Anti-Rickettsial Activity of Chitosan, Selenium, and Silver Nanoparticles: Efficacy in Vero Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultivation of R. conorii subsp. caspia
2.2. Nanoparticles Synthesis
2.3. Cell Viability
2.4. Treatment of Rickettsial Infection with Nanoparticles
2.5. RNA Extraction and Reverse-Transcriptase Quantitative PCR
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AgNPs | Silver nanoparticles |
| SeNPs | Selenium nanoparticles |
| CSNPs | Chitosan nanoparticles |
| ROS | Reactive oxygen species |
| SFG | Spotted fever group |
| TG | Typhus group |
References
- Boldiš, V.; Štrus, J.; Kocianová, E.; Tušek-Žnidarič, M.; Štefanidesová, K.; Schwarzová, K.; Kúdelová, M.; Sekeyová, Z.; Špitalská, E. Life cycle of Rickettsia slovaca in L929 cell line studied by quantitative real-time PCR and transmission electron microscopy. FEMS Microbiol. Lett. 2009, 293, 102–106. [Google Scholar] [CrossRef][Green Version]
- Silverman, D.J.; Santucci, L.A.; Sekeyova, Z. Heparin protects human endothelial cells infected by Rickettsia rickettsii. Infect. Immun. 1991, 59, 4505–4510. [Google Scholar] [CrossRef]
- Liu, D. Molecular Medical Microbiology, 2nd ed.; Academic Press: New York, NY, USA, 2015; Volume 3, pp. 2043–2056. ISBN 9780123971692. [Google Scholar]
- Sekeyová, Z.; Socolovschi, C.; Špitalská, E.; Kocianová, E.; Boldiš, V.; Diaz, M.Q.; Berthová, L.; Bohácsová, M.; Valáriková, J.; Fournier, P.E.; et al. Update on rickettsioses in Slovakia. Acta Virol. 2013, 57, 180–199. [Google Scholar] [CrossRef]
- Kim, H.K. Rickettsia-Host-Tick Interactions: Knowledge Advances and Gaps. Infect. Immun. 2022, 90, e0062121. [Google Scholar] [CrossRef] [PubMed]
- Shpynov, S.N.; Fournier, P.E.; Pozdnichenko, N.N.; Gumenuk, A.S.; Skiba, A.A. New approaches in the systematics of rickettsiae. New Microbes New Infect. 2018, 23, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, M.Y.; Stenos, J.; Bitam, I.; Fournier, P.E.; et al. Update on tick-borne rickettsioses around the world: A geographic approach. Clin. Microbiol. Rev. 2013, 26, 657–702. [Google Scholar] [CrossRef]
- Piotrowski, M.; Rymaszewska, A. Expansion of tick-borne rickettsioses in the world. Microorganisms 2020, 8, 1906. [Google Scholar] [CrossRef] [PubMed]
- Willson, R.; Zhao, Y.; Brosamer, K.; Pal, Y.; Blanton, L.; Arroyave, E.; Roach, C.; Walker, D.; Kourentzi, K.; Fang, R. Development of a rapid antigen-based lateral flow assay for tick-borne spotted fever rickettsioses. PLoS ONE 2025, 20, e0312819. [Google Scholar]
