Resistance of Acinetobacter baumannii Complex Clinical Isolates to Sulbactam–Durlobactam: A Systematic Review of Data from In Vitro Studies
Abstract
1. Introduction
2. Methods
2.1. Sources and Eligibility Criteria
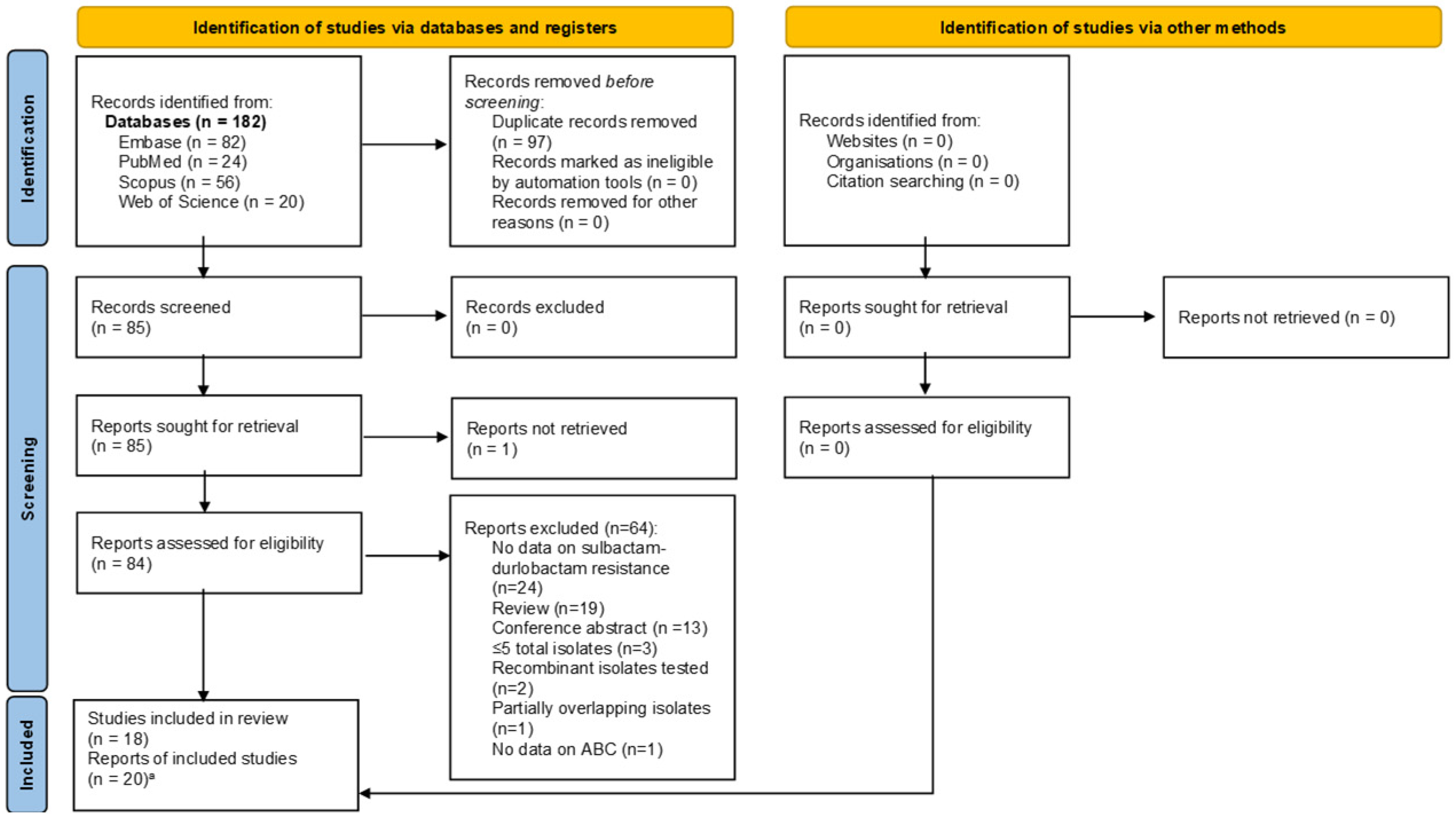
2.2. Search Strategy and Screening of Studies
2.3. Breakpoints of Susceptibility Testing
2.4. Data Extraction
2.5. Data Tabulation
3. Results
Selection of Relevant Articles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| A. baumannii | Acinetobacter baumannii |
| A. calcoaceticus | Acinetobacter calcoaceticus |
| A. nosocomialis | Acinetobacter nosocomialis |
| A. pittii | Acinetobacter pittii |
| ADC | Acinetobacter-derived cephalosporinases |
| β-lactamases | beta-lactamases |
| BL | β-lactam |
| BLI | β-lactamase inhibitor |
| CLSI | Clinical and Laboratory Standards Institute |
| CRAB | carbapenem-resistant A. baumannii |
| DOIs | digital object identifiers |
| FDA | Food and Drug Administration |
| HABP | hospital-acquired bacterial pneumonia |
| IMP | imipenemase |
| IV | intravenous |
| MBL | metallo-β-lactamase |
| MDR | multidrug-resistant |
| MIC | minimal inhibitory concentration |
| NDM | New Delhi metallo-β-lactamase |
| OXA | oxacillinase |
| PBP1 and PBP3 | penicillin-binding proteins 1 and 3 |
| PDR | pandrug-resistant |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| TEM | Temoniera β-lactamase |
| VABP | ventilator-associated bacterial pneumonia |
| VIM | Verona integron-encoded metallo-β-lactamase |
| XDR | extensively drug-resistant |
References
- Falagas, M.E.; Bliziotis, I.A.; Siempos, I.I. Attributable Mortality of Acinetobacter Baumannii Infections in Critically Ill Patients: A Systematic Review of Matched Cohort and Case-Control Studies. Crit. Care 2006, 10, R48. [Google Scholar] [CrossRef]
- Falagas, M.E.; Rafailidis, P.I. Attributable Mortality of Acinetobacter Baumannii: No Longer a Controversial Issue. Crit. Care 2007, 11, 134. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kopterides, P. Risk Factors for the Isolation of Multi-Drug-Resistant Acinetobacter Baumannii and Pseudomonas Aeruginosa: A Systematic Review of the Literature. J. Hosp. Infect. 2006, 64, 7–15. [Google Scholar] [CrossRef]
- Galani, I.; Papoutsaki, V.; Karaiskos, I.; Moustakas, N.; Galani, L.; Maraki, S.; Mavromanolaki, V.E.; Legga, O.; Fountoulis, K.; Platsouka, E.D.; et al. In Vitro Activities of Omadacycline, Eravacycline, Cefiderocol, Apramycin, and Comparator Antibiotics against Acinetobacter Baumannii Causing Bloodstream Infections in Greece, 2020–2021: A Multicenter Study. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Wang, D.; Hu, M.; Gu, Y.; Wang, M.; Hu, D.; Zhu, M.; Wang, M. In Vitro Activity of Ceftazidime/Avibactam, Imipenem/Relebactam and Meropenem/Vaborbactam Alone or in Combination with Polymyxin B against Carbapenem Resistant Acinetobacter Baumannii. J. Antibiot. 2023, 76, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Biedenbach, D.J.; Kazmierczak, K.; Bouchillon, S.K.; Sahm, D.F.; Bradford, P.A. In Vitro Activity of Aztreonam-Avibactam against a Global Collection of Gram-Negative Pathogens from 2012 and 2013. Antimicrob. Agents Chemother. 2015, 59, 4239–4248. [Google Scholar] [CrossRef]
