Beyond Microbiological Analysis: The Essential Role of Risk Assessment in Travel-Associated Legionnaires’ Disease Outbreak Investigations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting and Period
2.2. Case Definition and Diagnostic Criteria
2.3. Sample Collection
2.4. Microbiological and Chemical Analysis
Swab Samples
2.5. DNA Extraction and Whole Genome Sequencing
2.6. Whole Genome Sequencing Data Analysis
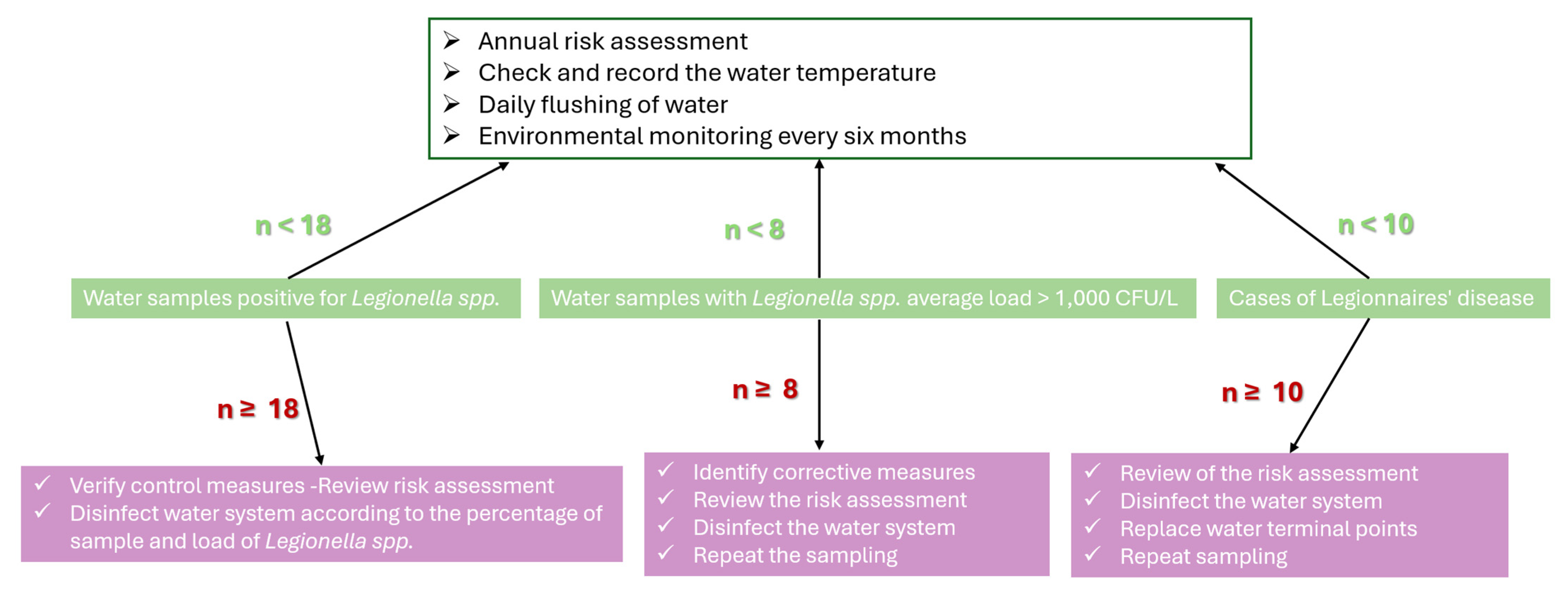
2.7. Risk Assessment (RA)
2.7.1. ECDC Facility Inspection Tool for TALD [63]
2.7.2. Greek National Public Health Authority (NPHA) Legionella Prevention and Management Checklist [27]
2.7.3. Structural and Water System Risk Scoring Tables [14]
2.8. Risk Assessment and Statistical Analysis
3. Results
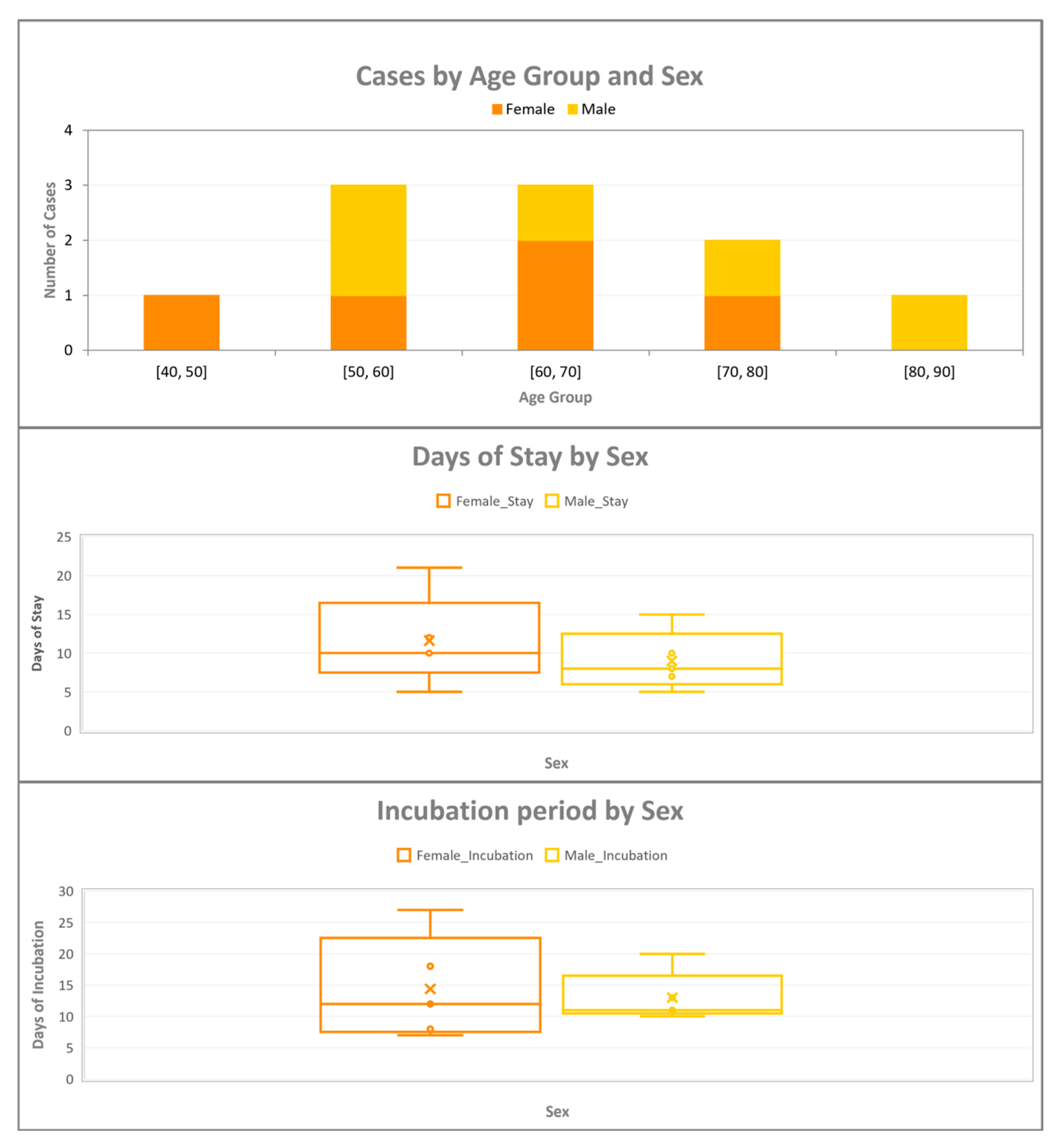
3.1. Descriptive Epidemiology of the Confirmed Cases
3.2. Microbiological Results
3.3. Association Between Total Coliforms and Presence of Legionella
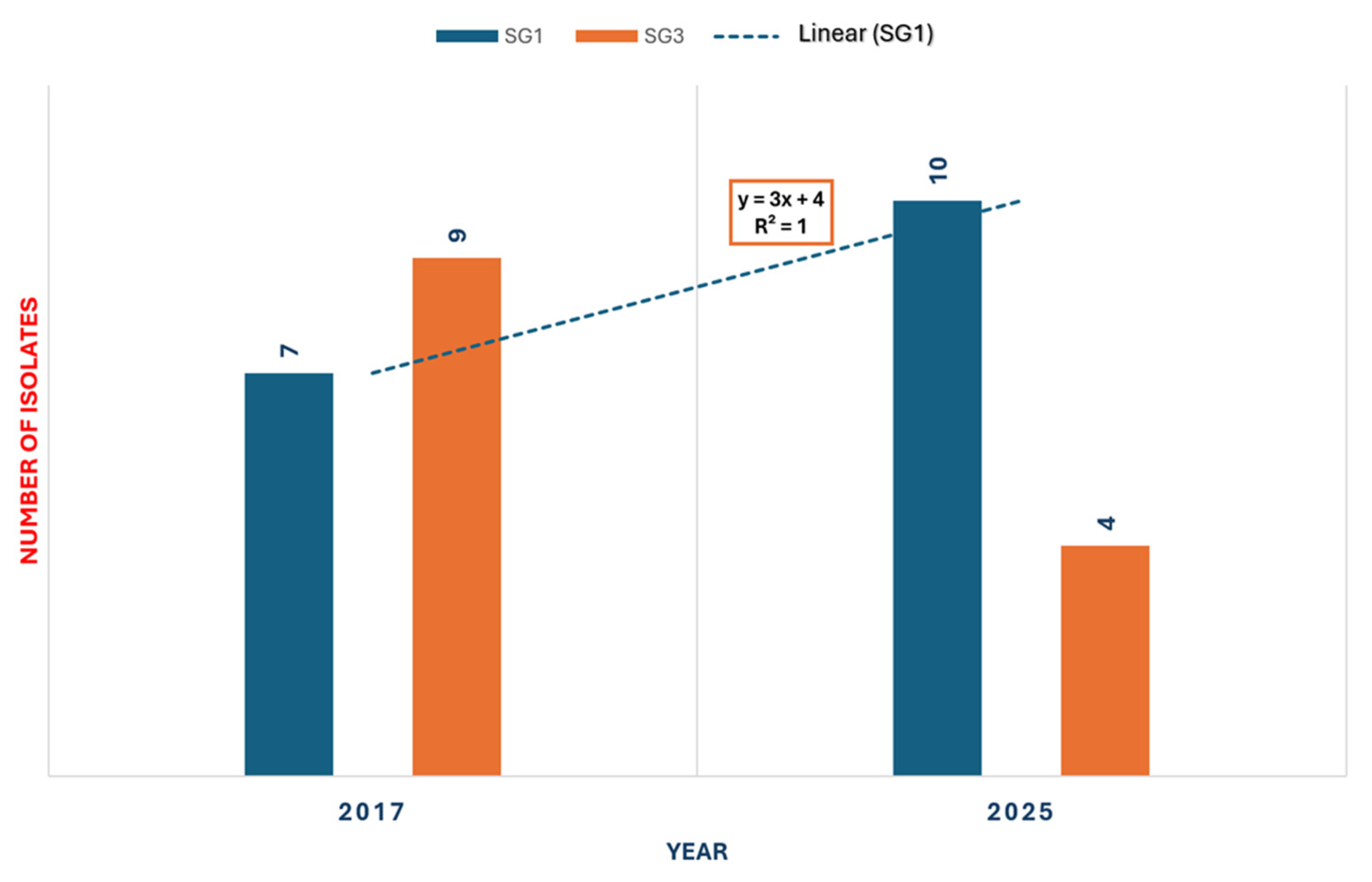
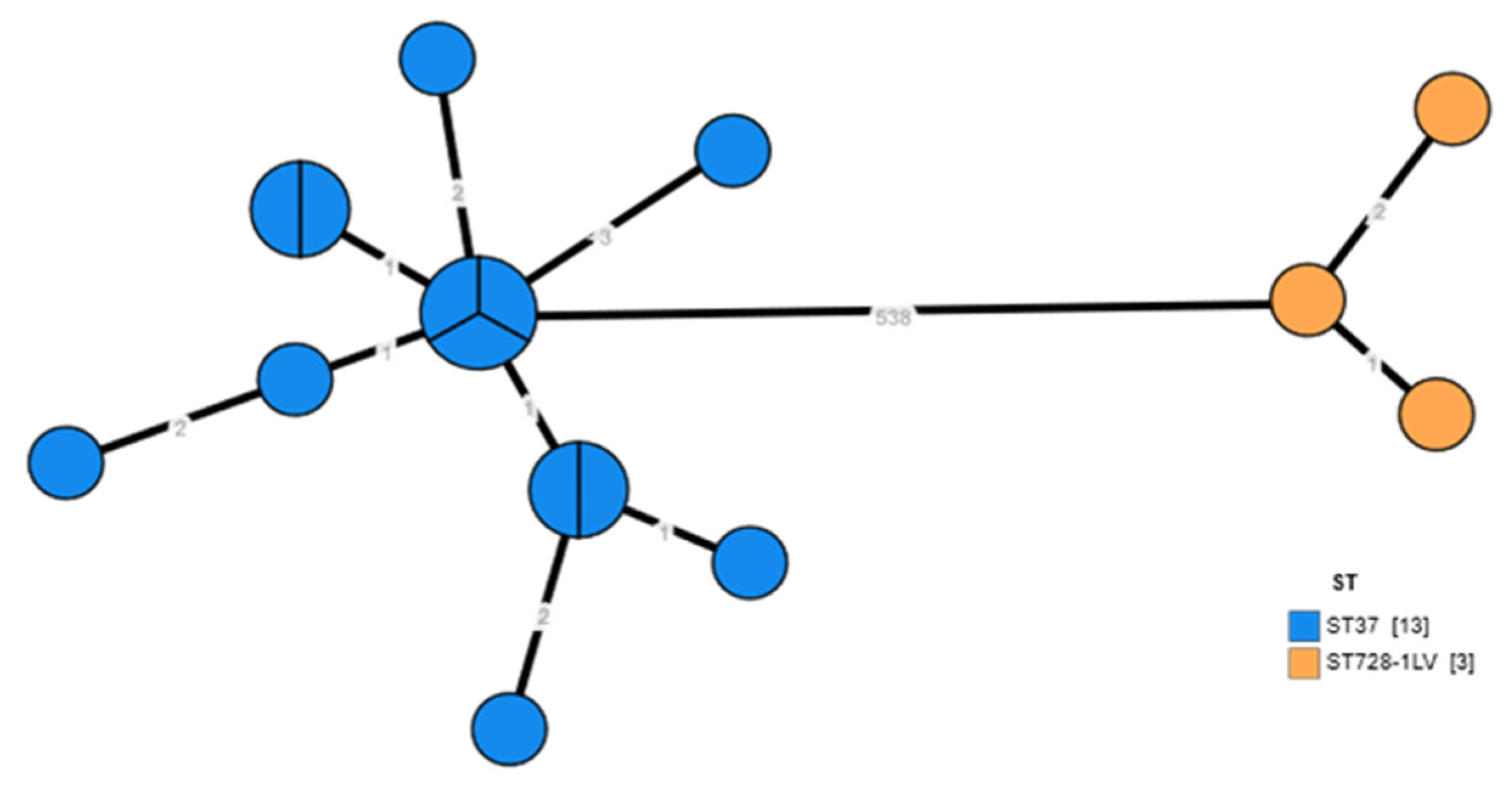
3.4. Whole Genome Sequencing of Environmental Isolates of L. pneumophila
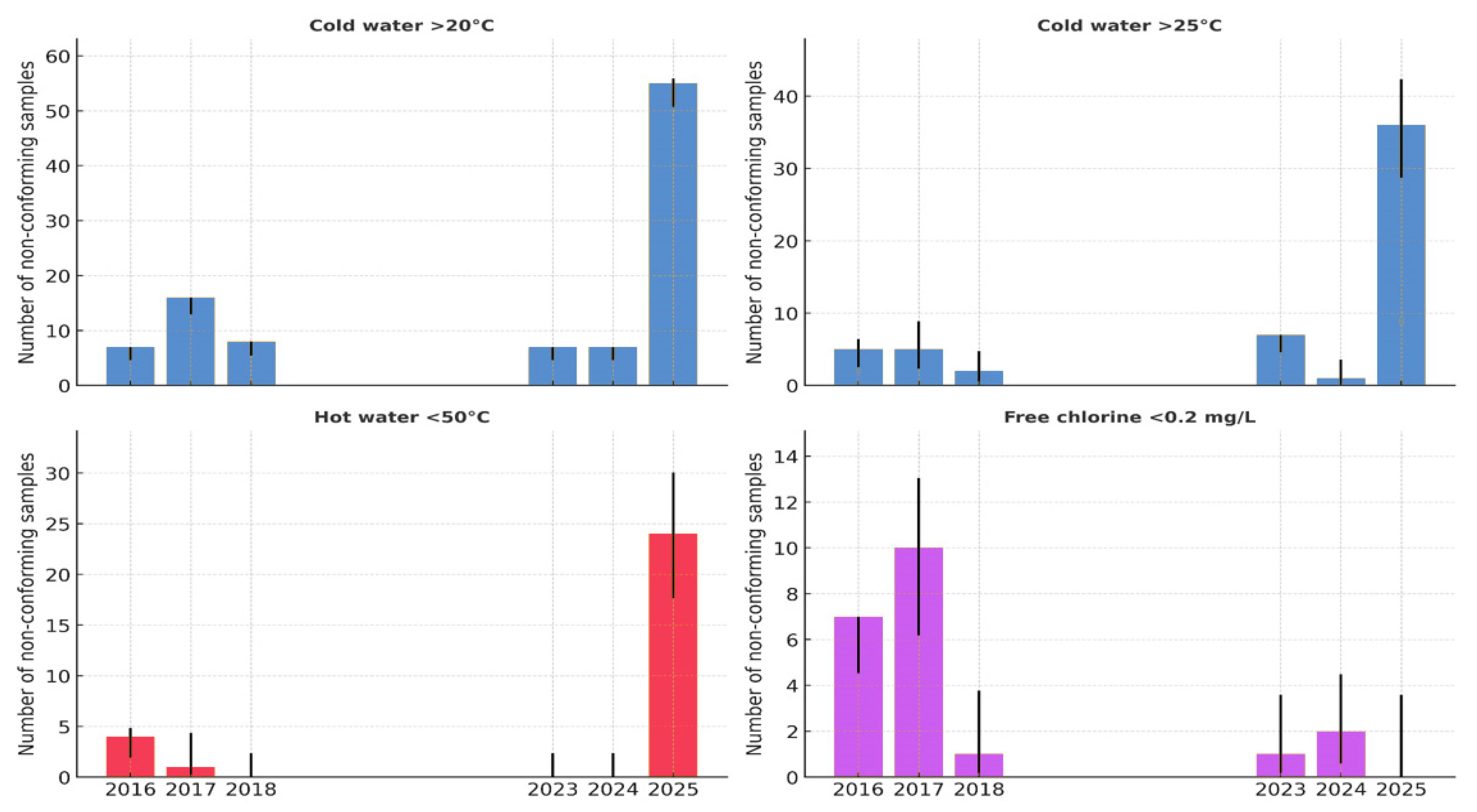
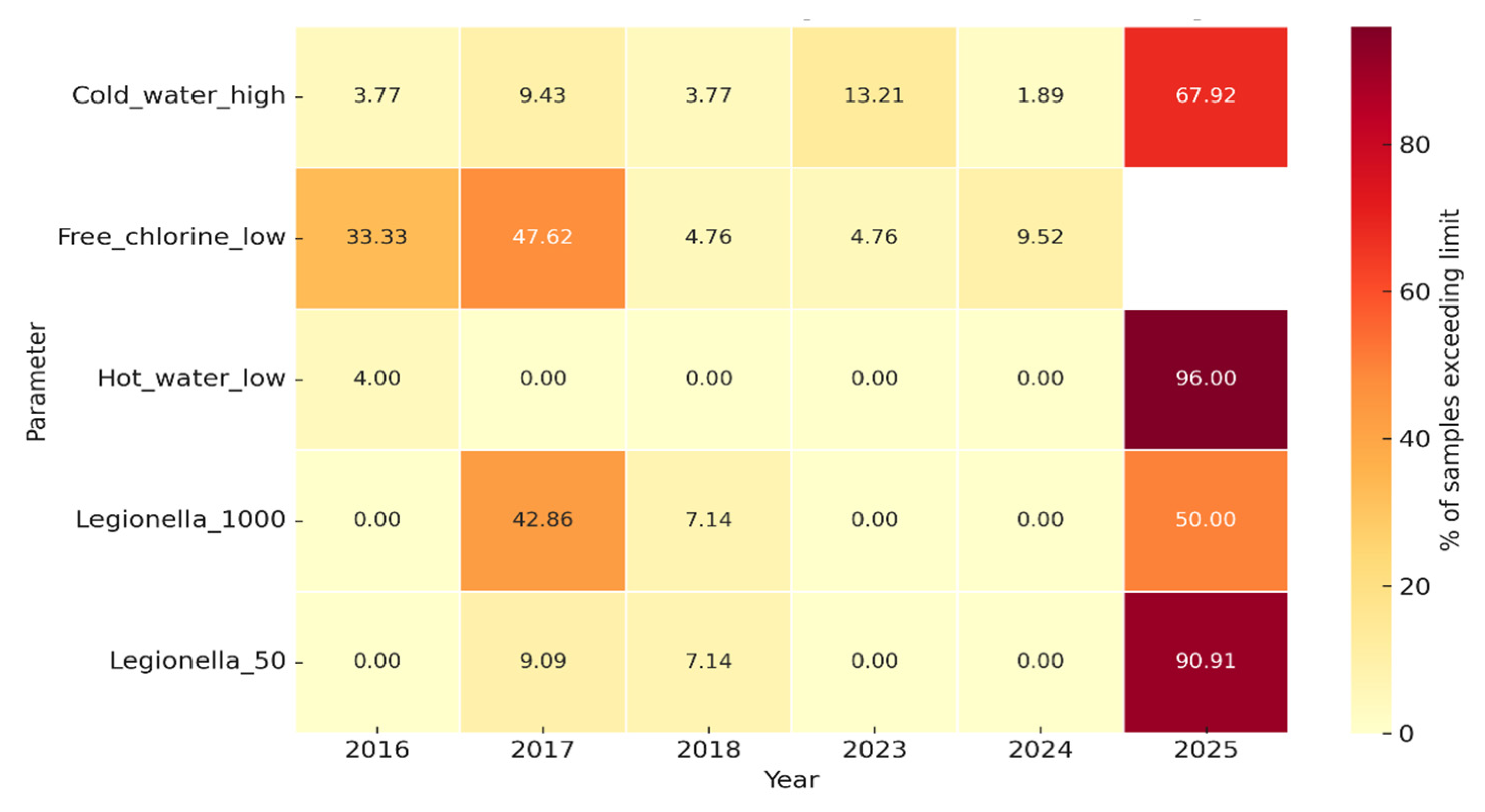
3.5. Physicochemical Parameters
3.6. Association Between Physicochemical Non-Compliance and Legionella Positivity
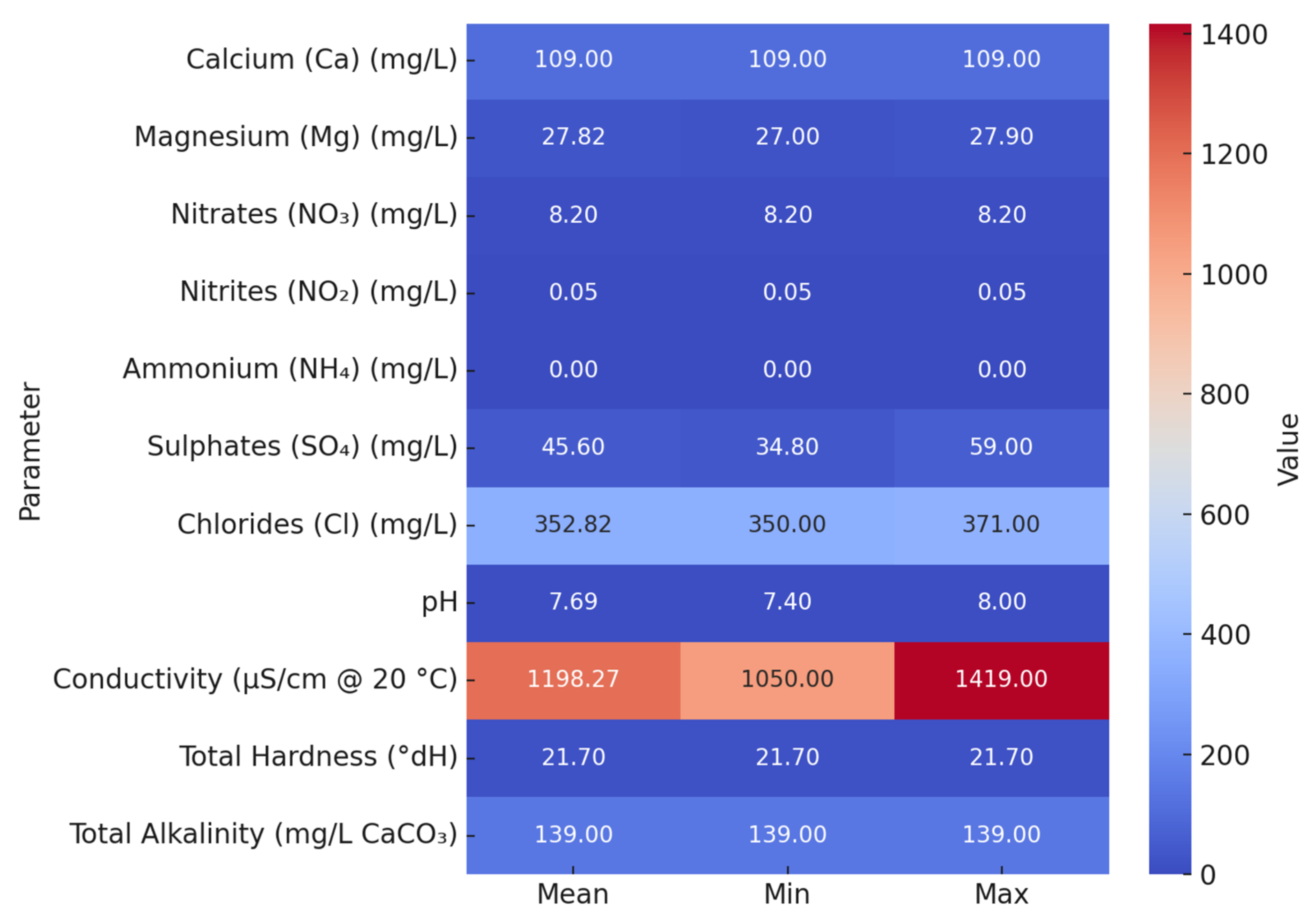
3.7. Chemical Composition of Selected Water Samples