- Velásquez, F.; Frazão, M.; Diez, A.; Villegas, F.; Álvarez-Bidwell, M.; Rivas-Pardo, J.; Vallejos-Vidal, E.; Reyes-Lopez, F.; Toro-Ascuy, D.; Ahumada, M.; et al. Salmon-IgM functionalized-PLGA nanosystem for florfenicol delivery as an antimicrobial strategy against Piscirickettsia salmonis. Nanomaterials 2024, 14, 1658. [Google Scholar] [CrossRef]
- Acedo-Valdez, M.; Grijalva-Chon, J.; Larios-Rodríguez, E.; Maldonado-Arce, A.; Mendoza-Cano, F.; Sánchez-Paz, J.; Castro-Longoria, R. Antibacterial effect of biosynthesized silver nanoparticles in Pacific white shrimp Litopenaeus vannamei (Boone) infected with necrotizing hepatopancreatitis bacterium (NHP-B). Lat. Am. J. Aquat. Res. 2017, 45, 421–430. [Google Scholar]
- Peresh, Y.Y.; Šoltys, K.; Kľúčar, Ľ.; Beke, G.; Kováčová, M.; Špitalský, Z.; Špitalská, E. Carbon nanodots as photosensitizer in photodynamic inactivation of Rickettsia slovaca. Photodiagnosis Photodyn. Ther. 2024, 50, 104402. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.M.; Ismael, E.; Shaalan, M. Evaluation of the effects of silver nanoparticles against experimentally induced necrotic enteritis in broiler chickens. Int. J. Nanomed. 2021, 16, 6783. [Google Scholar] [CrossRef] [PubMed]
- Doszpoly, A.; Shaalan, M.; El-Matbouli, M. Silver nanoparticles proved to be efficient antivirals in vitro against three highly pathogenic fish viruses. Viruses 2023, 15, 1689. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elala, N.M.; Shaalan, M.; Ali, S.E.; Younis, N.A. Immune responses and protective efficacy of diet supplementation with selenium nanoparticles against cadmium toxicity in Oreochromis niloticus. Aquac. Res. 2021, 52, 3677–3686. [Google Scholar] [CrossRef]
- Saleh, M.; Essawy, E.; Shaalan, M.; Osman, S.; Ahmed, F.; El-Matbouli, M. Therapeutic intervention with dietary chitosan nanoparticles alleviates fish pathological and molecular systemic inflammatory responses against infections. Mar. Drugs 2022, 20, 425. [Google Scholar] [CrossRef]
- Stenos, J.; Graves, S.R.; Unsworth, N.B. A highly sensitive and specific real-time PCR assay for the detection of spotted fever and typhus group rickettsiae. Am. J. Trop. Med. Hyg. 2005, 73, 1083–1085. [Google Scholar] [CrossRef]
- Social Science Statistics. Mann-Whitney U Test Calculator. 2025. Available online: https://www.socscistatistics.com/tests/mannwhitney/default2.aspx (accessed on 1 July 2025).
- Kuang, W.; Zhong, Q.; Ye, X.; Yan, Y.; Yang, Y.; Zhang, J.; Huang, L.; Tan, S.; Shi, Q. Antibacterial nanorods made of carbon quantum dots-ZnO under visible light irradiation. J. Nanosci. Nanotechnol. 2019, 19, 3982–3990. [Google Scholar] [CrossRef]
- Kang, J.; Dietz, M.; Hughes, K.; Xing, M.; Li, B. Silver nanoparticles present high intracellular and extracellular killing against Staphylococcus aureus. J. Antimicrob. Chemother. 2019, 74, 1578–1585. [Google Scholar] [CrossRef]
