Optimizing PRRSV Detection: The Impact of Sample Processing and Testing Strategies on Tongue Tips
Abstract
1. Introduction
2. Materials and Methods
2.1. Farm Selection Criteria
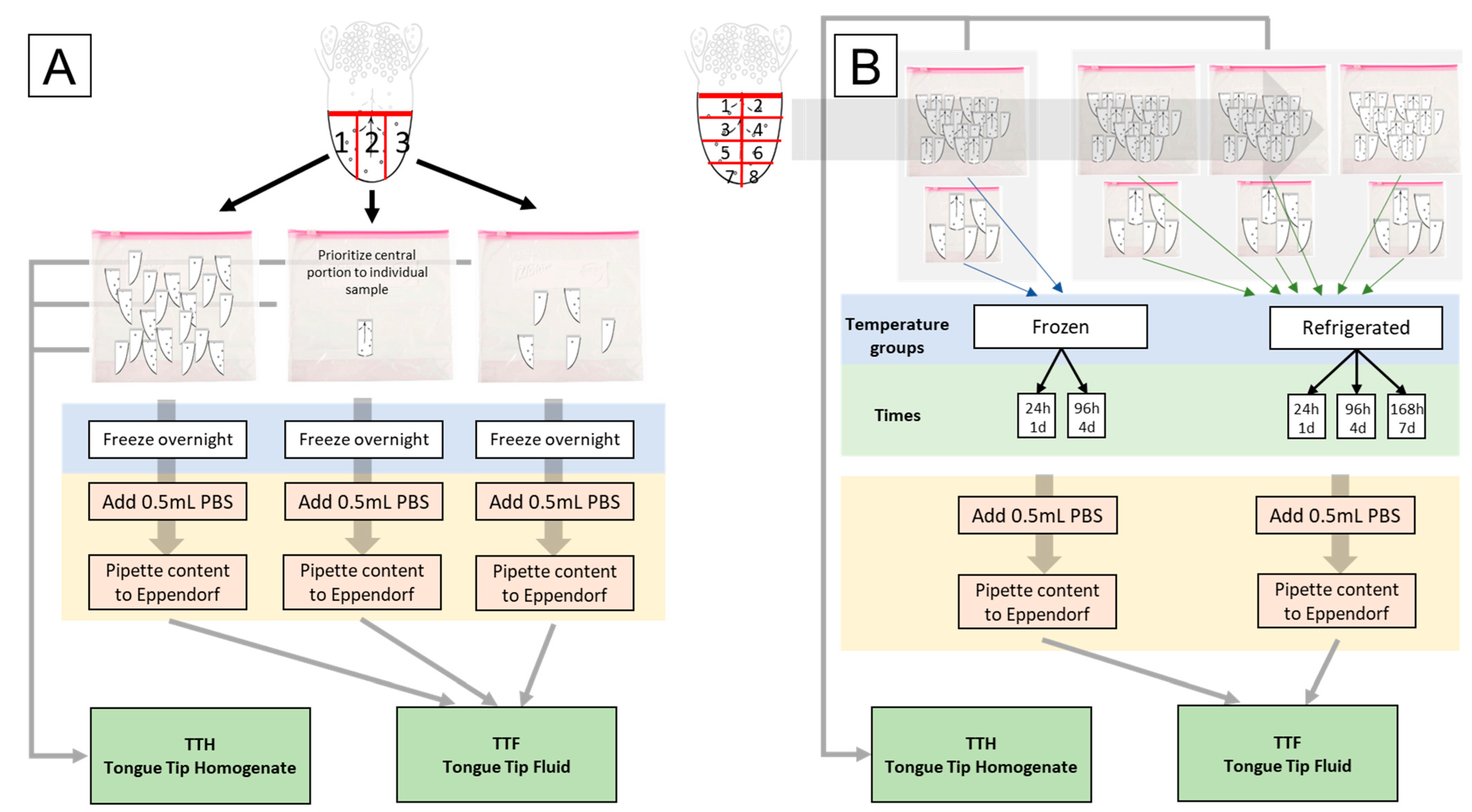
2.2. Sample Collection
2.3. Sample Processing, Submission, and Testing
2.4. Data Analysis
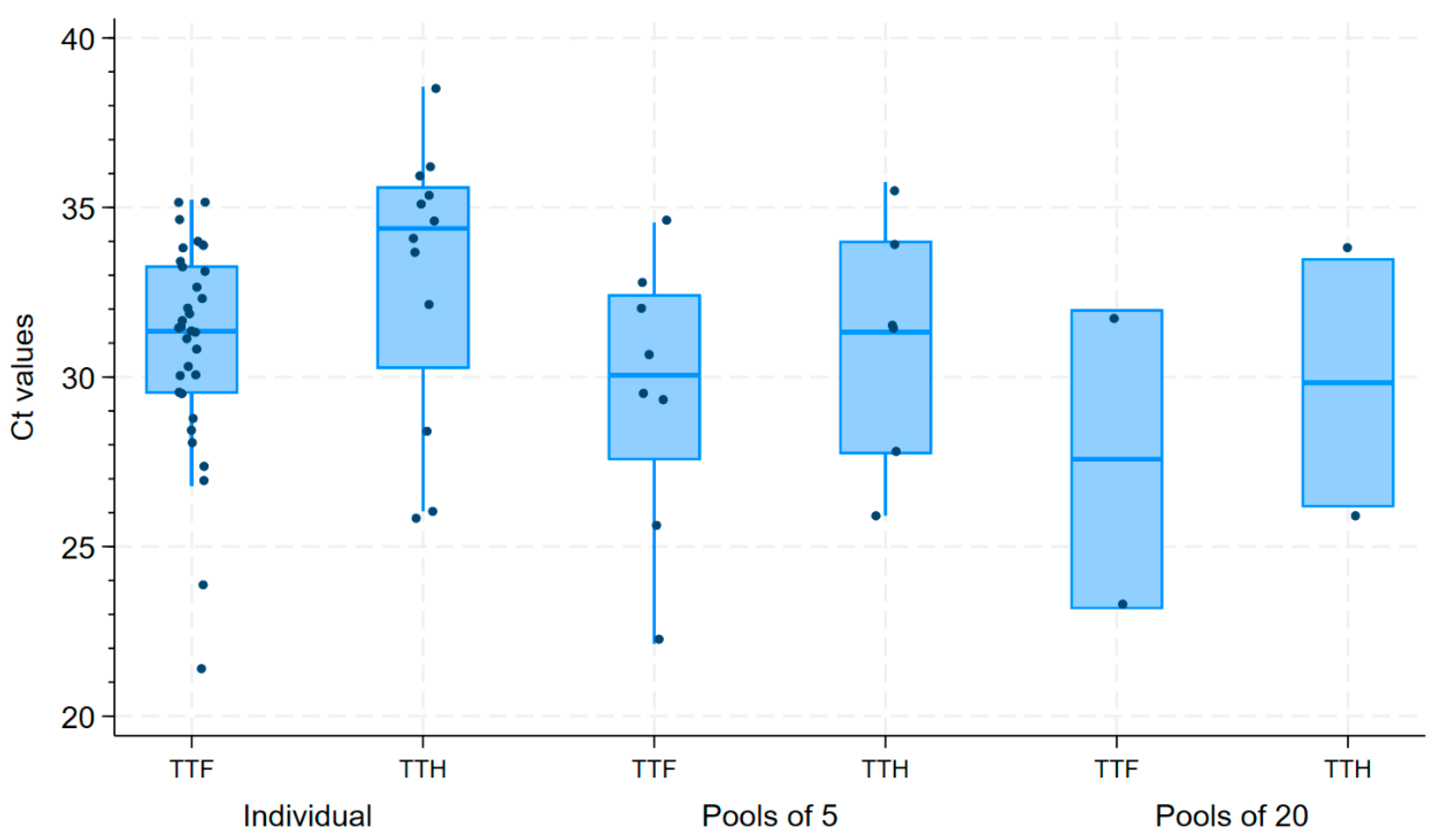
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stadejek, T.; Stankevicius, A.; Murtaugh, M.P.; Oleksiewicz, M.B. Molecular evolution of PRRSV in Europe: Current state of play. Vet. Microbiol. 2013, 165, 21–28. [Google Scholar] [CrossRef]
- VanderWaal, K.; Deen, J. Global trends in infectious diseases of swine. Proc. Natl. Acad. Sci. USA 2018, 115, 11495–11500. [Google Scholar] [CrossRef]
- Tian, K.; Yu, X.; Zhao, T.; Feng, Y.; Cao, Z.; Wang, C.; Hu, Y.; Chen, X.; Hu, D.; Tian, X.; et al. Emergence of Fatal PRRSV Variants: Unparalleled Outbreaks of Atypical PRRS in China and Molecular Dissection of the Unique Hallmark. PLoS ONE 2007, 2, e526. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, H. Porcine reproductive and respiratory syndrome in China. Virus Res. 2010, 154, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Osemeke, O.; Silva, G.S.; Corzo, C.A.; Kikuti, M.; Vadnais, S.; Yue, X.; Linhares, D.; Holtkamp, D. Economic impact of productivity losses attributable to porcine reproductive and respiratory syndrome virus in United States pork production, 2016–2020. Prev. Vet. Med. 2025, 244, 106627. [Google Scholar] [CrossRef] [PubMed]
- Christianson, W.T.; Joo, H.S. Porcine reproductive and respiratory syndrome: A review. J. Swine Health Prod. 1994, 2, 10–28. [Google Scholar]
- Pejsak, Z.; Stadejek, T.; Markowska-Daniel, I. Clinical signs and economic losses caused by porcine reproductive and respiratory syndrome virus in a large breeding farm. Vet. Microbiol. 1997, 55, 317–322. [Google Scholar] [CrossRef]
- Solano, G.I.; Segalés, J.; Collins, J.E.; Molitor, T.W.; Pijoan, C. Porcine reproductive and respiratory syndrome virus (PRRSv) interaction with Haemophilus parasuis. Vet. Microbiol. 1997, 55, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, S.; Li, L.; Lei, L.; Liu, Y.; Shi, W.; Wu, J.; Li, L.; Rong, F.; Xu, M.; et al. Secondary infection with Streptococcus suis serotype 7 increases the virulence of highly pathogenic porcine reproductive and respiratory syndrome virus in pigs. Virol. J. 2010, 7, 184. [Google Scholar] [CrossRef]
