Profile of Cytokines TNFα, IL-1β, IL-6, IL-4, and IL-10 in Relation to Disease Progression in a Patient with Advanced Liver Alveolar Echinococcosis and Non-Optimal Antiparasitic Treatment: Four-Year Follow-Up
Abstract
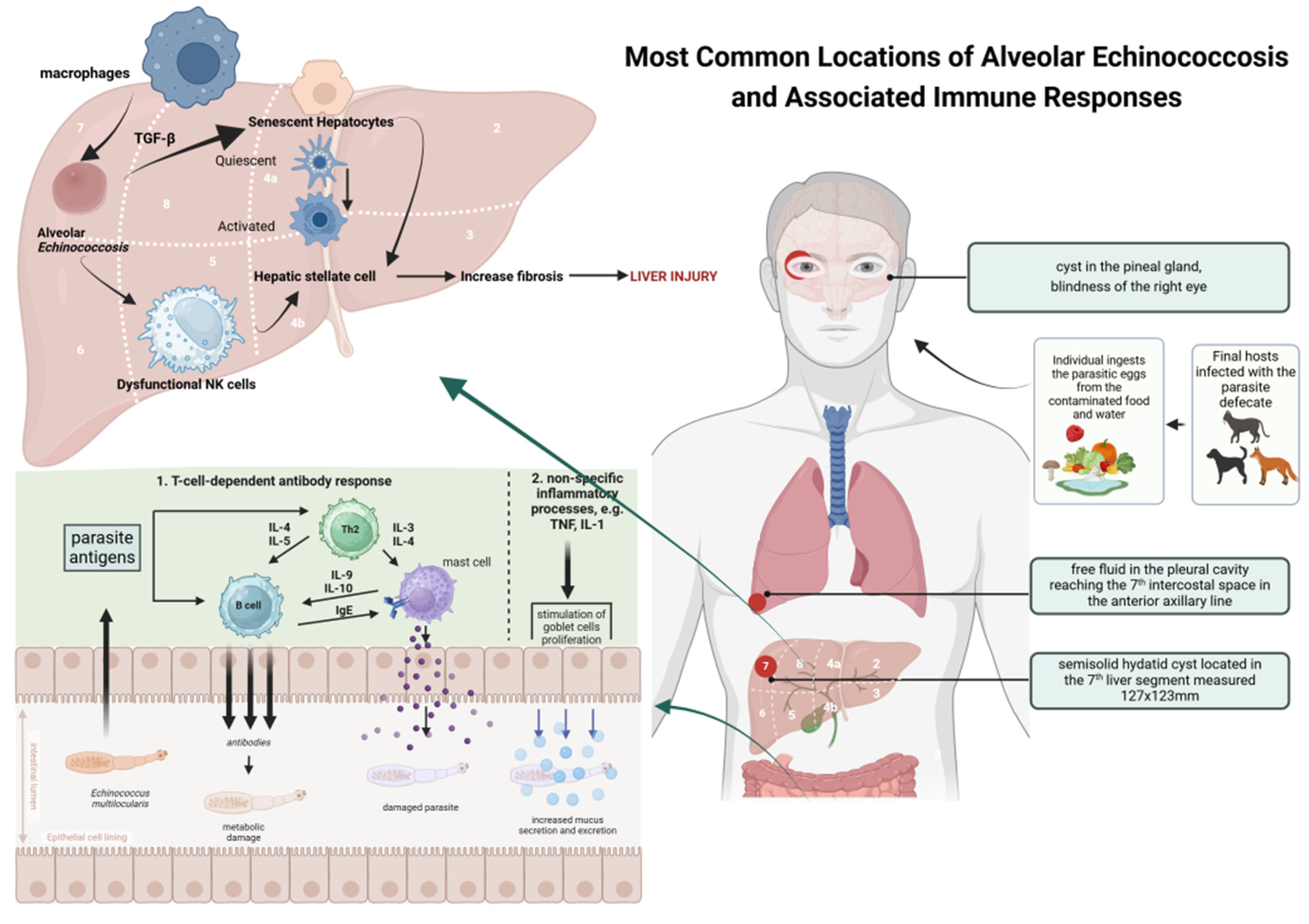
1. Introduction
2. Presentation of the Case
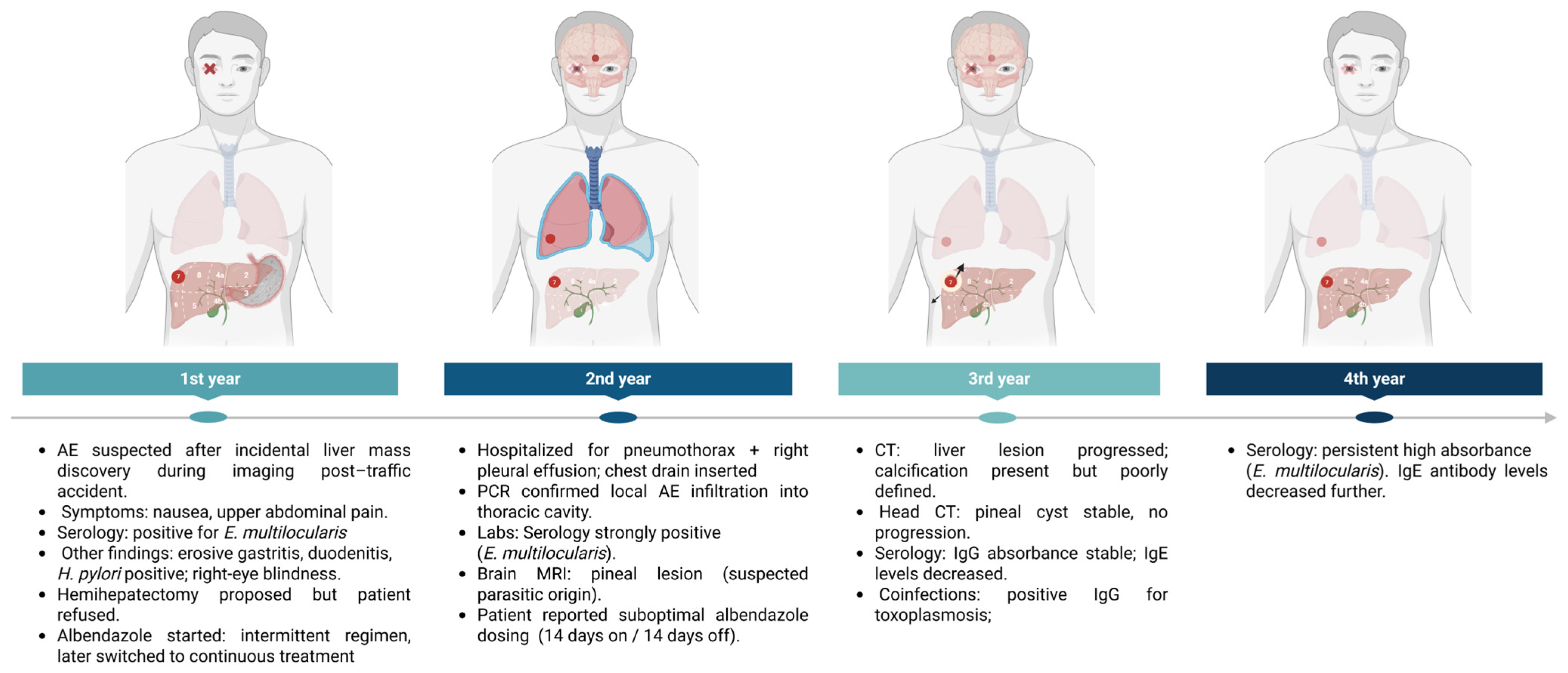
2.1. First Year of Diagnosis and Treatment of a Patient with Liver Alveolar Echinococcosis
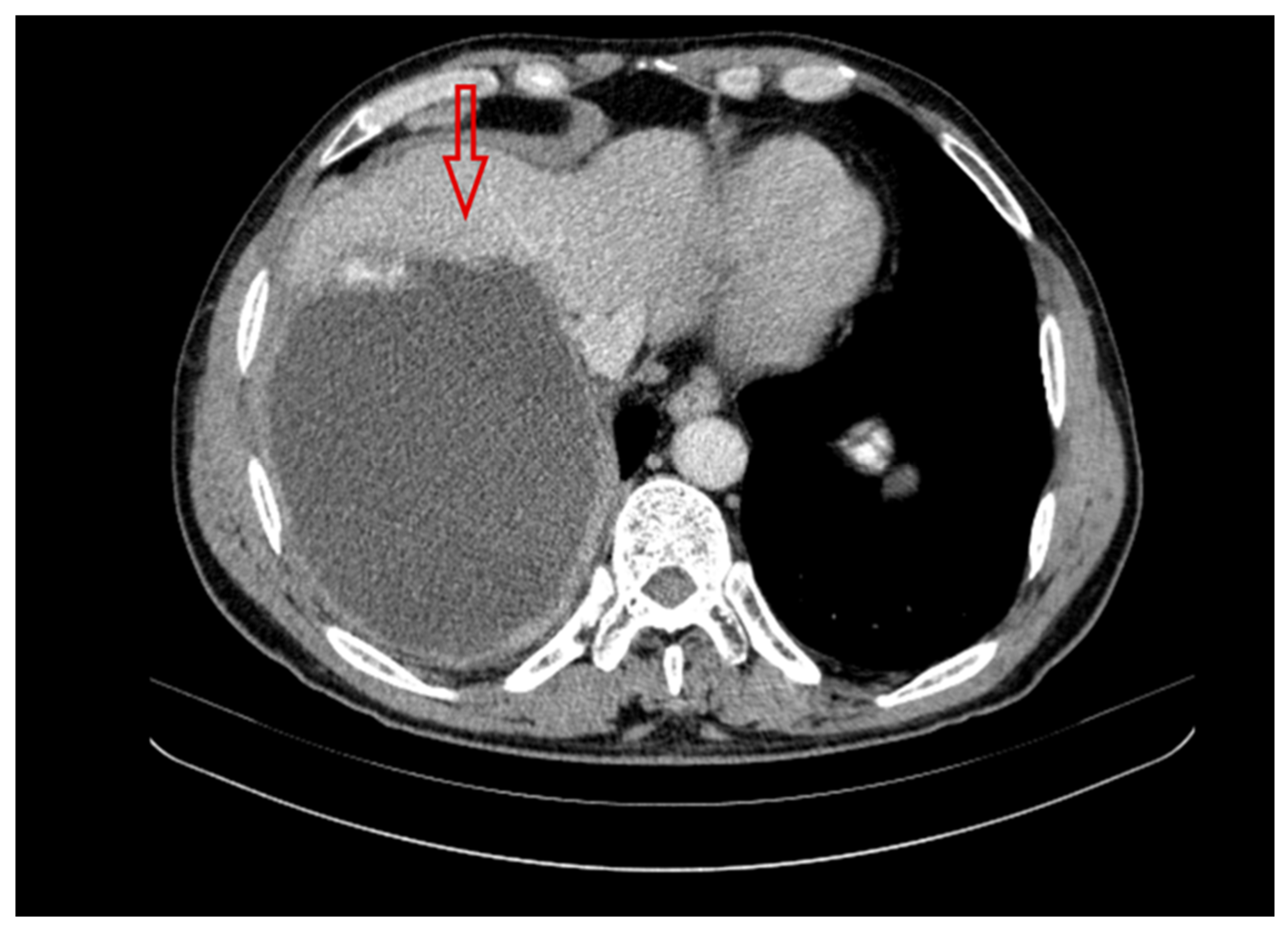
2.2. Second Year of Treatment and Observation
2.3. Third Year of Treatment and Observation
2.4. Fourth Year of Treatment and Observation
2.5. Patient’s Current Status (2025)
3. Materials and Methods
4. Discussion
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, H.; Vuitton, L.; Tuxun, T.; Li, J.; Vuitton, D.A.; Zhang, W.; McManus, D.P. Echinococcosis: Advances in the 21st Century. Clin. Microbiol. Rev. 2019, 32, e00075-18. [Google Scholar] [CrossRef]
- Kakamad, F.H.; Anwar, K.A.; Ahmed, H.K.; Habibullah, I.J.; Kaka Ali, H.H.; Nasralla, H.A.; Abdullah, H.O.; Tahir, S.H.; Kareem, H.O.; Hasan, A.H.; et al. Risk factors associated with human echinococcosis: A systematic review and meta-analysis. Front. Vet. Sci. 2024, 11, 1480579. [Google Scholar] [CrossRef]
- Jensenius, M.; Mørch, K.; Yaqub, S.; Halvorsen, D.S.; Reims, H.M.; Björk, I.G.; Røsok, B.I.; Oltmanns, G.; Helbak, K.; Øines, Ø.; et al. Alveolar echinococcosis. Tidsskr. Den Nor. Laegeforen. 2024, 144. [Google Scholar] [CrossRef]
- Deplazes, P.; Rinaldi, L.; Alvarez Rojas, C.A.; Torgerson, P.R.; Harandi, M.F.; Romig, T.; Antolova, D.; Schurer, J.J.; Lahmar, S.; Cringoli, G.; et al. Global Distribution of Alveolar and Cystic Echinococcosis. Adv. Parasitol. 2017, 95, 315–493. [Google Scholar] [CrossRef] [PubMed]