- Poulikakos, P.; Tansarli, G.S.; Falagas, M.E. Combination Antibiotic Treatment versus Monotherapy for Multidrug-Resistant, Extensively Drug-Resistant, and Pandrug-Resistant Acinetobacter Infections: A Systematic Review. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1675–1685. [Google Scholar] [CrossRef]
- Fahmy, G.; Abdel-Rahman, S.; Khalifa, R.; Mohamed, R. The Effect of Tigecycline-Usnic Acid Combination on Tigecycline-Non-Susceptible Acinetobacter Baumannii Clinical Isolates and the Role of Usnic Acid as an Adjuvant Efflux Pump Inhibitor. Microbes Infect. Dis. 2024, 5, 1471–1485. [Google Scholar] [CrossRef]
- Buyukyanbolu, E.; Argotsinger, J.; Beck, E.T.; Chamberland, R.R.; Clark, A.E.; Daniels, A.R.; Liesman, R.; Fisher, M.; Gialanella, P.; Hand, J.; et al. Activity of Ampicillin-Sulbactam, Sulbactam-Durlobactam, and Comparators against Acinetobacter Baumannii-Calcoaceticus Complex Strains Isolated from Respiratory and Bloodstream Sources: Results from ACNBio Study. Antimicrob. Agents Chemother. 2025, 69, e00379-25. [Google Scholar] [CrossRef]
- Falagas, M.E.; Vardakas, K.Z.; Roussos, N.S. Trimethoprim/Sulfamethoxazole for Acinetobacter Spp.: A Review of Current Microbiological and Clinical Evidence. Int. J. Antimicrob. Agents 2015, 46, 231–241. [Google Scholar] [CrossRef]
- Mack, A.R.; Hujer, A.M.; Mojica, M.F.; Taracila, M.A.; Feldgarden, M.; Haft, D.H.; Klimke, W.; Prasad, A.B.; Bonomo, R.A. β-Lactamase Diversity in Acinetobacter Baumannii. Antimicrob. Agents Chemother. 2025, 69, e00784-24. [Google Scholar] [CrossRef]
- Knight, D.; Dimitrova, D.D.; Rudin, S.D.; Bonomo, R.A.; Rather, P.N. Mutations Decreasing Intrinsic β-Lactam Resistance Are Linked to Cell Division in the Nosocomial Pathogen Acinetobacter Baumannii. Antimicrob. Agents Chemother. 2016, 60, 3751–3758. [Google Scholar] [CrossRef]
- Watkins, R.R.; Bonomo, R.A. Sulbactam-Durlobactam: A Step Forward in Treating Carbapenem-Resistant Acinetobacter Baumannii (CRAB) Infections. Clin. Infect. Dis. 2023, 76, S163–S165. [Google Scholar] [CrossRef]
- Pagano, M.; Martins, A.F.; Barth, A.L. Mobile Genetic Elements Related to Carbapenem Resistance in Acinetobacter Baumannii. Braz. J. Microbiol. 2016, 47, 785–792. [Google Scholar] [CrossRef]
- Gao, Y.; Li, H.; Chen, H.; Zhang, J.; Wang, R.; Wang, Z.; Wang, H. Origin, Phylogeny, and Transmission of the Epidemic Clone ST208 of Carbapenem-Resistant Acinetobacter Baumannii on a Global Scale. Microbiol. Spectr. 2022, 10, e0260421. [Google Scholar] [CrossRef]
- Zhang, X.; Li, F.; Awan, F.; Jiang, H.; Zeng, Z.; Lv, W. Molecular Epidemiology and Clone Transmission of Carbapenem-Resistant Acinetobacter Baumannii in ICU Rooms. Front. Cell. Infect. Microbiol. 2021, 11, 633817. [Google Scholar] [CrossRef]
- Al-Hassan, L.; Elbadawi, H.; Osman, E.; Ali, S.; Elhag, K.; Cantillon, D.; Wille, J.; Seifert, H.; Higgins, P.G. Molecular Epidemiology of Carbapenem-Resistant Acinetobacter Baumannii From Khartoum State, Sudan. Front. Microbiol. 2021, 12, 628736. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. New Drug Therapy Approvals 2023; U.S. Department of Health and Human Services, Food and Drug Administration: Silver Spring, MD, USA, 2024. Available online: https://www.fda.gov/media/175253/download (accessed on 15 October 2025).