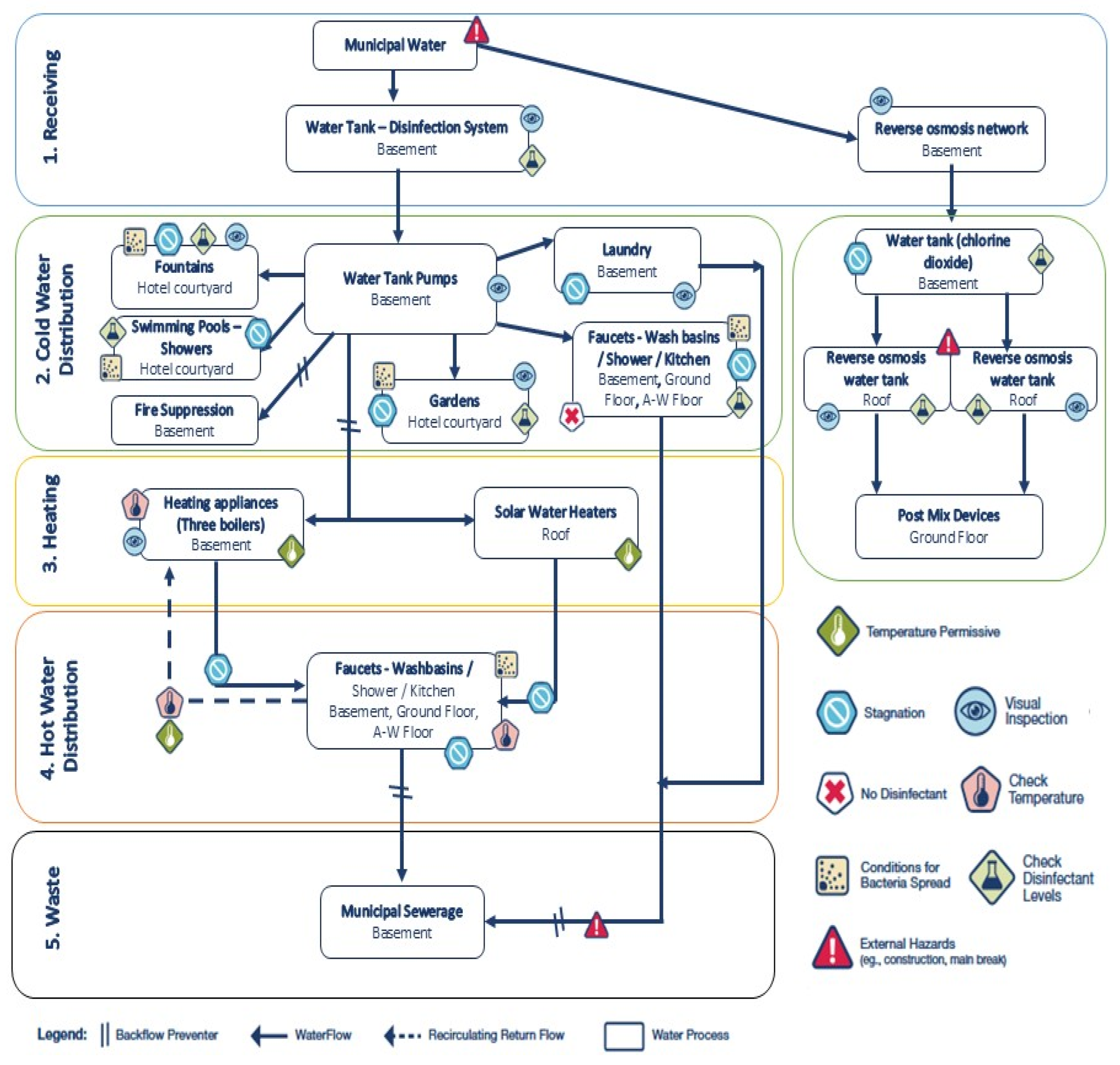
3.8. Dead Ends and Stagnant Lines Risk Assessment
3.9. Multi-Tool Risk Assessment Approach
3.9.1. ECDC Facility Inspection Tool for Travel-Associated Legionnaires’ Disease for Reducing Legionella Risk
3.9.2. Greek National Public Health Authority (NPHA) Legionella Prevention and Management Checklist
3.9.3. Structural and Water System Risk Scoring Tables
4. Discussion
4.1. Epidemiological Characteristics of Cases
4.2. Environmental Positivity and Threshold Limitations
4.3. Spatial Distribution
4.4. Physicochemical and Structural Risk Factors
4.5. Microbiological Indicators and Predictive Value
4.6. Added Value of Structured Risk Assessment
4.7. Comparison with Similar Cluster and Outbreak Investigations
4.8. Public Health Implications
4.9. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LD | Legionnaires’ disease |
| ELDSNet | European Legionnaires’ Disease Surveillance Network |
| TALD | Travel-Associated Legionnaires’ Disease |
| ECDC | European Centre for Disease Prevention and Control |
| CFU | Colony-forming unit |
| SG | Serogroup |
| PCR | Polymerase chain reaction |
| TVC | Total Viable Count |
| WSP | Water Safety Plan |
| RA | Risk Assessment |
| RPN | Risk Priority Number |
| WMP | Water Management Program |
References
