Climate and the Parasite Paradox: Tick–Host Networks Depend on Gradients of Environmental Overlap
Abstract
1. Introduction
2. Materials and Methods
2.1. Aim
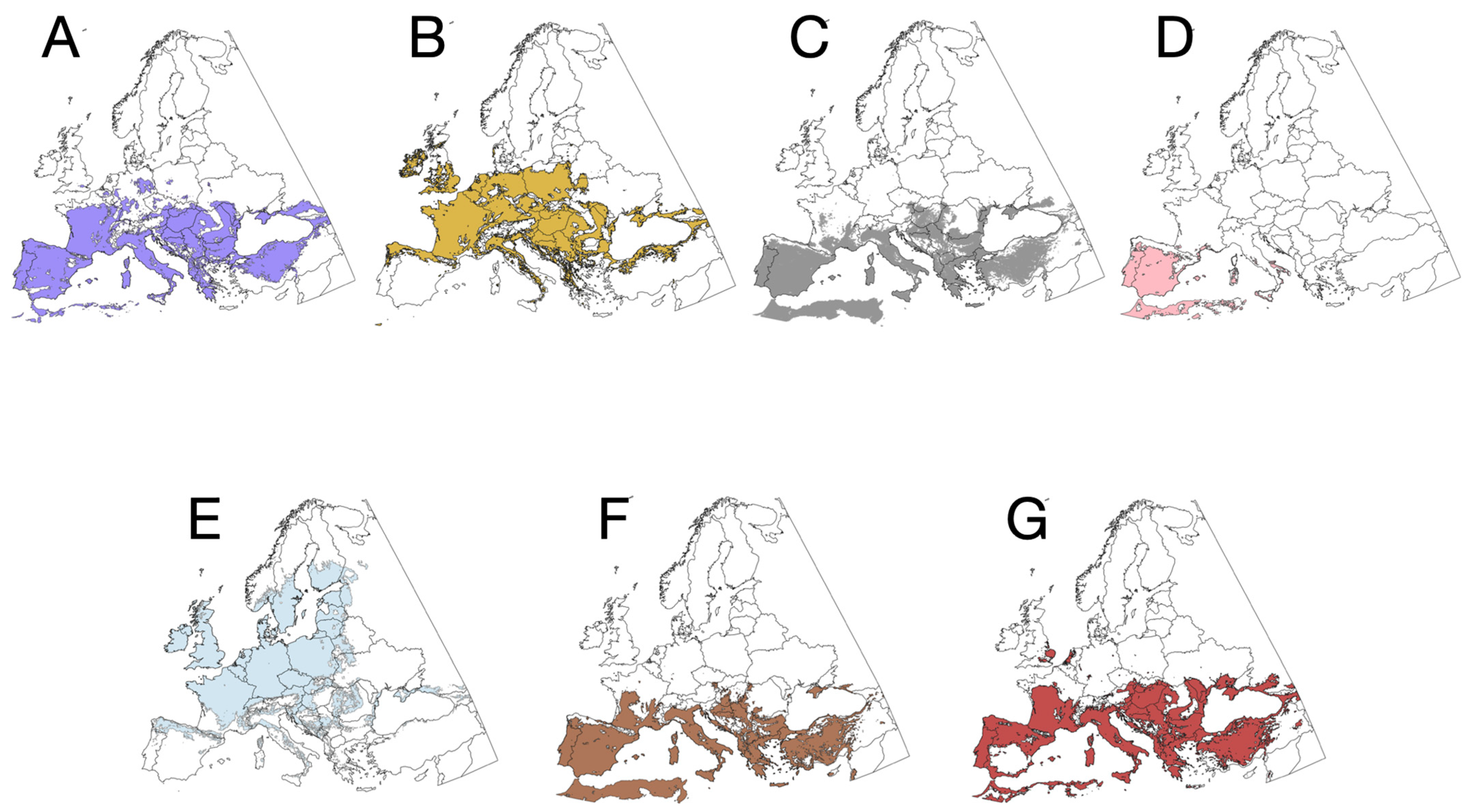
2.2. Sources of Data
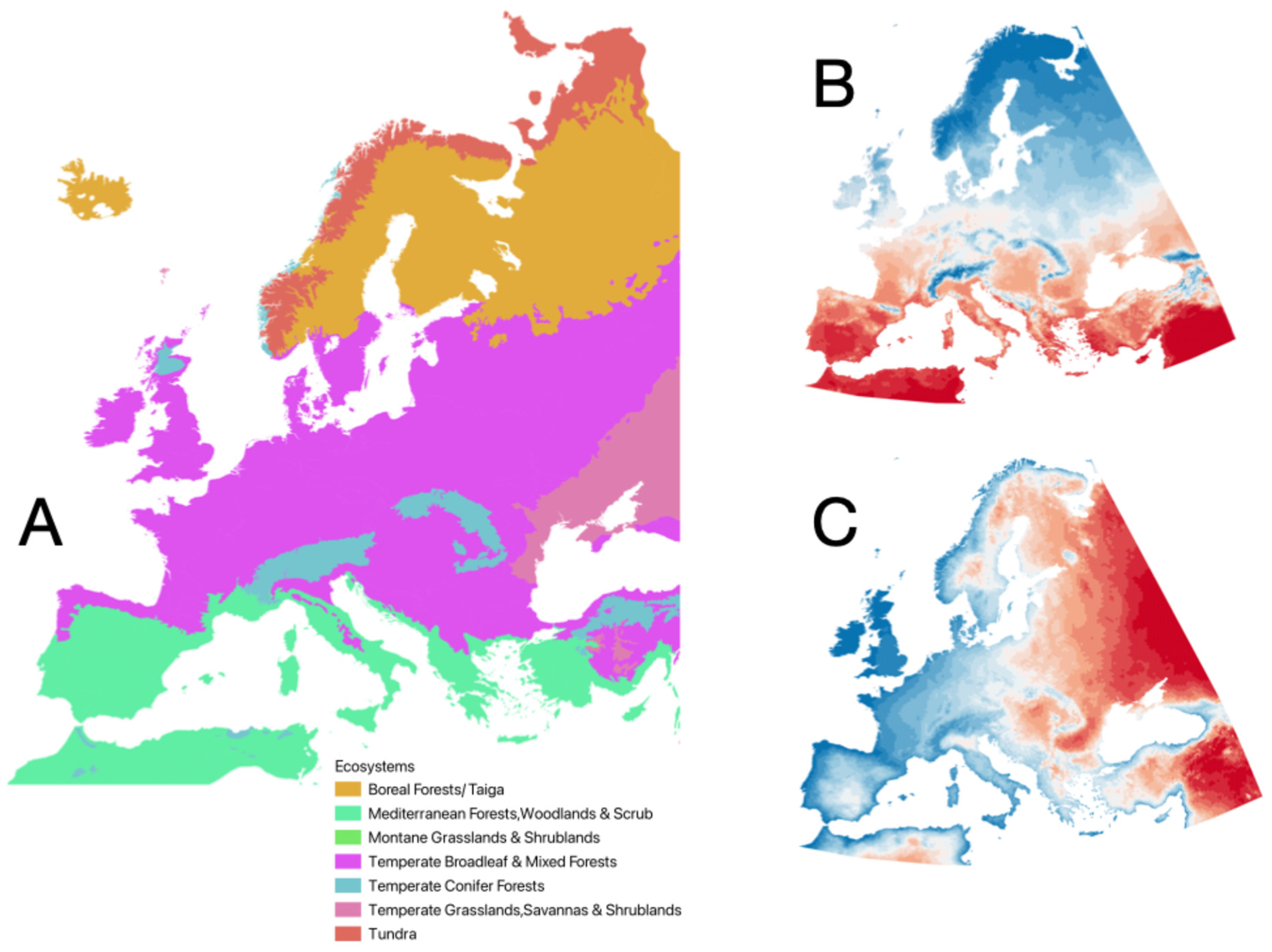
2.3. Modelling
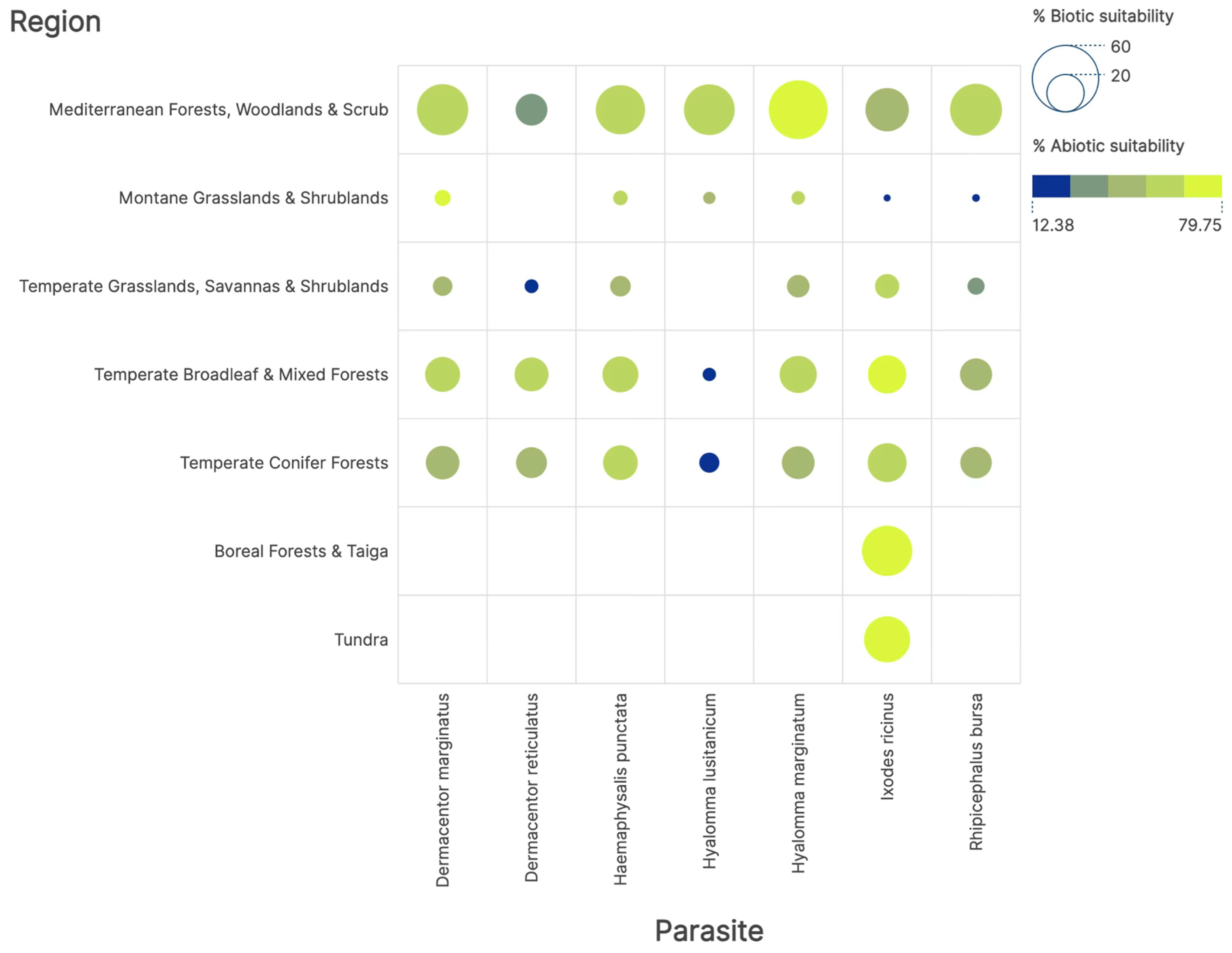
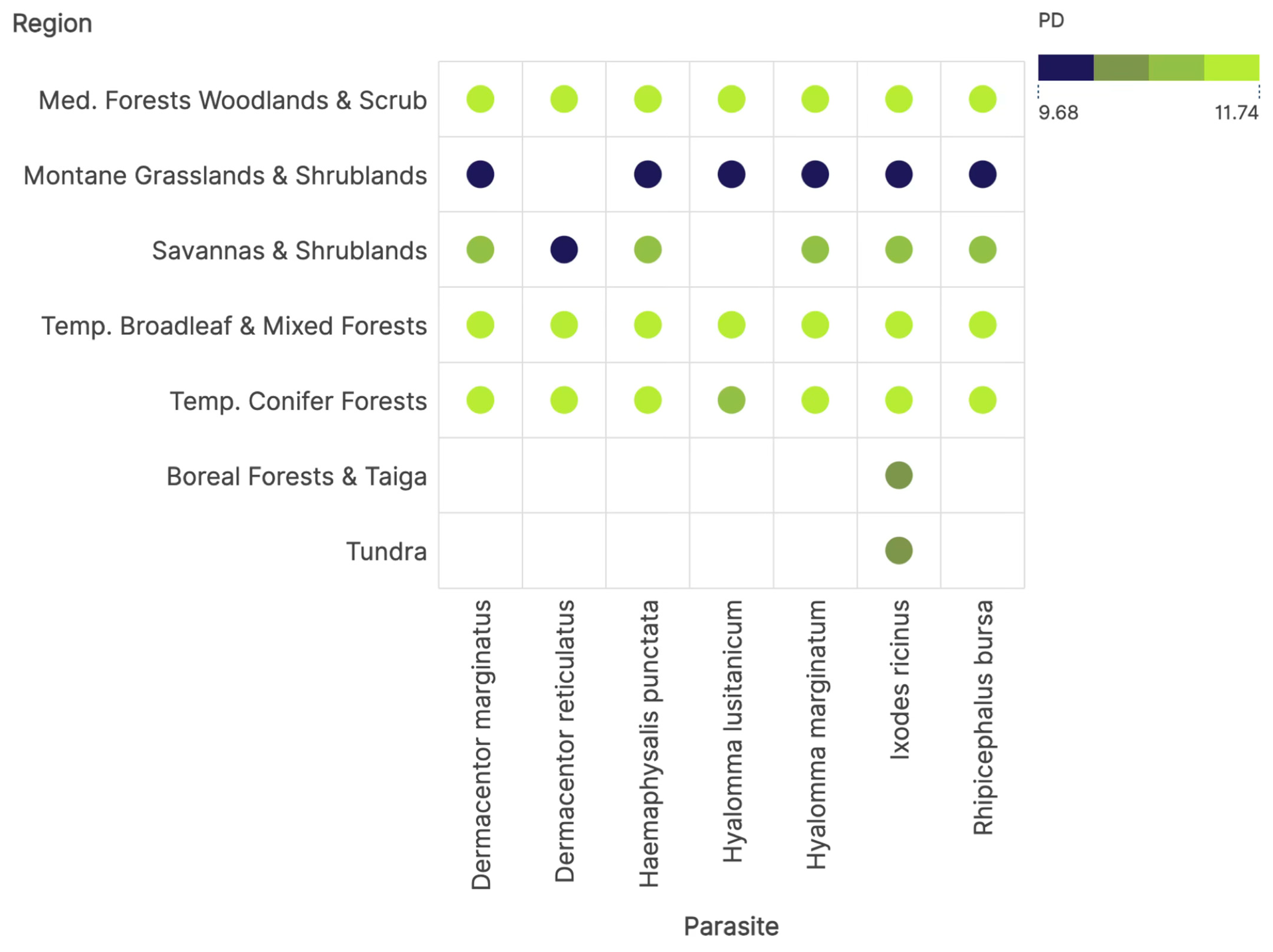
2.4. Measures of Niche Sharing Among Ticks and Vertebrates and Phylogenetic Diversity
2.5. Networks Construction
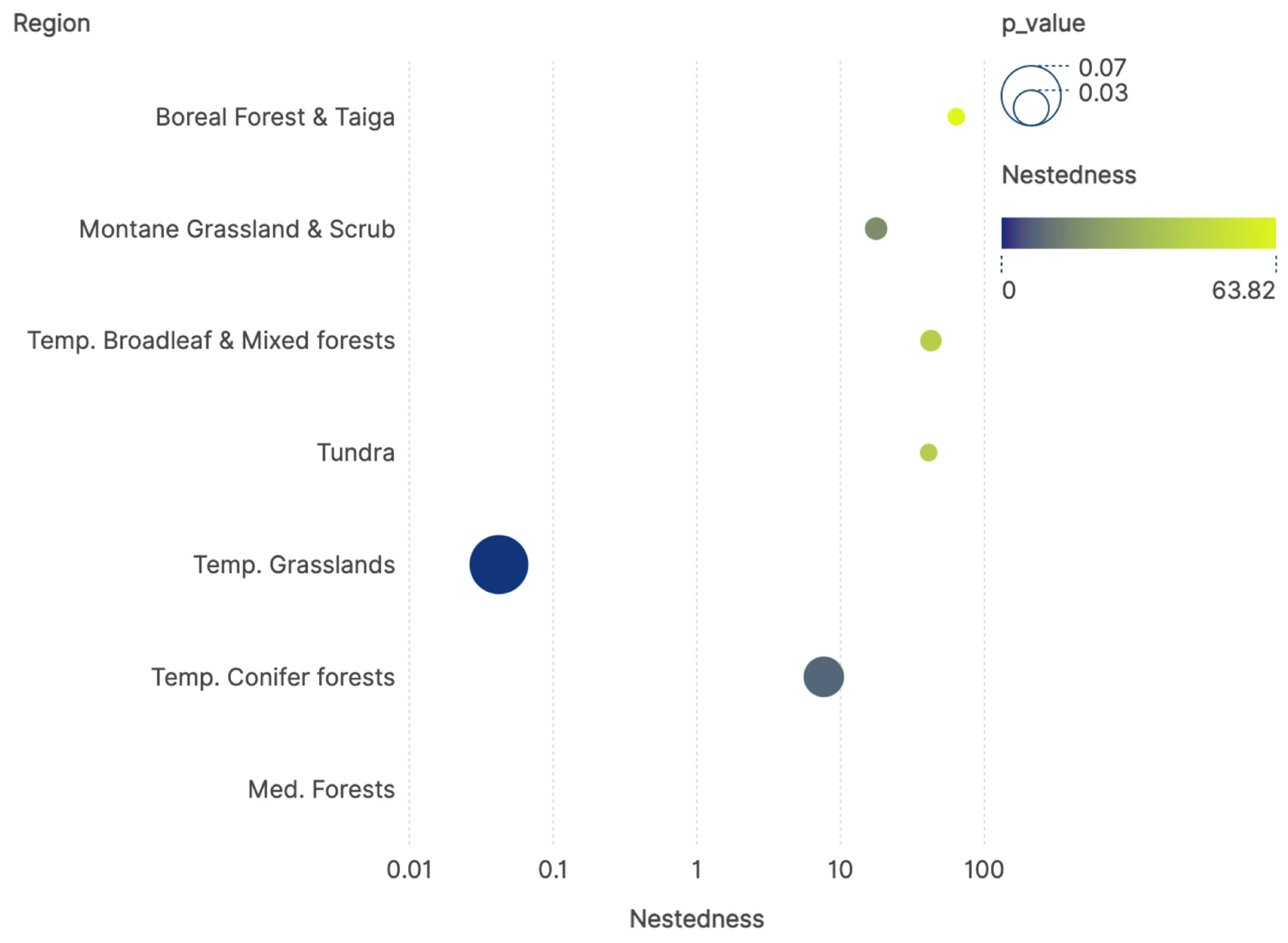
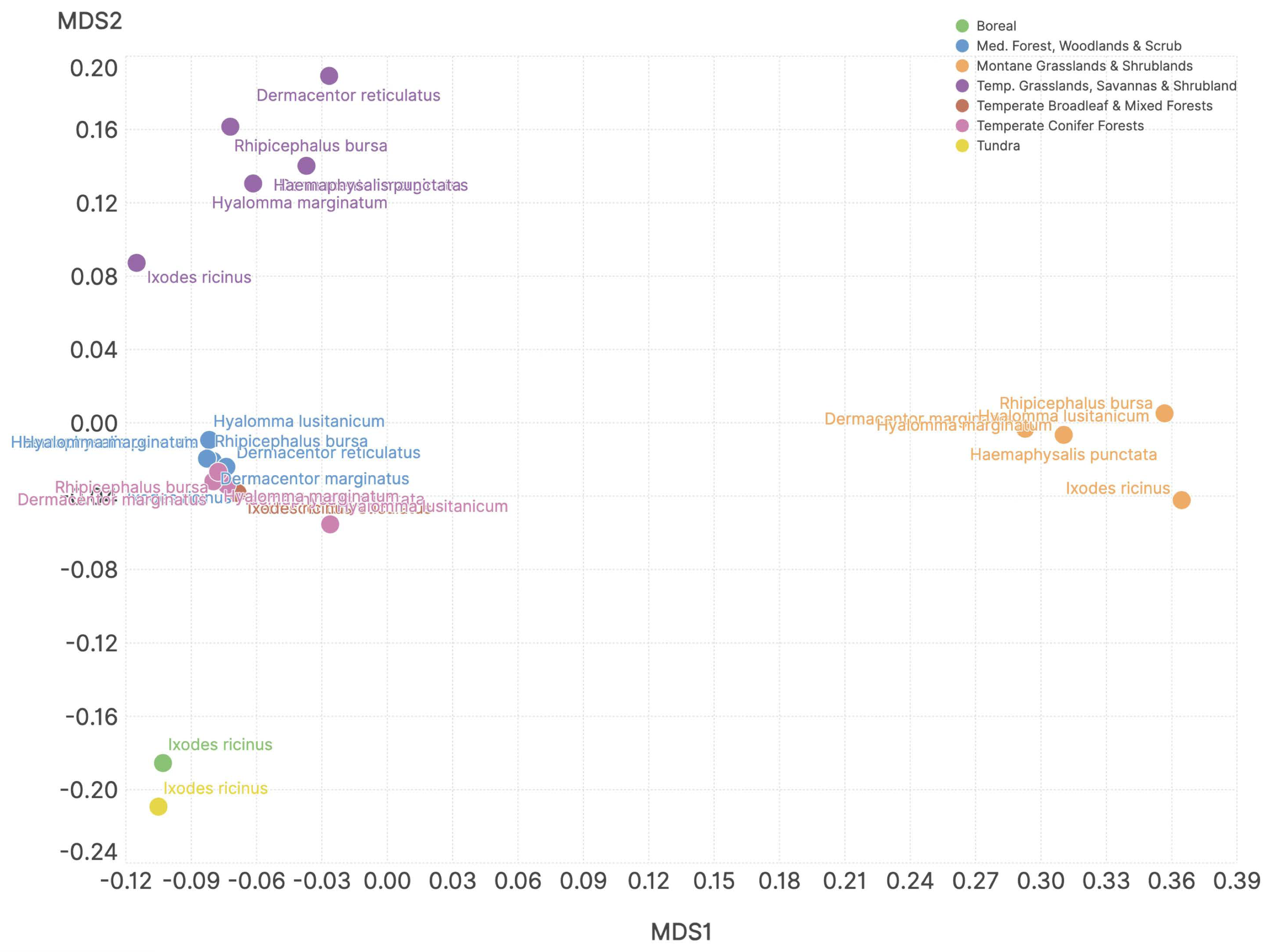
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDS | Multidimensional scaling |
| PCA | Principal Components Analysis |
| GBIF | Global Mammal Parasites Database |
| GAM | Generalized Additive Model |
| MARS | Multivariate Adaptive Regression Spline |
| SVM | Support Vector Machine |
References
- Poulin, R.; Morand, S. The diversity of parasites. Q. Rev. Biol. 2000, 75, 277–293. [Google Scholar] [CrossRef]
