Deep Mutational Scanning in Immunology: Techniques and Applications
Abstract
1. Introduction
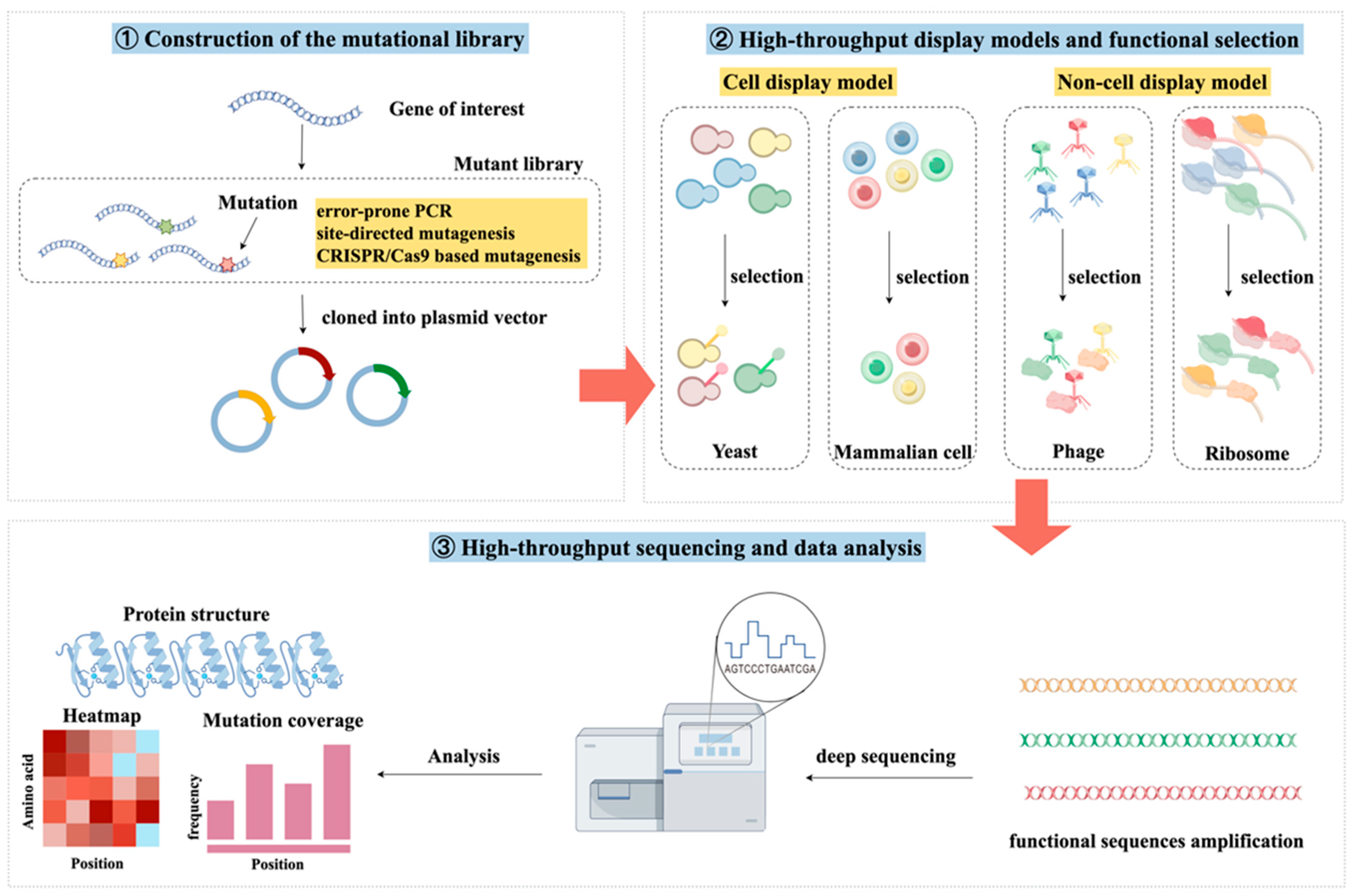
2. Deep Mutational Scanning Methods and Process
2.1. Construction of the Mutational Library
2.2. Functional Screening
2.3. High-Throughput Sequencing and Data Analysis
3. Application of Deep Mutational Scanning in Immunology
3.1. Antibody Engineering
3.2. Antigen Epitope Identification
3.3. Recognition by T Cell Receptors
4. Conclusions and Future Perspectives
4.1. Challenges and Limitations
4.2. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE2 | angiotensin converting enzyme 2 |
| ADA | anti-drug antibodies |
| Ang2 | angiopoietin 2 |
| CDR | complementarity determining region |
| DAF | dual action Fab |
| DMS | deep mutational scanning |
| ELISA | enzyme-linked immunosorbent assay |
| Fab | antigen-binding fragments |
| HCDR | heavy chain complementarity-determining region |
| HDR | homology-directed repair |
| HLA | human leukocyte antigen |
| IgG1 | immunoglobulin G1 |
| mAbs | monoclonal antibodies |
| MHC | major histocompatibility complex |
| PALs | programmed allelic series |
| PCR | polymerase chain reaction |
| PD-1 | programmed cell death protein-1 |
| RBD | receptor binding domain |
| scFv | single-chain variable fragments |
| SUNi | scalable and uniform nicking mutagenesis |
| TCR | T-cell receptor |
| TROP2 | trophoblast cell surface antigen 2 |
| TNF | tumor necrosis factor |
| TP53 | tumor protein p53 |
| PBMC | peripheral blood mononuclear cell |
| PTEN | phosphatase and tensin homolog |
| VEGF | vascular endothelial growth factor |
| VUS | variants of uncertain significance |
References
- Hietpas, R.T.; Jensen, J.D.; Bolon, D.N.A. Experimental illumination of a fitness landscape. Proc. Natl. Acad. Sci. USA 2011, 108, 7896–7901. [Google Scholar] [CrossRef] [PubMed]
- Ernst, A.; Gfeller, D.; Kan, Z.; Seshagiri, S.; Kim, P.M.; Bader, G.D.; Sidhu, S.S. Coevolution of PDZ domain–ligand interactions analyzed by high-throughput phage display and deep sequencing. Mol. Biosyst. 2010, 6, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Kinney, J.B.; McCandlish, D.M. Massively parallel assays and quantitative sequence–function relationships. Annu. Rev. Genom. Hum. Genet. 2019, 20, 99–127. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Li, X. Deep mutational scanning: A versatile tool in systematically mapping genotypes to phenotypes. Front. Genet. 2023, 14, 1087267. [Google Scholar] [CrossRef]
- Fowler, D.M.; Araya, C.L.; Fleishman, S.J.; Kellogg, E.H.; Stephany, J.J.; Baker, D.; Fields, S. High-resolution mapping of protein sequence-function relationships. Nat. Methods 2010, 7, 741–746. [Google Scholar] [CrossRef]
- Parkin, J.; Cohen, B. An overview of the immune system. Lancet 2001, 357, 1777–1789. [Google Scholar] [CrossRef]
- Van Beek, E.M.; Cochrane, F.; Barclay, A.N.; Berg, T.K.v.D. Signal regulatory proteins in the immune system. J. Immunol. 2005, 175, 7781–7787. [Google Scholar] [CrossRef]
- Weile, J.; Roth, F.P. Multiplexed assays of variant effects contribute to a growing genotype–phenotype atlas. Hum. Genet. 2018, 137, 665–678. [Google Scholar] [CrossRef]
- Burton, T.D.; Eyre, N.S. Applications of deep mutational scanning in virology. Viruses 2021, 13, 1020. [Google Scholar] [CrossRef]
- Kemble, H.; Nghe, P.; Tenaillon, O. Recent insights into the genotype–phenotype relationship from massively parallel genetic assays. Evol. Appl. 2019, 12, 1721–1742. [Google Scholar] [CrossRef]
