TREM-1 Interacts with Rotavirus Proteins and Drives Inflammatory Responses: A Combined Experimental and Computational Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Analysis of Public Transcriptomic Data
2.2. Cell and Virus Cultivation
2.3. Viral Titration
2.4. Cytokine Production in THP-1 Cells
2.5. Cytokine Production
2.6. Analysis of Cytopathic Effects
2.7. Statistical Analysis
2.8. In Silico Analysis of Putative Interactions Between TREM-1 and RV Proteins
2.9. Protein Modeling
2.10. Molecular Docking
2.11. Molecular Dynamics (MD)
2.12. Interaction Interfaces and Energetic Parameters of Protein–Protein Complexes
3. Results
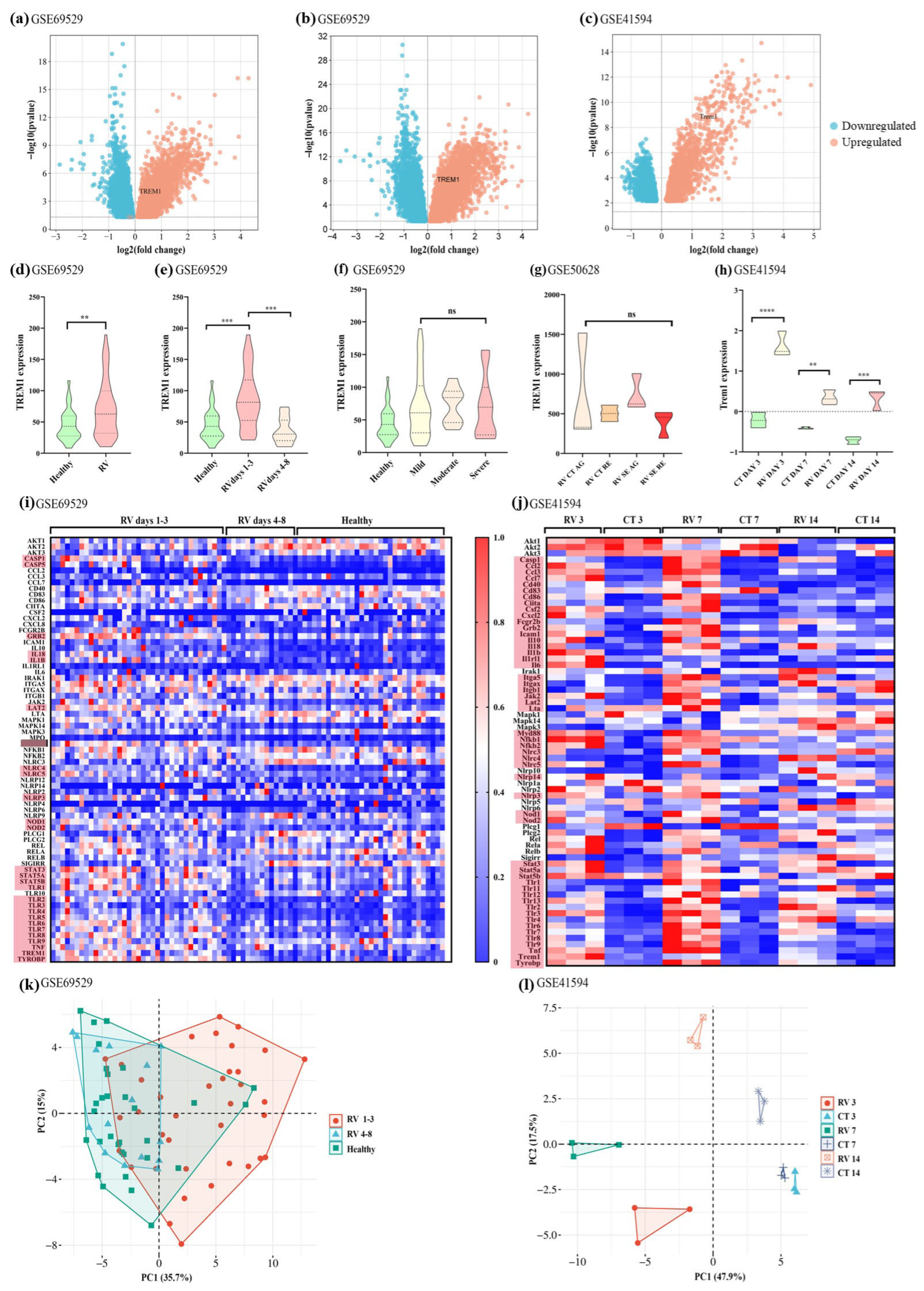
3.1. TREM-1 Pathway Is Upregulated in Mice and Children Infected with RV
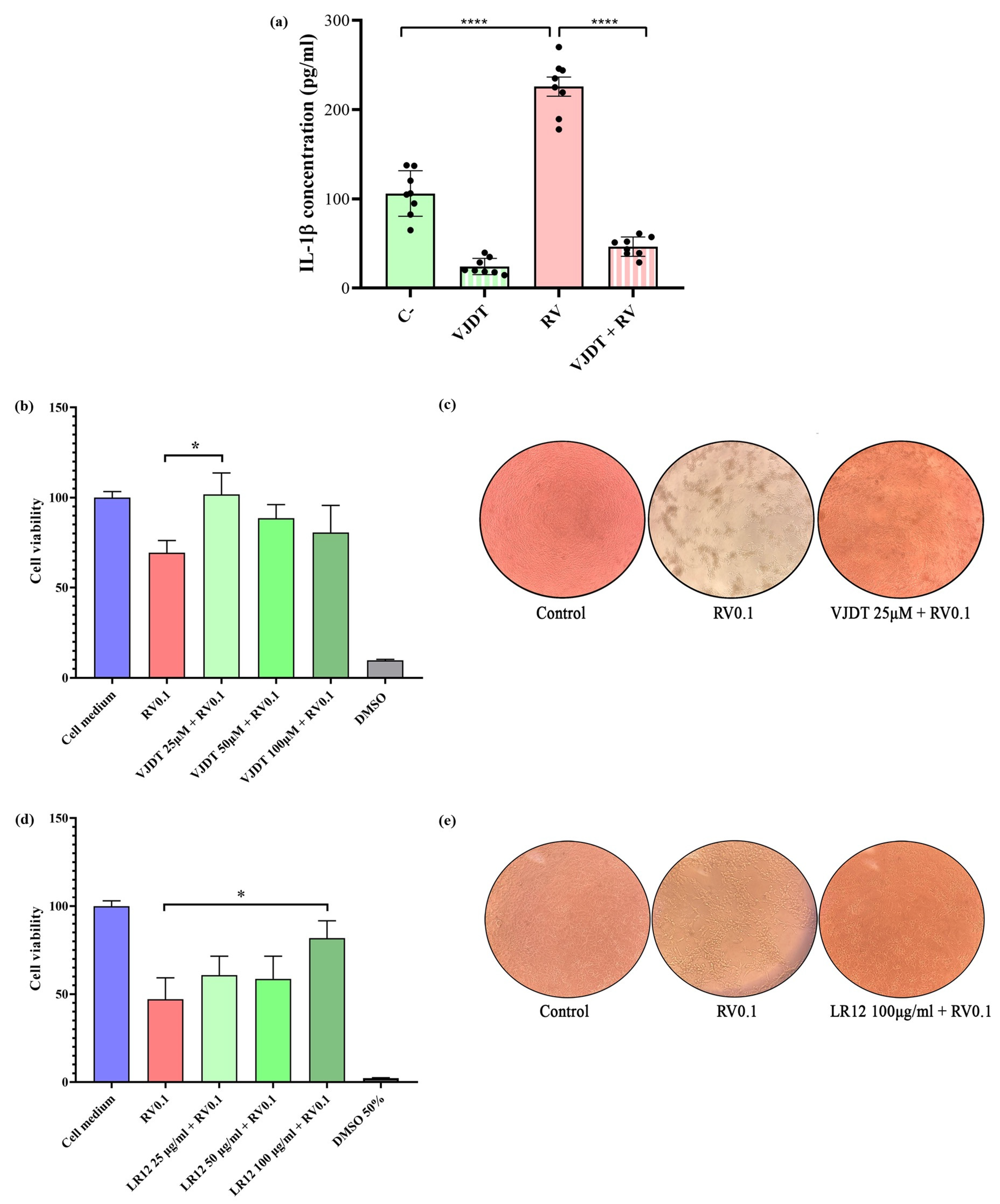
3.2. TREM-1 Inhibition Decreases the Production of Pro-Inflammatory Cytokines
3.3. TREM-1 Inhibition Decreases RV-Induced Cytopathic Effects
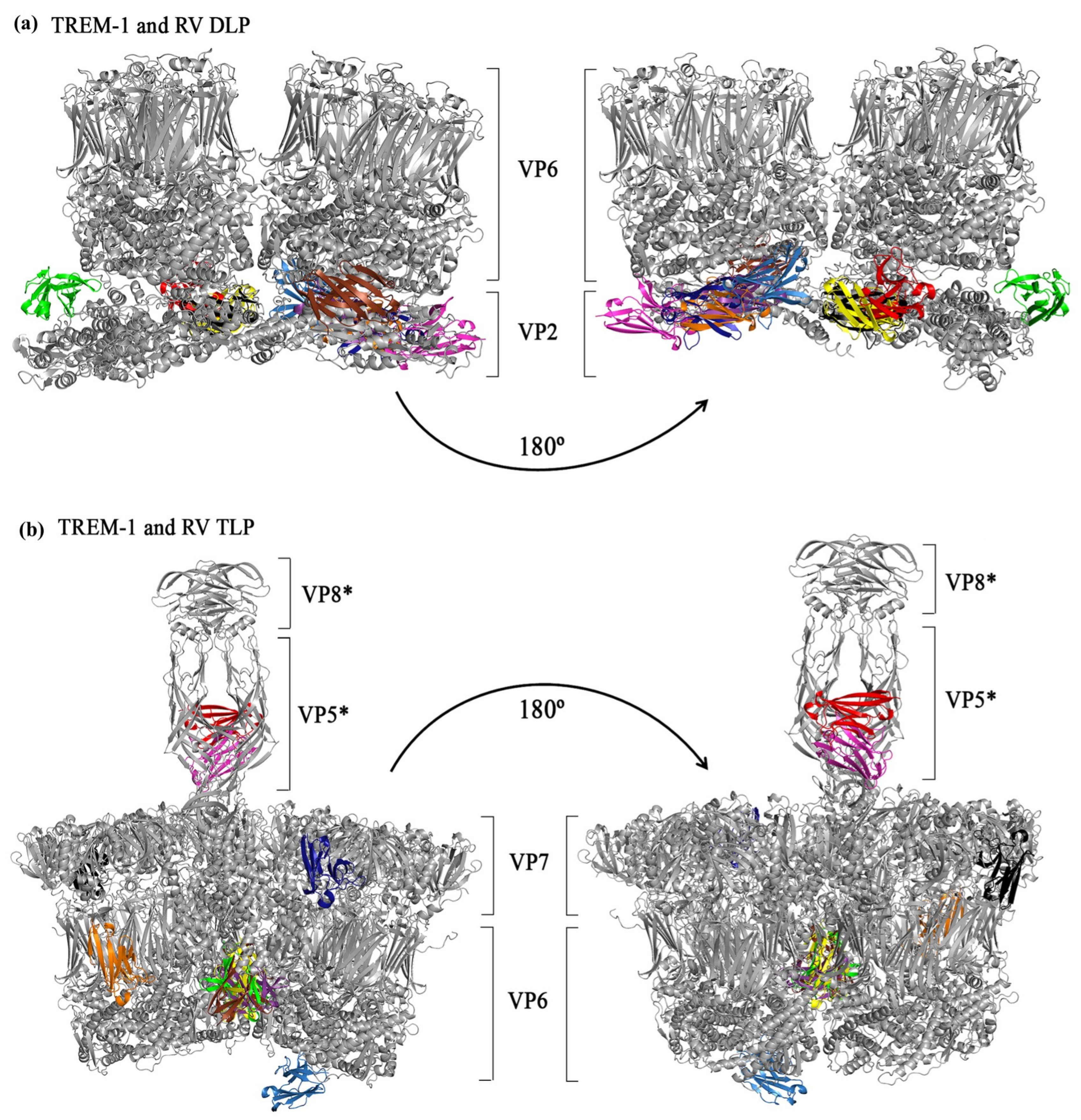
3.4. In Silico Simulations of the Interactions Between TREM-1 and RV Structural Proteins
3.5. In Silico Simulations of Interactions Between TREM-1 and the RV Enterotoxin
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DD | Diarrheal disease |
| AEBSF | 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride |
| AG | Acute |
| CT | Control |
| DEGs | Differentially expressed genes |
| DESOLV | Desolvation energy |
| DLP | Double-layered particle |
| DMSO | Dimethyl sulfoxide |
| ELE | Total electrostatic energy |
| ELISA | Enzyme-linked immunosorbent assay |
| FA_ATR | Attractive van der Waals forces |
| HBOND | Hydrogen bond potential |
| ICTV | International Committee on Taxonomy of Viruses |
| ISGs | Interferon-stimulated genes |
| Kd | Dissociation constant |
| LPS | Lipopolysaccharide |
| MD | Molecular dynamics |
| MM/GBSA | Molecular Mechanics–Generalized Born Surface Area |
| MOI | Multiplicity of infection |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide |
| PCA | Principal component analysis |
| PFU | Plaque-forming units |
| PYDOCK_TOT | Total energy |
| RE | Recovery |
| RMSD | Root mean square deviation |
| RV | Rotavirus |
| RVA | Rotavirus A |
