Abstract
Fungal infections (FIs) are widespread globally, affecting both immunocompromised and immunocompetent children, with varying clinical implications based on age and comorbidities. In immunocompromised children, particularly those with hematologic oncological conditions, FI leads to substantially longer hospital stays and increased in-hospital mortality, with reported rates ranging from 15% to 20%. Our study aims to analyze the epidemiological trends of fungal infections in the pediatric population within a specific region of Italy. We extracted ICD-9 codes related to fungal infections from hospital discharge records (HDRs) in the pediatric population of Veneto, located in the north-east of Italy, between 2007 and 2022. We included all children admitted to the hospital with a primary or secondary diagnosis during admission for other reasons. Data were stratified based on age, year, ward of admission, and type of diagnosis. Patients older than eighteen and HDRs related to a second admission within thirty days from the previous admission were excluded. A total of 1433 diagnoses were analyzed during the period, with 241 (16.8%) as main diagnoses and 1192 (83.2%) as secondary diagnoses. The overall hospitalization rate was 1084 cases/100,000 (1.69 cases/100,000 as primary diagnosis and 8.95 cases/100,000 as secondary). The hospitalization rate stratified for age was 11,055 cases/100,000 among infants younger than 1 year, 8.48 cases/100,000 among those aged 1-4 years, and 4.4 cases/100,000 among children older than 5. The more frequent infection was Candida spp. (62.8%), followed by Aspergillus spp. (14.6%) and skin mycosis (9.5%). Overall, the pediatric in-hospital case fatality rate due to FI was 2.09%. Our study elucidated the overall experience of fungal infections in the pediatric population of the Veneto region in Italy. Specifically, we underscored a relatively stable hospitalization rate for fungal diseases and a noteworthy mortality rate.
1. Background
Fungal infections (FIs) are common in all parts of the world, and in recent decades, many strategies have evolved, especially regarding the growing array of antifungal compounds. However, clinical situations could be differently related to the immune system of the patients: in immunocompromised children, especially with onco-hematological disease, invasive FIs are associated with significant increases in hospital lengths of stay and in-hospital mortality, with reported overall mortality between 15 and 20% for pediatric patients [1]. Among adult patients, the epidemiological trends are satisfactorily documented [2,3,4]. However, in the pediatric population, the epidemiology in the general population and population with comorbidities is still unclear [5,6,7,8].
Molds species, especially Aspergillus spp., are usually related to immunocompromised and cystic fibrosis patients. Aspergillus and other molds can be inhaled from the host’s environment and cause lung disease in the immunocompromised child and disseminate to other tissues. [9]. Regarding Candida spp. infections during pediatric age, the possible profiles can be represented by diaper dermatitis, dermatophytosis, oral candidiasis, candidemia, and invasive candidiasis, especially for immunocompromised patients [10,11]. For the last setting, the intensive care unit, post-surgical outcome, disruption of the gastrointestinal mucosa, and colonization of the central venous catheter are associated with a higher risk of Candida spp. infections [12,13]. Dissemination to secondary sites is reported in 10–20% of pediatric patients with candidemia, and severe sepsis or septic shock occurs in about 30%. For invasive candidemia, overall fatality rates range between 10% and 25% [12].
Our study aims to analyze the epidemiological trends of FIs in the pediatric population within a region of Italy.
2. Methods
2.1. Setting and Data Source
We conducted this retrospective study in the Veneto region, located in the north-east of Italy. In 2022, Veneto had an average population of 4.8 million, a mean age of 46.1 years, and 50.9% of its population were female [14]. In addition, the Italian National Health System is a publicly funded system primarily financed through general taxation and organized on a regional basis [15].
We analyzed the acute ordinary hospital discharge records (HDRs) of public hospitals from January 2007 to December 2022. HDRs include the following data: age, gender, type of the hospital, date of admission, length of stay from admission, comorbidities, and outcome. According to regional decree no. 118 dated December 23, 2016 [16], each HDR contained one primary diagnosis or first-listed diagnosis concerning the main condition identified during the patient’s hospital stay and up to five secondary diagnoses based on the diagnostic codes of the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM).