- Fowler, D.M.; Fields, S. Deep mutational scanning: A new style of protein science. Nat. Methods 2014, 11, 801–807. [Google Scholar] [CrossRef]
- Tee, K.L.; Wong, T.S. Polishing the craft of genetic diversity creation in directed evolution. Biotechnol. Adv. 2013, 31, 1707–1721. [Google Scholar] [CrossRef]
- Drummond, D.A.; Iverson, B.L.; Georgiou, G.; Arnold, F.H. Why High-error-rate Random Mutagenesis Libraries are Enriched in Functional and Improved Proteins. J. Mol. Biol. 2005, 350, 806–816. [Google Scholar] [CrossRef]
- Lim, B.N.; Choong, Y.S.; Ismail, A.; Glökler, J.; Konthur, Z.; Lim, T.S. Directed evolution of nucleotide-based libraries using lambda exonuclease. BioTechniques 2012, 53, 357–364. [Google Scholar] [CrossRef]
- Krumpe, L.R.; Schumacher, K.M.; McMahon, J.B.; Makowski, L.; Mori, T. Trinucleotide cassettes increase diversity of T7 phage-displayed peptide library. BMC Biotechnol. 2007, 7, 65. [Google Scholar] [CrossRef]
- Hanning, K.R.; Minot, M.; Warrender, A.K.; Kelton, W.; Reddy, S.T. Deep mutational scanning for therapeutic antibody engineering. Trends Pharmacol. Sci. 2022, 43, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Firnberg, E.; Ostermeier, M. PFunkel: Efficient, expansive, user-defined mutagenesis. PLoS ONE 2012, 7, e52031. [Google Scholar] [CrossRef] [PubMed]
- Kowalsky, C.A.; Faber, M.S.; Nath, A.; Dann, H.E.; Kelly, V.W.; Liu, L.; Shanker, P.; Wagner, E.K.; Maynard, J.A.; Chan, C.; et al. Rapid fine conformational epitope mapping using comprehensive mutagenesis and deep sequencing. J. Biol. Chem. 2015, 290, 26457–26470. [Google Scholar] [CrossRef] [PubMed]
- Mighell, T.L.; Toledano, I.; Lehner, B. SUNi mutagenesis: Scalable and uniform nicking for efficient generation of variant libraries. PLoS ONE 2023, 18, e0288158. [Google Scholar] [CrossRef]
- Oh, E.J.; Liu, R.; Liang, L.; Freed, E.F.; Eckert, C.A.; Gill, R.T. Multiplex evolution of antibody fragments utilizing a yeast surface display platform. ACS Synth. Biol. 2020, 9, 2197–2202. [Google Scholar] [CrossRef]
- Mason, D.M.; Weber, C.R.; Parola, C.; Meng, S.M.; Greiff, V.; Kelton, W.J.; Reddy, S.T. High-throughput antibody engineering in mammalian cells by CRISPR/Cas9-mediated homology-directed mutagenesis. Nucleic Acids Res. 2018, 46, 7436–7449. [Google Scholar] [CrossRef]
- Ahmed, S.; Bhasin, M.; Manjunath, K.; Varadarajan, R. Prediction of residue-specific contributions to binding and thermal stability using yeast surface display. Front. Mol. Biosci. 2022, 8, 800819. [Google Scholar] [CrossRef]
- Fujino, Y.; Fujita, R.; Wada, K.; Fujishige, K.; Kanamori, T.; Hunt, L.; Shimizu, Y.; Ueda, T. Robust in vitro affinity maturation strategy based on interface-focused high-throughput mutational scanning. Biochem. Biophys. Res. Commun. 2012, 428, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, C.M.; Juan, V.; Akamatsu, Y.; DuBridge, R.B.; Doan, M.; Ivanov, A.V.; Ma, Z.; Polakoff, D.; Razo, J.; Wilson, K.; et al. Deep mutational scanning of an antibody against epidermal growth factor receptor using mammalian cell display and massively parallel pyrosequencing. In mAbs; Taylor & Francis: Abingdon, UK, 2013; Volume 5, pp. 523–532. [Google Scholar]
- Koch, P.; Schmitt, S.; Heynisch, A.; Gumpinger, A.; Wüthrich, I.; Gysin, M.; Shcherbakov, D.; Hobbie, S.N.; Panke, S.; Held, M. Optimization of the antimicrobial peptide Bac7 by deep mutational scanning. BMC Biol. 2022, 20, 114. [Google Scholar] [CrossRef] [PubMed]