- Legionella (Legionnaires’ Disease and Pontiac Fever)|Legionella|CDC. Available online: https://www.cdc.gov/legionella/index.html (accessed on 14 September 2025).
- European Technical Guidelines for the Prevention, Control and Investigation of Infections Caused by Legionella Species|Safety and Health at Work EU-OSHA. Available online: https://osha.europa.eu/en/legislation/guidelines/european-technical-guidelines-prevention-control-and-investigation-infections-caused-legionella-species (accessed on 14 September 2025).
- Mouchtouri, V.A.; Rudge, J.W. Legionnaires’ Disease in Hotels and Passenger Ships: A Systematic Review of Evidence, Sources, and Contributing Factors. J. Travel. Med. 2015, 22, 325–337. [Google Scholar] [CrossRef]
- Doménech-Sánchez, A.; Laso, E.; Albertí, S. Determination of Legionella Spp. Prevalence in Spanish Hotels in Five Years. Are Tourists Really at Risk? Travel. Med. Infect. Dis. 2022, 46, 102269. [Google Scholar] [CrossRef]
- Anagnostopoulos, L.; Kourentis, L.; Papadakis, A.; Mouchtouri, V.A. Re-Starting the Cruise Sector during the COVID-19 Pandemic in Greece: Assessing Effectiveness of Port Contingency Planning. Int. J. Environ. Res. Public Health 2022, 19, 13262. [Google Scholar] [CrossRef] [PubMed]
- Kourentis, L.; Anagnostopoulos, L.; Tsinaris, Z.; Galanopoulos, A.P.; Van Reusel, D.; Van den Bogaert, R.; Helewaut, B.; Steenhout, I.; Helewaut, H.; Damman, D.; et al. Legionella Spp. Colonization on Non-Passenger Ships Calling at Belgian Ports. Med. Sci. Forum 2022, 13, 15. [Google Scholar] [CrossRef]