- Harada, A.; Xu, W.; Ono, K.; Tsutsuki, H.; Yahiro, K.; Sawa, T.; Niidome, T. Modification of silver nanoplates with cell-binding subunit of bacterial toxin and their antimicrobial activity against intracellular bacteria. ACS Appl. Bio Mater. 2023, 6, 3387–3394. [Google Scholar] [CrossRef]
- Liu, S.; Phillips, S.; Northrup, S.; Levi, N. The impact of silver nanoparticle-induced photothermal therapy and its augmentation of hyperthermia on breast cancer cells harboring intracellular bacteria. Pharmaceutics 2023, 15, 2466. [Google Scholar] [CrossRef]
- Ameen, F.; Alyahya, S.; Govarthanan, M.; Aljahdali, N.; Al-Enazi, N.; Alsamhary, K.; Alshehri, W.; Alwakeel, S.; Alharbi, S. Soil bacteria Cupriavidus sp. mediates the extracellular synthesis of antibacterial silver nanoparticles. J. Mol. Struct. 2020, 1202, 127233. [Google Scholar] [CrossRef]
- Ghodake, G.; Kim, M.; Sung, J.; Shinde, S.; Yang, J.; Hwang, K.; Kim, D. Extracellular synthesis and characterization of silver nanoparticles—Antibacterial activity against multidrug-resistant bacterial strains. Nanomaterials 2020, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Bharti, S.; Mukherji, S.; Mukherji, S. Extracellular synthesis of silver nanoparticles by Thiosphaera pantotropha and evaluation of their antibacterial and cytotoxic effects. 3 Biotech 2020, 10, 237. [Google Scholar] [CrossRef]
- Tripathi, N.; Goshisht, M. Recent advances and mechanistic insights into antibacterial activity, antibiofilm activity, and cytotoxicity of silver nanoparticles. ACS Appl. Bio Mater. 2022, 5, 1391–1463. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, B.; Parandhaman, T.; Das, S. Antibacterial effects of biosynthesized silver nanoparticles on surface ultrastructure and nanomechanical properties of gram-negative bacteria viz. Escherichia coli and Pseudomonas aeruginosa. ACS Appl. Mater. Interfaces 2016, 8, 4963–4976. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver nanoparticles and their antibacterial applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Yan, X.; He, B.; Liu, L.; Qu, G.; Shi, J.; Hu, L.; Jiang, G. Antibacterial mechanism of silver nanoparticles in Pseudomonas aeruginosa: Proteomics approach. Metallomics 2018, 10, 557–564. [Google Scholar] [CrossRef]
- Alam, H.; Khatoon, N.; Khan, M.; Husain, S.; Saravanan, M.; Sardar, M. Synthesis of selenium nanoparticles using probiotic bacteria Lactobacillus acidophilus and their enhanced antimicrobial activity against resistant bacteria. J. Clust. Sci. 2019, 31, 1003–1011. [Google Scholar] [CrossRef]