- Lass, A.; Szostakowska, B.; Kontogeorgos, I.; Korzeniewski, K.; Karamon, J.; Sulima, M.; Karanis, P. First detection of Echinococcus multilocularis in environmental water sources in endemic areas using capsule filtration and molecular detection methods. Water Res. 2019, 160, 466–474. [Google Scholar] [CrossRef]
- Lass, A.; Szostakowska, B.; Myjak, P.; Korzeniewski, K. The first detection of Echinococcus multilocular is DNA in environment al fruit, vegetable, and mushroom samples using nested PCR. Parasitol. Res. 2015, 114, 4023–4029. [Google Scholar] [CrossRef] [PubMed]
- Szostakowska, B.; Lass, A.; Kostyra, K.; Pietkiewicz, H.; Myjak, P. First finding of Echinococcus multilocularis DNA in soil: Preliminary survey in Varmia-Masuria Province, northeast Poland. Vet. Parasitol. 2014, 203, 73–79. [Google Scholar] [CrossRef]
- Stefaniak, M.; Derda, M.; Zmora, P.; Nowak, S.P. Risk Factors and the Character of Clinical Course of the Echinococcus multilocularis Infection in Patients in Poland. Pathogens 2023, 12, 199. [Google Scholar] [CrossRef]
- Available online: http://whqlibdoc.who.int/hq/2012/WHO_HTM_NTD_2012.1_eng.pdf (accessed on 17 September 2025).
- Casulli, A.; Barth, T.F.E.; Tamarozzi, F. Echinococcus multilocularis. Trends Parasitol. 2019, 35, 738–739. [Google Scholar] [CrossRef]
- Nahorski, W.L.; Knap, J.P.; Pawłowski, Z.S.; Krawczyk, M.; Polański, J.; Stefaniak, J.; Patkowski, W.; Szostakowska, B.; Pietkiewicz, H.; Grzeszczuk, A.; et al. Human alveolar echinococcosis in Poland: 1990–2011. PLoS Negl. Trop Dis. 2013, 7, e1986. [Google Scholar] [CrossRef]