- Legionnaires’ Disease—Symptoms & Causes—Mayo Clinic. Available online: https://www.mayoclinic.org/diseases-conditions/legionnaires-disease/symptoms-causes/syc-20351747 (accessed on 14 September 2025).
- Legionnaires’ Disease—Annual Epidemiological Report for 2021. Available online: https://www.ecdc.europa.eu/en/publications-data/legionnaires-disease-annual-epidemiological-report-2021 (accessed on 14 September 2025).
- Rota, M.C.; Bella, A.; Caporali, M.G.; Nicolau, A.; Drasar, V.; Ricci, M.L.; Scaturro, M.; Gumá, M.; Crespi, S. Travel-Associated Legionnaires’ Disease: Would Changing Cluster Definition Lead to the Prevention of a Larger Number of Cases? Epidemiol. Infect. 2018, 147, e62. [Google Scholar] [CrossRef]
- Fields, B.S.; Benson, R.F.; Besser, R.E. Legionella and Legionnaires’ Disease: 25 Years of Investigation. Clin. Microbiol. Rev. 2002, 15, 506–526. [Google Scholar] [CrossRef]
- Phin, N.; Parry-Ford, F.; Harrison, T.; Stagg, H.R.; Zhang, N.; Kumar, K.; Lortholary, O.; Zumla, A.; Abubakar, I. Epidemiology and Clinical Management of Legionnaires’ Disease. Lancet Infect. Dis. 2014, 14, 1011–1021. [Google Scholar] [CrossRef]
- Legionellosis. Available online: https://www.who.int/news-room/fact-sheets/detail/legionellosis (accessed on 4 October 2025).
