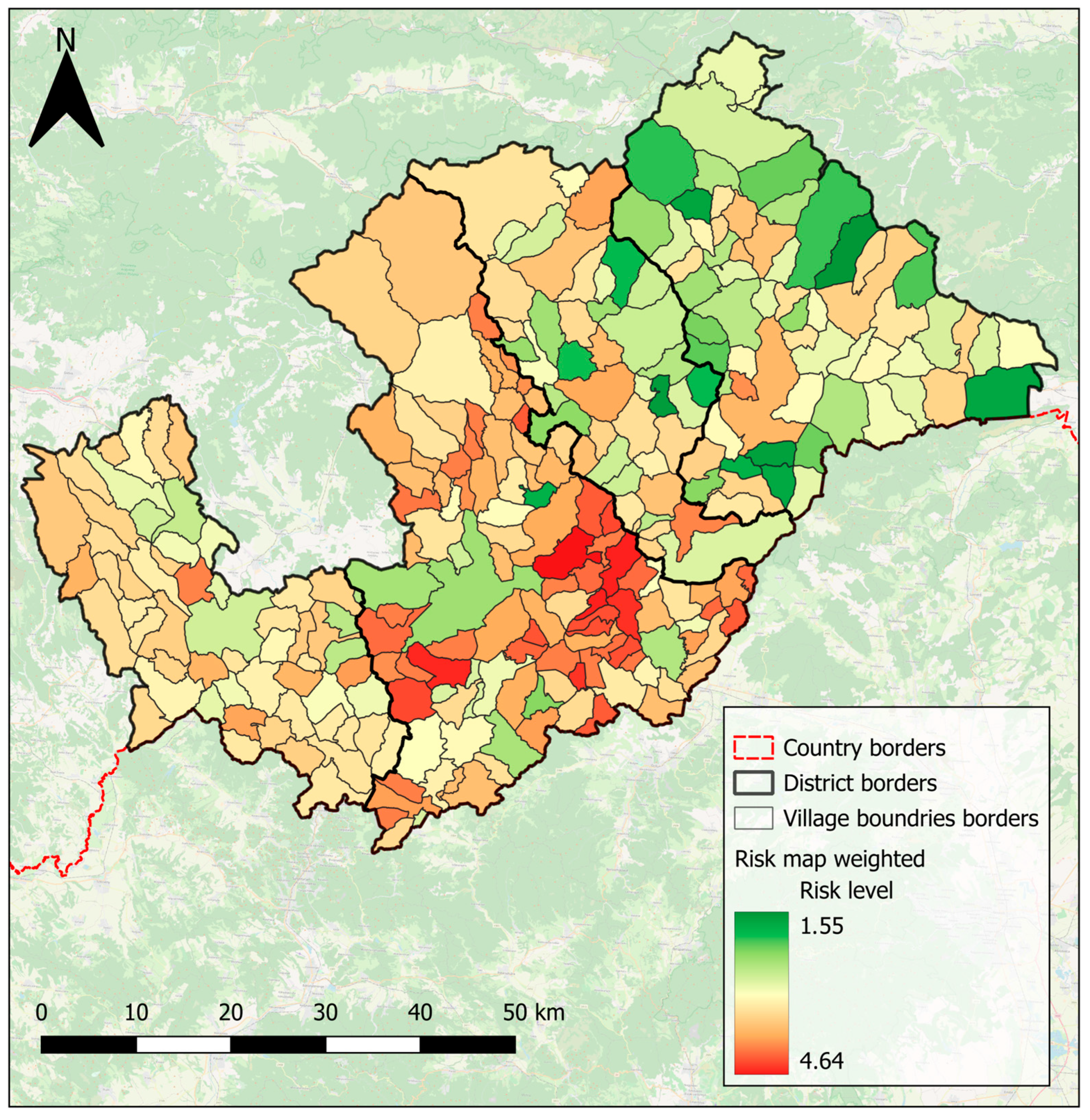
4.4. Risk Maps Highlight High-Risk Areas, and Inform Decision-Making in the Hungry Valleys
4.5. Implications of This Research for Other Marginalized Population Groups in Challenging Living Environments
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Endoparasites. ESCCAP UK. 2024. Available online: https://www.esccapuk.org.uk/page/Endoparasites/14/ (accessed on 12 December 2024).