- Traxlmayr, M.W.; Hasenhindl, C.; Hackl, M.; Stadlmayr, G.; Rybka, J.D.; Borth, N.; Grillari, J.; Rüker, F.; Obinger, C. Construction of a stability landscape of the CH3 domain of human IgG1 by combining directed evolution with high throughput sequencing. J. Mol. Biol. 2012, 423, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Lombardi, R.; Jung, J.S.; Schlatter, F.S.; Mei, A.; Mantuano, N.R.; Bieberich, F.; Hong, K.-L.; Kucharczyk, J.; Kapetanovic, E.; Aznauryan, E.; et al. High-throughput T cell receptor engineering by functional screening identifies candidates with enhanced potency and specificity. Immunity 2022, 55, 1953–1966.e10. [Google Scholar] [CrossRef]
- Abdelfattah, N.S.; Kula, T.; Elledge, S.J. T-Switch: A specificity-based engineering platform for developing safe and effective T cell therapeutics. Immunity 2024, 57, 2945–2958.e5. [Google Scholar] [CrossRef]
- Ledsgaard, L.; Ljungars, A.; Rimbault, C.; Sørensen, C.V.; Tulika, T.; Wade, J.; Wouters, Y.; McCafferty, J.; Laustsen, A.H. Advances in antibody phage display technology. Drug Discov. Today 2022, 27, 2151–2169. [Google Scholar] [CrossRef]
- Villemagne, D.; Jackson, R.; Douthwaite, J.A. Highly efficient ribosome display selection by use of purified components for in vitro translation. J. Immunol. Methods 2006, 313, 140–148. [Google Scholar] [CrossRef]
- Schofield, D.J.; Pope, A.R.; Clementel, V.; Buckell, J.; Chapple, S.D.; Clarke, K.F.; Conquer, J.S.; Crofts, A.M.; Crowther, S.R.; Dyson, M.R.; et al. Application of phage display to high throughput antibody generation and characterization. Genome Biol. 2007, 8, R254. [Google Scholar] [CrossRef]
- Araya, C.L.; Fowler, D.M. Deep mutational scanning: Assessing protein function on a massive scale. Trends Biotechnol. 2011, 29, 435–442. [Google Scholar] [CrossRef]
- Van Dijk, E.L.; Jaszczyszyn, Y.; Naquin, D.; Thermes, C. The third revolution in sequencing technology. Trends Genet. 2018, 34, 666–681. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Bollas, A.; Wang, Y.; Au, K.F. Nanopore sequencing technology, bioinformatics and applications. Nat. Biotechnol. 2021, 39, 1348–1365. [Google Scholar] [CrossRef] [PubMed]
- Fowler, D.M.; Araya, C.L.; Gerard, W.; Fields, S. Enrich: Software for analysis of protein function by enrichment and depletion of variants. Bioinformatics 2011, 27, 3430–3431. [Google Scholar] [CrossRef] [PubMed]
- Rubin, A.F.; Gelman, H.; Lucas, N.; Bajjalieh, S.M.; Papenfuss, A.T.; Speed, T.P.; Fowler, D.M. A statistical framework for analyzing deep mutational scanning data. Genome Biol. 2017, 18, 150. [Google Scholar] [CrossRef] [PubMed]
- Greaney, A.J.; Loes, A.N.; Crawford, K.H.; Starr, T.N.; Malone, K.D.; Chu, H.Y.; Bloom, J.D. Comprehensive mapping of mutations in the SARS-CoV-2 receptor-binding domain that affect recognition by polyclonal human plasma antibodies. Cell Host Microbe. 2021, 29, 463–476.e6. [Google Scholar] [CrossRef]
- Faure, A.J.; Schmiedel, J.M.; Baeza-Centurion, P.; Lehner, B. DiMSum: An error model and pipeline for analyzing deep mutational scanning data and diagnosing common experimental pathologies. Genome Biol. 2020, 21, 207. [Google Scholar] [CrossRef]
- Bloom, J.D. Software for the analysis and visualization of deep mutational scanning data. BMC Bioinform. 2015, 16, 168. [Google Scholar] [CrossRef]
- Dingens, A.S.; Haddox, H.K.; Overbaugh, J.; Bloom, J.D. Comprehensive mapping of HIV-1 escape from a broadly neutralizing antibody. Cell Host Microbe 2017, 21, 777–787.e4. [Google Scholar] [CrossRef]
- Lee, J.M.; Eguia, R.; Zost, S.J.; Choudhary, S.; Wilson, P.C.; Bedford, T.; Stevens-Ayers, T.; Boeckh, M.; Hurt, A.C.; Lakdawala, S.S.; et al. Mapping person-to-person variation in viral mutations that escape polyclonal serum targeting influenza hemagglutinin. eLife 2019, 8, e49324. [Google Scholar] [CrossRef]