- Chochlakis, D.; Sandalakis, V.; Keramarou, M.; Tselentis, Y.; Psaroulaki, A. Legionellosis: A Walk-through to Identification of the Source of Infection. Cent. Eur. J. Public Health 2017, 25, 235–239. [Google Scholar] [CrossRef] [PubMed]
- De Giglio, O.; Napoli, C.; Diella, G.; Fasano, F.; Lopuzzo, M.; Apollonio, F.; D’Ambrosio, M.; Campanale, C.; Triggiano, F.; Caggiano, G.; et al. Integrated Approach for Legionellosis Risk Analysis in Touristic-Recreational Facilities. Environ. Res. 2021, 202, 111649. [Google Scholar] [CrossRef]
- Cowgill, K.O.; Lucas, C.E.; Benson, R.F.; Chamany, S.; Brown, E.W.; Fields, B.S.; Feikin, D.R. Recurrence of Legionnaires Disease at a Hotel in the United States Virgin Islands over a 20-Year Period. Clin. Infect. Dis. 2005, 40, 1205–1207. [Google Scholar] [CrossRef] [PubMed]
- Legionnaires’ Disease in the Caribbean. An Outbreak Associated with a Resort Hotel—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/4062461/ (accessed on 14 September 2025).
- Legionnaires’ Disease: Guidance, Data and Analysis—GOV.UK. Available online: https://www.gov.uk/government/collections/legionnaires-disease-guidance-data-and-analysis (accessed on 14 September 2025).
- Leegionella Ltd.—Independent Public Health Microbiology Consultancy and Advisory Services. Available online: https://leegionella.co.uk/ (accessed on 14 September 2025).
- Clinical Overview of Legionnaires’ Disease|Legionella|CDC. Available online: https://www.cdc.gov/legionella/hcp/clinical-overview/index.html (accessed on 4 October 2025).
- Hayatimehr, S.; Mirkalantari, S.; Amirmozafari, N.; Jazi, F.M.; Moghadam, M.T. Virulence Genes and Biofilm Formation Among Legionella Pneumophila Isolates Collected from Hospital Water Sources. Curr. Microbiol. 2024, 81, 141. [Google Scholar] [CrossRef]
- Monistero, V.; Vicari, N.; Prati, P.; Bragoni, R.; Gazzola, A.; Sala, L.; Maisano, A.; Moroni, P.; Bronzo, V.; Luini, M.V.; et al. A Rapid and Reliable Method for Early Legionella Pneumophila Identification and Characterization in Support of the Epidemiology Study. Front. Microbiol. 2024, 15, 1452861. [Google Scholar] [CrossRef]
- Iliadi, V.; Staykova, J.; Iliadis, S.; Konstantinidou, I.; Sivykh, P.; Romanidou, G.; Vardikov, D.F.; Cassimos, D.; Konstantinidis, T.G. Legionella Pneumophila: The Journey from the Environment to the Blood. J. Clin. Med. 2022, 11, 6126. [Google Scholar] [CrossRef]
- Euser, S.M.; Pelgrim, M.; Den Boer, J.W. Legionnaires’ Disease and Pontiac Fever after Using a Private Outdoor Whirlpool Spa. Scand. J. Infect. Dis. 2010, 42, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Lüttichau, H.R.; Vinther, C.; Uldum, S.A.; Møller, J.; Faber, M.; Jensen, J.S. An Outbreak of Pontiac Fever among Children Following Use of a Whirlpool. Clin. Infect. Dis. 1998, 26, 1374–1378. [Google Scholar] [CrossRef] [PubMed]
- Gumá, M.; Drasar, V.; Santandreu, B.; Cano, R.; Afshar, B.; Nicolau, A.; Bennassar, M.; del Barrio, J.; Crespi, P.; Crespi, S. A Community Outbreak of Legionnaires’ Disease Caused by Outdoor Hot Tubs for Private Use in a Hotel. Front. Microbiol. 2023, 14, 1137470. [Google Scholar] [CrossRef]
- Nisar, M.A.; Ross, K.E.; Brown, M.H.; Bentham, R.; Whiley, H. Water Stagnation and Flow Obstruction Reduces the Quality of Potable Water and Increases the Risk of Legionelloses. Front. Environ. Sci. 2020, 8, 611611. [Google Scholar] [CrossRef]
- Papadakis, A.; Chochlakis, D.; Sandalakis, V.; Keramarou, M.; Tselentis, Y.; Psaroulaki, A. Legionella Spp. Risk Assessment in Recreational and Garden Areas of Hotels. Int. J. Environ. Res. Public Health 2018, 15, 598. [Google Scholar] [CrossRef]
- Mellou, K.; Mplougoura, A.; Mandilara, G.; Papadakis, A.; Chochlakis, D.; Psaroulaki, A.; Mavridou, A. Swimming Pool Regulations in the COVID-19 Era: Assessing Acceptability and Compliance in Greek Hotels in Two Consecutive Summer Touristic Periods. Water 2022, 14, 796. [Google Scholar] [CrossRef]
- Legionnaires’ Disease—HSE. Available online: https://www.hse.gov.uk/pubns/books/hsg274.htm (accessed on 14 September 2025).
- Legionella Control and Compliance Software|AquaAdept. Available online: https://www.legionella-software.co.uk/ (accessed on 14 September 2025).
- Technologies for Legionella Control in Premise Plumbing Systems|US EPA. Available online: https://www.epa.gov/ground-water-and-drinking-water/technologies-legionella-control-premise-plumbing-systems (accessed on 14 September 2025).
- Singh, R.; Hamilton, K.A.; Rasheduzzaman, M.; Yang, Z.; Kar, S.; Fasnacht, A.; Masters, S.V.; Gurian, P.L. Managing Water Quality in Premise Plumbing: Subject Matter Experts’ Perspectives and a Systematic Review of Guidance Documents. Water 2020, 12, 347. [Google Scholar] [CrossRef]
- Falkinham, J.O.; Pruden, A.; Edwards, M. Opportunistic Premise Plumbing Pathogens: Increasingly Important Pathogens in Drinking Water. Pathogens 2015, 4, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Cullom, A.C.; Martin, R.L.; Song, Y.; Williams, K.; Williams, A.; Pruden, A.; Edwards, M.A. Critical Review: Propensity of Premise Plumbing Pipe Materials to Enhance or Diminish Growth of Legionella and Other Opportunistic Pathogens. Pathogens 2020, 9, 957. [Google Scholar] [CrossRef] [PubMed]
- European Legionnaires’ Disease Surveillance Network (ELDSNet)—Operating Procedures. Available online: https://www.ecdc.europa.eu/en/publications-data/european-legionnaires-disease-surveillance-network-eldsnet-operating-procedures (accessed on 14 September 2025).
- Hadjichristodoulou, C.; Mouchtouri, V.; Vaitsi, V.; Kapoula, C.; Vousoureli, A.; Kalivitis, I.; Chervoni, J.; Papastergiou, P.; Vasilogiannakopoulos, A.; Daniilidis, V.D.; et al. Management of Environmental Health Issues for the 2004 Athens Olympic Games: Is Enhanced Integrated Environmental Health Surveillance Needed in Every Day Routine Operation? BMC Public Health 2006, 6, 306. [Google Scholar] [CrossRef] [PubMed]
- Manage a Site Investigation for Travel-Associated Legionnaires Disease—GOV.UK. Available online: https://www.gov.uk/government/publications/report-a-site-investigation-for-travel-associated-legionnaires-disease (accessed on 14 September 2025).
- CDC Laboratory Guidance for Processing Environmental Samples|LD Investigations|CDC. Available online: https://www.cdc.gov/investigate-legionella/php/publications/lab-procedures-manual.html (accessed on 14 September 2025).
- Renwick, D.V.; Heinrich, A.; Weisman, R.; Arvanaghi, H.; Rotert, K. Potential Public Health Impacts of Deteriorating Distribution System Infrastructure. J. Am. Water Works Assoc. 2019, 111, 42–53. [Google Scholar] [CrossRef]
- LeChevallier, M.W.; Prosser, T.; Stevens, M. Opportunistic Pathogens in Drinking Water Distribution Systems—A Review. Microorganisms 2024, 12, 916. [Google Scholar] [CrossRef]
- Papadakis, A.; Koufakis, E.; Chaidoutis, E.A.; Chochlakis, D.; Psaroulaki, A. Comparative Risk Assessment of Legionella Spp. Colonization in Water Distribution Systems Across Hotels, Passenger Ships, and Healthcare Facilities During the COVID-19 Era. Water 2025, 17, 2149. [Google Scholar] [CrossRef]
- Yao, X.H.; Shen, F.; Hao, J.; Huang, L.; Keng, B. A Review of Legionella Transmission Risk in Built Environments: Sources, Regulations, Sampling, and Detection. Front. Public Health 2024, 12, 1415157. [Google Scholar] [CrossRef]
- Papadakis, A.A.; Tsirigotakis, I.; Katranitsa, S.; Donousis, C.; Papalexis, P.; Keramydas, D.; Chaidoutis, E.; Georgakopoulou, V.E.; Spandidos, D.A.; Constantinidis, T.C. Assessing the Impact of the COVID-19 Pandemic Health Protocols on the Hygiene Status of Swimming Pools of Hotel Units. Med. Int. 2023, 3, 32. [Google Scholar] [CrossRef]
- Papadakis, A.; Chochlakis, D.; Koufakis, E.; Carayanni, V.; Psaroulaki, A. Recreational Water Safety in Hotels: Lessons from the COVID-19 Pandemic and the Way Forward for a Safe Aquatic Environment. Tour. Hosp. 2024, 5, 1167–1181. [Google Scholar] [CrossRef]
- Legionella Outbreak Toolbox. Available online: https://legionnaires.ecdc.europa.eu/?pid=202 (accessed on 15 September 2025).