- Lion, S.; Gandon, S. Evolution of spatially structured host-parasite interactions. J. Evol. Biol. 2015, 28, 10–28. [Google Scholar] [CrossRef]
- Hawley, D.M.; Gibson, A.K.; Townsend, A.K.; Craft, M.E.; Stephenson, J.F. Bidirectional interactions between host social behaviour and parasites arise through ecological and evolutionary processes. Parasitology 2021, 148, 274–288. [Google Scholar] [CrossRef]
- Brandt, M.; Fischer-Blass, B.; Heinze, J.; Foitzik, S. Population structure and the co-evolution between social parasites and their hosts. Mol. Ecol. 2007, 16, 2063–2078. [Google Scholar] [CrossRef]
- Ebert, D.; Fields, P.D. Host-parasite co-evolution and its genomic signature. Nature reviews. Genetics 2020, 21, 754–768. [Google Scholar] [CrossRef]
- McLennan, D.A.; Brooks, D.R. Complex histories of speciation and dispersal in communities: A re-analysis of some Australian bird data using BPA. J. Biogeogr. 2002, 29, 1055–1066. [Google Scholar] [CrossRef]
- Wilkinson, D.M. The parable of Green Mountain: Ascension Island, ecosystem construction and ecological fitting: Guest Editorial. J. Biogeogr. 2004, 31, 1–4. [Google Scholar] [CrossRef]
- Brooks, D.R.; Ferrao, A.L. The historical biogeography of co-evolution: Emerging infectious diseases are evolutionary accidents waiting to happen: Guest Editorial. J. Biogeogr. 2005, 32, 1291–1299. [Google Scholar] [CrossRef]
- Agosta, S.J. On ecological fitting, plant–insect associations, herbivore host shifts, and host plant selection. Oikos (Cph. Den.) 2006, 114, 556–565. [Google Scholar] [CrossRef]
- Brooks, D.R.; León-Règagnon, V.; McLennan, D.A.; Zelmer, D. Ecological fitting as a determinant of the community structure of platyhelminth parasites of anurans. Ecology 2006, 87 (Suppl. S7), S76–S85. [Google Scholar] [CrossRef] [PubMed]