| SE | Seizure |
| sTREM-1 | Soluble triggering receptor expressed on myeloid cells 1 |
| TCID50 | 50% tissue culture infectious dose |
| TLP | Triple-layered particle |
| TREM-1 | Triggering receptor expressed on myeloid cells 1 |
| VDW | Van der Waals energy |
| ΔG | Gibbs free energy |
References
- Kyu, H.H.; Vongpradith, A.; Dominguez, R.-M.V.; Ma, J.; Albertson, S.B.; Novotney, A.; Khalil, I.A.; Troeger, C.E.; Doxey, M.C.; Ledesma, J.R.; et al. Global, regional, and national age-sex-specific burden of diarrhoeal diseases, their risk factors, and aetiologies, 1990–2021, for 204 countries and territories: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Infect. Dis. 2025, 25, 519–536. [Google Scholar] [CrossRef]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Bányai, K.; Estes, M.K.; Martella, V.; Parashar, U.D. Viral gastroenteritis. Lancet 2018, 392, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Burnett, E.; Jonesteller, C.L.; Tate, J.E.; Yen, C.; Parashar, U.D. Global Impact of Rotavirus Vaccination on Childhood Hospitalizations and Mortality From Diarrhea. J. Infect. Dis. 2017, 215, 1666–1672. [Google Scholar] [CrossRef]
- Tate, J.E.; Burton, A.H.; Boschi-Pinto, C.; Parashar, U.D. Global, Regional, and National Estimates of Rotavirus Mortality in Children <5 Years of Age, 2000–2013. Clin. Infect. Dis. 2016, 62 (Suppl. 2), S96–S105. [Google Scholar] [CrossRef] [PubMed]
- Matthijnssens, J.; Ciarlet, M.; McDonald, S.M.; Attoui, H.; Bányai, K.; Brister, J.R.; Buesa, J.; Esona, M.D.; Estes, M.K.; Gentsch, J.R.; et al. Uniformity of rotavirus strain nomenclature proposed by the Rotavirus Classification Working Group (RCWG). Arch. Virol. 2011, 156, 1397–1413. [Google Scholar] [CrossRef]
- Crawford, S.E.; Ding, S.; Greenberg, H.B.; Estes, M.K. Rotaviruses. In Fields Virology: RNA Viruses, 7th ed.; Knipe, D.M., Howley, P.M., Cohen, J.I., Damania, B., Enquist, L., Freed, E.O., Wheelan, S.P.J., Eds.; Wolters Kluwer Health: Filadélfia, PA, USA, 2022. [Google Scholar]
- International Committee on Taxonomy of Viruses. Taxonomy Browser. 2025. Available online: https://ictv.global/taxonomy (accessed on 10 September 2025).