2.2. Population and Acute Admissions for Fungal Infection
We included all HDRs related to children aged 0 to 18 years old either admitted to the hospital with a primary diagnosis of fungal infection (FI) or with a secondary diagnosis of FI during admission for other reasons.
The volume of acute admissions for fungal infection diagnosis is analyzed in terms of the number of admissions and related dynamics recorded in the period of 2007–2022.
Hospital admissions for FI were identified by the ICD-9-CM diagnoses reported in the Supplementary Materials. Considering the previous epidemiology, ICD-9-CM diagnoses related to endemic mycosis were not considered. Based on adjunctive ICD-9-CM diagnosis (see Supplementary Materials), all cases were grouped into three main categories: (i) neonatal infection, (ii) immunocompromised children, or (iii) other children. The categories included all admissions related to ICD-9-CM associated with all diagnoses of fungal infection in children (ICD-9-CM).
Patients older than eighteen and HDRs related to a second admission within thirty days from the previous admission were excluded.
2.3. Hospitalization Rate and Length of Stay
Based on the total number of hospital admissions concerning Veneto child residents each year, annual hospitalization rates were calculated by dividing the annual number of hospitalizations by the size of the resident population (source Demo Istat) [17], and expressing the rates as hospitalizations per 100,000 population. Further, the population was stratified according to the diagnosis in prematurity, solid organ transplantation (SOT), onco-hematological disease, and cystic fibrosis; consequently, the incidence rate of FI was estimated for each comorbidity.
The length of hospital stays was calculated as the days elapsing between the dates of admission and discharge, and the mean hospital stay was calculated. The case fatality rate (CFR) was calculated by dividing the number of in-hospital deaths by the number of patients hospitalized with a diagnosis of FI, expressed as a percentage.
Secondary analysis was epidemiology of FI pre- and post-SARS-CoV-2 pandemic.
2.4. Estimated Costs
The estimated direct cost to the health care system for FI-associated hospital admission was calculated using the diagnosis-related groups (DRGs) of hospitalized patients. The DRGs depend on the patient’s ICD classification at the time of their discharge from the hospital, their age and gender, and the consumption of resources during their hospital stay. According to the DRG-based reimbursement system, every hospitalized patient belongs to a group of diagnostically homogeneous cases. Patients within each category are therefore similar in clinical terms and are expected to require the same level of hospital resources. Veneto’s expenses per DRG are defined in the regional tariff schedule. Costs were expressed in euros (€) [18]. As a result, patients in the same DRG are assigned the same reimbursement charges. All hospital stays were analyzed considering, for the same patients, only the first hospital admission. Any case of secondary hospitalization, transfer to other acute care institutions, and admission to rehabilitation institutions, associated with the same patient, was removed from the initial dataset for the years 2007 to 2022.
2.5. Statistical Analysis
Categorical variables were represented with frequencies and percentages, while continuous variables were summarized as means, standard deviations (std), medians, minimum–maximum values, and interquartile ranges (IQRs). Chi-square tests (or Fisher’s exact tests) and Student’s t-tests (or Wilcoxon signed-rank tests) were employed to assess differences in HDR variables between groups.
Significant trends over the period considered were assessed by conducting a Joinpoint regression, estimating the annual percent changes (APCs) and the average APC (AAPCs), a summary measure of the trend over a given fixed interval [19]. An AAPC of zero coincides with the hypothesis of a trend that is neither increasing nor decreasing.
The 95% confidence intervals (95% CIs) were calculated as appropriate. A p-value < 0.05 was considered significant. All data manipulations, analyses, and visualizations were performed using Python 3.8.18 and R 4.2.2.