- Papp-Wallace, K.M.; McLeod, S.M.; Miller, A.A. Durlobactam, a Broad-Spectrum Serine β-Lactamase Inhibitor, Restores Sulbactam Activity Against Acinetobacter Species. Clin. Infect. Dis. 2023, 76, S194–S201. [Google Scholar] [CrossRef] [PubMed]
- Krizova, L.; Poirel, L.; Nordmann, P.; Nemec, A. TEM-1 β-Lactamase as a Source of Resistance to Sulbactam in Clinical Strains of Acinetobacter Baumannii. J. Antimicrob. Chemother. 2013, 68, 2786–2791. [Google Scholar] [CrossRef] [PubMed]
- Principe, L.; Di Bella, S.; Conti, J.; Perilli, M.; Piccirilli, A.; Mussini, C.; Decorti, G. Acinetobacter Baumannii Resistance to Sulbactam/Durlobactam: A Systematic Review. Antibiotics 2022, 11, 1793. [Google Scholar] [CrossRef] [PubMed]
- Barnes, M.D.; Kumar, V.; Bethel, C.R.; Moussa, S.H.; O’Donnell, J.; Rutter, J.D.; Good, C.E.; Hujer, K.M.; Hujer, A.M.; Marshall, S.H.; et al. Targeting Multidrug-Resistant Acinetobacter Spp.: Sulbactam and the Diazabicyclooctenone β-Lactamase Inhibitor ETX2514 as a Novel Therapeutic Agent. mBio 2019, 10, e00159-19. [Google Scholar] [CrossRef] [PubMed]
- Dorazio, A.J. Comparative in Vitro Activity of Sulbactam with Avibactam or Durlobactam against Carbapenem-Resistant Acinetobacter Baumannii. JAC-Antimicrob. Resist. 2025, 7, dlaf098. [Google Scholar] [CrossRef]
- Durand-Réville, T.F.; Guler, S.; Comita-Prevoir, J.; Chen, B.; Bifulco, N.; Huynh, H.; Lahiri, S.; Shapiro, A.B.; McLeod, S.M.; Carter, N.M.; et al. ETX2514 Is a Broad-Spectrum β-Lactamase Inhibitor for the Treatment of Drug-Resistant Gram-Negative Bacteria Including Acinetobacter Baumannii. Nat. Microbiol. 2017, 2, 17104. [Google Scholar] [CrossRef]
- Findlay, J.; Poirel, L.; Bouvier, M.; Nordmann, P. In Vitro Activity of Sulbactam-Durlobactam against Carbapenem-Resistant Acinetobacter Baumannii and Mechanisms of Resistance. J. Glob. Antimicrob. Resist. 2022, 30, 445–450. [Google Scholar] [CrossRef]
- Iovleva, A.; McElheny, C.L.; Fowler, E.L.; Cober, E.; Herc, E.S.; Arias, C.A.; Hill, C.; Baum, K.; Fowler, V.G.; Chambers, H.F.; et al. In Vitro Activity of Sulbactam-Durlobactam against Colistin-Resistant and/or Cefiderocol-Non-Susceptible, Carbapenem-Resistant Acinetobacter Baumannii Collected in U.S. Hospitals. Antimicrob. Agents Chemother. 2024, 68, e01258-23. [Google Scholar] [CrossRef]
- Karlowsky, J.A.; Hackel, M.A.; McLeod, S.M.; Miller, A.A. In Vitro Activity of Sulbactam-Durlobactam against Global Isolates of Acinetobacter Baumannii—Calcoaceticus Complex Collected from 2016 to 2021. Antimicrob. Agents Chemother. 2022, 66, e0078122. [Google Scholar] [CrossRef]
- Kaye, K.S.; McLeod, S.M.; O’Donnell, J.P.; Altarac, D. Sulbactam–Durlobactam for Infections Caused by Acinetobacter Baumannii–Calcoaceticus Complex—Authors’ Reply. Lancet Infect. Dis. 2023, 23, e275–e276. [Google Scholar] [CrossRef]
- McLeod, S.M.; Moussa, S.H.; Hackel, M.A.; Miller, A.A. In Vitro Activity of Sulbactam-Durlobactam against Acinetobacter Baumannii- Calcoaceticus Complex Isolates Collected Globally in 2016 and 2017. Antimicrob. Agents Chemother. 2020, 64, e02534-19. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.A.; Moussa, S.H.; McLeod, S.M. Characterization of Acinetobacter Baumannii-Calcoaceticus Complex Isolates and Microbiological Outcome for Patients Treated with Sulbactam-Durlobactam in a Phase 3 Trial (ATTACK). Antimicrob. Agents Chemother. 2024, 68, e0169823. [Google Scholar] [CrossRef]