- Vocabulary.com. Endoparasite. Available online: https://www.vocabulary.com/dictionary/endoparasite (accessed on 13 December 2024).
- Chumley, H.S. Intestinal Worms and Parasites. In The Color Atlas and Synopsis of Family Medicine, 3rd ed.; Usatine, R.P., Smith, M.A., Mayeaux, E.J., Jr., Chumley, H.S., Eds.; McGraw Hill: New York, NY, USA, 2019. [Google Scholar]
- WHO. Soil-transmitted helminthiasis: Number of children treated 2007–2008: Update on the 2010 global target. Wkly. Epidemiol. Rec. 2010, 85, 141–148. [Google Scholar]
- Joint Monitoring Programme for Water Supply, Sanitation and Hygiene by World Health Organization/UNICEF (JMP). Estimates for Drinking Water, Sanitation and Hygiene Services by Country (2000–2022). Available online: https://data.unicef.org/resources/jmp-report-2023 (accessed on 1 July 2024).
- Neglected Tropical Diseases. WHO. Available online: https://www.who.int/health-topics/neglected-tropical-diseases#tab=tab_2 (accessed on 13 December 2024).
- Papajová, I.; Šoltys, J. Nematode Infections Spread in Slovakia, a European Temperate Region. In Helminthiasis; Okwa, O.O., Ed.; IntechOpen: London, UK, 2020; ISBN 978178985335. [Google Scholar]
- Ihnacik, L.; Šmigová, J.; Šoltys, J.; Bobíková, D.; Kuzevičová, Ž.; Schusterová, I.; Papajová, I. The survey of soil-transmitted helminth species abundance in Slovakia with an emphasis on parameters important for their distribution. Front. Med. 2022, 9, 1043313. [Google Scholar] [CrossRef] [PubMed]
- Mušinka, A.; Škobla, D.; Hurrle, J.; Matlovičová, K.; Kling, J. Atlas of Roma Communities in Slovakia 2013; UNDP: Bratislava, Slovakia, 2014. [Google Scholar]
- Ihnacik, L.; Papajová, I.; Šmigová, J.; Brussel, M.; Manga, M.; Papaj, J.; Schusterová, I.; Anthonj, C. A Transect Through the Living Environments of Slovakia’s Roma Population: Urban, Sub-Urban, and Rural Settlements, and Exposure to Environmental and Water-Related Health Risks. Int. J. Environ. Res. Public Health 2025, 22, 988. [Google Scholar] [CrossRef] [PubMed]
- Ravasz, A.; Kovács, Ľ.; Markovič, F. Atlas Rómskych Komunít 2019 [Atlas of Roma Communities 2019]; VEDA: Bratislava, Slovakia, 2020; Available online: https://www.academia.edu/109355562/Atlas_r%C3%B3mskych_komun%C3%ADt_2019 (accessed on 1 December 2024).
