Cytokine Profiling of Exudates from Periapical Lesions and the Efficacy of CXCL10 as a Healing Marker
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Comprehensive Analysis of Cytokine Production in Apical Exudate
2.3. Collection of Bone Marrow Mononuclear Cells
2.4. Migration Assay
2.5. Statistical Analysis
3. Results
3.1. Cytokine Profiling of Periapical Exudate of Patients
3.2. Changes in Cytokine Profiling During Treatment
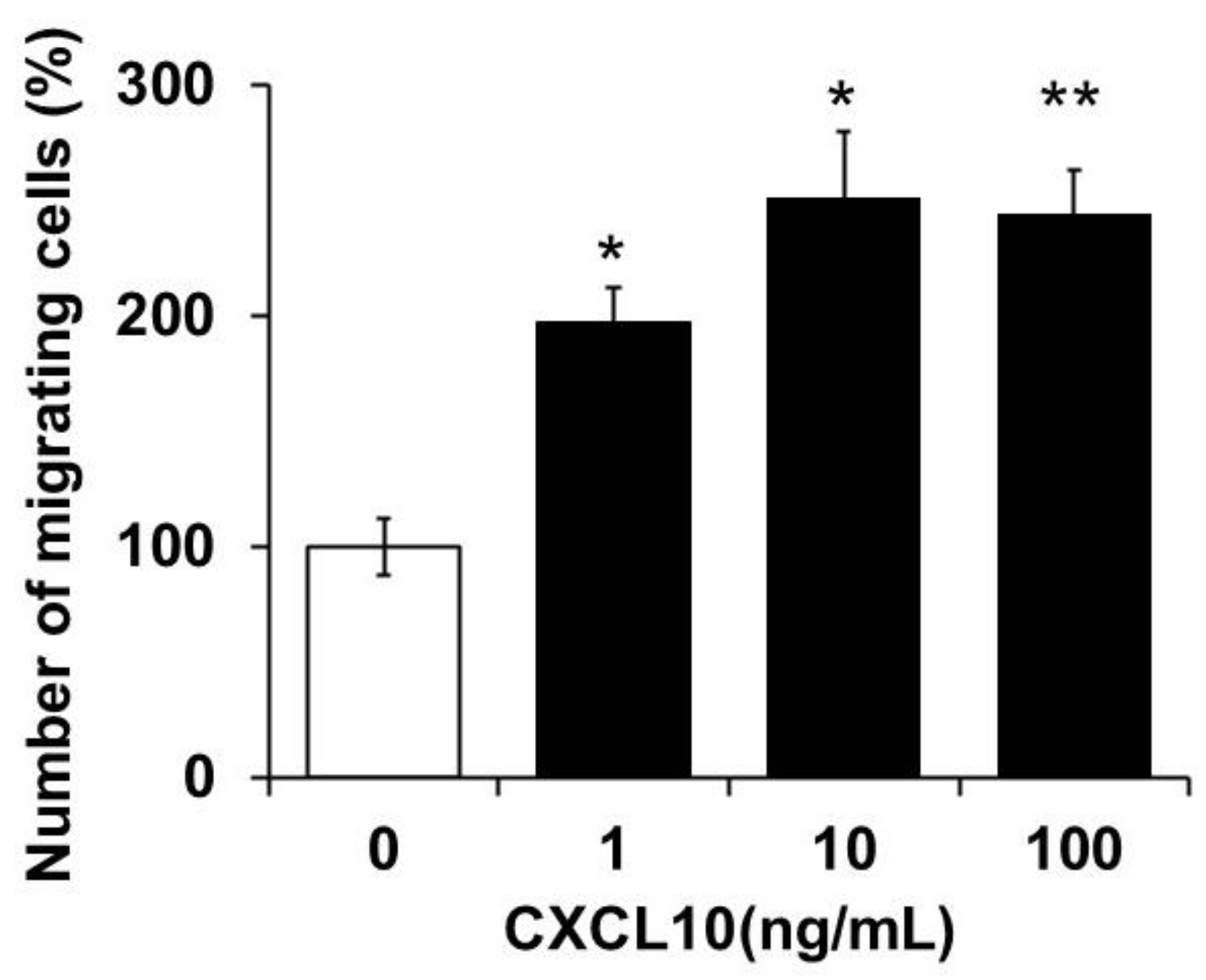
3.3. Chemotactic Activity of Monocytes by CXCL10
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMMC | bone marrow-derived mononuclear cells |
| IL | interleukin |
| G-CSF | granulocyte colony-stimulating factor |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| IFN-γ | interferon gamma |
| MCP | monocyte chemoattractant protein |
| MIP | macrophage inflammatory protein |
| TNF | tumor necrosis factor |
| AP | apical periodontitis |
| RAP | refractory apical periodontitis |
| RCT | root canal treatment |
References
- Tibúrcio-Machado, C.S.; Michelon, C.; Zanatta, F.B.; Gomes, M.S.; Marin, J.A.; Bier, C.A. The global prevalence of apical periodontitis: A systematic review and meta-analysis. Int. Endod. J. 2021, 54, 712–735. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Cao, L.; Song, W.; Yang, C.; Tang, Q.; Yuan, Z. Interaction between apical periodontitis and systemic disease. Int. J. Mol. Med. 2023, 52, 60. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.N. On the causes of persistent apical periodontitis: A review. Int. Endod. J. 2006, 39, 249–281. [Google Scholar] [CrossRef] [PubMed]
- Yu, V.S.; Khin, L.W.; Hsu, C.S.; Yee, R.; Messer, H.H. Risk score algorithm for treatment of persistent apical periodontitis. J. Dent. Res. 2014, 93, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Bronzato, J.D.; Davidian, M.E.; de Castro, M.; de-Jesus-Soares, A.; Ferraz, C.C.; Almeida, J.F.; Marciano, M.A.; Gomes, B.P.F.A. Bacteria and virulence factors in periapical lesions associated with teeth following primary and secondary root canal treatment. Int. Endod. J. 2021, 54, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.; Wragg, N.M.; Shariatzadeh, M.; Wilson, S.L. The use of platelet-rich plasma (PRP) for the management of non-union fractures. Curr. Osteoporos. Rep. 2021, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Luster, A.D.; Ravetch, J.V. Biochemical characterization of a gamma interferon-inducible cytokine (IP-10). J. Exp. Med. 1987, 166, 1084–1097. [Google Scholar] [CrossRef] [PubMed]
- Rotondi, M.; Chiovato, L.; Romagnani, S.; Serio, M.; Romagnani, P. Role of chemokines in endocrine autoimmune diseases. Endocr. Rev. 2007, 28, 492–520. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.B.; Ha, H.; Kim, H.N.; Lee, J.H.; Kim, H.S.; Lee, S.; Kim, H.M.; Kim, J.Y.; Kim, H.H.; Song, Y.W.; et al. Reciprocal cross-talk between RANKL and interferon-gamma-inducible protein 10 is responsible for bone-erosive experimental arthritis. Arthritis Rheum. 2008, 58, 1332–1342. [Google Scholar] [CrossRef]
- Yamakawa, M.; Ouhara, K.; Kajiya, M.; Munenaga, S.; Kittaka, M.; Yamasaki, S.; Takeda, K.; Takeshita, K.; Mizuno, N.; Fujita, T.; et al. Porphyromonas gingivalis infection exacerbates the onset of rheumatoid arthritis in SKG mice. Clin. Exp. Immunol. 2016, 186, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, H.N.; Kim, K.O.; Jin, W.J.; Lee, S.; Kim, H.H.; Ha, H.; Lee, Z.H. CXCL10 promotes osteolytic bone metastasis by enhancing cancer outgrowth and osteoclastogenesis. Cancer Res. 2012, 72, 3175–3186. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, E.T.; Gomes, B.P.; Ferraz, C.C.; Sousa, E.L.; Teixeira, F.B.; Souza-Filho, F.J. Microorganisms from canals of root-filled teeth with periapical lesions. Int Endod. J. 2003, 36, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Ribeiro, M.; De-Jesus-Soares, A.; Zaia, A.A.; Ferraz, C.C.; Almeida, J.F.; Gomes, B.P. Quantification of lipoteichoic acid contents and cultivable bacteria at the different phases of the endodontic retreatment. J. Endod. 