- Moussa, S.H.; Shapiro, A.B.; McLeod, S.M.; Iyer, R.; Carter, N.M.; Tsai, Y.-K.; Siu, L.K.; Miller, A.A. Molecular Drivers of Resistance to Sulbactam-Durlobactam in Contemporary Clinical Isolates of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2023, 67, e0066523. [Google Scholar] [CrossRef] [PubMed]
- Nodari, C.S.; Santos, F.F.; Kurihara, M.N.L.; Valiatti, T.B.; Cayô, R.; Gales, A.C. In Vitro Activity of Sulbactam/Durlobactam against Extensively Drug-Resistant Acinetobacter Baumannii Isolates Belonging to South American Major Clones. J. Glob. Antimicrob. Resist. 2021, 25, 363–366. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.; Tanudra, A.; Chen, A.; Newman, J.; McLeod, S.M.; Tommasi, R. In Vivo Dose Response and Efficacy of the β-Lactamase Inhibitor, Durlobactam, in Combination with Sulbactam against the Acinetobacter Baumannii-Calcoaceticus Complex. Antimicrob. Agents Chemother. 2024, 68, e0080023. [Google Scholar] [CrossRef]
- Petropoulou, D.; Siopi, M.; Vourli, S.; Pournaras, S. Activity of Sulbactam-Durlobactam and Comparators Against a National Collection of Carbapenem-Resistant Acinetobacter Baumannii Isolates From Greece. Front. Cell. Infect. Microbiol. 2022, 11, 814530. [Google Scholar] [CrossRef]
- Santerre Henriksen, A.; Jeannot, K.; Oliver, A.; Perry, J.D.; Pletz, M.W.; Stefani, S.; Morrissey, I.; Longshaw, C.; ARTEMIS Study Investigators; Willinger, B.; et al. In Vitro Activity of Cefiderocol against European Pseudomonas Aeruginosa and Acinetobacter Spp., Including Isolates Resistant to Meropenem and Recent β-Lactam/β-Lactamase Inhibitor Combinations. Microbiol. Spectr. 2024, 12, e0383623. [Google Scholar] [CrossRef]
- Segatore, B.; Piccirilli, A.; Cherubini, S.; Principe, L.; Alloggia, G.; Mezzatesta, M.L.; Salmeri, M.; Di Bella, S.; Migliavacca, R.; Piazza, A.; et al. In Vitro Activity of Sulbactam–Durlobactam against Carbapenem-Resistant Acinetobacter Baumannii Clinical Isolates: A Multicentre Report from Italy. Antibiotics 2022, 11, 1136. [Google Scholar] [CrossRef]
- Seifert, H.; Müller, C.; Stefanik, D.; Higgins, P.G.; Miller, A.; Kresken, M. In Vitro Activity of Sulbactam/Durlobactam against Global Isolates of Carbapenem-Resistant Acinetobacter Baumannii. J. Antimicrob. Chemother. 2020, 75, 2616–2621. [Google Scholar] [CrossRef]
- Terrier, C.L. Multidrug-Resistant Gram-Negative Clinical Isolates with Reduced Susceptibility/Resistance to Cefiderocol: Which Are the Best Present and Future Therapeutic Alternatives? Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 339–354. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, Y.; Jia, P.; Zhu, Y.; Zhang, J.; Zhang, G.; Deng, J.; Hackel, M.; Bradford, P.A.; Reinhart, H. In Vitro Activity of Sulbactam/Durlobactam against Clinical Isolates of Acinetobacter Baumannii Collected in China. J. Antimicrob. Chemother. 2020, 75, 1833–1839. [Google Scholar] [CrossRef]