- Huang, T.; Holden, J.; Heath, D.; O’Brien-Simpson, N.; O’Connor, A. Engineering highly effective antimicrobial selenium nanoparticles through control of particle size. Nanoscale 2019, 11, 14937–14951. [Google Scholar] [CrossRef]
- Ruiz-Fresneda, M.; Schaefer, S.; Hübner, R.; Fahmy, K.; Merroun, M. Exploring antibacterial activity and bacterial-mediated allotropic transition of differentially coated selenium nanoparticles. ACS Appl. Mater. Interfaces 2023, 15, 29958–29970. [Google Scholar] [CrossRef]
- Han, H.; Patel, K.; Kwak, J.; Jun, S.; Jang, T.; Lee, S.; Knowles, J.; Kim, H.; Lee, H.; Lee, J. Selenium nanoparticles as candidates for antibacterial substitutes and supplements against multidrug-resistant bacteria. Biomolecules 2021, 11, 1028. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.; Fan, S.; Dai, C.; Yu, B.; Wang, P.; Qu, Y. Antibacterial properties and mechanism of selenium nanoparticles synthesized by Providencia sp. DCX. Environ. Res. 2020, 194, 110630. [Google Scholar] [CrossRef]
- Bužková, A.; Hochvaldová, L.; Večeřová, R.; Malina, T.; Petr, M.; Kašlík, J.; Kvítek, L.; Kolář, M.; Panáček, A.; Prucek, R. Selenium nanoparticles: Influence of reducing agents on particle stability and antibacterial activity at biogenic concentrations. Nanoscale 2025, 13. [Google Scholar] [CrossRef]
- Vahdati, M.; Moghadam, T. Synthesis and characterization of selenium nanoparticles-lysozyme nanohybrid system with synergistic antibacterial properties. Sci. Rep. 2020, 10, 510. [Google Scholar] [CrossRef]
- Huang, T.; Holden, J.; Reynolds, E.; Heath, D.; O’Brien-Simpson, N.; O’Connor, A. Multifunctional antimicrobial polypeptide-selenium nanoparticles combat drug-resistant bacteria. ACS Appl. Mater. Interfaces 2020, 12, 50. [Google Scholar] [CrossRef]
- Fahmy, N.; Abdel-Kareem, M.; Ahmed, H.; Helmy, M.; Mahmoud, E. Evaluation of the antibacterial and antibiofilm effect of mycosynthesized silver and selenium nanoparticles and their synergistic effect with antibiotics on nosocomial bacteria. Microb. Cell Fact. 2025, 24, 6. [Google Scholar] [CrossRef]
- Zaki, N.; Hafez, M. Enhanced antibacterial effect of ceftriaxone sodium-loaded chitosan nanoparticles against intracellular Salmonella typhimurium. AAPS Pharm. Sci. Tech. 2012, 13, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Razei, A.; Cheraghali, A.; Saadati, M.; Ramandi, M.; Panahi, Y.; Hajizade, A.; Siadat, S.; Behrouzi, A. Gentamicin-loaded chitosan nanoparticles improve its therapeutic effects on Brucella-Infected J774A.1 murine cells. Galen Med. J. 2019, 8, e1296. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Liu, X.; Li, Y.; Xu, Q.; Yang, L.; Gao, F. Nitrogen-phosphorous co-doped carbonized chitosan nanoparticles for chemotherapy and ROS-mediated immunotherapy of intracellular Staphylococcus aureus infection. Carbohydr. Polym. 2023, 315, 121013. [Google Scholar] [CrossRef] [PubMed]