2016, 42, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Mariano, L.S.; Nakamura-Silva, R.; Macedo, L.M.; Oliveira-Silva, M.; Goulart, R.D.; Pelisson, M.; Vespero, E.C.; Silva-Sousa, Y.T.C.; Pitondo-Silva, A. Identification and antimicrobial susceptibility profile of bacteria isolated from primary endodontic infections. Braz. Oral. Res. 2024, 38, e024. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Ribeiro, M.; Arruda-Vasconcelos, R.; Louzada, L.M.; Dos Santos, D.G.; Andreote, F.D.; Gomes, B.P. Microbiological analysis of endodontically treated teeth with apical periodontitis before and after endodontic retreatment. Clin. Oral. Investig. 2021, 25, 2017–2027. [Google Scholar] [CrossRef] [PubMed]
- Chianca, G.C.; Fendeler, C.C.; Junior, S.P.; Pereira, G.L.; Póvoa, H.C.; Antunes, L.A.; Antunes, L.S.; Iorio, N.L.P.P. Whole-genome amplification as a tool to improve bacterial detection by PCR in microbiological samples after endodontic treatment. Front. Oral Health 2025, 6, 1520945. [Google Scholar] [CrossRef] [PubMed]
- Łysakowska, M.E.; Ciebiada-Adamiec, A.; Sienkiewicz, M.; Sokołowski, J.; Banaszek, K. The cultivable microbiota of primary and secondary infected root canals, their susceptibility to antibiotics and association with the signs and symptoms of infection. Int. Endod. J. 2016, 49, 422–430. [Google Scholar] [CrossRef] [PubMed]
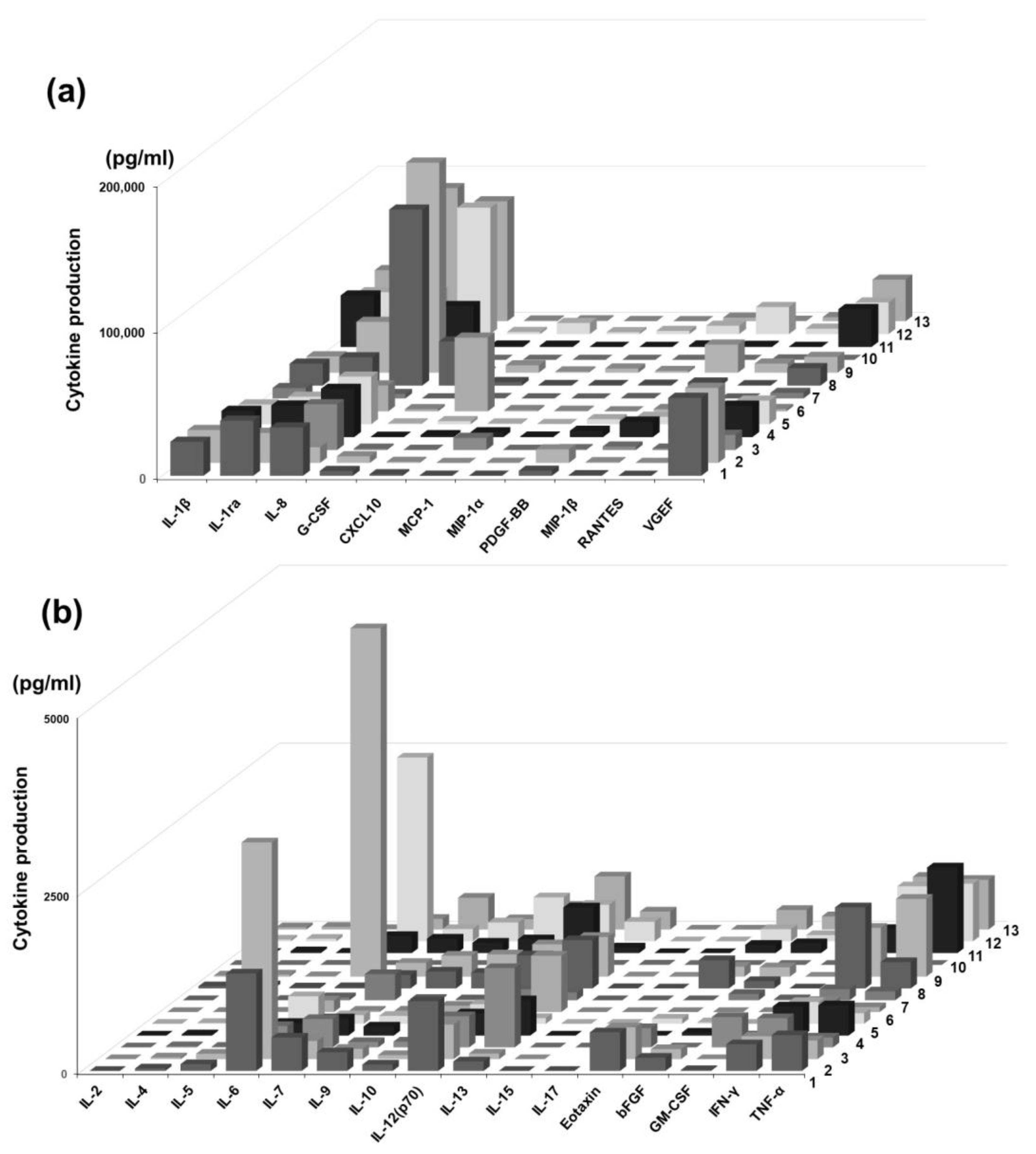

| Pt | Age | Sex | Site | Date | Diag. | Healing Outcomes | Systemic Comorbidities |
|---|---|---|---|---|---|---|---|
| 1 | 49 | ♀ | 46 | first visit | acute apical periodontitis | Healing of the periapical lesion | Cleft palate |
| 2 | 43 | ♂ | 12 | first visit | acute apical periodontitis or redicular cyst | Healing of the periapical lesion | |
| 3 | 68 | ♂ | 22 | first visit | acute apical periodontitis | Extraction | Pharyngeal cancer |
| 4 | 53 | ♀ | 12 | first visit | acute apical periodontitis | Healing of the periapical lesion | bronchial asthma |
| 5 | 67 | ♀ | 24 | first visit | acute apical periodontitis | Healing of the periapical lesion | cerebral infarction |
| 6 | 58 | ♀ | 11 | first visit | chronic apical periodontitis | Healing of the periapical lesion | diabetes mellitus |