- Epe, C.; Meuwissen, M.; Stoye, M.; Schnieder, T. Transmission trials, ITS2-PCR and RAPD-PCR show identity of Toxocara canis isolates from red fox and dog. Vet. Parasitol. 1999, 84, 101–112. [Google Scholar] [CrossRef]
- Antolová, D.; Reiterová, K.; Miterpáková, M.; Stanko, M.; Dubinský, P. Circulation of Toxocara spp. in suburban and rural ecosystems in the Slovak Republic. Vet. Parasitol. 2004, 126, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.C.; Rohen, M.; Epe, C.; Schnieder, T. Prevalence of endoparasites in stray and fostered dogs and cats in Northern Germany. Parasitol. Res. 2012, 111, 849–857. [Google Scholar] [CrossRef]
- Anthonj, C.; Setty, K.E.; Ezbakhe, F.; Manga, M.; Hoeser, C. A systematic review of water, sanitation and hygiene among Roma communities in Europe: Situation analysis, cultural context, and obstacles to improvement. Int. J. Hyg. Environ. Health 2020, 226, 113506. [Google Scholar] [CrossRef]
- Hakl, R.; Odkladal, M. 30 Rokov Slovenska: Fenomén Hladových Dolín Nedokázala Doteraz Vyriešiť Žiadna Vláda. Available online: https://www.aktuality.sk/clanok/07JHFad/30-rokov-slovenska-fenomen-hladovych-dolin-nedokazala-doteraz-vyriesit-ziadna-vlada/ (accessed on 28 November 2024).