- Desselberger, U. Rotaviruses. Virus Res. 2014, 190, 75–96. [Google Scholar] [CrossRef]
- Mathieu, M.; Petitpas, I.; Navaza, J.; Lepault, J.; Kohli, E.; Pothier, P.; Prasad, B.V.; Cohen, J.; Rey, F.A. Atomic structure of the major capsid protein of rotavirus: Implications for the architecture of the virion. EMBO J. 2001, 20, 1485–1497. [Google Scholar] [CrossRef]
- McClain, B.; Settembre, E.; Temple, B.R.; Bellamy, A.R.; Harrison, S.C. X-ray crystal structure of the rotavirus inner capsid particle at 3.8 A resolution. J. Mol. Biol. 2010, 397, 587–599. [Google Scholar] [CrossRef]
- Settembre, E.C.; Chen, J.Z.; Dormitzer, P.R.; Grigorieff, N.; Harrison, S.C. Atomic model of an infectious rotavirus particle. EMBO J. 2011, 30, 408–416. [Google Scholar] [CrossRef]
- Shah, P.N.M.; Gilchrist, J.B.; Forsberg, B.O.; Burt, A.; Howe, A.; Mosalaganti, S.; Wan, W.; Radecke, J.; Chaban, Y.; Sutton, G.; et al. Characterization of the rotavirus assembly pathway in situ using cryoelectron tomography. Cell Host Microbe 2023, 31, 604–615.e604. [Google Scholar] [CrossRef]
- Ciarlet, M.; Crawford, S.E.; Cheng, E.; Blutt, S.E.; Rice, D.A.; Bergelson, J.M.; Estes, M.K. VLA-2 (alpha2beta1) integrin promotes rotavirus entry into cells but is not necessary for rotavirus attachment. J. Virol. 2002, 76, 1109–1123. [Google Scholar] [CrossRef]
- Isa, P.; Arias, C.F.; López, S. Role of sialic acids in rotavirus infection. Glycoconj. J. 2006, 23, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Isa, P.; Realpe, M.; Romero, P.; López, S.; Arias, C.F. Rotavirus RRV associates with lipid membrane microdomains during cell entry. Virology 2004, 322, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ramelot, T.A.; Huang, P.; Liu, Y.; Li, Z.; Feizi, T.; Zhong, W.; Wu, F.T.; Tan, M.; Kennedy, M.A.; et al. Glycan Specificity of P [19] Rotavirus and Comparison with Those of Related P Genotypes. J. Virol. 2016, 90, 9983–9996. [Google Scholar] [CrossRef]
- López, S.; Arias, C.F. Multistep entry of rotavirus into cells: A Versaillesque dance. Trends Microbiol. 2004, 12, 271–278. [Google Scholar] [CrossRef]
- Pang, L.L.; Wang, M.X.; Sun, X.M.; Yuan, Y.; Qing, Y.; Xin, Y.; Zhang, J.Y.; Li, D.D.; Duan, Z.J. Glycan binding patterns of human rotavirus P [10] VP8* protein. Virol. J. 2018, 15, 161. [Google Scholar] [CrossRef]
- Ramani, S.; Hu, L.; Venkataram Prasad, B.V.; Estes, M.K. Diversity in Rotavirus-Host Glycan Interactions: A “Sweet” Spectrum. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 263–273. [Google Scholar] [CrossRef]
- Venkataram Prasad, B.V.; Shanker, S.; Muhaxhiri, Z.; Choi, J.M.; Atmar, R.L.; Estes, M.K. Chapter 3.1—Structural Biology of Noroviruses. In Viral Gastroenteritis; Svensson, L., Desselberger, U., Greenberg, H.B., Estes, M.K., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 329–354. [Google Scholar]
- Yoder, J.D.; Trask, S.D.; Vo, T.P.; Binka, M.; Feng, N.; Harrison, S.C.; Greenberg, H.B.; Dormitzer, P.R. VP5* rearranges when rotavirus uncoats. J. Virol. 2009, 83, 11372–11377. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Guillon, A.; Szyczew, A.J.; Kiefel, M.J.; Coulson, B.S.; von Itzstein, M.; Blanchard, H. Crystallization and preliminary X-ray diffraction analysis of the carbohydrate-recognizing domain (VP8*) of bovine rotavirus strain NCDV. Struct. Biol. Cryst. Commun. 2008, 64, 509–511. [Google Scholar] [CrossRef]
- WHO. Rotavirus vaccines. In The Immunological Basis for Immunization Series; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Gómez-Rial, J.; Sánchez-Batán, S.; Rivero-Calle, I.; Pardo-Seco, J.; Martinón-Martínez, J.M.; Salas, A.; Martinón-Torres, F. Rotavirus infection beyond the gut. Infect. Drug Resist. 2019, 12, 55–64. [Google Scholar] [CrossRef]
- Sastri, N.P.; Crawford, S.E.; Estes, M.K. Chapter 2.4—Pleiotropic Properties of Rotavirus Nonstructural Protein 4 (NSP4) and Their Effects on Viral Replication and Pathogenesis. In Viral Gastroenteritis; Svensson, L., Desselberger, U., Greenberg, H.B., Estes, M.K., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 145–174. [Google Scholar]
- Mohammed, F.S.; Uysal, İ.; Sevindik, M. A review on antiviral plants effective against different virus types. Prospect. Pharm. Sci. 2023, 21, 1–21. [Google Scholar] [CrossRef]
- Jiang, L.; Tang, A.; Song, L.; Tong, Y.; Fan, H. Advances in the development of antivirals for rotavirus infection. Front. Immunol. 2023, 14, 1041149. [Google Scholar] [CrossRef] [PubMed]
- La Frazia, S.; Ciucci, A.; Arnoldi, F.; Coira, M.; Gianferretti, P.; Angelini, M.; Belardo, G.; Burrone, O.R.; Rossignol, J.F.; Santoro, M.G. Thiazolides, a new class of antiviral agents effective against rotavirus infection, target viral morphogenesis, inhibiting viroplasm formation. J. Virol. 2013, 87, 11096–11106. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, X.; Wu, J.; Lin, X.; Chen, R.; Lu, C.; Song, X.; Leng, Q.; Li, Y.; Kuang, X.; et al. ML241 Antagonizes ERK 1/2 Activation and Inhibits Rotavirus Proliferation. Viruses 2024, 16, 623. [Google Scholar] [CrossRef]
- Bassetto, M.; Van Dycke, J.; Neyts, J.; Brancale, A.; Rocha-Pereira, J. Targeting the Viral Polymerase of Diarrhea-Causing Viruses as a Strategy to Develop a Single Broad-Spectrum Antiviral Therapy. Viruses 2019, 11, 173. [Google Scholar] [CrossRef]
- Holloway, G.; Coulson, B.S. Innate cellular responses to rotavirus infection. J. Gen. Virol. 2013, 94, 1151–1160. [Google Scholar] [CrossRef]
- Angel, J.; Franco, M.A.; Greenberg, H.B. Rotavirus immune responses and correlates of protection. Curr. Opin. Virol. 2012, 2, 419–425. [Google Scholar] [CrossRef]
- Broquet, A.H.; Hirata, Y.; McAllister, C.S.; Kagnoff, M.F. RIG-I/MDA5/MAVS are required to signal a protective IFN response in rotavirus-infected intestinal epithelium. J. Immunol. 2011, 186, 1618–1626. [Google Scholar] [CrossRef]
- Schoggins, J.W.; MacDuff, D.A.; Imanaka, N.; Gainey, M.D.; Shrestha, B.; Eitson, J.L.; Mar, K.B.; Richardson, R.B.; Ratushny, A.V.; Litvak, V.; et al. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature 2014, 505, 691–695. [Google Scholar] [CrossRef]
- Sen, A.; Pruijssers, A.J.; Dermody, T.S.; García-Sastre, A.; Greenberg, H.B. The early interferon response to rotavirus is regulated by PKR and depends on MAVS/IPS-1, RIG-I, MDA-5, and IRF3. J. Virol. 2011, 85, 3717–3732. [Google Scholar] [CrossRef]