- Agosta Salvatore, J.; Klemens, J.A. Ecological fitting by phenotypically flexible genotypes: Implications for species associations, community assembly and evolution: Ecological fitting. Ecol. Lett. 2008, 11, 1123–1134. [Google Scholar] [CrossRef]
- Holt, R.D. Bringing the Hutchinsonian niche into the 21st century: Ecological and evolutionary perspectives. Proc. Natl. Acad. Sci. USA 2009, 106 (Suppl. S2), 19659–19665. [Google Scholar] [CrossRef]
- Radtke, A.; McLennan, D.A.; Brooks, D.R. Resource tracking in North American Telorchis spp. (Digenea: Plagiorchiformes: Telorchidae). J. Parasitol. 2002, 88, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Broennimann, O.; Petitpierre, B.; Chevalier, M.; González-Suárez, M.; Jeschke, J.M.; Rolland, J.; Gray, S.M.; Bacher, S.; Guisan, A. Distance to native climatic niche margins explains establishment success of alien mammals. Nat. Commun. 2021, 12, 2353. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, G.; Mouillot, D.; Poulin, R. Testing the niche apportionment hypothesis with parasite communities: Is random assortment always the rule? Parasitology 2006, 132, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Wiens, J.J.; Ackerly, D.D.; Allen, A.P.; Anacker, B.L.; Buckley, L.B.; Cornell, H.V.; Damschen, E.I.; Jonathan Davies, T.; Grytnes, J.A.; Harrison, S.P.; et al. Niche conservatism as an emerging principle in ecology and conservation biology. Ecol. Lett. 2010, 13, 1310–1324. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Guglielmone, A.A.; Nava, S. Worldwide host associations of the tick genus Ixodes suggest relationships based on environmental sharing rather than on co-phylogenetic events. Parasites Vectors 2023, 16, 75. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; de la Fuente, J.; Cabezas-Cruz, A. Functional redundancy and ecological innovation shape the circulation of tick-transmitted pathogens. Front. Cell. Infect. Microbiol. 2017, 7, 234. [Google Scholar] [CrossRef]
- Kim, H.K. Rickettsia-host-tick interactions: Knowledge advances and gaps. Infect. Immun. 2022, 90, e0062121. [Google Scholar] [CrossRef]
- Loss, S.R.; Noden, B.H.; Hamer, G.L.; Hamer, S.A. A quantitative synthesis of the role of birds in carrying ticks and tick-borne pathogens in North America. Oecologia 2016, 182, 947–959. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; de la Fuente, J. Machine learning algorithms for the evaluation of risk by tick-borne pathogens in Europe. Ann. Med. 2024, 56, 2405074. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.R.; Pappalardo, P.; Huang, S.; Byers, J.E.; Farrell, M.J.; Gehman, A.; Ghai, R.R.; Haas, S.E.; Han, B.; Park, A.W.; et al. Global Mammal Parasite Database version 2.0. Ecology 2017, 98, 1476. [Google Scholar] [CrossRef]
- GBIF Occurrence Download. List of Downloads of Records Used in the Current Paper (Including DOI and Date). Available online: https://doi.org/10.15468/dl.smu5d3 (accessed on 23 December 2024).