- Ústredie Práce, Sociálnych vecí a Rodiny (UPSVR). Nezamestnanost’—Mesačné Štatistiky. Available online: https://www.upsvr.gov.sk/statistiky/nezamestnanost-mesacnestatistiky/2024.html?page_id=1335212 (accessed on 28 November 2024).
- Statistical Office of the Slovak Republic (SO SR). SOBD 2021—Sčítanie Obyvateľov, Domov a Bytov [Census of Inhabitants, Houses and Apartments]. Available online: https://www.scitanie.sk/ (accessed on 28 November 2024).
- Papajová, I.; Bystrianska, J.; Giboda, M.; Becker, S.L.; Utzinger, J.; Marti, H. Intestinal parasites in segregated minority communities of Slovakia: Results from a cross-sectional survey in children. Acta Trop. 2021, 214, 105783. [Google Scholar] [CrossRef]
- Dudlová, A.; Juris, P.; Jurišová, S.; Jarcuska, P.; Krcméry, V. Epidemiology and geographical distribution of gastrointestinal parasitic infection in humans in Slovakia. Helminthologia 2016, 53, 309–317. [Google Scholar] [CrossRef][Green Version]
- Mehlhorn, H. Human Parasites; Springer: Cham, Switzerland, 2016. [Google Scholar][Green Version]
- Rawla, P.; Sharma, S. Enterobius Vermicularis; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK536974/ (accessed on 6 November 2024).[Green Version]