- Norman, R.A.; Ambrosetti, F.; Bonvin, A.M.J.J.; Colwell, L.J.; Kelm, S.; Kumar, S.; Krawczyk, K. Computational approaches to therapeutic antibody design: Established methods and emerging trends. Brief. Bioinform. 2020, 21, 1549–1567. [Google Scholar] [CrossRef]
- Olson, C.A.; Wu, N.C.; Sun, R. A comprehensive biophysical description of pairwise epistasis throughout an entire protein domain. Curr. Biol. 2014, 24, 2643–2651. [Google Scholar] [CrossRef] [PubMed]
- Otwinowski, J.; McCandlish, D.M.; Plotkin, J.B. Inferring the shape of global epistasis. Proc. Natl. Acad. Sci. USA. 2018, 115, E7550–E7558. [Google Scholar] [CrossRef] [PubMed]
- Koenig, P.; Lee, C.V.; Sanowar, S.; Wu, P.; Stinson, J.; Harris, S.F.; Fuh, G. Deep Sequencing-guided Design of a High Affinity Dual Specificity Antibody to Target Two Angiogenic Factors in Neovascular Age-related Macular Degeneration. J. Biol. Chem. 2015, 290, 21773–21786. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Zhang, C. Prediction of immunogenicity for humanized and full human therapeutic antibodies. PLoS ONE 2020, 15, e0238150. [Google Scholar] [CrossRef]
- Sarri, C.A.; Papadopoulos, G.E.; Papa, A.; Tsakris, A.; Pervanidou, D.; Baka, A.; Politis, C.; Billinis, C.; Hadjichristodoulou, C.; Mamuris, Z.; et al. Amino acid signatures in the HLA class II peptide-binding region associated with protection/susceptibility to the severe West Nile Virus disease. PLoS ONE 2018, 13, e0205557. [Google Scholar] [CrossRef]
- Sivelle, C.; Sierocki, R.; Lesparre, Y.; Lomet, A.; Quintilio, W.; Dubois, S.; Correia, E.; Moro, A.M.; Maillère, B.; Nozach, H. Combining deep mutational scanning to heatmap of HLA class II binding of immunogenic sequences to preserve functionality and mitigate predicted immunogenicity. Front. Immunol. 2023, 14, 1197919. [Google Scholar] [CrossRef]
- Narayanan, K.K.; Procko, E. Deep mutational scanning of viral glycoproteins and their host receptors. Front. Mol. Biosci. 2021, 8, 636660. [Google Scholar] [CrossRef]
- Greaney, A.J.; Starr, T.N.; Gilchuk, P.; Zost, S.J.; Binshtein, E.; Loes, A.N.; Hilton, S.K.; Huddleston, J.; Eguia, R.; Crawford, K.H.; et al. Complete mapping of mutations to the SARS-CoV-2 spike receptor-binding domain that escape antibody recognition. Cell Host Microbe 2021, 29, 44–57.e9. [Google Scholar] [CrossRef]
- Greaney, A.J.; Starr, T.N.; Barnes, C.O.; Weisblum, Y.; Schmidt, F.; Caskey, M.; Gaebler, C.; Cho, A.; Agudelo, M.; Finkin, S.; et al. Mapping mutations to the SARS-CoV-2 RBD that escape binding by different classes of antibodies. Nat. Commun. 2021, 12, 4196. [Google Scholar] [CrossRef]
- Javanmardi, K.; Segall-Shapiro, T.H.; Chou, C.-W.; Boutz, D.R.; Olsen, R.J.; Xie, X.; Xia, H.; Shi, P.-Y.; Johnson, C.D.; Annapareddy, A.; et al. Antibody escape and cryptic cross-domain stabilization in the SARS-CoV-2 Omicron spike protein. Cell Host Microbe 2022, 30, 1242–1254.e6. [Google Scholar] [CrossRef] [PubMed]
- Starr, T.N.; Greaney, A.J.; Hilton, S.K.; Ellis, D.; Crawford, K.H.D.; Dingens, A.S.; Navarro, M.J.; Bowen, J.E.; Tortorici, M.A.; Walls, A.C.; et al. Deep mutational scanning of SARS-CoV-2 receptor binding domain reveals constraints on folding and ACE2 binding. Cell 2020, 182, 1295–1310.e20. [Google Scholar] [CrossRef]
- Dadonaite, B.; Crawford, K.H.; Radford, C.E.; Farrell, A.G.; Yu, T.C.; Hannon, W.W.; Zhou, P.; Andrabi, R.; Burton, D.R.; Liu, L.; et al. A pseudovirus system enables deep mutational scanning of the full SARS-CoV-2 spike. Cell 2023, 186, 1263–1278.e20. [Google Scholar] [CrossRef]