- Sulima, M.; Nahorski, W.; Gorycki, T.; Wołyniec, W.; Wąż, P.; Felczak-Korzybska, I.; Szostakowska, B.; Sikorska, K. Ultrasound images in hepatic alveolar echinococcosis and clinical stage of the disease. Adv. Med. Sci. 2019, 64, 324–330. [Google Scholar] [CrossRef]
- Batçık, O.E.; Öğrenci, A.; Koban, O.; Ekşi, M.S.; Bilge, T. Cerebral Alveolar Echinococcosis Concomitant with Liver and Lung Lesions in a Young Adult Patient: Case Report and Literature Review. Turk. Parazitol. Derg. 2016, 40, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Reuss, A.M.; Wulf, M.A.; Oertel, M.F.; Bozinov, O.; Henzi, A.; Kaelin, M.B.; Reinehr, M.; Grimm, F.; Rushing, E.J. An immunocompetent farmer with isolated cerebral alveolar echinococcosis: Illustrative case. J. Neurosurg. Case Lessons 2021, 1, CASE2187. [Google Scholar] [CrossRef]
- Bakhsh, A.; Siddiqui, K.M.A.; Taraif, S. Primary hydatid cyst of pineal region of brain: A case report from Saudi Arabia. Asian J. Neurosurg. 2017, 12, 314–317. [Google Scholar] [CrossRef]
- Guo, C.; Zhu, R.; Qiu, J.; Zhu, L.; Yang, L. Subretinal echinococcosis: A case report. BMC Ophthalmol. 2017, 17, 185. [Google Scholar] [CrossRef]
- Gottstein, B.; Saucy, F.; Deplazes, P.; Reichen, J.; Demierre, G.; Busato, A.; Zuercher, C.; Pugin, P. Is high prevalence of Echinococcus multilocularis in wild and domestic animals associated with disease incidence in humans? Emerg. Infect. Dis. 2001, 7, 408–412. [Google Scholar] [CrossRef]