- Štrkolcová, G.; Fiľakovská Bobáková, D.; Kaduková, M.; Schreiberová, A.; Klein, D.; Halán, M.; Urbančíková, I. Intestinal parasitic infections in children from marginalised Roma communities: Prevalence and risk factors. BMC Infect. Dis. 2024, 24, 596. [Google Scholar] [CrossRef] [PubMed]
- Štrkolcová, G.; Mravcová, K.; Barbušinová, E.; Mucha, R.; Várady, M.; Goldová, M. Prevalence of intestinal parasites in children living in various living conditions in Slovakia. J. Pediatr. Perinatol. Child Health 2019, 3, 174–185. [Google Scholar] [CrossRef]
- Ihnacik, L.; Šmigová, J.; Papajová, I. Identification of risk factors and high-risk areas for transmission of intestinal parasites in model locality in Slovakia. Sci. Rep. 2025, 15, 25811. [Google Scholar] [CrossRef]
- Pipiková, J.; Papajová, I.; Šoltys, J.; Schusterová, I. Occurrence of the most common helminth infections among children in the Eastern Slovak Republic. Public Health 2017, 150, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Pullan, R.L.; Bethony, J.M.; Geiger, S.M.; Cundill, B.; Correa-Oliveira, R.; Quinnell, R.J.; Brooker, S. Human helminth co-infection: Analysis of spatial patterns and risk factors in a Brazilian community. PLoS Negl. Trop. Dis. 2008, 2, e352. [Google Scholar] [CrossRef]