3. Results
3.1. Hospitalization for FI
From 2007 to 2022 a total of 1433 admissions due to FI were recorded. The overall hospitalization rate was 10,839 per 100,000 in the pediatric population. Stratifying for age, 51.1 % were under one year of age (110,552 per 100,000), 29.3 % were between 1 and 9 years old (6625 per 100,000), and 19.6% were between 10 and 18 years old (4426 per 100,000) (Figure 1). The majority of episodes were presented during the center years of the study (2010–2014), with a peak in 2012 (13,600 per 100,000 cases) and a subsequent decreasing incidence rate of FI. The negative trend in hospitalization rate resulted significantly after 2011 (APC = −4.79, p-value = 0.0012) (Figure 2).
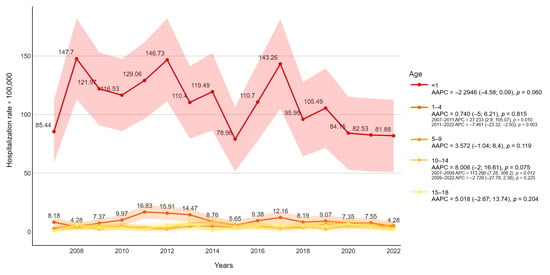
Figure 1.
Fungal infection-related hospitalization rate (per 100,000 inhabitants) trends were stratified for age with average annual percentage changes (AAPCs) and 95% confidence intervals.

Figure 2.
Overall fungal infection-related hospitalization rate (per 100,000 inhabitants) trends with average annual percentage changes (AAPCs) and 95% confidence intervals are presented.
The inpatient rate by gender was 9536 admissions per 100,000 males and 11,053 admissions per 100,000 females.
Patients were also stratified by comorbidities showing an incidence rate that was different for each category. About 9.8% of FI-hospitalized children had a history of prematurity, 3.2% of solid organ transplantation, 18.8% of onco-hematological conditions, and 0.6% of cystic fibrosis.
The median annual rates of hospitalization stratified for comorbidities were 242,424/100,000 for patients with a history of prematurity; 140,3509/100,000 for people with a history of organ transplantation; 596,491/100,000 for patients with an onco-hematological history; and 26,315/100,000 for persons with a history of cystic fibrosis (Figure 3). For all patients with comorbidities, the hospitalization rate was significantly higher (p-value < 0.015) than those who did not present with comorbidities.
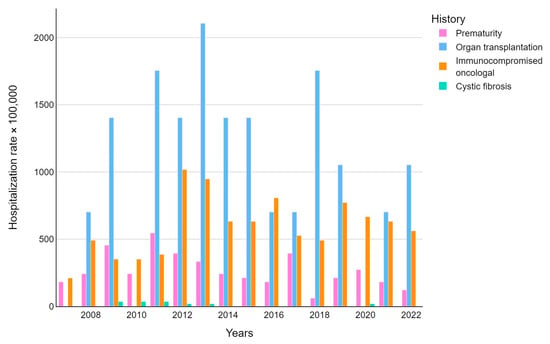
Figure 3.
Fungal infection-related hospitalization rate (per 100,000 inhabitants) trends were stratified by disease.
Regarding the length of hospitalization, the overall average was 14,7 days (median 6 days). Length of stay was significantly higher in 10–14-year-old patients (mean 27.8 days, median 11) and during the COVID-19 pandemic (18.2 versus 14.2, p-value = 0.0021). The trend analysis revealed an increasing pattern in the length of stay (AAPC = 6.17, p-value < 0.001) (Figure 4).

Figure 4.
Length of stay trends for fungal infection with average annual percentage changes (AAPCs) and 95% confidence intervals are presented.
Stratifying for comorbidities, patients with a history of solid organ transplantation had the longest length of hospitalization (49.8 days), followed by patients with a history of cystic fibrosis (38.6 days) and patients with onco-hematological disease (26.1 days).
3.2. DRG Analysis
In the analyzed period, most of the diagnosis was represented by candidiasis (50.2%, ICD-9-CM from 11,281 to 11,289), neonatal Candida (12.1%, ICD-9-CM 7717), and aspergillosis infection (10.5%, ICD-9-CM 1173 and 4846). The complete list of diagnoses is reported in Table 1.