- Dadonaite, B.; Brown, J.; McMahon, T.E.; Farrell, A.G.; Asarnow, D.; Stewart, C.; Logue, J.; Murrell, B.; Chu, H.Y.; Veesler, D.; et al. Full-spike deep mutational scanning helps predict the evolutionary success of SARS-CoV-2 clades. bioRxiv 2023. [Google Scholar] [CrossRef]
- Sourisseau, M.; Lawrence, D.J.P.; Schwarz, M.C.; Storrs, C.H.; Veit, E.C.; Bloom, J.D.; Evans, M.J. Deep mutational scanning comprehensively maps how Zika envelope protein mutations affect viral growth and antibody escape. J. Virol. 2019, 93, e01291-19. [Google Scholar] [CrossRef]
- Yang, M.; Dent, M.; Lai, H.; Sun, H.; Chen, Q. Immunization of Zika virus envelope protein domain III induces specific and neutralizing immune responses against Zika virus. Vaccine 2017, 35, 4287–4294. [Google Scholar] [CrossRef]
- Stettler, K.; Beltramello, M.; Espinosa, D.A.; Graham, V.; Cassotta, A.; Bianchi, S.; Vanzetta, F.; Minola, A.; Jaconi, S.; Mele, F.; et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science 2016, 353, 823–826. [Google Scholar] [CrossRef]
- Wu, N.C.; Young, A.P.; Al-Mawsawi, L.Q.; Olson, C.A.; Feng, J.; Qi, H.; Chen, S.-H.; Lu, I.-H.; Lin, C.-Y.; Chin, R.G.; et al. High-throughput profiling of influenza A virus hemagglutinin gene at single-nucleotide resolution. Sci. Rep. 2014, 4, 4942. [Google Scholar] [CrossRef]
- Doud, M.B.; Bloom, J.D. Accurate measurement of the effects of all amino-acid mutations on influenza hemagglutinin. Viruses 2016, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Thyagarajan, B.; Bloom, J.D. The inherent mutational tolerance and antigenic evolvability of influenza hemagglutinin. eLife 2014, 3, e03300. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Huddleston, J.; Doud, M.B.; Hooper, K.A.; Wu, N.C.; Bedford, T.; Bloom, J.D. Deep mutational scanning of hemagglutinin helps predict evolutionary fates of human H3N2 influenza variants. Proc. Natl. Acad. Sci. USA 2018, 115, E8276–E8285. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A.M.; Ayres, C.M.; Medina-Cucurella, A.V.; Whitehead, T.A.; Baker, B.M. Enhanced T cell receptor specificity through framework engineering. Front. Immunol. 2024, 15, 1345368. [Google Scholar] [CrossRef] [PubMed]
- Foote, J.; Eisen, H.N. Breaking the affinity ceiling for antibodies and T cell receptors. Proc. Natl. Acad. Sci. USA 2000, 97, 10679–10681. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kranz, D.M. Subtle changes at the variable domain interface of the T-cell receptor can strongly increase affinity. J. Biol. Chem. 2018, 293, 1820–1834. [Google Scholar] [CrossRef]
- Harris, D.T.; Wang, N.; Riley, T.P.; Anderson, S.D.; Singh, N.K.; Procko, E.; Baker, B.M.; Kranz, D.M. Deep mutational scans as a guide to engineering high affinity T cell receptor interactions with peptide-bound major histocompatibility complex. J. Biol. Chem. 2016, 291, 24566–24578. [Google Scholar] [CrossRef]
- Calis, J.J.A.; De Boer, R.J.; Keşmir, C. Degenerate T-cell recognition of peptides on MHC molecules creates large holes in the T-cell repertoire. PLoS Comput. Biol. 2012, 8, e1002412. [Google Scholar] [CrossRef]
- Armstrong, K.M.; Piepenbrink, K.H.; Baker, B.M. Conformational changes and flexibility in T-cell receptor recognition of peptide–MHC complexes. Biochem. J. 2008, 415, 183–196. [Google Scholar] [CrossRef]
- Mason, D. A very high level of crossreactivity is an essential feature of the T-cell receptor. Immunol. Today 1998, 19, 395–404. [Google Scholar] [CrossRef]