- Ross, A.G.; Olveda, R.M.; McManus, D.P.; Harn, D.A.; Chy, D.; Li, Y.; Tallo, V.; Ng, S.K. Risk factors for human helminthiases in rural Philippines. Int. J. Infect. Dis. 2017, 54, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Papajová, I.; Blišťan, P.; Ihnacik, L.; Šmigová, J.; Šoltys, J.; Kovanič, Ľ.; Blišťanová, M. Atlas Výskytu Závažných Endoparazitárnych Ochorení u Ľudí v Okrese Rožňava, 1st ed.; Fakulta Berg TUKE; Parazitologický ústav SAV v.v.i.: Košice, Slovakia, 2023; ISBN 978-80-553-4404-1. [Google Scholar]
- Blišťan, P.; Papajová, I.; Ihnacik, L.; Kovanič, Ľ.; Blišťanová, M.; Šmigová, J.; Šoltys, J. Atlas Máp Oblastí s Rizikom Výskytu Endoparazitov v Košickom Samosprávnom Kraji, 1st ed.; Fakulta Berg TUKE; Parazitologický ústav SAV v.v.i.: Košice, Slovakia, 2023; ISBN 978-80-553-4405-8. [Google Scholar]
- Lin, C.-H.; Wen, T.-H. How spatial epidemiology helps understand infectious human disease transmission. Trop. Med. Infect. Dis. 2022, 7, 164. [Google Scholar] [CrossRef]

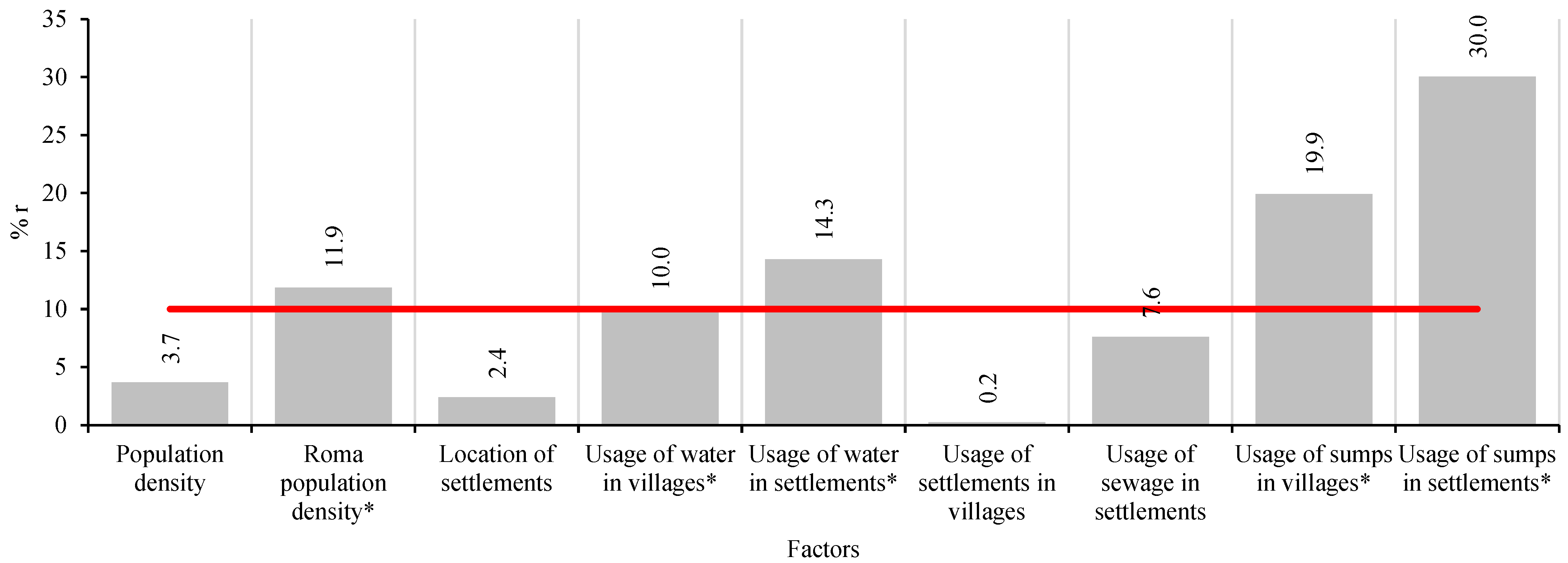
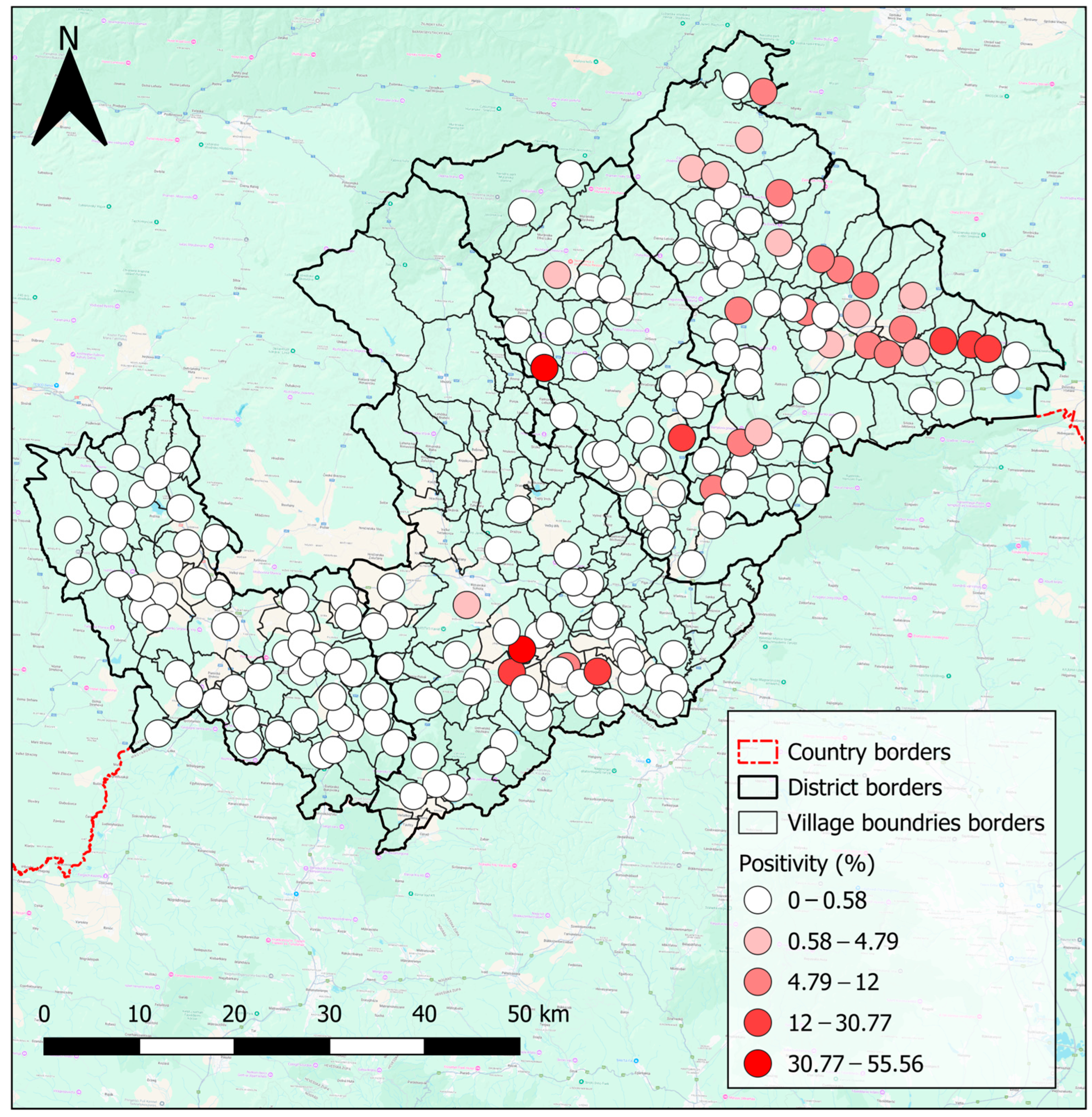
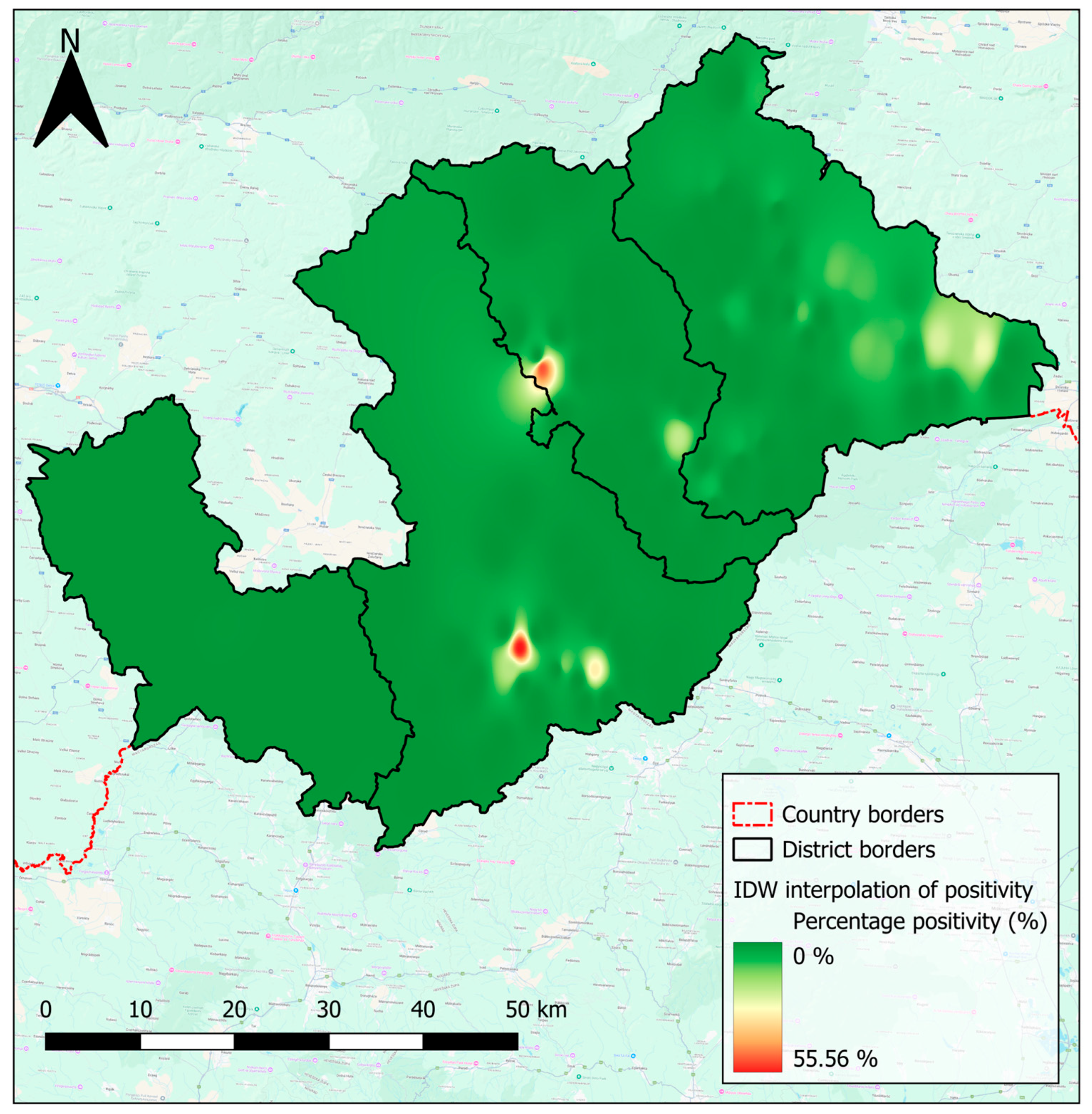