- Grüner, B.; Peters, L.; Hillenbrand, A.; Voßberg, P.; Schweiker, J.; Rollmann, E.G.; Rodriguez, L.H.; Blumhardt, J.; Burkert, S.; Kern, P.; et al. Echinococcus multilocularis specific antibody, systemic cytokine, and chemokine levels, as well as antigen-specific cellular responses in patients with progressive, stable, and cured alveolar echinococcosis: A 10-year follow-up. PLoS Negl. Trop. Dis. 2022, 16, e0010099. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, X.; Liu, J.; Liu, Y.; Zhao, C.; Cai, H.; Lei, W.; Ma, J.; Fan, H.; Zhou, J.; et al. The correlations between Th1 and Th2 cytokines in human alveolar echinococcosis. BMC Infect. Dis. 2020, 20, 414. [Google Scholar] [CrossRef]
- Al-Qahtani, A.A.; Alhamlan, F.S.; Al-Qahtani, A.A. Pro-Inflammatory and Anti-Inflammatory Interleukins in Infectious Diseases: A Comprehensive Review. Trop. Med. Infect. Dis. 2024, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Naik, M.I.; Tenguria, R.K.; Haq, E. Detection of serum cytokines before and after pharmacological and surgical treatment in patients with cystic echinococcosis. J. Helminthol. 2016, 90, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Sadr, S.; Charbgoo, A.; Borji, H.; Hajjafari, A. Interactions between innate immunity system and Echinococcus granulosus: Permission for vaccine development. Ser. Med. Sci. 2022, 3, 1–18. [Google Scholar]
- Sulima, M.; Wołyniec, W.; Oładakowska-Jedynak, U.; Patkowski, W.; Wasielak, N.; Witczak-Malinowska, K.; Borys, S.; Nahorski, W.; Wroczyńska, A.; Szostakowska, B.; et al. Liver Transplantation for Incurable Alveolar Echinococcosis: An Analysis of Patients Hospitalized in Department of Tropical and Parasitic Diseases in Gdynia. Transplant. Proc. 2016, 48, 1708–1712. [Google Scholar] [CrossRef]