Table 1.
Fungal infection hospitalization distribution according to age.
Candidiasis and neonatal Candida were prevalently represented among children younger than one year old (59.97% and 23.91, respectively), compared to aspergillosis infection among patients between 10 and 14 years old (28.93%).
The overall case fatality rate (CRF) was 2.094% (30 patients, p-value < 0.0001) lower for children < one year old (0.820), and higher for patients between 10 and 14 years old (4.403%). Mortality was significantly higher during the COVID-19 pandemic rather than in the previous years (3483 versus 1867). Overall, the Joinpoint regression found no trend (AAPC = 4.45, 95%CI −3.32; 1025); however, the 2019–2022 APC resulted in significant positive results (52.78, p-value = 0.042), highlighting a fast growth in CFR after 2020 (Figure 5).
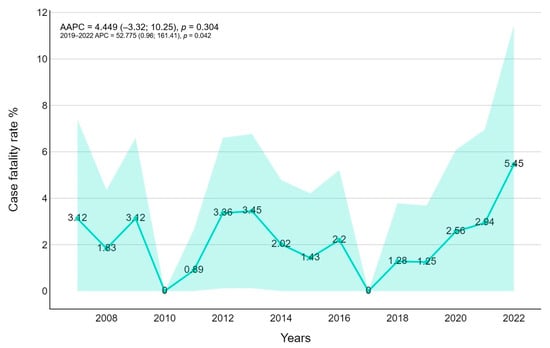
Figure 5.
Case fatality trends for fungal infection with average annual percentage changes (AAPCs) and 95% confidence intervals are presented.
Further, CFR was higher for patients with onco-hematological comorbidities (5926%) and post-solid organ transplantation (26,087%). In contrast, prematurity and cystic fibrosis were associated with lower CFR (0.709% and 0%, respectively).
3.3. Estimated Cost
The overall estimated hospitalization cost for FI was EUR 46,128 (median 27,768) with a mean estimated daily cost of 5659 euros/day (median 4082). The median cost was stable through the years (AAPC = 0.0018) with the highest value in 2014 (7489 euros/day), but without any peek during the COVID-19 pandemic. The average cost was 5504 euros/day for patients with a history of prematurity, 4791 euros/day for immunocompromised patients, 3951 euros/day for solid organ transplantation, and 3843 euros/day for cystic fibrosis. According to ICD-9-CM, the higher cost was associated with pneumonia due to non-aspergillosis (ICD-9-CM 4847 and 4848 with 8539 euros/day). Furthermore, surgical DRGs were associated with higher daily costs (8370 euros/day) (Figure 6).

Figure 6.
Hospitalization cost trends for fungal infection with average annual percentage changes (AAPCs) and 95% confidence intervals are presented.
All FI features and outcome assessments are reported in Table 2.

Table 2.
Fungal infection features and outcome assessments.
4. Discussion
Based on HDRs, the present retrospective study analyzed hospitalizations for FI in the Veneto region, Italy, for the period of 2007–2022 in the pediatric population. Admission for FI is mainly concentrated in the first year of age, followed by those who are 1–9 years old and 10–18 years old. Candida spp. is a documented infection in the neonatal phase of life with a variable incidence and mortality ranging from 12% to 37% in high-income countries (HICs) and from 8.9% to 75% in low- and middle-income countries (LMICs) [20], especially for prematurity with early gestational age and low weight [21]. However, in our study, we also analyzed changes in hospitalizations for FI from 2007 to 2022. Over this period, there was a slight reduction in hospitalizations for FI, with a progressive reduction from 13.6 in 2012 to 6.88 in 2022. A possible explanation for this reduction trend could be the use of antifungal prophylaxis in specific settings, such as neonatal intensive care units and onco-hematology departments where antifungal prophylaxis protocols are often applied. Compared to other studies in the literature, Marya et al. reported a hospitalization rate between 3,65 and 5.56/100,000 cases between 2000 and 2005 for Candida spp. infection [22] and a similar hospitalization rate is also analyzed in other studies [23]. For mold infections, the hospitalization rate is reported to be from 1.7 to 3.4 per million persons between 2000 and 2013 in adult studies [24]. Unfortunately, data are scarce in the literature for the pediatric population: Zaoutis et al. described the incidence of candidemia as 43 cases per 100,000 hospital admissions of children [1] and as 437/100,000 cases for invasive aspergillosis among immunocompromised pediatric admissions in the United States [25]. Both studies described a stable trend throughout the analyzed period.