- Tousignant, S.J.P.; Perez, A.; Morrison, R. Comparison between the 2013-2014 and 2009-2012 annual porcine reproductive and respiratory syndrome virus epidemics in a cohort of sow herds in the United States. Can. Vet. J. La Rev. Vet. Can. 2015, 56, 1087–1089. [Google Scholar]
- Morrison Swine Health Monitoring Project (MSHMP). PRRS Cumulative Incidence. 2025. Available online: https://mshmp.umn.edu/reports#Charts (accessed on 1 September 2025).
- Holtkamp, D.J.; Torremorrell, M.; Corzo, C.A.; Linhares, D.C.L.; Almeida, M.N.; Yeske, P.; Polson, D.D.; Becton, L.; Snelson, H.; Donovan, T.; et al. Proposed modifications to porcine reproductive and respiratory syndrome virus herd classification. J. Swine Health Prod. 2021, 29, 261–270. Available online: https://www.aasv.org/shap/issues/v29n5/v29n5p261.pdf (accessed on 1 September 2025). [CrossRef]
- Holtkamp, D.J.; Polson, D.D.; Torremorell, M.; Morrison, B.; Classen, D.M.; Becton, L.; Henry, S.; Rodibaugh, M.T.; Rowland, R.R.; Snelson, H.; et al. Terminology for classifying the porcine reproductive and respiratory syndrome virus (PRRSV) status of swine herds. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 2011, 39, 101–112. [Google Scholar]
- Kikuti, M.; Melini, C.M.; Yue, X.; Culhane, M.; Corzo, C.A. Postmortem Sampling in Piglet Populations: Unveiling Specimens Accuracy for Porcine Reproductive and Respiratory Syndrome Detection. Pathogens 2024, 13, 649. [Google Scholar] [CrossRef]
- Baliellas, J.; Novell, E.; Enric-Tarancón, V.; Vilalta, C.; Fraile, L. Porcine Reproductive and Respiratory Syndrome Surveillance in breeding Herds and Nurseries Using Tongue Tips from Dead Animals. Vet. Sci. 2021, 8, 259. [Google Scholar] [CrossRef] [PubMed]
- Machado, I.F.; Magalhães, E.S.; Poeta Silva, A.P.S.; Moraes, D.C.A.; Cezar, G.; Mil-Homens, M.P.; Osemeke, O.H.; Paiva, R.; Moura, C.A.A.; Gauger, P.; et al. Porcine reproductive and respiratory syndrome virus RNA detection in tongue tips from dead animals. Front. Vet. Sci. 2022, 9, 993442. [Google Scholar] [CrossRef]