- Zalacain, M.; Achard, P.; Llanos, A.; Morrissey, I.; Hawser, S.; Holden, K.; Toomey, E.; Davies, D.; Leiris, S.; Sable, C.; et al. Meropenem-ANT3310, a Unique β-Lactam-β-Lactamase Inhibitor Combination with Expanded Antibacterial Spectrum against Gram-Negative Pathogens Including Carbapenem-Resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2024, 68, e0112023. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Entasis Therapeutics a Phase I, Double-Blind, Randomized, Placebo-Controlled Study to Evaluate the Safety, Tolerability and Pharmacokinetics of Intravenous ETX2514 Administered in Healthy Subjects. 2017. Available online: https://clinicaltrials.gov/study/NCT02971423 (accessed on 28 June 2025).
- Entasis Therapeutics Evaluation of the Pharmacokinetics, Safety, and Tolerability of Intravenous ETX2514 and Sulbactam Administered Concurrently to Subjects with Various Degrees of Renal Impairment and Healthy Matched Control Subjects. 2018. Available online: https://www.clinicaltrials.gov/study/NCT03310463 (accessed on 28 June 2025).
- Entasis Therapeutics a Phase I Study to Determine and Compare Plasma, Epithelial Lining Fluid, and Alveolar Macrophage Concentrations of Intravenous ETX2514 and Sulbactam Administered to Healthy Adult Subjects. 2017. Available online: https://clinicaltrials.gov/study/NCT03303924 (accessed on 28 June 2025).
- Innoviva Specialty Therapeutics a Multicenter, Open-Label, Phase 1b Study to Assess the Pharmacokinetics, Safety, and Tolerability of Sulbactam-Durlobactam in Hospitalized Pediatric Patients from Birth to <18 Years Who Are Receiving Systemic Antibiotic Therapy for Suspected or Confirmed Acinetobacter Baumannii-Calcoaceticus Complex Infection. 2025. Available online: https://www.clinicaltrials.gov/study/NCT06801223 (accessed on 28 June 2025).
- Entasis Therapeutics a Double-Blind, Randomized, Placebo-Controlled Study to Evaluate the Safety and Efficacy of Intravenous Sulbactam-ETX2514 in the Treatment of Hospitalized Adults with Complicated Urinary Tract Infections, Including Acute Pyelonephritis. 2020. Available online: https://clinicaltrials.gov/study/NCT03445195 (accessed on 28 June 2025).
- Sagan, O.; Yakubsevitch, R.; Yanev, K.; Fomkin, R.; Stone, E.; Hines, D.; O’Donnell, J.; Miller, A.; Isaacs, R.; Srinivasan, S. Pharmacokinetics and Tolerability of Intravenous Sulbactam-Durlobactam with Imipenem-Cilastatin in Hospitalized Adults with Complicated Urinary Tract Infections, Including Acute Pyelonephritis. Antimicrob. Agents Chemother. 2020, 64, e01506-19. [Google Scholar] [CrossRef] [PubMed]
- Kaye, K.S.; Shorr, A.F.; Wunderink, R.G.; Du, B.; Poirier, G.E.; Rana, K.; Miller, A.; Lewis, D.; O’Donnell, J.; Chen, L.; et al. Efficacy and Safety of Sulbactam-Durlobactam versus Colistin for the Treatment of Patients with Serious Infections Caused by Acinetobacter Baumannii-Calcoaceticus Complex: A Multicentre, Randomised, Active-Controlled, Phase 3, Non-Inferiority Clinical Trial (ATTACK). Lancet Infect. Dis. 2023, 23, 1072–1084. [Google Scholar] [CrossRef]
- Innoviva Specialty Therapeutics a Single-Arm, Open-Label, Prospective, Observational Study to Assess the Safety of Sulbactam-Durlobactam, Including the Risk of Hypersensitivity Reactions (Including Anaphylaxis) in Participants with Acinetobacter Baumannii-Calcoaceticus Complex Infection. 2025. Available online: https://clinicaltrials.gov/study/NCT06746883 (accessed on 29 June 2025).