- Michonneau, F.; Brown, J.W.; Winter, D.J. Rotl: An R package to interact with the Open Tree of Life data. Meth. Ecol. Evol. 2016, 7, 1476–1481. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 6 July 2024).
- Abatzoglou, J.; Dobrowski, S.; Parks, S. TerraClimate, a high-resolution global dataset of monthly climate and climatic water balance from 1958–2015. Sci. Data 2018, 5, 170191. [Google Scholar] [CrossRef] [PubMed]
- Scharlemann, J.P.; Benz, D.; Hay, S.I.; Purse, B.V.; Tatem, A.J.; Wint, G.W.; Rogers, D.J. Global data for ecology and epidemiology: A novel algorithm for temporal Fourier processing MODIS data. PLoS ONE 2008, 3, e1408. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Alexander, N.; Wint, G.R. Perspectives on modelling the distribution of ticks for large areas: So far so good? Parasites Vectors 2016, 9, 179. [Google Scholar] [CrossRef]
- Calabrese, J.M.; Certain, G.; Kraan, C.; Dormann, C.F. Stacking species distribution models and adjusting bias by linking them to macroecological models. Glob. Ecol. Biogeog. 2014, 23, 99–112. [Google Scholar] [CrossRef]
- Schmitt, S.; Pouteau, R.; Justeau, D.; De Boissieu, F.; Birnbaum, P. ssdm: An r package to predict distribution of species richness and composition based on stacked species distribution models. Meth. Ecol. Evol. 2017, 8, 1795–1803. [Google Scholar] [CrossRef]
- Pearse, W.; Davies, J.; Wolkovich, E. How to define, use, and interpret Pagel’s lambda (lambda) in ecology and evolution. Glob. Ecol. Biogeogr. 2023, 34, e70012. [Google Scholar] [CrossRef]
- Tzeng, J.; Lu, H.H.; Li, W.H. Multidimensional scaling for large genomic data sets. BMC Bioinform. 2008, 9, 179. [Google Scholar] [CrossRef]
- Moreira, E.F.; Boscolo, D.; Viana, B.F. Spatial heterogeneity regulates plant-pollinator networks across multiple landscape scales. PLoS ONE 2015, 10, e0123628. [Google Scholar] [CrossRef]
- Cantor, M.; Pires, M.M.; Marquitti, F.M.; Raimundo, R.L.; Sebastián-González, E.; Coltri, P.P.; Perez, S.I.; Barneche, D.R.; Brandt, D.Y.; Nunes, K.; et al. Nestedness across biological scales. PLoS ONE 2017, 12, e0171691. [Google Scholar] [CrossRef]
- Yan, C. Nestedness interacts with subnetwork structures and interconnection patterns to affect community dynamics in ecological multilayer networks. J. Anim. Ecol. 2022, 91, 738–751. [Google Scholar] [CrossRef]
- Qi, X.; Mei, G. Network Resilience: Definitions, Approaches, and Applications. J. King Saud. Univ. –Comput. Inf. Sciences. 2023, 36, 101882. [Google Scholar] [CrossRef]
- Vanwambeke, S.O.; Sumilo, D.; Bormane, A.; Lambin, E.F.; Randolph, S.E. Landscape predictors of tick-borne encephalitis in Latvia: Land cover, land use, and land ownership. Vector Borne Zoonotic Dis. 2010, 10, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Tardy, O.; Acheson, E.S.; Bouchard, C.; Chamberland, É.; Fortin, A.; Ogden, N.H.; Leighton, P.A. Mechanistic movement models to predict geographic range expansions of ticks and tick-borne pathogens: Case studies with Ixodes scapularis and Amblyomma americanum in eastern North America. Ticks Tick. Borne Dis. 2023, 14, 102161. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Sprong, H.; Cabezas-Cruz, A.; de la Fuente, J.; Ramo, A.; Coipan, E.C. Nested coevolutionary networks shape the ecological relationships of ticks, hosts, and the Lyme disease bacteria of the Borrelia burgdorferi (s.l.) complex. Parasit. Vectors 2016, 9, 517. [Google Scholar] [CrossRef] [PubMed]
- Mysterud, A.; Hügli, C.; Viljugrein, H. Tick infestation on medium-large-sized mammalian hosts: Are all equally suitable to Ixodes ricinus adults? Parasites Vectors 2021, 14, 254. [Google Scholar] [CrossRef]