- Pott, J.; Stockinger, S.; Torow, N.; Smoczek, A.; Lindner, C.; McInerney, G.; Bäckhed, F.; Baumann, U.; Pabst, O.; Bleich, A.; et al. Age-dependent TLR3 expression of the intestinal epithelium contributes to rotavirus susceptibility. PLoS Pathog. 2012, 8, e1002670. [Google Scholar] [CrossRef]
- Zhu, S.; Ding, S.; Wang, P.; Wei, Z.; Pan, W.; Palm, N.W.; Yang, Y.; Yu, H.; Li, H.B.; Wang, G.; et al. Nlrp9b inflammasome restricts rotavirus infection in intestinal epithelial cells. Nature 2017, 546, 667–670. [Google Scholar] [CrossRef]
- Dantas, P.; Matos, A.O.; da Silva Filho, E.; Silva-Sales, M.; Sales-Campos, H. Triggering receptor expressed on myeloid cells-1 (TREM-1) as a therapeutic target in infectious and noninfectious disease: A critical review. Int. Rev. Immunol. 2020, 39, 188–202. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Matos, A.; Dos Santos Dantas, P.H.; Figueira Marques Silva-Sales, M.; Sales-Campos, H. The role of the triggering receptor expressed on myeloid cells-1 (TREM-1) in non-bacterial infections. Crit. Rev. Microbiol. 2020, 46, 237–252. [Google Scholar] [CrossRef]
- Matos, A.O.; Dantas, P.; Silva-Sales, M.; Sales-Campos, H. TREM-1 isoforms in bacterial infections: To immune modulation and beyond. Crit. Rev. Microbiol. 2021, 47, 290–306. [Google Scholar] [CrossRef]
- Amrun, S.N.; Tan, J.J.L.; Rickett, N.Y.; Cox, J.A.; Lee, B.; Griffiths, M.J.; Solomon, T.; Perera, D.; Ooi, M.H.; Hiscox, J.A.; et al. TREM-1 activation is a potential key regulator in driving severe pathogenesis of enterovirus A71 infection. Sci. Rep. 2020, 10, 3810. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.R.; To, R.K.; Spector, S.A. TREM-1 Protects HIV-1-Infected Macrophages from Apoptosis through Maintenance of Mitochondrial Function. mBio 2019, 10, 10-1128. [Google Scholar] [CrossRef]
- Hyun, J.; McMahon, R.S.; Lang, A.L.; Edwards, J.S.; Badilla, A.D.; Greene, M.E.; Stone, G.W.; Pallikkuth, S.; Stevenson, M.; Dykxhoorn, D.M.; et al. HIV and HCV augments inflammatory responses through increased TREM-1 expression and signaling in Kupffer and Myeloid cells. PLoS Pathog. 2019, 15, e1007883. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Belcaid, M.; Nerurkar, V.R. Identification of host genes leading to West Nile virus encephalitis in mice brain using RNA-seq analysis. Sci. Rep. 2016, 6, 26350. [Google Scholar] [CrossRef]
- Mohamadzadeh, M.; Coberley, S.S.; Olinger, G.G.; Kalina, W.V.; Ruthel, G.; Fuller, C.L.; Swenson, D.L.; Pratt, W.D.; Kuhns, D.B.; Schmaljohn, A.L. Activation of triggering receptor expressed on myeloid cells-1 on human neutrophils by marburg and ebola viruses. J. Virol. 2006, 80, 7235–7244. [Google Scholar] [CrossRef]
- Mukhamedova, N.; Hoang, A.; Dragoljevic, D.; Dubrovsky, L.; Pushkarsky, T.; Low, H.; Ditiatkovski, M.; Fu, Y.; Ohkawa, R.; Meikle, P.J.; et al. Exosomes containing HIV protein Nef reorganize lipid rafts potentiating inflammatory response in bystander cells. PLoS Pathog. 2019, 15, e1007907. [Google Scholar] [CrossRef]
- Yuan, Z.; Fan, X.; Staitieh, B.; Bedi, C.; Spearman, P.; Guidot, D.M.; Sadikot, R.T. HIV-related proteins prolong macrophage survival through induction of Triggering receptor expressed on myeloid cells-1. Sci. Rep. 2017, 7, 42028. [Google Scholar] [CrossRef]
- Bosco, M.C.; Pierobon, D.; Blengio, F.; Raggi, F.; Vanni, C.; Gattorno, M.; Eva, A.; Novelli, F.; Cappello, P.; Giovarelli, M.; et al. Hypoxia modulates the gene expression profile of immunoregulatory receptors in human mature dendritic cells: Identification of TREM-1 as a novel hypoxic marker in vitro and in vivo. Blood 2011, 117, 2625–2639. [Google Scholar] [CrossRef]
- Bouchon, A.; Dietrich, J.; Colonna, M. Cutting edge: Inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J. Immunol. 2000, 164, 4991–4995. [Google Scholar] [CrossRef]
- Chen, L.C.; Laskin, J.D.; Gordon, M.K.; Laskin, D.L. Regulation of TREM expression in hepatic macrophages and endothelial cells during acute endotoxemia. Exp. Mol. Pathol. 2008, 84, 145–155. [Google Scholar] [CrossRef]
- Gingras, M.C.; Lapillonne, H.; Margolin, J.F. TREM-1, MDL-1, and DAP12 expression is associated with a mature stage of myeloid development. Mol. Immunol. 2002, 38, 817–824. [Google Scholar] [CrossRef]
- Rigo, I.; McMahon, L.; Dhawan, P.; Christakos, S.; Yim, S.; Ryan, L.K.; Diamond, G. Induction of triggering receptor expressed on myeloid cells (TREM-1) in airway epithelial cells by 1,25(OH)2 vitamin D3. Innate Immun. 2012, 18, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Schmausser, B.; Endrich, S.; Beier, D.; Moran, A.P.; Burek, C.J.; Rosenwald, A.; Rieckmann, P.; Müller-Hermelink, H.K.; Eck, M. Triggering receptor expressed on myeloid cells-1 (TREM-1) expression on gastric epithelium: Implication for a role of TREM-1 in Helicobacter pylori infection. Clin. Exp. Immunol. 2008, 152, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Tammaro, A.; Derive, M.; Gibot, S.; Leemans, J.C.; Florquin, S.; Dessing, M.C. TREM-1 and its potential ligands in non-infectious diseases: From biology to clinical perspectives. Pharmacol. Ther. 2017, 177, 81–95. [Google Scholar] [CrossRef]
- Bleharski, J.R.; Kiessler, V.; Buonsanti, C.; Sieling, P.A.; Stenger, S.; Colonna, M.; Modlin, R.L. A role for triggering receptor expressed on myeloid cells-1 in host defense during the early-induced and adaptive phases of the immune response. J. Immunol. 2003, 170, 3812–3818. [Google Scholar] [CrossRef]
- Boufenzer, A.; Carrasco, K.; Jolly, L.; Brustolin, B.; Di-Pillo, E.; Derive, M.; Gibot, S. Potentiation of NETs release is novel characteristic of TREM-1 activation and the pharmacological inhibition of TREM-1 could prevent from the deleterious consequences of NETs release in sepsis. Cell. Mol. Immunol. 2021, 18, 452–460. [Google Scholar] [CrossRef]
- Dower, K.; Ellis, D.K.; Saraf, K.; Jelinsky, S.A.; Lin, L.L. Innate immune responses to TREM-1 activation: Overlap, divergence, and positive and negative cross-talk with bacterial lipopolysaccharide. J. Immunol. 2008, 180, 3520–3534. [Google Scholar] [CrossRef]