- Autier, B.; Gottstein, B.; Millon, L.; Ramharter, M.; Gruener, B.; Bresson-Hadni, S.; Dion, S.; Robert-Gangneux, F. Alveolar echinococcosis in immunocompromised hosts. Clin. Microbiol. Infect. 2023, 29, 593–599. [Google Scholar] [CrossRef]
- Wang, J.; Marreros, N.; Rufener, R.; Hemphill, A.; Gottstein, B.; Lundström-Stadelmann, B. Short communication: Efficacy of albendazole in Echinococcus multilocularis-infected mice depends on the functional immunity of the host. Exp. Parasitol. 2020, 219, 108013. [Google Scholar] [CrossRef]
- Wang, J.; Gottstein, B. Immunoregulation in larval Echinococcus multilocularis infection. Parasite Immunol. 2016, 38, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Ludikhuize, J.; Dongelmans, D.A.; Smorenburg, S.M.; Gans-Langelaar, M.; de Jonge, E.; de Rooij, S.E. How nurses and physicians judge their own quality of care for deteriorating patients on medical wards: Self-assessment of quality of care is suboptimal. Crit. Care Med. 2012, 40, 2982–2986. [Google Scholar] [CrossRef] [PubMed]
- Su, X.E.; Wu, S.H.; He, H.F.; Lin, C.L.; Lin, S.; Weng, P.Q. The effect of multimodal care based on Peplau’s interpersonal relationship theory on postoperative recovery in lung cancer surgery: A retrospective analysis. BMC Pulm. Med. 2024, 24, 59. [Google Scholar] [CrossRef]
- Kern, P.; Wen, H.; Sato, N.; Vuitton, D.A.; Gruener, B.; Shao, Y.; Delabrousse, E.; Kratzer, W.; Bresson-Hadni, S. WHO classification of alveolar echinococcosis: Principles and application. Parasitol. Int. 2006, 55, S283–S287. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Informal Working Group on Echinococcosis (WHO-IWGE). Available online: https://iris.who.int/bitstream/handle/10665/381674/9789240110472-eng.pdf (accessed on 17 September 2025).