- Papp-Wallace, K.M.; Shapiro, A.B.; Becka, S.A.; Zeiser, E.T.; LiPuma, J.J.; Lane, D.J.; Panchal, R.G.; Mueller, J.P.; O’Donnell, J.P.; Miller, A.A. In Vitro Antibacterial Activity and In Vivo Efficacy of Sulbactam-Durlobactam against Pathogenic Burkholderia Species. Antimicrob. Agents Chemother. 2021, 65, e01930-20. [Google Scholar] [CrossRef]
- Tamma, P.D.; Heil, E.L.; Justo, J.A.; Mathers, A.J.; Satlin, M.J.; Bonomo, R.A. Infectious Diseases Society of America 2024 Guidance on the Treatment of Antimicrobial-Resistant Gram-Negative Infections. Clin. Infect. Dis. 2024, 79, ciae403. [Google Scholar] [CrossRef] [PubMed]
| Author a | Year | Isolates | N | β-Lactamase Genes (Number of Isolates) | MIC Value or Range (mg/L) | MIC50 (mg/L) | MIC90 (mg/L) | Resistance % (I: %) b [Breakpoint] c |
|---|---|---|---|---|---|---|---|---|
| Buyukyanbolu [10] | 2025 | A. baumannii | 523 | OXA-23 (8), OXA-66 (8), ADC-73 (7), TEM-1 (3), ADC-56 (2), OXA-72 (2), ADC-222 (2), OXA-24 (2), OXA-95 (2), NDM-1 (2), NDM-5 (2), OXA-23 [M1X] (1), OXA-66 [T10K] (1), ADC-76 (1), OXA-58 (1), OXA-68 (1) | ≤0.125–>64 | 2 | 4 | 1.2 (I: 1.9) |
| Iovleva [27] | 2024 | A. baumannii | 87 | OXA-82 (65), OXA-23 (59), OXA-66 (8), OXA-95 (6), OXA-24 (5), OXA-83 (3), OXA-51 (2), OXA-223 (2), OXA-72 (1), OXA-113 (1), OXA-166 (1) | 0.5–64 | 2 | 8 | 4.6 (I: 2) |
| Miller [31] Kaye [29] | 2024 2023 | A. baumannii complex | 175 | OXA-23 (8), OXA-66 (6), TEM-1 (5), ADC-30 (3), ADC-73 (3), ADC-115 (1), ADC-6-like (1), OXA-80 (1), OXA-71 (1) | 0.25–32 | 2 | 4 | 4.6 d |
| Senterre-Henriksen [36] | 2024 | Acinetobacter spp. | 501 | OXA (202), MBL (3), OXA + MBL (9), OXA + ESBL (4), ESBL + KPC (1), No Acquired β-lactamase (8) | NA | NA | 4 | 3 (I: 0) |
| Moussa [32] Karlowsky [28] | 2023 2022 | All A. baumannii A. calcoaceticus A. nosocomialis A. pittii | 5032 4038 55 296 638 | OXA-23 (34), NDM-1 (32), OXA-66 (32), ADC-30 (11), ADC-73 (11), OXA-24 (9), OXA-58 (6), ADC-25 (5), OXA-69 (5), ADC-169 (4), OXA-402 (4), CTX-M-15 (3), ADC-26 (3), CARB-2 (3), OXA-94 (3), TEM-1 (19), VEB-1 (2), ADC-176 (2), ADC-30-like (2), ADC-99-like (2), ADC-43-like (2), OXA-533-like (2), OXA-64 (2), OXA-71 (2), ADC-11 (1), ADC-131-like (1), ADC-152 (1), ADC-163 (1), ADC-169-like (1), ADC-176-like (1), ADC-18 (1), ADC-181 (1), ADC-214 (1), ADC-216 (1), ADC-39 (1), ADC-43 (1), ADC-5 (1), ADC-53 (1), ADC-6-like (1), ADC-7-like (1), ADC-73-like (1), ADC-76 (1), ADC-80 (1), ADC-82 (1), ADC-91 (1), ADC-97-like (1), CARB-16 (1), OXA-10 (1), OXA-121 (1), OXA-132 (1), OXA-23+ (1), OXA-259 (1), OXA-407 (1), OXA-500 (1), OXA-51 (1), OXA-65 (1), OXA-66+ (1), OXA-68 (1), OXA-70 (1), OXA-72 (1), OXA-820 (1), OXA-83 (1), OXA-91 (1), PER-1 (1), PER-7 (1), VIM-4 (1) | ≤0.03–>64 ≤0.03–>64 0.12–2 ≤0.03–8 ≤0.03–32 | 1 1 0.5 0.5 0.5 | 2 2 1 1 2 | 1.7 d 2 d 0 0.3 d 0.6 d |