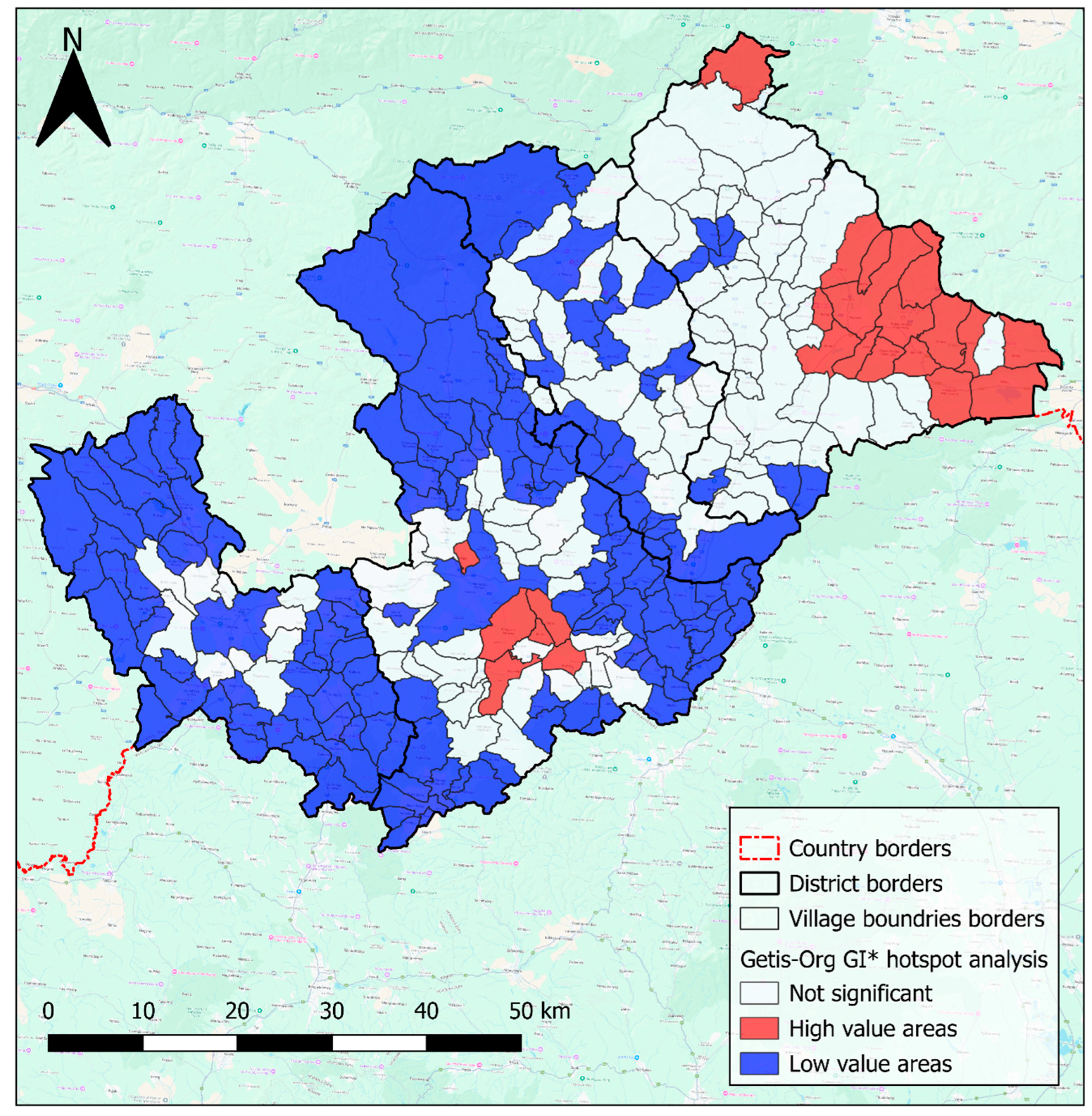

| Eggs/Cysts | Overall | Ethnicity | Sex | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Roma | Non-Roma | Men | Women | ||||||
| n * | % | n * | % | n * | % | n * | % | ||
| A. lumbricoides | 159 | 146 | 91.82 | 13 | 8.18 | 82 | 51.57 | 77 | 48.43 |
| T. trichiura | 20 | 18 | 90.00 | 2 | 10.00 | 14 | 70.00 | 6 | 30.00 |
| E. vermicularis | 14 | 13 | 92.86 | 1 | 7.14 | 10 | 71.42 | 4 | 28.57 |
| n | nposit | % (95% CI) | COR (95% CI) | χ2 | p-Value | ||
|---|---|---|---|---|---|---|---|
| Overall | 3816 | 193 | 5.06 (4.38–5.80) | - | - | - | |
| Nationality | Roma | 1302 | 176 | 13.52 (11.70–15.49) | 22.95 (13.88–37.95) | 294.56 | <0.01 * |
| Non-Roma a | 2514 | 17 | 0.68 (0.39–1.08) | 0.04 (0.02–0.07) | - | - | |
| Sex | Men | 1972 | 102 | 5.17 (4.24–6.25) | 1.05 (0.78–1.40) | 0.11 | 0.74 |
| Women a | 1844 | 91 | 4.93 (3.99–6.02) | 0.95 (0.71–1.27) | - | - | |
| Environment | Rural | 1819 | 124 | 6.82 (5.70–8.07) | 2.04 (1.51–2.76) | 22.40 | <0.01 * |
| Urban a | 1997 | 69 | 3.46 (2.69–4.35) | 0.48 (0.36–0.66) | - | - | |
| n | nposit | % (95% CI) | ||
|---|---|---|---|---|
| Age groups | Infants (0–1) | 220 | 14 | 6.36 (3.52–10.44) |