- Netea, M.G.; Azam, T.; Ferwerda, G.; Girardin, S.E.; Kim, S.H.; Dinarello, C.A. Triggering receptor expressed on myeloid cells-1 (TREM-1) amplifies the signals induced by the NACHT-LRR (NLR) pattern recognition receptors. J. Leukoc. Biol. 2006, 80, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Prüfer, S.; Weber, M.; Sasca, D.; Teschner, D.; Wölfel, C.; Stein, P.; Stassen, M.; Schild, H.; Radsak, M.P. Distinct signaling cascades of TREM-1, TLR and NLR in neutrophils and monocytic cells. J. Innate Immun. 2014, 6, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Radsak, M.P.; Salih, H.R.; Rammensee, H.G.; Schild, H. Triggering receptor expressed on myeloid cells-1 in neutrophil inflammatory responses: Differential regulation of activation and survival. J. Immunol. 2004, 172, 4956–4963. [Google Scholar] [CrossRef] [PubMed]
- Schenk, M.; Bouchon, A.; Seibold, F.; Mueller, C. TREM-1--expressing intestinal macrophages crucially amplify chronic inflammation in experimental colitis and inflammatory bowel diseases. J. Clin. Investig. 2007, 117, 3097–3106. [Google Scholar] [CrossRef]
- Denning, N.L.; Aziz, M.; Murao, A.; Gurien, S.D.; Ochani, M.; Prince, J.M.; Wang, P. Extracellular CIRP as an endogenous TREM-1 ligand to fuel inflammation in sepsis. JCI Insight 2020, 5, e134172. [Google Scholar] [CrossRef]
- Fu, L.; Han, L.; Xie, C.; Li, W.; Lin, L.; Pan, S.; Zhou, Y.; Li, Z.; Jin, M.; Zhang, A. Identification of Extracellular Actin As a Ligand for Triggering Receptor Expressed on Myeloid Cells-1 Signaling. Front. Immunol. 2017, 8, 917. [Google Scholar] [CrossRef]
- Read, C.B.; Kuijper, J.L.; Hjorth, S.A.; Heipel, M.D.; Tang, X.; Fleetwood, A.J.; Dantzler, J.L.; Grell, S.N.; Kastrup, J.; Wang, C.; et al. Cutting Edge: Identification of neutrophil PGLYRP1 as a ligand for TREM-1. J. Immunol. 2015, 194, 1417–1421. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.; Salcedo, R.; Mivechi, N.F.; Trinchieri, G.; Horuzsko, A. The proinflammatory myeloid cell receptor TREM-1 controls Kupffer cell activation and development of hepatocellular carcinoma. Cancer Res. 2012, 72, 3977–3986. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]
- Lei, C.; Yang, J.; Hu, J.; Sun, X. On the Calculation of TCID(50) for Quantitation of Virus Infectivity. Virol. Sin. 2021, 36, 141–144. [Google Scholar] [CrossRef]
- Davis, B.D.; Dulbecco, R.; Eisen, H.N.; Ginsberg, H.S.; Wood, W.B. Nature of viruses. Microbiology 1972, 437, 1044–1053. [Google Scholar]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Ge, Y.; Mansell, A.; Ussher, J.E.; Brooks, A.E.; Manning, K.; Wang, C.J.; Taylor, J.A. Rotavirus NSP4 Triggers Secretion of Proinflammatory Cytokines from Macrophages via Toll-Like Receptor 2. J. Virol. 2013, 87, 11160–11167. [Google Scholar] [CrossRef] [PubMed]
- Graham, K.L.; Fleming, F.E.; Halasz, P.; Hewish, M.J.; Nagesha, H.S.; Holmes, I.H.; Takada, Y.; Coulson, B.S. Rotaviruses interact with alpha4beta7 and alpha4beta1 integrins by binding the same integrin domains as natural ligands. J. Gen. Virol. 2005, 86, 3397–3408. [Google Scholar] [CrossRef]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making protein folding accessible to all. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef]
- Williams, C.J.; Headd, J.J.; Moriarty, N.W.; Prisant, M.G.; Videau, L.L.; Deis, L.N.; Verma, V.; Keedy, D.A.; Hintze, B.J.; Chen, V.B.; et al. MolProbity: More and better reference data for improved all-atom structure validation. Protein Sci. A Publ. Protein Soc. 2018, 27, 293–315. [Google Scholar] [CrossRef]
- Jones, G.; Jindal, A.; Ghani, U.; Kotelnikov, S.; Egbert, M.; Hashemi, N.; Vajda, S.; Padhorny, D.; Kozakov, D. Elucidation of protein function using computational docking and hotspot analysis by ClusPro and FTMap. Biol. Crystallogr. 2022, 78, 690–697. [Google Scholar] [CrossRef]
- Kozakov, D.; Beglov, D.; Bohnuud, T.; Mottarella, S.E.; Xia, B.; Hall, D.R.; Vajda, S. How good is automated protein docking? Proteins 2013, 81, 2159–2166. [Google Scholar] [CrossRef]
- Kozakov, D.; Hall, D.R.; Xia, B.; Porter, K.A.; Padhorny, D.; Yueh, C.; Beglov, D.; Vajda, S. The ClusPro web server for protein-protein docking. Nat. Protoc. 2017, 12, 255–278. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Sukhwal, A.; Sowdhamini, R. Oligomerisation status and evolutionary conservation of interfaces of protein structural domain superfamilies. Mol. Biosyst. 2013, 9, 1652–1661. [Google Scholar] [CrossRef]
- Laskowski, R.A. PDBsum1: A standalone program for generating PDBsum analyses. Protein Sci. 2022, 31, e4473. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Weng, G.; Wang, E.; Wang, Z.; Liu, H.; Zhu, F.; Li, D.; Hou, T. HawkDock: A web server to predict and analyze the protein-protein complex based on computational docking and MM/GBSA. Nucleic Acids Res. 2019, 47, W322–W330. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.C.; Rodrigues, J.P.; Kastritis, P.L.; Bonvin, A.M.; Vangone, A. PRODIGY: A web server for predicting the binding affinity of protein-protein complexes. Bioinformatics 2016, 32, 3676–3678. [Google Scholar] [CrossRef]
- Moal, I.H.; Jiménez-García, B.; Fernández-Recio, J. CCharPPI web server: Computational characterization of protein-protein interactions from structure. Bioinformatics 2015, 31, 123–125. [Google Scholar] [CrossRef]
- Roy, A.A.; Dhawanjewar, A.S.; Sharma, P.; Singh, G.; Madhusudhan, M.S. Protein Interaction Z Score Assessment (PIZSA): An empirical scoring scheme for evaluation of protein-protein interactions. Nucleic Acids Res. 2019, 47, W331–W337. [Google Scholar] [CrossRef]
- Bessho, K.; Shanmukhappa, K.; Sheridan, R.; Shivakumar, P.; Mourya, R.; Walters, S.; Kaimal, V.; Dilbone, E.; Jegga, A.G.; Bezerra, J.A. Integrative genomics identifies candidate microRNAs for pathogenesis of experimental biliary atresia. BMC Syst. Biol. 2013, 7, 104. [Google Scholar] [CrossRef]
- Tsuge, M.; Oka, T.; Yamashita, N.; Saito, Y.; Fujii, Y.; Nagaoka, Y.; Yashiro, M.; Tsukahara, H.; Morishima, T. Gene expression analysis in children with complex seizures due to influenza A(H1N1)pdm09 or rotavirus gastroenteritis. J. Neurovirol. 2014, 20, 73–84. [Google Scholar] [CrossRef]