- Bowerman, N.A.; Crofts, T.S.; Chlewicki, L.; Do, P.; Baker, B.M.; Garcia, K.C.; Kranz, D.M. Engineering the binding properties of the T cell receptor: Peptide: MHC ternary complex that governs T cell activity. Mol. Immunol. 2009, 46, 3000–3008. [Google Scholar] [CrossRef][Green Version]
- Holler, P.D.; Holman, P.O.; Shusta, E.V.; O’Herrin, S.; Wittrup, K.D.; Kranz, D.M. In vitro evolution of a T cell receptor with high affinity for peptide/MHC. Proc. Natl. Acad. Sci. USA 2000, 97, 5387–5392. [Google Scholar] [CrossRef]
- Cole, D.K.; Sami, M.; Scott, D.R.; Rizkallah, P.J.; Borbulevych, O.Y.; Todorov, P.T.; Moysey, R.K.; Jakobsen, B.K.; Boulter, J.M.; Baker, B.M.; et al. Increased peptide contacts govern high affinity binding of a modified TCR whilst maintaining a native pMHC docking mode. Front. Immunol. 2013, 4, 168. [Google Scholar] [CrossRef]
- Matuszewski, S.; E Hildebrandt, M.; Ghenu, A.-H.; Jensen, J.D.; Bank, C. A statistical guide to the design of deep mutational scanning experiments. Genetics 2016, 204, 77–87. [Google Scholar] [CrossRef]
- Tang, S.; Kim, P.S. A high-affinity human PD-1/PD-L2 complex informs avenues for small-molecule immune checkpoint drug discovery. Proc. Natl. Acad. Sci. USA 2019, 116, 24500–24506. [Google Scholar] [CrossRef] [PubMed]
- Findlay, G.M.; Daza, R.M.; Martin, B.; Zhang, M.D.; Leith, A.P.; Gasperini, M.; Janizek, J.D.; Huang, X.; Starita, L.M.; Shendure, J. Accurate classification of BRCA1 variants with saturation genome editing. Nature 2018, 562, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Starita, L.M.; Young, D.L.; Islam, M.; O Kitzman, J.; Gullingsrud, J.; Hause, R.J.; Fowler, D.M.; Parvin, J.D.; Shendure, J.; Fields, S. Massively parallel functional analysis of BRCA1 RING domain variants. Genetics 2015, 200, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Kotler, E.; Shani, O.; Goldfeld, G.; Lotan-Pompan, M.; Tarcic, O.; Gershoni, A.; Hopf, T.A.; Marks, D.S.; Oren, M.; Segal, E. A systematic p53 mutation library links differential functional impact to cancer mutation pattern and evolutionary conservation. Mol. Cell 2018, 71, 178–190.e8. [Google Scholar]
- Matreyek, K.A.; Starita, L.M.; Stephany, J.J.; Martin, B.; Chiasson, M.A.; Gray, V.E.; Kircher, M.; Khechaduri, A.; Dines, J.N.; Hause, R.J.; et al. Multiplex assessment of protein variant abundance by massively parallel sequencing. Nat. Genet. 2018, 50, 874–882. [Google Scholar] [CrossRef]
- Mighell, T.L.; Evans-Dutson, S.; O’Roak, B.J. A saturation mutagenesis approach to understanding PTEN lipid phosphatase activity and genotype-phenotype relationships. Am. J. Hum. Genet. 2018, 102, 943–955. [Google Scholar] [CrossRef]
- Funk, J.S.; Klimovich, M.; Drangenstein, D.; Pielhoop, O.; Hunold, P.; Borowek, A.; Noeparast, M.; Pavlakis, E.; Neumann, M.; Balourdas, D.-I.; et al. Deep CRISPR mutagenesis characterizes the functional diversity of TP53 mutations. Nat. Genet. 2025, 57, 140–153. [Google Scholar] [CrossRef]
- Livesey, B.J.; Marsh, J.A. Variant effect predictor correlation with functional assays is reflective of clinical classification performance. Genome Biol. 2025, 26, 104. [Google Scholar] [CrossRef]
- Bennett, G.; Karbassi, I.; Chen, W.; Harrison, S.M.; Lebo, M.S.; Meng, L.; Nagan, N.; Rigobello, R.; Rehm, H.L. Distinct rates of VUS reclassification are observed when subclassifying VUS by evidence level. Genet. Med. 2025, 27, 101400. [Google Scholar] [CrossRef]
- Blutt, S.E.; Estes, M.K. Organoid models for infectious disease. Annu. Rev. Med. 2022, 73, 167–182. [Google Scholar] [CrossRef]