- Case Definitions—Guidance for the Public Health Management of Legionnaires’ Disease—Version 2—Guidance for the Public Health Management of Legionnaires’ Disease—Publications—Public Health Scotland. Available online: https://publichealthscotland.scot/publications/guidance-for-the-public-health-management-of-legionnaires-disease/guidance-for-the-public-health-management-of-legionnaires-disease-version-2/case-definitions/ (accessed on 15 September 2025).
- Legionnaires’ Disease: Case Definitions—GOV.UK. Available online: https://www.gov.uk/government/publications/legionnaires-disease-clinical-case-definitions/legionnaires-disease-case-definitions (accessed on 15 September 2025).
- Legionellosis Surveillance and Trends|Legionella|CDC. Available online: https://www.cdc.gov/legionella/php/surveillance/index.html (accessed on 15 September 2025).
- About the Data: Case Definitions|LD Investigations|CDC. Available online: https://www.cdc.gov/investigate-legionella/php/data-research/case-definitions.html (accessed on 15 September 2025).
- Legionnaires. Available online: https://www.cste.org/page/Legionnaires (accessed on 15 September 2025).
- ISO 11731:2017; Water Quality—Enumeration of Legionella. International Organization for Standardization: Geneva, Switzerland. Available online: https://www.iso.org/standard/61782.html (accessed on 15 September 2025).
- ISO 11731-2:2004; Water Quality—Detection and Enumeration of Legionella—Part 2: Direct Membrane Filtration Method for Waters with Low Bacterial Counts. International Organization for Standardization: Geneva, Switzerland. Available online: https://www.iso.org/standard/32326.html (accessed on 15 September 2025).
- ISO 9308-1:2014; Water Quality—Enumeration of Escherichia coli and Coliform Bacteria—Part 1: Membrane Filtration Method for Waters with Low Bacterial Background Flora. International Organization for Standardization: Geneva, Switzerland. Available online: https://www.iso.org/standard/55832.html (accessed on 15 September 2025).
- ISO 6222:1999; Water Quality—Enumeration of Culturable Micro-Organisms—Colony Count by Inoculation in a Nutrient Agar Culture Medium. International Organization for Standardization: Geneva, Switzerland. Available online: https://www.iso.org/standard/28960.html (accessed on 15 September 2025).
- ISO 7899-2:2000; Water Quality—Detection and Enumeration of Intestinal Enterococci—Part 2: Membrane Filtration Method. International Organization for Standardization: Geneva, Switzerland. Available online: https://www.iso.org/standard/14854.html (accessed on 15 September 2025).
- Standard Methods for the Examination of Water & Wastewater|Hach—Downloads. Available online: https://my.hach.com/standard-methods-for-the-examination-of-water-wastewater/product-downloads?id=59428492840 (accessed on 15 September 2025).
- ISO 19458:2006; Water Quality—Sampling for Microbiological Analysis. International Organization for Standardization: Geneva, Switzerland. Available online: https://www.iso.org/standard/33845.html (accessed on 15 September 2025).
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data; Scientific Research Publishing: Wuhan, China, 2010; Available online: https://www.scirp.org/reference/referencespapers?referenceid=2781642 (accessed on 10 October 2025).
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Moran-Gilad, J.; Prior, K.; Yakunin, E.; Harrison, T.G.; Underwood, A.; Lazarovitch, T.; Valinsky, L.; Lück, C.; Krux, F.; Agmon, V.; et al. Design and Application of a Core Genome Multilocus Sequence Typing Scheme for Investigation of Legionnaires’ Disease Incidents. Euro Surveill. 2015, 20, 21186. [Google Scholar] [CrossRef] [PubMed]
- Toolkit: Developing a Legionella Water Management Program|Control Legionella|CDC. Available online: https://www.cdc.gov/control-legionella/php/toolkit/wmp-toolkit.html (accessed on 15 September 2025).
- Samuelsson, J.; Hallström, L.P.; Marrone, G.; Dias, J.G. Legionnaires’ Disease in the EU/EEA*: Increasing Trend from 2017 to 2019. Eurosurveillance 2023, 28, 2200114. [Google Scholar] [CrossRef]
- Epi InfoTM|CDC. Available online: https://www.cdc.gov/epiinfo/index.html (accessed on 15 September 2025).
- MedCalc’s Relative Risk Calculator. Available online: https://www.medcalc.org/en/calc/relative_risk.php (accessed on 15 September 2025).
- Dettori, M.; Arghittu, A.; Deiana, G.; Castiglia, P.; Azara, A. The Revised European Directive 2020/2184 on the Quality of Water Intended for Human Consumption. A Step Forward in Risk Assessment, Consumer Safety and Informative Communication. Environ. Res. 2022, 209, 112773. [Google Scholar] [CrossRef]
- Directive—2020/2184—EN—EUR-Lex. Available online: https://eur-lex.europa.eu/eli/dir/2020/2184/oj/eng (accessed on 12 October 2025).
- Implementing Water Safety Plans in the European Region. Available online: https://www.who.int/europe/activities/implementing-water-safety-plans-in-the-european-region (accessed on 12 October 2025).
- Marras, L.; Bertolino, G.; Sanna, A.; Carraro, V.; Coroneo, V. Legionella Spp. Monitoring in the Water Supply Systems of Accommodation Facilities in Sardinia, Italy: A Two-Year Retrospective Analysis. Int. J. Environ. Res. Public Health 2023, 20, 6722. [Google Scholar] [CrossRef]
- Di Onofrio, V.; Pagano, M.; Santulli, M.; Rossi, A.; Liguori, R.; Di Dio, M.; Liguori, G. Contamination of Hotel Water Distribution Systems by Legionella Species: Environmental Surveillance in Campania Region, South Italy. Microorganisms 2023, 11, 1840. [Google Scholar] [CrossRef]
- Di Dio, M.; Santulli, M.; Pagano, M.; Rossi, A.M.; Liguori, R.; Liguori, G.; Di Onofrio, V. 4-Year Study in Monitoring the Presence of Legionella in the Campania Region’s Healthcare Facilities. Hygiene 2025, 5, 16. [Google Scholar] [CrossRef]
- Domenech-Sanchez, A.; Laso, E.; Berrocal, C.I.; Albertí, S. Environmental Surveillance of Legionella in Tourist Facilities of the Balearic Islands, Spain, 2006 to 2010 and 2015 to 2018. Euro Surveill. 2022, 27, 2100769. [Google Scholar] [CrossRef]
- Abu Khweek, A.; Amer, A.O. Factors Mediating Environmental Biofilm Formation by Legionella Pneumophila. Front. Cell Infect. Microbiol. 2018, 8, 38. [Google Scholar] [CrossRef]
- Khairullah, A.R.; Nuradji, H.; Nurjanah, D.; Dharmayanti, N.L.P.I.; Kusuma Wardhani, B.W.; Wibowo, S.; Moses, I.B.; Ariani Kurniasih, D.A.; Fauziah, I.; Kusala, M.K.J.; et al. Legionnaires’ Disease: A Review of Emerging Public Health Threats. Int. J. One Health 2025, 11, 62–77. [Google Scholar] [CrossRef]
- Sylvestre, É.; Charron, D.; Lefebvre, X.; Bedard, E.; Prévost, M. Leveraging Regulatory Monitoring Data for Quantitative Microbial Risk Assessment of Legionella Pneumophila in Cooling Towers. Sci. Total Environ. 2025, 975, 179293. [Google Scholar] [CrossRef]
- Armstrong, T.W.; Haas, C.N. A Quantitative Microbial Risk Assessment Model for Legionnaires’ Disease: Animal Model Selection and Dose-Response Modeling. Risk Anal. 2007, 27, 1581–1596. [Google Scholar] [CrossRef]
- Buse, H.Y.; Schoen, M.E.; Ashbolt, N.J. Legionellae in Engineered Systems and Use of Quantitative Microbial Risk Assessment to Predict Exposure. Water Res. 2012, 46, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, W.J.; Hammes, F. Growth of Legionella during COVID-19 Lockdown Stagnation. Environ. Sci. 2021, 7, 10–15. [Google Scholar] [CrossRef]
- Kunz, J.M.; Hannapel, E.; Vander Kelen, P.; Hils, J.; Hoover, E.R.; Edens, C. Effects of the COVID-19 Pandemic on Legionella Water Management Program Performance across a United States Lodging Organization. Int. J. Environ. Res. Public Health 2023, 20, 6885. [Google Scholar] [CrossRef] [PubMed]
- Sopena, N.; Pedro-Botet, L.; Mateu, L.; Tolschinsky, G.; Rey-Joly, C.; Sabrià, M. Community-Acquired Legionella Pneumonia in Elderly Patients: Characteristics and Outcome. J. Am. Geriatr. Soc. 2007, 55, 114–119. [Google Scholar] [CrossRef]
- Fernández, J.A.; López, P.; Orozco, D.; Merino, J. Clinical Study of an Outbreak of Legionnaire’s Disease in Alcoy, Southeastern Spain. Eur. J. Clin. Microbiol. Infect. Dis. 2002, 21, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Increasing Rates of Legionnaires’ Disease in the EU/EEA. Available online: https://www.ecdc.europa.eu/en/news-events/increasing-rates-legionnaires-disease-eueea (accessed on 15 September 2025).