- DeBerg, H.A.; Zaidi, M.B.; Altman, M.C.; Khaenam, P.; Gersuk, V.H.; Campos, F.D.; Perez-Martinez, I.; Meza-Segura, M.; Chaussabel, D.; Banchereau, J.; et al. Shared and organism-specific host responses to childhood diarrheal diseases revealed by whole blood transcript profiling. PLoS ONE 2018, 13, e0192082. [Google Scholar] [CrossRef]
- Kelker, M.S.; Foss, T.R.; Peti, W.; Teyton, L.; Kelly, J.W.; Wüthrich, K.; Wilson, I.A. Crystal Structure of Human Triggering Receptor Expressed on Myeloid Cells 1 (TREM-1) at 1.47Å. J. Mol. Biol. 2004, 342, 1237–1248. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Matos, A.; Dos Santos Dantas, P.H.; Colmenares, M.T.C.; Sartori, G.R.; Silva-Sales, M.; Da Silva, J.H.M.; Neves, B.J.; Andrade, C.H.; Sales-Campos, H. The CDR3 region as the major driver of TREM-1 interaction with its ligands, an in silico characterization. Comput. Struct. Biotechnol. J. 2023, 21, 2579–2590. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.S.; Kim, S.E.; Heo, J.Y.; Lee, M.E.; Kim, H.M.; Paik, S.G.; Lee, H.; Lee, J.O. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 2007, 130, 1071–1082. [Google Scholar] [CrossRef]
- Fortin, C.F.; Lesur, O.; Fulop, T., Jr. Effects of TREM-1 activation in human neutrophils: Activation of signaling pathways, recruitment into lipid rafts and association with TLR4. Int. Immunol. 2007, 19, 41–50. [Google Scholar] [CrossRef]
- Murao, A.; Arif, A.; Brenner, M.; Denning, N.L.; Jin, H.; Takizawa, S.; Nicastro, B.; Wang, P.; Aziz, M. Extracellular CIRP and TREM-1 axis promotes ICAM-1-Rho-mediated NETosis in sepsis. FASEB J. 2020, 34, 9771–9786. [Google Scholar] [CrossRef]
- Chen, N.; Jin, J.; Qiao, B.; Gao, Z.; Tian, Y.; Ping, J. JNK kinase promotes inflammatory responses by inducing the expression of the inflammatory amplifier TREM1 during influenza a virus infection. Virus Res. 2025, 356, 199577. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.; Schuster, S.; Zysset, D.; Rihs, S.; Dickgreber, N.; Schürch, C.; Riether, C.; Siegrist, M.; Schneider, C.; Pawelski, H.; et al. TREM-1 deficiency can attenuate disease severity without affecting pathogen clearance. PLoS Pathog. 2014, 10, e1003900. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, W.; Miao, Q.; Shi, S.; Wei, B.; Luo, L.; Cai, B. CCR2+TREM-1+ monocytes promote natural killer T cell dysfunction contributing towards HBV disease progression. Immunol. Res. 2024, 72, 938–947. [Google Scholar] [CrossRef]
- Wu, X.; Cai, B.; Lu, W.; Fu, Y.; Wei, B.; Niu, Q.; Su, Z.; Li, Y.; Wang, L. HBV upregulated triggering receptor expressed on myeloid cells-1 (TREM-1) expression on monocytes participated in disease progression through NF-Kb pathway. Clin. Immunol. 2021, 223, 108650. [Google Scholar] [CrossRef] [PubMed]
- Alipanah-Lechner, N.; Hurst-Hopf, J.; Delucchi, K.; Swigart, L.; Willmore, A.; LaCombe, B.; Dewar, R.; Lane, H.C.; Lallemand, P.; Liu, K.D.; et al. Novel subtypes of severe COVID-19 respiratory failure based on biological heterogeneity: A secondary analysis of a randomized controlled trial. Crit. Care 2024, 28, 56. [Google Scholar] [CrossRef]
- de Nooijer, A.H.; Grondman, I.; Lambden, S.; Kooistra, E.J.; Janssen, N.A.F.; Kox, M.; Pickkers, P.; Joosten, L.A.B.; van de Veerdonk, F.L.; Derive, M.; et al. Increased sTREM-1 plasma concentrations are associated with poor clinical outcomes in patients with COVID-19. Biosci. Rep. 2021, 41, BSR20210940. [Google Scholar] [CrossRef]
- Fan, R.; Cheng, Z.; Huang, Z.; Yang, Y.; Sun, N.; Hu, B.; Hou, P.; Liu, B.; Huang, C.; Liu, S. TREM-1, TREM-2 and their association with disease severity in patients with COVID-19. Ann. Med. 2023, 55, 2269558. [Google Scholar] [CrossRef]
- Paranga, T.G.; Pavel-Tanasa, M.; Constantinescu, D.; Plesca, C.E.; Petrovici, C.; Miftode, I.L.; Moscalu, M.; Cianga, P.; Miftode, E.G. Comparison of C-reactive protein with distinct hyperinflammatory biomarkers in association with COVID-19 severity, mortality and SARS-CoV-2 variants. Front. Immunol. 2023, 14, 1213246. [Google Scholar] [CrossRef]
- Colmenares, M.T.C.; Matos, A.O.; Dantas, P.; Neto, J.; Neves, B.J.; Gardinassi, L.G.A.; Silva-Sales, M.; Sales-Campos, H. TREM-1 as a Potential Coreceptor in Norovirus Pathogenesis: Insights from Transcriptomic Analysis and Molecular Docking. ACS Omega 2025, 10, 4881–4895. [Google Scholar] [CrossRef] [PubMed]
- Vinolo, E.; Maillefer, M.; Jolly, L.; Colné, N.; Meiffren, G.; Carrasco, K.; Derive, M. The potential of targeting TREM-1 in IBD. Adv. Pharmacol. 2024, 101, 301–330. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Matos, A.; Henrique Dos Santos Dantas, P.; Rodrigues do Carmo Neto, J.; Contreras Colmenares, M.T.; Felice, A.G.; de Castro Soares, S.; Silva-Sales, M.; Sales-Campos, H. Uncovering the role of TREM-1 in celiac disease: In silico insights into the recognition of gluten-derived peptides and inflammatory mechanisms. Comput. Biol. Med. 2025, 189, 109981. [Google Scholar] [CrossRef] [PubMed]
- Altay, F.A.; Elaldi, N.; Şentürk, G.; Altin, N.; Gözel, M.G.; Albayrak, Y.; Şencan, İ. Serum sTREM-1 level is quite higher in Crimean Congo Hemorrhagic Fever, a viral infection. J. Med. Virol. 2016, 88, 1473–1478. [Google Scholar] [CrossRef]
- Ruiz-Pacheco, J.A.; Vivanco-Cid, H.; Izaguirre-Hernández, I.Y.; Estrada-García, I.; Arriaga-Pizano, L.; Chacón-Salinas, R.; Fonseca-Coronado, S.; Vaughan, G.; Tovar, K.R.; Rivera-Osorio, M.P.; et al. TREM-1 modulation during early stages of dengue virus infection. Immunol. Lett. 2014, 158, 183–188. [Google Scholar] [CrossRef]
- Carrasco, K.; Boufenzer, A.; Jolly, L.; Le Cordier, H.; Wang, G.; Heck, A.J.; Cerwenka, A.; Vinolo, E.; Nazabal, A.; Kriznik, A.; et al. TREM-1 multimerization is essential for its activation on monocytes and neutrophils. Cell. Mol. Immunol. 2019, 16, 460–472. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Piña, V.; Soares-Schanoski, A.; Rodríguez-Rojas, A.; Del Fresno, C.; García, F.; Vallejo-Cremades, M.T.; Fernández-Ruiz, I.; Arnalich, F.; Fuentes-Prior, P.; López-Collazo, E. Metalloproteinases shed TREM-1 ectodomain from lipopolysaccharide-stimulated human monocytes. J. Immunol. 2007, 179, 4065–4073. [Google Scholar] [CrossRef]
- Weiss, G.; Lai, C.; Fife, M.E.; Grabiec, A.M.; Tildy, B.; Snelgrove, R.J.; Xin, G.; Lloyd, C.M.; Hussell, T. Reversal of TREM-1 ectodomain shedding and improved bacterial clearance by intranasal metalloproteinase inhibitors. Mucosal Immunol. 2017, 10, 1021–1030. [Google Scholar] [CrossRef]