- Starita, L.M.; Ahituv, N.; Dunham, M.J.; Kitzman, J.O.; Roth, F.P.; Seelig, G.; Shendure, J.; Fowler, D.M. Variant interpretation: Functional assays to the rescue. Am. J. Hum. Genet. 2017, 101, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Doudna, J.; Charpentier, E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 2014, 346, 6213. [Google Scholar] [CrossRef] [PubMed]
- Rees, H.A.; Liu, D.R. Base editing: Precision chemistry on the genome and transcriptome of living cells. Nat. Rev. Genet. 2018, 19, 770–788. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wei, J.J.; Sabatini, D.M.; Lander, E.S. Genetic screens in human cells using the CRISPR-Cas9 system. Science 2014, 343, 80–84. [Google Scholar] [CrossRef]
- Hart, T.; Chandrashekhar, M.; Aregger, M.; Steinhart, Z.; Brown, K.R.; MacLeod, G.; Mis, M.; Zimmermann, M.; Fradet-Turcotte, A.; Sun, S.; et al. High-resolution CRISPR screens reveal fitness genes and genotype-specific cancer liabilities. Cell 2015, 163, 1515–1526. [Google Scholar] [CrossRef]
- Sadhu, M.J.; Bloom, J.S.; Day, L.; Siegel, J.J.; Kosuri, S.; Kruglyak, L. Highly parallel genome variant engineering with CRISPR–Cas9. Nat. Genet. 2018, 50, 510–514. [Google Scholar] [CrossRef]
- Hanna, R.E.; Hegde, M.; Fagre, C.R.; DeWeirdt, P.C.; Sangree, A.K.; Szegletes, Z.; Griffith, A.; Feeley, M.N.; Sanson, K.R.; Baidi, Y.; et al. Massively parallel assessment of human variants with base editor screens. Cell 2021, 184, 1064–1080.e20. [Google Scholar] [CrossRef]
- Ménoret, S.; Tesson, L.; Remy, S.; Usal, C.; Ouisse, L.-H.; Brusselle, L.; Chenouard, V.; Anegon, I. Advances in transgenic animal models and techniques. Transgenic Res. 2017, 26, 703–708. [Google Scholar] [CrossRef]
- Houdebine, L.M. Transgenic animal models in biomedical research. In Target Discovery and Validation Reviews and Protocols: Volume 1, Emerging Strategies for Targets and Biomarker Discovery; Humana Press: Totowa, NJ, USA, 2007; pp. 163–202. [Google Scholar]
- Cervenak, J.; Kurrle, R.; Kacskovics, I. Accelerating antibody discovery using transgenic animals overexpressing the neonatal Fc receptor as a result of augmented humoral immunity. Immunol. Rev. 2015, 268, 269–287. [Google Scholar] [CrossRef]
- Shakweer, W.M.E.; Krivoruchko, A.Y.; Dessouki, S.; Khattab, A.A. A review of transgenic animal techniques and their applications. J. Genet. Eng. Biotechnol. 2023, 21, 55. [Google Scholar] [CrossRef]
- Yuki, K.; Cheng, N.; Nakano, M.; Kuo, C.J. Organoid models of tumor immunology. Trends Immunol. 2020, 41, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Bar-Ephraim, Y.E.; Kretzschmar, K.; Clevers, H. Organoids in immunological research. Nat. Rev. Immunol. 2020, 20, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yang, L.; Lacko, L.A.; Chen, S. Human organoid models to study SARS-CoV-2 infection. Nat. Methods 2022, 19, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Bruskin, S.; Ishkin, A.; Nikolsky, Y.; Nikolskaya, T.; Piruzian, E. Analysis of Transcriptomic and Proteomic Data in Immune-Mediated Diseases. In Computational Biology and Applied Bioinformatics; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar]
- Franciosa, G.; Kverneland, A.H.; Jensen, A.W.P.; Donia, M.; Olsen, J.V. Proteomics to study cancer immunity and improve treatment. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2023; Volume 45, pp. 241–251. [Google Scholar]
- Berge, T.; Eriksson, A.; Brorson, I.S.; Høgestøl, E.A.; Berg-Hansen, P.; Døskeland, A.; Mjaavatten, O.; Bos, S.D.; Harbo, H.F.; Berven, F. Quantitative proteomic analyses of CD4+ and CD8+ T cells reveal differentially expressed proteins in multiple sclerosis patients and healthy controls. Clin. Proteom. 2019, 16, 19. [Google Scholar] [CrossRef]