- Myjak, P.; Nahorski, W.; Pietkiewicz, H.; von Nickisch-Rosenegk, M.; Stolarczyk, J.; Kacprzak, E.; Felczak-Korzybska, I.; Szostakowska, B.; Lucius, R. Molecular confirmation of human alveolar echinococcosis in Poland. Clin. Infect. Dis. 2003, 37, e121–e125. [Google Scholar] [CrossRef]
- Muftuoglu, G.; Cicik, E.; Ozdamar, A.; Yetik, H.; Ozkan, S. Vitreoretinal surgery for a subretinal hydatid cyst. Am. J. Ophthalmol. 2001, 132, 435–437. [Google Scholar] [CrossRef]
- Narang, S.; Kochhar, S.; Punia, R.S.; Kumar, R.; Sambhav, K.; Sood, S. Submacular hydatid cyst: A case report. Retin. Cases Brief Rep. 2010, 4, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Kahveci, R.; Sanli, A.M.; Gurer, B.; Sekerci, Z. Orbital hydatid cyst. J. Neurosurg. Pediatr. 2012, 9, 42–44. [Google Scholar] [CrossRef]
- Peters, L.; Jiang, W.; Eberhardt, N.; Hagemann, J.B.; Grüner, B.; Tappe, D. 18FDG-PET/CT-Scans and Biomarker Levels Predicting Clinical Outcome in Patients with Alveolar Echinococcosis-A Single-Center Cohort Study with 179 Patients. Pathogens 2023, 12, 1041. [Google Scholar] [CrossRef]
- Caprio, V.; Badimon, L.; Di Napoli, M.; Fang, W.H.; Ferris, G.R.; Guo, B.; Iemma, R.S.; Liu, D.; Zeinolabediny, Y.; Slevin, M. pCRP-mCRP Dissociation Mechanisms as Potential Targets for the Development of Small-Molecule Anti-Inflammatory Chemotherapeutics. Front. Immunol. 2018, 9, 1089. [Google Scholar] [CrossRef] [PubMed]
- Weingartner, M.; Stücheli, S.; Jebbawi, F.; Gottstein, B.; Beldi, G.; Lundström-Stadelmann, B.; Wang, J. Albendazole reduces hepatic inflammation and endoplasmic reticulum-stress in a mouse model of chronic Echinococcus multilocularis infection. PLoS Negl. Trop. Dis. 2022, 16, e0009192. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Patient Hospitalization at Four Time Points | |||
|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | |
| Em spp. IgG | 2.226 | 2.663 | 2.362 | 2.252 |
| Em2+ | 1.939 | 2.573 | 2.414 | 2.288 |
| Echinococcus WB IgG | Positive profile P3 | |||
| IgE IU/mL | 6672 | - | 2123 | 1533 |
| Parameters | Patient of the Treatment and Observation at Four Time Points | Reference Value | |||
|---|---|---|---|---|---|
| Stage of disease [PNM scale] # | PN0M | PN1M0 | PN1M0 | PN1M0 | - |
| Pharmacotheray | Albendazol | Albendazol | Albendazol | Albendazol | - |
| Surgical treatment | - | Drainage of right pleural cavity | - | - | - |
| Radical surgical treatment | - | - | - | - | - |
| Transplantation | - | - | - | - | - |
| BMI | 26.5 | 25.5 | 27.7 | 27.7 | 18.5–24.9 |