| 7 | 42 | ♂ | 26 | first visit | chronic apical periodontitis or redicular cyst | Root resection | |
| 8 | 82 | ♀ | 22 | first visit | chronic apical periodontitis | Apicoectomy | rheumatoid arthritis |
| 9 | 59 | ♂ | 16 | first visit | chronic apical periodontitis | Healing of the periapical lesion | hyper tension |
| 10 | 63 | ♂ | 36 | first visit | chronic apical periodontitis | Healing of the periapical lesion | |
| 11 | 79 | ♀ | 13 | first visit | chronic apical periodontitis | Healing of the periapical lesion | diabetes mellitus, hyper tension |
| 12 | 66 | ♀ | 13 | first visit | chronic apical periodontitis | Healing of the periapical lesion | |
| 13 | 69 | ♂ | 16 | first visit | chronic apical periodontitis | Healing of the periapical lesion | stomach cancer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouhara, K.; Taniguchi, Y.; Zhai, R.; Takeda, K.; Fujimori, R.; Kuwahara, N.; Ueda, S.; Hou, Y.; Honoka, N.; Shimizu, M.; et al. Cytokine Profiling of Exudates from Periapical Lesions and the Efficacy of CXCL10 as a Healing Marker. Pathogens 2025, 14, 1013. https://doi.org/10.3390/pathogens14101013
Ouhara K, Taniguchi Y, Zhai R, Takeda K, Fujimori R, Kuwahara N, Ueda S, Hou Y, Honoka N, Shimizu M, et al. Cytokine Profiling of Exudates from Periapical Lesions and the Efficacy of CXCL10 as a Healing Marker. Pathogens. 2025; 14(10):1013. https://doi.org/10.3390/pathogens14101013
Chicago/Turabian StyleOuhara, Kazuhisa, Yuri Taniguchi, Ruoqi Zhai, Katsuhiro Takeda, Ryousuke Fujimori, Naoya Kuwahara, Shoya Ueda, Yitong Hou, Nomi Honoka, Masaru Shimizu, and et al. 2025. "Cytokine Profiling of Exudates from Periapical Lesions and the Efficacy of CXCL10 as a Healing Marker" Pathogens 14, no. 10: 1013. https://doi.org/10.3390/pathogens14101013
APA StyleOuhara, K., Taniguchi, Y., Zhai, R., Takeda, K., Fujimori, R., Kuwahara, N., Ueda, S., Hou, Y., Honoka, N., Shimizu, M., Kono, S., Iwata, T., Matsuda, S., & Mizuno, N. (2025). Cytokine Profiling of Exudates from Periapical Lesions and the Efficacy of CXCL10 as a Healing Marker. Pathogens, 14(10), 1013. https://doi.org/10.3390/pathogens14101013