- Melini, C.M.; Kikuti, M.; Yue, X.; Paploski, I.A.D.; Canturri, A.; Rossow, S.; Leuwerke, B.; Stone, S.; Corzo, C.A. Assessment of Diagnostic Value of Post Mortem Tongue Tip Fluids for Disease Detection in Growing Pigs. Animals 2025, 15, 2434. [Google Scholar] [CrossRef]
- Tousignant, S.J.P.; Perez, A.M.; Lowe, J.F.; Yeske, P.E.; Morrison, R.B. Temporal and spatial dynamics of porcine reproductive and respiratory syndrome virus infection in the United States. Am. J. Vet. Res. 2015, 76, 70–76. [Google Scholar] [CrossRef]
- Perez, A.M.; Alba, A.; Goede, D.; McCluskey, B.; Morrison, R. Monitoring the Spread of Swine Enteric Coronavirus Diseases in the United States in the Absence of a Regulatory Framework. Front. Vet. Sci. 2016, 3, 18. [Google Scholar] [CrossRef]
- Vilalta, C.; Arruda, A.G.; Tousignant, S.J.P.; Valdes-Donoso, P.; Muellner, P.; Muellner, U.; Alkhamis, M.A.; Morrison, R.B.; Perez, A.M. A Review of Quantitative Tools Used to Assess the Epidemiology of Porcine Reproductive and Respiratory Syndrome in U.S. Swine Farms Using Dr. Morrison’s Swine Health Monitoring Program Data. Front. Vet. Sci. 2017, 4, 94. [Google Scholar] [CrossRef] [PubMed]
- StataCorp. Stata Statistical Software: Release 18; StataCorp LLC: College Station, TX, USA, 2023. [Google Scholar]
- Trevisan, G.; Linhares, L.C.M.; Crim, B.; Dubey, P.; Schwartz, K.J.; Burrough, E.R.; Main, R.G.; Sundberg, P.; Thurn, M.; Lages, P.T.F.; et al. Macroepidemiological aspects of porcine reproductive and respiratory syndrome virus detection by major United States veterinary diagnostic laboratories over time, age group, and specimen. PLoS ONE 2019, 14, e0223544. [Google Scholar] [CrossRef] [PubMed]
- Machado, I.F.; Osemeke, O.H.; Doolittle, K.; Moura, C.A.A.; Galina Pantoja, L.; Trevisan, G.; Gauger, P.; Linhares, D.C.L. Effect of Time and Temperature on the Detection of PRRSV RNA and Endogenous Internal Sample Control in Porcine Tongue Fluids. Vet. Sci. 2025, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Kikuti, M.; Vilalta, C.; Sanhueza, J.; Melini, C.M.; Corzo, C.A. Porcine reproductive and respiratory syndrome prevalence and processing fluids use for diagnosis in United States breeding herds. Front. Vet. Sci. 2022, 9, 953918. [Google Scholar] [CrossRef]