- Jolly, L.; Carrasco, K.; Salcedo-Magguilli, M.; Garaud, J.J.; Lambden, S.; van der Poll, T.; Mebazaa, A.; Laterre, P.F.; Gibot, S.; Boufenzer, A.; et al. sTREM-1 is a specific biomarker of TREM-1 pathway activation. Cell. Mol. Immunol. 2021, 18, 2054–2056. [Google Scholar] [CrossRef] [PubMed]
- Bruneel, F.; Tubach, F.; Mira, J.P.; Houze, S.; Gibot, S.; Huisse, M.G.; Megarbane, B.; Choquet, C.; Corne, P.; Peytel, E.; et al. Imported falciparum malaria in adults: Host- and parasite-related factors associated with severity. The French prospective multicenter PALUREA cohort study. Intensive Care Med. 2016, 42, 1588–1596. [Google Scholar] [CrossRef]
- Horst, S.A.; Linnér, A.; Beineke, A.; Lehne, S.; Höltje, C.; Hecht, A.; Norrby-Teglund, A.; Medina, E.; Goldmann, O. Prognostic value and therapeutic potential of TREM-1 in Streptococcus pyogenes- induced sepsis. J. Innate Immun. 2013, 5, 581–590. [Google Scholar] [CrossRef]
- Kozik, J.H.; Trautmann, T.; Carambia, A.; Preti, M.; Lütgehetmann, M.; Krech, T.; Wiegard, C.; Heeren, J.; Herkel, J. Attenuated viral hepatitis in Trem1-/- mice is associated with reduced inflammatory activity of neutrophils. Sci. Rep. 2016, 6, 28556. [Google Scholar] [CrossRef]
- Chen, S.-M.; Lin, C.-P.; Tsai, J.-D.; Chao, Y.-H.; Sheu, J.-N. The Significance of Serum and Fecal Levels of Interleukin-6 and Interleukin-8 in Hospitalized Children with Acute Rotavirus and Norovirus Gastroenteritis. Pediatr. Neonatol. 2014, 55, 120–126. [Google Scholar] [CrossRef]
- Di Fiore, I.J.M.; Holloway, G.; Coulson, B.S. Innate immune responses to rotavirus infection in macrophages depend on MAVS but involve neither the NLRP3 inflammasome nor JNK and p38 signaling pathways. Virus Res. 2015, 208, 89–97. [Google Scholar] [CrossRef]
- Gómez-Rial, J.; Curras-Tuala, M.J.; Rivero-Calle, I.; Rodríguez-Tenreiro, C.; Redondo-Collazo, L.; Gómez-Carballa, A.; Pardo-Seco, J.; Salas, A.; Martinón-Torres, F. Rotavirus intestinal infection induces an oral mucosa cytokine response. PLoS ONE 2018, 13, e0195314. [Google Scholar] [CrossRef]
- Hagbom, M.; Hellysaz, A.; Istrate, C.; Nordgren, J.; Sharma, S.; de-Faria, F.M.; Magnusson, K.E.; Svensson, L. The 5-HT(3) Receptor Affects Rotavirus-Induced Motility. J. Virol. 2021, 95, e0075121. [Google Scholar] [CrossRef]
- Jiang, B.; Snipes-Magaldi, L.; Dennehy, P.; Keyserling, H.; Holman, R.C.; Bresee, J.; Gentsch, J.; Glass, R.I. Cytokines as Mediators for or Effectors against Rotavirus Disease in Children. Clin. Vaccine Immunol. 2003, 10, 995–1001. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, K.; Kim, W. Genipin inhibits rotavirus-induced diarrhea by suppressing viral replication and regulating inflammatory responses. Sci. Rep. 2020, 10, 15836. [Google Scholar] [CrossRef]
- Deal, E.M.; Jaimes, M.C.; Crawford, S.E.; Estes, M.K.; Greenberg, H.B. Rotavirus structural proteins and dsRNA are required for the human primary plasmacytoid dendritic cell IFNalpha response. PLoS Pathog. 2010, 6, e1000931. [Google Scholar] [CrossRef]
- Hagbom, M.; Istrate, C.; Engblom, D.; Karlsson, T.; Rodriguez-Diaz, J.; Buesa, J.; Taylor, J.A.; Loitto, V.M.; Magnusson, K.E.; Ahlman, H.; et al. Rotavirus stimulates release of serotonin (5-HT) from human enterochromaffin cells and activates brain structures involved in nausea and vomiting. PLoS Pathog. 2011, 7, e1002115. [Google Scholar] [CrossRef]
- Hakim, M.S.; Ding, S.; Chen, S.; Yin, Y.; Su, J.; van der Woude, C.J.; Fuhler, G.M.; Peppelenbosch, M.P.; Pan, Q.; Wang, W. TNF-α exerts potent anti-rotavirus effects via the activation of classical NF-κB pathway. Virus Res. 2018, 253, 28–37. [Google Scholar] [CrossRef]
- Boraschi, D. What Is IL-1 for? The Functions of Interleukin-1 Across Evolution. Front. Immunol. 2022, 13, 872155. [Google Scholar] [CrossRef]
- Nishimoto, N.; Kishimoto, T. Interleukin 6: From bench to bedside. Nat. Clin. Pract. Rheumatol. 2006, 2, 619–626. [Google Scholar] [CrossRef]
- Aggeletopoulou, I.; Kalafateli, M.; Tsounis, E.P.; Triantos, C. Exploring the role of IL-1β in inflammatory bowel disease pathogenesis. Front. Med. 2024, 11, 1307394. [Google Scholar] [CrossRef]
- Alhendi, A.; Naser, S.A. The dual role of interleukin-6 in Crohn’s disease pathophysiology. Front. Immunol. 2023, 14, 1295230. [Google Scholar] [CrossRef]
- Kandil, H.M.; Berschneider, H.M.; Argenzio, R.A. Tumour necrosis factor alpha changes porcine intestinal ion transport through a paracrine mechanism involving prostaglandins. Gut 1994, 35, 934–940. [Google Scholar] [CrossRef]
- Oprins, J.C.; Meijer, H.P.; Groot, J.A. TNF-alpha potentiates the ion secretion induced by muscarinic receptor activation in HT29cl.19A cells. Am. J. Physiol. Cell Physiol. 2000, 278, C463–C472. [Google Scholar] [CrossRef]
- Roth, J.; De Souza, G.E. Fever induction pathways: Evidence from responses to systemic or local cytokine formation. Braz. J. Med. Biol. Res. 2001, 34, 301–314. [Google Scholar] [CrossRef]
- Mota, C.M.D.; Madden, C.J. Neural circuits mediating circulating interleukin-1β-evoked fever in the absence of prostaglandin E2 production. Brain Behav. Immun. 2022, 103, 109–121. [Google Scholar] [CrossRef]
- Lopez, S.; Arias, C.F. Early steps in rotavirus cell entry. Curr. Top. Microbiol. Immunol. 2006, 309, 39–66. [Google Scholar] [CrossRef]
- Venkataram Prasad, B.V.; Shanker, S.; Hu, L.; Choi, J.M.; Crawford, S.E.; Ramani, S.; Czako, R.; Atmar, R.L.; Estes, M.K. Structural basis of glycan interaction in gastroenteric viral pathogens. Curr. Opin. Virol. 2014, 7, 119–127. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Isa, P.; Sánchez-San Martin, C.; Pérez-Vargas, J.; Espinosa, R.; Arias, C.F.; López, S. Different rotavirus strains enter MA104 cells through different endocytic pathways: The role of clathrin-mediated endocytosis. J. Virol. 2010, 84, 9161–9169. [Google Scholar] [CrossRef]
- Arias, C.F.; Silva-Ayala, D.; López, S. Rotavirus entry: A deep journey into the cell with several exits. J. Virol. 2015, 89, 890–893. [Google Scholar] [CrossRef]