- Rollins, N.J.; Brock, K.P.; Poelwijk, F.J.; Stiffler, M.A.; Gauthier, N.P.; Sander, C.; Marks, D.S. Inferring protein 3D structure from deep mutation scans. Nat. Genet. 2019, 51, 1170–1176. [Google Scholar] [CrossRef]
| Display Type | Characteristic | Advantages | Limitation | Refs. |
|---|---|---|---|---|
| Yeast display | The target fragment (such as an antibody fragment, receptor or antigen mutant) is anchored and fused to the yeast cell surface so that it is fixed on the yeast surface for display. |
|
| [22] |
| Mammalian display | Rely on viral or plasmid vectors to introduce mutants into cells one by one, and display them inside cells or on the surface through transmembrane anchoring or secretion capture. |
|
| [16,23,24] |
| Phage display | The target protein (usually an antibody fragment) is fused and expressed on the phage coat protein, and screening is achieved through phage proliferation and selection. |
|
| [16] |
| Ribosome display | Generating ribosome-mRNA-protein complexes through stop-codon-free translation enables genotype-phenotype coupling, which is then screened through ligand binding and high-throughput sequencing. |
|
| [16] |
| Application | Challenge | Potential Solutions | Refs. |
|---|---|---|---|
| Antibody optimization | Some mutations may significantly improve antigen binding (high affinity), but may also disrupt antibody expression or structure, making it difficult to assess in vivo function. | Combine structural modeling to predict conformational stability and verify antibody function through organoid models or other in vivo experiments. | [16,42] |
| Antigen escape | Epitope regions are complex and constantly mutate under host immune pressure. A single cell-based or cell-free screening platform may not truly reflect the infection process (it cannot simulate the interaction between immune cells and pathways). | Design multi-site combination mutations and verify escape variants in combination with organoid or animal models. | [49,53,82] |
| TCR recognition | TCR–MHC interactions are highly dependent on MHC context and peptide conformation. | Designing multi-MHC parallel DMS, combining structural simulation and functional screening. | [63] |
| Complex or poorly defined immune molecules | Unable to build a function-dependent screening system, making it difficult to quantify mutation function through high-throughput. | Joint proteome/transcriptome prediction functional modules. | [11,84] |
| Clinical diagnosis | Lack of large-scale immunology database combined with DMS research, the immune system is highly personalized. | Combined database cross-analysis. | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, C.; Jia, S.; Li, Y.; Li, J. Deep Mutational Scanning in Immunology: Techniques and Applications. Pathogens 2025, 14, 1027. https://doi.org/10.3390/pathogens14101027
Shao C, Jia S, Li Y, Li J. Deep Mutational Scanning in Immunology: Techniques and Applications. Pathogens. 2025; 14(10):1027. https://doi.org/10.3390/pathogens14101027
Chicago/Turabian StyleShao, Chengwei, Siyue Jia, Yue Li, and Jingxin Li. 2025. "Deep Mutational Scanning in Immunology: Techniques and Applications" Pathogens 14, no. 10: 1027. https://doi.org/10.3390/pathogens14101027
APA StyleShao, C., Jia, S., Li, Y., & Li, J. (2025). Deep Mutational Scanning in Immunology: Techniques and Applications. Pathogens, 14(10), 1027. https://doi.org/10.3390/pathogens14101027