- Osemeke, O.H.; Machado, I.; De Conti, E.; Musskopf, M.; Mil-Homens, M.P.; Stutzman, S.; Guo, B.; Petznick, T.; Silva, G.D.-S.-E.; Gauger, P.; et al. Optimizing Tongue Fluid Sampling and Testing Protocols for Enhanced PRRSV Isolation from Perinatal Swine Mortalities. Viruses 2025, 17, 102. [Google Scholar] [CrossRef] [PubMed]
- Munguía-Ramírez, B.; Armenta-Leyva, B.; Henao-Díaz, A.; Cheng, T.-Y.; Zhang, J.; Rawal, G.; Ye, F.; Giménez-Lirola, L.; Zimmerman, J.J. Effect of extrinsic factors on the detection of PRRSV and a porcine-specific internal sample control in serum, oral fluid, and fecal specimens tested by RT-rtPCR. J. Vet. Diagnostic Investig. 2023, 35, 375–384. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture. National Agricultural Statistics Service 2022 Census of Agriculture; United States Department of Agriculture: Washington, DC, USA, 2022. [Google Scholar]

| Phase | Farm | Storage Time | Refrigerated | Frozen | |||||
|---|---|---|---|---|---|---|---|---|---|
| Pools of 5 | Pools of 20 | Individual | Pools of 5 | Pools of 20 | |||||
| Phase 1 | 1 | 1d | - | - | F/H | F/H | F/H | ||
| 4d | - | - | - | - | - | ||||
| 7d | - | - | - | - | - | ||||
| 2 | 1d | - | - | F/H | F/H | F/H | |||
| 4d | - | - | - | - | - | ||||
| 7d | - | - | - | - | - | ||||
| 3 | 1d | - | - | F/H | F/H | F/H | |||
| 4d | - | - | - | - | - | ||||
| 7d | - | - | - | - | - | ||||
| 4 | 1d | - | - | F/H | F/H | F/H | |||
| 4d | - | - | - | - | - | ||||
| 7d | - | - | - | - | - | ||||
| 5 | 1d | - | - | F/H | F/H | F/H | |||
| 4d | - | - | - | - | - | ||||
| 7d | - | - | - | - | - | ||||
| Phase 2 | 6 | 1d | F/H | F/H | - | F/H | F/H | ||
| 4d | F/H | F/H | - | - | - | ||||
| 7d | F/H | F/H | - | - | - | ||||
| 7 | 1d | F/H | F/H | - | - | - | |||
| 4d | F/H | F/H | - | F/H | F/H | ||||
| 7d | F/H | F/H | - | - | - | ||||
| Farm | Proportion (n/N) of Positive Samples | ||||||
|---|---|---|---|---|---|---|---|
| Tongue Tip Fluid | Tongue Tip Homogenate | ||||||
| Individual | Pools of 5 | Pool of 20 | Individual | Pools of 5 | Pool of 20 | ||
| 1 | 5% (1/20) | 0% (0/4) | 0% (0/1) | 0% (0/20) | 0% (0/4) | 0% (0/1) | |
| 2 | 45% (9/20) | 75% (3/4) | 100% (1/1) | 15% (3/20) | 50% (2/4) | 100% (1/1) | |
| 3 | 20% (4/20) | 25% (1/4) | 0% (0/1) | 0% (0/20) | 0% (0/4) | 0% (0/1) | |