- Cuadras, M.A.; Bordier, B.B.; Zambrano, J.L.; Ludert, J.E.; Greenberg, H.B. Dissecting rotavirus particle-raft interaction with small interfering RNAs: Insights into rotavirus transit through the secretory pathway. J. Virol. 2006, 80, 3935–3946. [Google Scholar] [CrossRef]
- Cuadras, M.A.; Greenberg, H.B. Rotavirus infectious particles use lipid rafts during replication for transport to the cell surface in vitro and in vivo. Virology 2003, 313, 308–321. [Google Scholar] [CrossRef]
- Martínez, M.A.; López, S.; Arias, C.F.; Isa, P. Gangliosides have a functional role during rotavirus cell entry. J. Virol. 2013, 87, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Ehehalt, R. Cholesterol, lipid rafts, and disease. J. Clin. Investig. 2002, 110, 597–603. [Google Scholar] [CrossRef]
- Guerrero, C.A.; Méndez, E.; Zárate, S.; Isa, P.; López, S.; Arias, C.F. Integrin alpha(v)beta(3) mediates rotavirus cell entry. Proc. Natl. Acad. Sci. USA 2000, 97, 14644–14649. [Google Scholar] [CrossRef]
- Sánchez-San Martín, C.; López, T.; Arias, C.F.; López, S. Characterization of rotavirus cell entry. J. Virol. 2004, 78, 2310–2318. [Google Scholar] [CrossRef]
- Ball, J.M.; Tian, P.; Zeng, C.Q.; Morris, A.P.; Estes, M.K. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science 1996, 272, 101–104. [Google Scholar] [CrossRef]
- Begum, N.A.; Ishii, K.; Kurita-Taniguchi, M.; Tanabe, M.; Kobayashi, M.; Moriwaki, Y.; Matsumoto, M.; Fukumori, Y.; Azuma, I.; Toyoshima, K.; et al. Mycobacterium bovis BCG cell wall-specific differentially expressed genes identified by differential display and cDNA subtraction in human macrophages. Infect. Immun. 2004, 72, 937–948. [Google Scholar] [CrossRef]
- Zhang, M.; Zeng, C.Q.; Morris, A.P.; Estes, M.K. A functional NSP4 enterotoxin peptide secreted from rotavirus-infected cells. J. Virol. 2000, 74, 11663–11670. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, T.; Tatsumi, M.; Tsutsumi, H. Virulence-associated genome mutations of murine rotavirus identified by alternating serial passages in mice and cell cultures. J. Virol. 2014, 88, 5543–5558. [Google Scholar] [CrossRef]

| Protein Modeling | ||||
| Genotypes | P[4] | P[8] | P[9] | SA-11 |
| Genbank ID | BBA27072.1 | BBE28644.1 | ATI14959.1 | ABH10616.1 |
| Amino acids | 248–479 | 248–479 | 248–479 | 247–479 |
| Molprobity score | 0.87 | 1.0 | 1.24 | 1.15 |
| Clashscore | 1.37 | 2.2 | 4.14 | 2.47 |
| Poor rotamers | 0.49% | 0.49% | 0.98% | 0.99% |
| Favored rotamers | 99.02% | 99.02% | 98.53% | 97.03% |
| Ramachandran outliers | 0.43% | 0.43% | 0.43% | 0.43% |
| Ramachandran favored | 98.27% | 98.27% | 97.84% | 97.39% |
| Bad bonds | 0% | 0% | 0% | 0% |
| Bad angles | 0.19% | 0.23% | 0.16% | 0.27% |
| Molecular Docking | ||||
| Complex | P[4]/TREM-1 | P[8]/TREM-1 | P[9]/TREM-1 | SA-11/ITGA4 |
| ΔG (kcal/mol) | −46.2 | −67.8 | −73.0 | −112.0 |
| Kd (M) (36.5 °C) | 2.3 × 10−7 | 2.3 × 10−7 | 2.8 × 10−8 | 4 × 10−15 |
| PYDOCK_TOT (−60 a −5) | −47.957 | −42.631 | −36.571 | −66.499 |
| ELE (−60 a 0) | −13.13 | −16.422 | −16.433 | −15.808 |
| HBOND (−15 a −1) | −4.37 | −8.55 | −5.03 | 0.0 |
| VDW (−200 a −50) | −78.941 | −89.175 | −104.721 | −185.153 |
| FA_ATR (−100 a −20) | −43.377 | −56.54 | −62.045 | −125.133 |
| DESOLV (−30 a 20) | −26.932 | −17.291 | −9.666 | −32.176 |
| Z-score (binder: >1.000) | 1.802 | 1.442 | 1.757 | 1.547 |
| Protein Modeling | ||||
| Genotypes | E1 | E2 | E3 | BOV |
| Genbank ID | AYJ18795.1 | AWH62678.1 | ALJ03222.1 | K03384.1 |
| Molprobity score | 2.17 | 1.86 | 2.00 | 2.17 |
| Clashscore | 3.46 | 2.07 | 3.77 | 2.4 |
| Poor rotamers | 4.24% | 3.09% | 3.03% | 5.49% |
| Favored rotamers | 90.91% | 90.12% | 92.12% | 90.85% |
| Ramachandran outliers | 2.89% | 2.31% | 2.89% | 3.47% |
| Ramachandran favored | 89.60% | 91.33% | 92.49% | 88.44% |
| Bad bonds | 0% | 0% | 0% | 0% |
| Bad angles | 0.36% | 0.57% | 0.41% | 0.26% |
| Molecular Docking | ||||
| Complex | E1/TREM-1 | E2/TREM-1 | E3/TREM-1 | BOV/TLR2 |
| ΔG (kcal/mol) | −76.66 | −74.79 | −59.38 | −42.15 |
| Kd (M) (36.5 °C) | 1.4 × 10−8 | 2.4 × 10−9 | 9.6 × 10−8 | 3.8 × 10−11 |
| PYDOCK_TOT (−60 a −5) | −45.782 | −48.499 | −60.849 | −54.45 |
| ELE (−60 a 0) | −17.75 | −16.016 | −7.766 | −6.262 |
| HBOND (−15 a −1) | −3.83 | −5.38 | −1.81 | −6.0 |
| VDW (−200 a −50) | −114.961 | −119.72 | −97.915 | −128.306 |
| FA_ATR (−100 a −20) | −70.462 | −75.283 | −51.673 | −71.156 |
| DESOLV (−30 a 20) | −16.536 | −20.511 | −43.291 | −35.358 |
| Z-score (binder > 1.000) | 1.162 | 1.950 | 1.750 | 1.900 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira Matos, A.; do Carmo Neto, J.R.; Franco, F.C.; Dietz, J.d.C.; Dantas, P.H.d.S.; Felice, A.G.; Luchs, A.; de Oliveira, M.A.P.; da Silva, A.C.G.; de Castro Soares, S.; et al. TREM-1 Interacts with Rotavirus Proteins and Drives Inflammatory Responses: A Combined Experimental and Computational Approach. Pathogens 2025, 14, 1029. https://doi.org/10.3390/pathogens14101029
de Oliveira Matos A, do Carmo Neto JR, Franco FC, Dietz JdC, Dantas PHdS, Felice AG, Luchs A, de Oliveira MAP, da Silva ACG, de Castro Soares S, et al. TREM-1 Interacts with Rotavirus Proteins and Drives Inflammatory Responses: A Combined Experimental and Computational Approach. Pathogens. 2025; 14(10):1029. https://doi.org/10.3390/pathogens14101029
Chicago/Turabian Stylede Oliveira Matos, Amanda, José Rodrigues do Carmo Neto, Fernanda Craveiro Franco, Jefferson do Carmo Dietz, Pedro Henrique dos Santos Dantas, Andrei Giacchetto Felice, Adriana Luchs, Milton Adriano Pelli de Oliveira, Artur Christian Garcia da Silva, Siomar de Castro Soares, and et al. 2025. "TREM-1 Interacts with Rotavirus Proteins and Drives Inflammatory Responses: A Combined Experimental and Computational Approach" Pathogens 14, no. 10: 1029. https://doi.org/10.3390/pathogens14101029
APA Stylede Oliveira Matos, A., do Carmo Neto, J. R., Franco, F. C., Dietz, J. d. C., Dantas, P. H. d. S., Felice, A. G., Luchs, A., de Oliveira, M. A. P., da Silva, A. C. G., de Castro Soares, S., da Fonseca, S. G., Ribeiro-Dias, F., Neves, B. J., Andrade, C. H., Silva-Sales, M., & Sales-Campos, H. (2025). TREM-1 Interacts with Rotavirus Proteins and Drives Inflammatory Responses: A Combined Experimental and Computational Approach. Pathogens, 14(10), 1029. https://doi.org/10.3390/pathogens14101029