| Kids (2–6) | 1128 | 70 | 6.21 (4.86–7.77) | |
| Adolescents (7–18) | 1186 | 85 | 7.17 (5.76–8.78) | |
| Productive (19–66) | 1146 | 23 | 2.01 (1.27–2.99) | |
| Post-productive (>66) | 136 | 1 | 0.74 (0.00–4.02) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ihnacik, L.; Šmigová, J.; Anthonj, C.; Papajová, I. Prevalence and Risk Mapping of Intestinal Parasites in the “Hungry Valleys” Region of Slovakia. Pathogens 2025, 14, 966. https://doi.org/10.3390/pathogens14100966
Ihnacik L, Šmigová J, Anthonj C, Papajová I. Prevalence and Risk Mapping of Intestinal Parasites in the “Hungry Valleys” Region of Slovakia. Pathogens. 2025; 14(10):966. https://doi.org/10.3390/pathogens14100966
Chicago/Turabian StyleIhnacik, Lukáš, Júlia Šmigová, Carmen Anthonj, and Ingrid Papajová. 2025. "Prevalence and Risk Mapping of Intestinal Parasites in the “Hungry Valleys” Region of Slovakia" Pathogens 14, no. 10: 966. https://doi.org/10.3390/pathogens14100966
APA StyleIhnacik, L., Šmigová, J., Anthonj, C., & Papajová, I. (2025). Prevalence and Risk Mapping of Intestinal Parasites in the “Hungry Valleys” Region of Slovakia. Pathogens, 14(10), 966. https://doi.org/10.3390/pathogens14100966