- O’Neill, X.; White, A.; Gortázar, C.; Ruiz-Fons, F. The impact of host abundance on the epidemiology of tick-borne infection. Bull. Math. Biol. 2023, 85, 30. [Google Scholar] [CrossRef] [PubMed]
- Levi, T.; Keesing, F.; Holt, R.D.; Barfield, M.; Ostfeld, R.S. Quantifying dilution and amplification in a community of hosts for tick-borne pathogens. Ecol. Appl. 2016, 26, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Fernández-Ruiz, N. Is composition of vertebrates an indicator of the prevalence of tick-borne pathogens? Infect. Ecol. Epidemiol. 2022, 12, 2025647. [Google Scholar] [CrossRef]
- Coipan, C.E.; van Duijvendijk, G.L.A.; Hofmeester, T.R.; Takumi, K.; Sprong, H. The genetic diversity of Borrelia afzelii is not maintained by the diversity of the rodent hosts. Parasit. Vectors. 2018, 11, 454. [Google Scholar] [CrossRef]
- Jaarsma, R.I.; Sprong, H.; Takumi, K.; Kazimirova, M.; Silaghi, C.; Mysterud, A.; Rudolf, I.; Beck, R.; Földvári, G.; Tomassone, L.; et al. Anaplasma phagocytophilum evolves in geographical and biotic niches of vertebrates and ticks. Parasit. Vectors. 2019, 12, 328. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Binder, L.C.; Nava, S.; Szabó, M.P.J.; Labruna, M.B. Exploring the ecological and evolutionary relationships between Rickettsia and hard ticks in the Neotropical region. Ticks Tick-Borne Dis. 2021, 12, 101754. [Google Scholar] [CrossRef]
- Fabri, N.D.; Sprong, H.; Heesterbeek, H. The circulation of Anaplasma phagocytophilum ecotypes is associated with community composition of vertebrate hosts. Ecosphere 2022, 13, e4243. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Sprong, H.; Wijburg, S.R. A crucial nexus: Phylogenetic versus ecological support of the life-cycle of Ixodes ricinus (Ixodoidea: Ixodidae) and Borrelia spp. amplification. Curr. Res. Parasitol. Vector-Borne Dis. 2024, 6, 100198. [Google Scholar] [CrossRef]
- Heffernan, C. Climate change and multiple emerging infectious diseases. Vet. J. 2018, 234, 43–47. [Google Scholar] [CrossRef]
- de Souza, W.M.; Weaver, S.C. Effects of climate change and human activities on vector-borne diseases. Nat. Rev. Microbiol. 2024, 22, 476–491. [Google Scholar] [CrossRef]
- Williams, P.C.; Bartlett, A.W.; Howard-Jones, A.; McMullan, B.; Khatami, A.; Britton, P.N.; Marais, B.J. Impact of climate change and biodiversity collapse on the global emergence and spread of infectious diseases. J. Paediatr. Child Health 2021, 57, 1811–1818. [Google Scholar] [CrossRef] [PubMed]
- King, K.C.; Hall, M.D.; Wolinska, J. Infectious disease ecology and evolution in a changing world. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2023, 378, 20220002. [Google Scholar] [CrossRef] [PubMed]
- Araujo, S.B.; Braga, M.P.; Brooks, D.R.; Agosta, S.J.; Hoberg, E.P.; von Hartenthal, F.W.; Boeger, W.A. Understanding host-switching by ecological fitting. PLoS ONE 2015, 210, e0139225. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estrada-Peña, A. Climate and the Parasite Paradox: Tick–Host Networks Depend on Gradients of Environmental Overlap. Pathogens 2025, 14, 1025. https://doi.org/10.3390/pathogens14101025
Estrada-Peña A. Climate and the Parasite Paradox: Tick–Host Networks Depend on Gradients of Environmental Overlap. Pathogens. 2025; 14(10):1025. https://doi.org/10.3390/pathogens14101025
Chicago/Turabian StyleEstrada-Peña, Agustín. 2025. "Climate and the Parasite Paradox: Tick–Host Networks Depend on Gradients of Environmental Overlap" Pathogens 14, no. 10: 1025. https://doi.org/10.3390/pathogens14101025
APA StyleEstrada-Peña, A. (2025). Climate and the Parasite Paradox: Tick–Host Networks Depend on Gradients of Environmental Overlap. Pathogens, 14(10), 1025. https://doi.org/10.3390/pathogens14101025