| McLeod [30] | 2020 | A. baumannii complex A. baumannii A. calcoaeceticus A. nosocomialis A. pittii | 1722 1420 10 60 232 | OXA-23 (17), OXA-66 (15), NDM-1 (11), TEM-1 (8), ADC-25 (5), ADC-73 (5), ADC-30 (4), OXA-24 (4), OXA-58 (3), ADC-152 (S341T) (2), ADC-169 (2), CARB-2 (2), OXA-64 (2), OXA-69 (2) OXA-132 (2), OXA-402 (2), ADC-5 (1), ADC-5 (G239S, N341T) (1), ADC-7-like (1), ADC-26 (1), ADC-50 (1), ADC-53 (A236V) (1), ADC-80 (V119E) (1), ADC-82 (1), ADC-97-like (1), ADC-99-like (1), ADC-176 (1), CTX-M-15 (1), OXA-64 (1) OXA-65 (1), OXA-70 (1), OXA-71 (1), OXA-83 (1), OXA-91(1), OXA-94 (2), PER-7(1), subclass B3 MBL (1), VEB-1 (1) | ≤0.03–>64 ≤0.03–>64 0.12–1 0.12–4 0.12–4 | 1 1 0.5 0.5 0.5 | 2 4 1 1 2 | 2.3 d NA |
| Yang [40] | 2020 | A. baumannii | 982 | NA | ≤0.03–>64 | 1 | 2 | 2.1 d |
| Durand-Réville [25] | 2017 | A. baumannii | 84 | OXA-23 (49), OXA-66 (48), TEM-1 (41), ADC-30 (18), ADC-73 (18), OXA-40 (7), ADC-82 (6), OXA-72 (6), OXA-65 (5), OXA-113 (5), ADC-ETX1 e (4), ADC-76 (4), OXA-68 (4), ADC-11 (3), OXA-10/69 (3), OXA-58 (3),SHV-5 (3), ADC-ETX29 (2), ADC-25 e (2), ADC-79 (2), ADC-ETX15 (2), OXA-20 (2), OXA-51 (2), OXA-64 (2), OXA-65 (2),OXA-71 (2), OXA-82 (2), OXA-132 (2), PER-1 (2), GES-12 (1), IMP-4(b) (1), ADC-1 (1), ADC-26 (1), ADC-80 (1), ADC-87 e (1), ADC-ETX3 f (1), ADC-ETX5 f (1), ADC-ETX7 (1), ADC-ETX8 (1), ADC-ETX9 (1), ADC-ETX10 e (1), ADC-ETX12 e (1), ADC-ETX13 (1), ADC-ETX17 (1), ADC-ETX18 (1), ADC-ETX19 e (1), ADC-ETX20 (1), ADC-ETX21 (1), ADC-ETX22 (1), ADC-ETX26 (1), ADC-ETX27 (1), ADC-ETX33 (1), OXA-40 (1), similar to ADC-52 (1), OXA-69 (1), OXA-73 (1), OXA-94 (1), OXA-100 (1), OXA-109 (1), OXA-172 (1), OXA-398 (1), OXA-ARC2597 (1), OXA-ARC2598 (1), OXA-ARC2719 (1), OXA-ARC3488 (1), OXA-ARC3489 (1), PER-unq (1), SHV-12 (1) | 0.25–16 | 2 | 4 | 1.2 (I: 7.1) |
| Author a | Year | Isolates | N | β-Lactamase Genes (Number of Isolates) | MIC Value or Range (mg/L) | MIC50 (mg/L) | MIC90 (mg/L) | Resistance % (I: %) b [Breakpoint] c |
|---|---|---|---|---|---|---|---|---|
| Doragio [24] | 2025 | CRAB | 58 | OXA-51-like (58), OXA-23-like (49), ADC-30 (20), ADC-73 (20), TEM-1D (18), OXA-24-like (9), ADC-33 (5), ADC-56 (4), ADC-222 (4), ADC-268 (2), ADC-103 (1), ADC-229 (1), OXA-50-like (1), PAO (1) | 0.5–32 | 2 | 8 | 5.2 (I: 12) |
| Zalacain [41] | 2024 | CRAB | 340 | NA | 0.25–>32 | 2 | 4 | 3.8 (I: 3.2) |
| Petropoulou [35] | 2022 | CRAB | 190 | TEM-1 + NDM-1 + ADC-73 + OXA-23 + OXA-66 (1), TEM-1 + ADC-73 + OXA-23 + OXA-66 (1), ADC-188 + OXA-23 + OXA-66 (1) | 0.5–>64 | 4 | 8 | 1.6 (I: 10.5) |
| Segatore [37] | 2022 | CRAB | 141 | ADC-25 + OXA-20 + OXA-58 + OXA-66 (7), ADC-25 + OXA-20 + OXA-58 (4) | 0.06–>128 | 0.5 | 4 | 5 (I: 2.8) |