| RR mmHg | 125/80 | 120/70 | 125/75 | 100/60 | <120/80 |
| Hb g/dL | 13 | 11.3 | 15.1 | 14.3 | 13.5–17.5 |
| Hematocrit% | 40.3 | 34.4 | 43.8 | 42.5 | 41–53 |
| PLT K/μL | 214 | 342 | 197 | 241 | 150–400 |
| WBC K/μL | 4.66 | 8.45 | 4.96 | 5.26 | 4.0–10.0 |
| Neutrophils% | 56 | 73 | 61 | 58.6 | 40–70 |
| Lymphocytes% | 28 | 15 | 26 | 30 | 20–45 |
| Monocytes% | 13 | 11 | 11 | 9.9 | 2–10 |
| Eosinophils% | 3 | 1 | 2 | 1.3 | 1–6 |
| CRP mg/L ESR mm/1h | 13.6 28 | 64.33 80 | 9.54 20 | 12.5 20 | <5 <15 |
| ALAT U/L | 13 | 13 | 12 | 12 | <40 |
| ASPAT U/L | 18 | 14 | 19 | 17 | <40 |
| GGTP U/L | 84 | - | 23 | 27 | <60 |
| ALP U/L | 155 | 83 | 74 | 83 | 40–129 |
| Parameters | Serum Cytokine Levels | |||
|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | |
| TNFα pg/mL | 2.31 | 1.88 | 2.05 | 1.63 |
| IL1β pg/mL | 5.7 | 3.26 | 2.1 | 1.44 |
| IL6 pg/mL | 8.14 | 7.36 | 4.1 | 2.24 |
| IL4 pg/mL | 1.36 | 2.45 | 3.73 | 4.56 |
| IL10 pg/mL | 0.00 | 1.597 | 1.504 | 1.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zorena, K.; Sulima, M.; Szostakowska, B.; Siewert, B.; Sikorska, K. Profile of Cytokines TNFα, IL-1β, IL-6, IL-4, and IL-10 in Relation to Disease Progression in a Patient with Advanced Liver Alveolar Echinococcosis and Non-Optimal Antiparasitic Treatment: Four-Year Follow-Up. Pathogens 2025, 14, 957. https://doi.org/10.3390/pathogens14100957
Zorena K, Sulima M, Szostakowska B, Siewert B, Sikorska K. Profile of Cytokines TNFα, IL-1β, IL-6, IL-4, and IL-10 in Relation to Disease Progression in a Patient with Advanced Liver Alveolar Echinococcosis and Non-Optimal Antiparasitic Treatment: Four-Year Follow-Up. Pathogens. 2025; 14(10):957. https://doi.org/10.3390/pathogens14100957
Chicago/Turabian StyleZorena, Katarzyna, Małgorzata Sulima, Beata Szostakowska, Barbara Siewert, and Katarzyna Sikorska. 2025. "Profile of Cytokines TNFα, IL-1β, IL-6, IL-4, and IL-10 in Relation to Disease Progression in a Patient with Advanced Liver Alveolar Echinococcosis and Non-Optimal Antiparasitic Treatment: Four-Year Follow-Up" Pathogens 14, no. 10: 957. https://doi.org/10.3390/pathogens14100957
APA StyleZorena, K., Sulima, M., Szostakowska, B., Siewert, B., & Sikorska, K. (2025). Profile of Cytokines TNFα, IL-1β, IL-6, IL-4, and IL-10 in Relation to Disease Progression in a Patient with Advanced Liver Alveolar Echinococcosis and Non-Optimal Antiparasitic Treatment: Four-Year Follow-Up. Pathogens, 14(10), 957. https://doi.org/10.3390/pathogens14100957