- Trousil, J.; Dal, N.; Fenaroli, F.; Schlachet, I.; Kubíčková, P.; Janoušková, O.; Pavlova, E.; Škorič, M.; Trejbalová, K.; Pavliš, O.; et al. Antibiotic-loaded amphiphilic chitosan nanoparticles target macrophages and kill an intracellular pathogen. Small 2022, 18, e2201853. [Google Scholar] [CrossRef]
- Menichetti, A.; Mavridi-Printezi, A.; Mordini, D.; Montalti, M. Effect of size, shape and surface functionalization on the antibacterial activity of silver nanoparticles. J. Funct. Biomater. 2023, 14, 244. [Google Scholar] [CrossRef]
- Ali, M.; Shareef, A. Antibacterial activity of silver nanoparticles derived from extracellular extract of Enterococcus aerogenes against dental disease bacteria isolated. Regen. Eng. Transl. Med. 2023, 10, 68–77. [Google Scholar] [CrossRef]
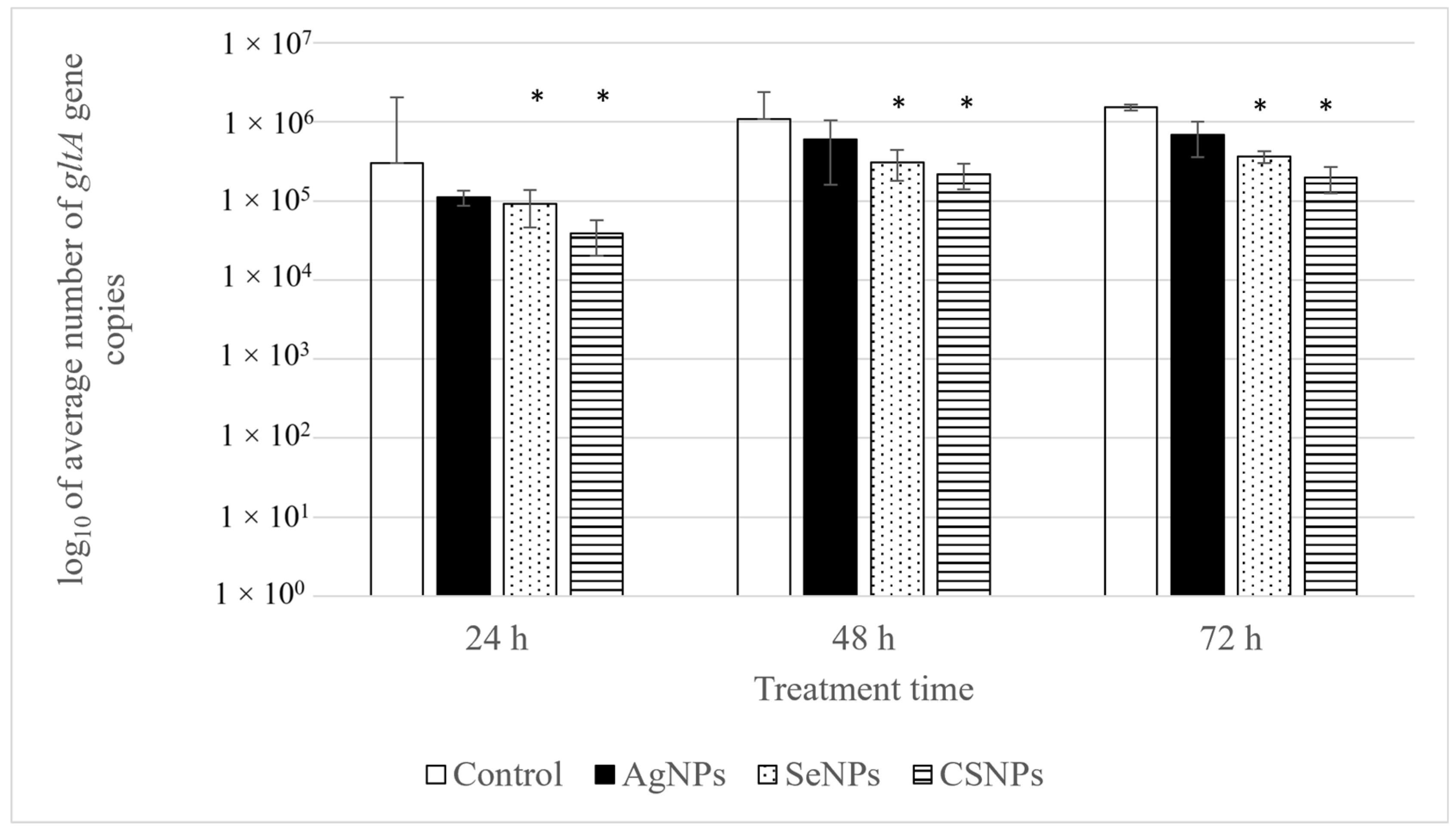
| Treatment Time (h) | Bacterial Load in Untreated Cells (Average Number of gltA Gene Copies/μL) | Bacterial Load in Treated Cells (Average Number of gltA Gene Copies/μL) | R (%) |
|---|---|---|---|
| CSNPs | |||
| 24 | 3.00 × 105 | 3.86 × 104 | 87.14 |
| 48 | 1.08 × 106 | 2.20 × 105 | 79.67 |
| 72 | 1.53 × 106 | 1.98 × 105 | 87.09 |
| SeNPs | |||
| 24 | 3.00 × 105 | 9.13 × 104 | 69.61 |
| 48 | 1.08 × 106 | 3.10 × 105 | 71.27 |
| 72 | 1.53 × 106 | 3.65 × 105 | 76.20 |
| AgNPs | |||
| 24 | 3.00 × 105 | 1.11 × 105 | 63.11 |
| 48 | 1.08 × 106 | 6.00 × 105 | 44.50 |
| 72 | 1.53 × 106 | 6.86 × 105 | 55.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peresh, Y.-Y.; Špitalský, Z.; Shaalan, M.; Špitalská, E. Anti-Rickettsial Activity of Chitosan, Selenium, and Silver Nanoparticles: Efficacy in Vero Cells. Pathogens 2025, 14, 885. https://doi.org/10.3390/pathogens14090885
Peresh Y-Y, Špitalský Z, Shaalan M, Špitalská E. Anti-Rickettsial Activity of Chitosan, Selenium, and Silver Nanoparticles: Efficacy in Vero Cells. Pathogens. 2025; 14(9):885. https://doi.org/10.3390/pathogens14090885
Chicago/Turabian StylePeresh, Yevheniy-Yuliy, Zdenko Špitalský, Mohamed Shaalan, and Eva Špitalská. 2025. "Anti-Rickettsial Activity of Chitosan, Selenium, and Silver Nanoparticles: Efficacy in Vero Cells" Pathogens 14, no. 9: 885. https://doi.org/10.3390/pathogens14090885
APA StylePeresh, Y.-Y., Špitalský, Z., Shaalan, M., & Špitalská, E. (2025). Anti-Rickettsial Activity of Chitosan, Selenium, and Silver Nanoparticles: Efficacy in Vero Cells. Pathogens, 14(9), 885. https://doi.org/10.3390/pathogens14090885