| Nodari [33] | 2021 | CRAB | 112 | OXA-24/40-like (48), OXA-23 (34), OXA-23 + OXA-24/40-like (17), OXA-143-like (10), OXA-23 + OXA-143-like (2), OXA-58 (1) (75 of the above isolates were also TEM-1 positive) | ≤0.25–4 | 1 | 1 | 0 (I: 0) |
| Seifert [38] | 2020 | CRAB | 246 | OXA-23-like (184), OXA-40-like (47), OXA-58-like (3), IMP-26 (1), NDM-1 (3), OXA-51 (7), OXA-237 (1) | 0.25–128 | 1 | 2 | 2.4 (I: 1.2) |
| Barnes [23] | 2019 | CRAB | 26 | NA | 0.25–4 | 2 | 2 | 0 (I: 0) |
| Author a | Year | Isolates | N | β-Lactamase Genes (Number of Isolates) | MIC Value or Range (mg/L) | MIC50 (mg/L) | MIC90 (mg/L) | Resistance % (I: %) b [Breakpoint] c |
|---|---|---|---|---|---|---|---|---|
| Le Terrier [39] | 2023 | A. baumannii | 11 | OXA-23 (9), PER-7 (5), PER-1 (3), NDM-1 (2), NDM-5 (1) | 0.25–128 | 1 | 128 | 27.3 (I: 0) |
| O’Donnell [34] | 2023 | A. baumannii | 10 | ADC-5 (1), ADC-11 (1), ADC-30 (2), ADC-33 (1), ADC-80 (1), ADC-82 (2), ADC-99 [N379S] (1), ADC-176 (1), ADC-214 [T341S] (1), OXA-23 (5), OXA-64 (1), OXA-65 (1), OXA-66 (2), OXA-66 [K42] (1), OXA-69 (1), OXA-72 (3), OXA-82 (1), OXA-83 (1), OXA-94 (1), OXA-259 (1), TEM-1 (4) | 0.5–16 | 1 | 8 | 10 (I: 20) |
| Findlay [26] | 2022 | A. baumannii | 100 | OXA-23 (73), OXA-72 (10), OXA-40 (6), OXA-58 (5), OXA-24 (1), NDM-1 (4), NDM-5 (1) | 0.06–64 | 4 | 16 | 15 (I: 4) |
| Barnes [23] | 2019 | A. baumannii | 72 | ADC (71), OXA-69-like (70), TEM (29), OXA-58-like (9), OXA-23-like (8), PER (2) In the 4 non-susceptible strains: ADC-25 + OXA-66 + TEM-1 (3), ADC-79 + OXA-66 + OXA-69 + TEM-1 (1) | 0.5–32 | 1 | 2 | 1.4 (I: 4.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falagas, M.E.; Romanos, L.T.; Ragias, D.; Filippou, C. Resistance of Acinetobacter baumannii Complex Clinical Isolates to Sulbactam–Durlobactam: A Systematic Review of Data from In Vitro Studies. Pathogens 2025, 14, 1062. https://doi.org/10.3390/pathogens14101062
Falagas ME, Romanos LT, Ragias D, Filippou C. Resistance of Acinetobacter baumannii Complex Clinical Isolates to Sulbactam–Durlobactam: A Systematic Review of Data from In Vitro Studies. Pathogens. 2025; 14(10):1062. https://doi.org/10.3390/pathogens14101062
Chicago/Turabian StyleFalagas, Matthew E., Laura T. Romanos, Dimitrios Ragias, and Charalampos Filippou. 2025. "Resistance of Acinetobacter baumannii Complex Clinical Isolates to Sulbactam–Durlobactam: A Systematic Review of Data from In Vitro Studies" Pathogens 14, no. 10: 1062. https://doi.org/10.3390/pathogens14101062
APA StyleFalagas, M. E., Romanos, L. T., Ragias, D., & Filippou, C. (2025). Resistance of Acinetobacter baumannii Complex Clinical Isolates to Sulbactam–Durlobactam: A Systematic Review of Data from In Vitro Studies. Pathogens, 14(10), 1062. https://doi.org/10.3390/pathogens14101062