- Jain, N.; Krygowska, A.M. Legionnaire’s Looms: Europe’s Wake-up Call to Enhance Vigilance in Detection and Reporting. New Microbes New Infect. 2023, 55, 101194. [Google Scholar] [CrossRef]
- Viasus, D.; Gaia, V.; Manzur-Barbur, C.; Carratalà, J. Legionnaires’ Disease: Update on Diagnosis and Treatment. Infect. Dis. Ther. 2022, 11, 973–986. [Google Scholar] [CrossRef]
- Gleason, J.A.; Cohn, P.D. A Review of Legionnaires’ Disease and Public Water Systems—Scientific Considerations, Uncertainties and Recommendations. Int. J. Hyg. Environ. Health 2022, 240, 113906. [Google Scholar] [CrossRef]
- Rothberg, M.B.; Imrey, P.B.; Guo, N.; Deshpande, A.; Higgins, T.L.; Lindenauer, P.K. A Risk Model to Identify Legionella among Patients Admitted with Community-acquired Pneumonia: A Retrospective Cohort Study. J. Hosp. Med. 2022, 17, 624. [Google Scholar] [CrossRef] [PubMed]
- Bellew, S.; Grijalva, C.G.; Williams, D.J.; Anderson, E.J.; Wunderink, R.G.; Zhu, Y.; Waterer, G.W.; Bramley, A.M.; Jain, S.; Edwards, K.M.; et al. Pneumococcal and Legionella Urinary Antigen Tests in Community-Acquired Pneumonia: Prospective Evaluation of Indications for Testing. Clin. Infect. Dis. 2019, 68, 2026–2033. [Google Scholar] [CrossRef] [PubMed]
- Gorzynski, J.; Wee, B.; Llano, M.; Alves, J.; Cameron, R.; McMenamin, J.; Smith, A.; Lindsay, D.; Fitzgerald, J.R. Epidemiological Analysis of Legionnaires’ Disease in Scotland: A Genomic Study. Lancet Microbe 2022, 3, e835–e845. [Google Scholar] [CrossRef] [PubMed]
- Barskey, A.E.; Lackraj, D.; Tripathi, P.S.; Lee, S.; Smith, J.; Edens, C. Travel-Associated Cases of Legionnaires’ Disease in the United States, 2015–2016. Travel. Med. Infect. Dis. 2020, 40, 101943. [Google Scholar] [CrossRef]
- Marston, B.J. Surveillance for Legionnaires’ Disease. Arch. Intern. Med. 1994, 154, 2417. [Google Scholar] [CrossRef]
- Van Heijnsbergen, E.; Schalk, J.A.C.; Euser, S.M.; Brandsema, P.S.; Den Boer, J.W.; De Roda Husman, A.M. Confirmed and Potential Sources of Legionella Reviewed. Environ. Sci. Technol. 2015, 49, 4797–4815. [Google Scholar] [CrossRef]
- Montagna, M.T.; De Giglio, O.; Cristina, M.L.; Napoli, C.; Pacifico, C.; Agodi, A.; Baldovin, T.; Casini, B.; Coniglio, M.A.; D’Errico, M.M.; et al. Evaluation of Legionella Air Contamination in Healthcare Facilities by Different Sampling Methods: An Italian Multicenter Study. Int. J. Environ. Res. Public Health 2017, 14, 670. [Google Scholar] [CrossRef]
- Bentham, R.H.; Broadbent, C.R. A Model for Autumn Outbreaks of Legionnaires’ Disease Associated with Cooling Towers, Linked to System Operation and Size. Epidemiol. Infect. 1993, 111, 287–295. [Google Scholar] [CrossRef]
- Kyritsi, M.A.; Mouchtouri, V.A.; Katsioulis, A.; Kostara, E.; Nakoulas, V.; Hatzinikou, M.; Hadjichristodoulou, C. Legionella Colonization of Hotel Water Systems in Touristic Places of Greece: Association with System Characteristics and Physicochemical Parameters. Int. J. Environ. Res. Public Health 2018, 15, 2707. [Google Scholar] [CrossRef]
- Papadakis, A.; Keramarou, M.; Chochlakis, D.; Sandalakis, V.; Mouchtouri, V.A.; Psaroulaki, A. Legionella Spp. Colonization in Water Systems of Hotels Linked with Travel-Associated Legionnaires’ Disease. Water 2021, 13, 2243. [Google Scholar] [CrossRef]
- Papageorgiou, K.; Chronis, E.; Tzouanopoulos, A.; Steris, V.; Koutsopoulos, D.; Tzavaras, I.; Paraskevopoulos, K.; Karolidis, S. Prevalence of Legionella Spp. in the Water Distribution Systems of Northern Greece. Eur. J. Environ. Public Health 2023, 7, em0147. [Google Scholar] [CrossRef]
- Winn, W.C. Legionnaires Disease: Historical Perspective. Clin. Microbiol. Rev. 1988, 1, 60–81. [Google Scholar] [CrossRef]
- Beauté, J.; Robesyn, E.; Jong, B. De Legionnaires’ Disease in Europe: All Quiet on the Eastern Front? Eur. Respir. J. 2013, 42, 1454–1458. [Google Scholar] [CrossRef]
- Arrigo, I.; Galia, E.; Fasciana, T.; Diquattro, O.; Tricoli, M.R.; Serra, N.; Palermo, M.; Giammanco, A. Four-Year Environmental Surveillance Program of Legionella Spp. in One of Palermo’s Largest Hospitals. Microorganisms 2022, 10, 764. [Google Scholar] [CrossRef]
- Scaturro, M.; Lanni, A.; Mancini, F.; Girolamo, A.; Fillo, S.; Ciammaruconi, A.; Lista, F.; Cocuzza, C.E.; Musumeci, R.; Ginevra, C.; et al. Antimicrobial Susceptibility and Epidemiological Types of Legionella Pneumophila Human Isolates from Italy (1987–2020). J. Glob. Antimicrob. Resist. 2025, 41, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Uldum, S.A.; Schjoldager, L.G.; Baig, S.; Cassell, K. A Tale of Four Danish Cities: Legionella Pneumophila Diversity in Domestic Hot Water and Spatial Variations in Disease Incidence. Int. J. Environ. Res. Public Health 2022, 19, 2530. [Google Scholar] [CrossRef]
- Moll, H.; Sonesson, A.; Jantzen, E.; Marre, R.; Zähringer, U. Identification of 27-Oxo-Octacosanoic Acid and Heptacosane-1,27-Dioic Acid in Legionella Pneumophila. FEMS Microbiol. Lett. 1992, 97, 1–6. [Google Scholar] [CrossRef][Green Version]
- Fasciana, T.; Mascarella, C.; Distefano, S.A.; Calà, C.; Capra, G.; Rampulla, A.; Di Carlo, P.; Palermo, M.; Giammanco, A. Cluster of Legionnaires’ Disease in an Italian Prison. Int. J. Environ. Res. Public Health 2019, 16, 2062. [Google Scholar] [CrossRef]
- Kowalczyk, B.; Petzold, M.; Kaczyński, Z.; Szuster-Ciesielska, A.; Luchowski, R.; Gruszecki, W.I.; Fuchs, B.; Galuska, C.E.; Choma, A.; Tarasiuk, J.; et al. Lipopolysaccharide of Legionella Pneumophila Serogroup 1 Facilitates Interaction with Host Cells. Int. J. Mol. Sci. 2023, 24, 14602. [Google Scholar] [CrossRef]
- Controlling Legionella in Potable Water Systems|Control Legionella|CDC. Available online: https://www.cdc.gov/control-legionella/php/toolkit/potable-water-systems-module.html (accessed on 15 September 2025).