| 4 | 0% (0/20) | 0% (0/4) | 0% (0/1) | 0% (0/20) | 0% (0/4) | 0% (0/1) | |
| 5 | 100% (20/20) | 100% (4/4) | 100% (1/1) | 45% (9/20) | 100% (4/4) | 100% (1/1) | |
| Tongue tip fluid | Individual testing | Pool of 5 | Individual testing | Pool of 20 | |||||||
| Positive | Negative | Positive | Negative | ||||||||
| Positive | 8 | 3 | Positive | 2 | 1 | ||||||
| Negative | 0 | 9 | Negative | 0 | 1 | ||||||
| Sensitivity | 72.7% | Specificity | 100.0% | Sensitivity | 66.7% | Specificity | 100.0% | ||||
| PPV | 100.0% | NPV | 75.0% | PPV | 100.0% | NPV | 50.0% | ||||
| Agreement | 85.0% | Agreement | 75.0% | ||||||||
| Tongue tissue homogenate | Individual testing | Pool of 5 | Individual testing | Pool of 20 | |||||||
| Positive | Negative | Positive | Negative | ||||||||
| Positive | 5 | 0 | Positive | 2 | 0 | ||||||
| Negative | 1 | 14 | Negative | 0 | 3 | ||||||
| Sensitivity | 100.0% | Specificity | 93.3% | Sensitivity | 100.0% | Specificity | 100.0% | ||||
| PPV | 83.3% | NPV | 100.0% | PPV | 100.0% | NPV | 100.0% | ||||
| Agreement | 95.0% | Agreement | 100.0% | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paploski, I.A.D.; Kikuti, M.; Yue, X.; Melini, C.M.; Canturri, A.; Rossow, S.; Corzo, C.A. Optimizing PRRSV Detection: The Impact of Sample Processing and Testing Strategies on Tongue Tips. Pathogens 2025, 14, 1028. https://doi.org/10.3390/pathogens14101028
Paploski IAD, Kikuti M, Yue X, Melini CM, Canturri A, Rossow S, Corzo CA. Optimizing PRRSV Detection: The Impact of Sample Processing and Testing Strategies on Tongue Tips. Pathogens. 2025; 14(10):1028. https://doi.org/10.3390/pathogens14101028
Chicago/Turabian StylePaploski, Igor A. D., Mariana Kikuti, Xiaomei Yue, Claudio Marcello Melini, Albert Canturri, Stephanie Rossow, and Cesar A. Corzo. 2025. "Optimizing PRRSV Detection: The Impact of Sample Processing and Testing Strategies on Tongue Tips" Pathogens 14, no. 10: 1028. https://doi.org/10.3390/pathogens14101028
APA StylePaploski, I. A. D., Kikuti, M., Yue, X., Melini, C. M., Canturri, A., Rossow, S., & Corzo, C. A. (2025). Optimizing PRRSV Detection: The Impact of Sample Processing and Testing Strategies on Tongue Tips. Pathogens, 14(10), 1028. https://doi.org/10.3390/pathogens14101028