- Rakić, A.; Lušić, D.V.; Savičević, A.J. Influence of Metal Concentration and Plumbing Materials on Legionella Contamination. Microorganisms 2022, 10, 1051. [Google Scholar] [CrossRef]
- Xi, H.; Ross, K.E.; Hinds, J.; Molino, P.J.; Whiley, H. Efficacy of Chlorine-Based Disinfectants to Control Legionella within Premise Plumbing Systems. Water Res. 2024, 259, 121794. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, J.; Wu, J.; Weir, M.H.; Xi, C. Prevalence of Legionella in a Public Building Water Plumbing System During COVID-19 Lockdown. Environ. Health 2023, 1, 352. [Google Scholar] [CrossRef] [PubMed]
- Kahn, R.; Derado, G.; Hannapel, E.J.; Vander Kelen, P.; Kunz, J.M.; Edens, C. Factors Associated with Legionella Detection in the Water Systems of National Lodging Organization Facilities with Water Management Programs in the United States. Int. J. Environ. Res. Public Health 2024, 21, 939. [Google Scholar] [CrossRef]
- Brousseau, N.; Lévesque, B.; Guillemet, T.A.; Cantin, P.; Gauvin, D.; Giroux, J.P.; Gingras, S.; Proulx, F.; Côté, P.A.; Dewailly, E. Contamination of Public Whirlpool Spas: Factors Associated with the Presence of Legionella Spp., Pseudomonas Aeruginosa and Escherichia Coli. Int. J. Environ. Health Res. 2013, 23, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Benkel, D.H.; McClure, E.M.; Woolard, D.; Rullan, J.V.; Miller, G.B.; Jenkins, S.R.; Hershey, J.H.; Benson, R.F.; Pruckler, J.M.; Brown, E.W.; et al. Outbreak of Legionnaires’ Disease Associated with a Display Whirlpool Spa. Int. J. Epidemiol. 2000, 29, 1092–1098. [Google Scholar] [CrossRef][Green Version]
- Coetzee, N.; Duggal, H.; Hawker, J.; Ibbotson, S.; Harrison, T.G.; Phin, N.; Laza-Stanca, V.; Johnston, R.; Iqbal, Z.; Rehman, Y.; et al. An Outbreak of Legionnaires’ Disease Associated with a Display Spa Pool in Retail Premises, Stoke-on-Trent, United Kingdom, July 2012. Eurosurveillance 2012, 17, 20271. [Google Scholar] [CrossRef]
- Borella, P.; Montagna, M.T.; Romano-Spica, V.; Stampi, S.; Stancanelli, G.; Triassi, M.; Neglia, R.; Marchesi, I.; Fantuzzi, G.; Tatòt, D.; et al. Legionella Infection Risk from Domestic Hot Water. Emerg. Infect. Dis. 2004, 10, 457. [Google Scholar] [CrossRef]
- De Filippis, P.; Mozzetti, C.; Messina, A.; D’Alò, G.L. Data on Legionella Prevalence and Water Quality in Showers of Retirement Homes and Group Homes in the Province of Rome, Lazio Region, Italy. Data Brief. 2018, 19, 2364–2373. [Google Scholar] [CrossRef]
- De Filippis, P.; Mozzetti, C.; Messina, A.; D’Alò, G.L. Prevalence of Legionella in Retirement Homes and Group Homes Water Distribution Systems. Sci. Total Environ. 2018, 643, 715–724. [Google Scholar] [CrossRef]
- Leoni, E.; Catalani, F.; Marini, S.; Dallolio, L. Legionellosis Associated with Recreational Waters: A Systematic Review of Cases and Outbreaks in Swimming Pools, Spa Pools, and Similar Environments. Int. J. Environ. Res. Public Health 2018, 15, 1612. [Google Scholar] [CrossRef]
- Hatanaka, N.; Xu, B.; Yasugi, M.; Morino, H.; Tagishi, H.; Miura, T.; Shibata, T.; Yamasaki, S. Chlorine Dioxide Is a More Potent Antiviral Agent against SARS-CoV-2 than Sodium Hypochlorite. J. Hosp. Infect. 2021, 118, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Padhi, R.K.; Subramanian, S.; Satpathy, K.K. Formation, Distribution, and Speciation of DBPs (THMs, HAAs, ClO2−, and ClO3−) during Treatment of Different Source Water with Chlorine and Chlorine Dioxide. Chemosphere 2019, 218, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Włodyka-Bergier, A.; Bergier, T. Influence of the Use of an Additional Oxidant (Chlorine Dioxide) in Water Treatment on Swimming Pool Water Quality. Energies 2022, 15, 5054. [Google Scholar] [CrossRef]
| Parameter | Description |
|---|---|
| Pipe material | PVC |
| Number of boilers | 3 × 2000 L |
| Hot water circulation | Throughout the building via a circulation pump |
| Heating system | Gas boiler with solar panel assistance |
| Seasonal operation | May–October |
| Dead ends | 5 (27.8%) |
| Stagnant lines | 13 (72.2%) |
| Backflow prevention devices | Installed, compliant with NF EN 1717 |
| Year | Total Samples (n) | ≥50 CFU/L Positive (n, %) [95% CI] | ≥1000 CFU/L Positive (n, %) [95% CI] |
|---|---|---|---|
| 2016 | 12 | 0 (0.00%) [0.00–26.46] | 0 (0.00%) [0.00–26.46] |
| 2017 | 29 | 10 (31.03%) [7.3–49.2] | 6 (20.69%) [9.84–38.39] |
| 2018 | 14 | 0 (0.00%) [0.0–21.4] | 0 (0.00%) [0.0–21.4] |
| 2023 | 13 | 0 (0.00%) [0.00–24.71] | 0 (0.00%) [0.00–24.71] |
| 2024 | 13 | 0 (0.00%) [0.00–24.71] | 0 (0.00%) [0.00–24.71] |
| 2025 (Pre-Intervention) | 68 | 14 (20.59%) [12.0–32.5] | 5 (7.35%) [2.4–16.3] |
| 2025 (Post-Intervention) | 32 | 0 (0%) [0.0–10.9] | 0 (0.00%) [0.0–10.9] |
| Total (2016–2025) | 181 | 23 (12.71%) [7.86–17.56] | 11 (6.08%) [2.60–9.56] |
| Parameter | Legionella + | Legionella − | Total | Relative Risk (95% CI) | p-Value |
|---|---|---|---|---|---|
| Hot water < 55 °C | 5 | 12 | 17 | 1.81 (0.75–4.35) | 0.20 |
| Hot water ≥ 55 °C | 10 | 53 | 63 | - | – |
| Free chlorine < 0.2 mg/L | 4 | 17 | 21 | 1.09 (0.41–2.92) | 0.85 |
| Free chlorine ≥ 0.2 mg/L | 11 | 48 | 59 | - | – |
| Domain | Key Requirements | Compliance | Comments |
|---|---|---|---|
| 1. Personnel and responsibility | Appointed responsible person; staff training; external contractors competent | No | No designated person; no staff training |
| 2. Control measures | Potable supply; hot water 50–60 °C; cold water < 25 °C; biocides monitored | Partial | Public water supply; chlorine dioxide in 2025; temperature monitoring only from late May 2025; no biocide monitoring |
| 3. Other risk factors | Flushing outlets; cleaning showerheads; no dead-legs; no corrosion | No | No flushing; no showerhead maintenance; stagnant pipework present; visible corrosion at outlets |
| 4. Cleaning and disinfection | Annual calorifier/tank cleaning; seasonal disinfection; filters/softeners maintained | Partial | Calorifiers and tanks cleaned; no filters; no written SOPs; incomplete network disinfection |
| 5. Surveillance and documentation | Written control programme; logbooks; risk assessment every 2 years; independent audit | No | No written programme, logbooks, or audit |
| 6. Particular water systems | Spa pools; cooling towers; other high-risk systems | Not applicable | No spa pools or cooling towers; outdoor showers, irrigation and fountains present but not monitored |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadakis, A.; Koufakis, E.; Nakoulas, V.; Kourentis, L.; Manouras, T.; Kokkinomagoula, A.; Ntoula, A.; Malliarou, M.; Gerakoudis, K.; Tsilipounidaki, K.; et al. Beyond Microbiological Analysis: The Essential Role of Risk Assessment in Travel-Associated Legionnaires’ Disease Outbreak Investigations. Pathogens 2025, 14, 1059. https://doi.org/10.3390/pathogens14101059
Papadakis A, Koufakis E, Nakoulas V, Kourentis L, Manouras T, Kokkinomagoula A, Ntoula A, Malliarou M, Gerakoudis K, Tsilipounidaki K, et al. Beyond Microbiological Analysis: The Essential Role of Risk Assessment in Travel-Associated Legionnaires’ Disease Outbreak Investigations. Pathogens. 2025; 14(10):1059. https://doi.org/10.3390/pathogens14101059
Chicago/Turabian StylePapadakis, Antonios, Eleftherios Koufakis, Vasileios Nakoulas, Leonidas Kourentis, Theodore Manouras, Areti Kokkinomagoula, Artemis Ntoula, Maria Malliarou, Kyriazis Gerakoudis, Katerina Tsilipounidaki, and et al. 2025. "Beyond Microbiological Analysis: The Essential Role of Risk Assessment in Travel-Associated Legionnaires’ Disease Outbreak Investigations" Pathogens 14, no. 10: 1059. https://doi.org/10.3390/pathogens14101059
APA StylePapadakis, A., Koufakis, E., Nakoulas, V., Kourentis, L., Manouras, T., Kokkinomagoula, A., Ntoula, A., Malliarou, M., Gerakoudis, K., Tsilipounidaki, K., Chochlakis, D., & Psaroulaki, A. (2025). Beyond Microbiological Analysis: The Essential Role of Risk Assessment in Travel-Associated Legionnaires’ Disease Outbreak Investigations. Pathogens, 14(10), 1059. https://doi.org/10.3390/pathogens14101059