In-hospital case fatality rate occurred in roughly 2.09% of cases. Considering age-related mortality, a higher case fatality rate has been observed in patients between 5 and 14 years old (4.4%) despite a relatively lower number of infections than neonatal age (0.82%). These data are considerably related to the higher number of oncological and solid organ transplantation patients. Stratifying for the pathogen, we noted higher CFR for Aspergillus spp. infections (14%) and relatively lower CFR for Candida spp. in particular for neonatal patients (0.6%). A similar high CFR for Aspergillus spp. is reported by the oncological pediatric population [25], indicating the seriousness of Aspergillus spp. infection in this population. However, in our analysis, an increasing trend of mortality was reported after the SARS-CoV-2 pandemic in 2020 (Figure 5) similar to the hospitalization length stay (Figure 6): Muthu suggested early-onset mold infections after COVID-19 and a more severe COVID-19 course in mold pneumonia co-infections in the adult population [26]. The potential causality of a prior viral infection remains unestablished. Some authors suggest a possible role for co-isolated infections in enhancing disease virulence, as well as an association between Aspergillus colonization and increased COVID-19 mortality [27,28]. Additionally, environmental factors could play a role, particularly in construction and renovation activities within hospitals or nearby areas. In Italy, and specifically in our region, a national building renovation plan has been underway since 2020, which might contribute to an increased number and severity of infections [29].
However, the real causality of these events is still unclear and further data are required.
In economic terms, the mean daily value of acute inpatient admission is EUR 565 with a maximum of EUR 13,063. The direct costs are not comparable with other studies for different values and situations [25].
Our study has several limitations. The first is the reliance on HDRs from the Veneto region, as these data are primarily used as an administrative tool and only secondarily used for healthcare purposes. Accessing complete medical histories was not feasible within the scope of this research. As a result, our classification depends on the diagnosis codes recorded in the HDRs, which are still coded using the ICD-9-CM system in the Veneto region. In addition to possible miscoding, we emphasize that our study may also be susceptible to underestimation of cases of fungal infection due to other etiologic agents or co-infection with other etiologic agents. Furthermore, HDR data do not contain any information about the microbiological, diagnostic, and therapeutic information, limiting the possible evaluation of the patient.
5. Conclusions
In summary, we reported a trend of FI hospitalizations in the Italian region from 2007 to 2022 in the pediatric population showing a general decrease despite persistently high case fatality rates, which increased following the COVID-19 pandemic. FI was more frequently observed in children under one year of age, primarily due to Candida species, while adolescents were more affected by Aspergillus and other mold infections. Significant differences are presented in hospitalizations for FI for comorbidities with higher impacts in terms of length of stay and CRF in oncological patients and those who had undergone solid organ transplantation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens14010093/s1, File S1: List of CODES ICD-9-CM DIAGNOSIS and CODES ICD-9-CM COMORBIDITIES.
Author Contributions
Conceptualization: L.C. and C.C.; methodology: L.C. and C.C.; software: C.C.; validation: L.C. and C.C.; formal analysis: C.C. investigation: L.C. and C.C.; resources: M.S. and S.C.; data curation: L.C and C.C.; writing—original draft preparation: L.C and C.C.; writing—review and editing: D.D. and C.G.; visualization: D.D. and V.B. supervision: C.G., D.D, and V.B project administration: L.C. and V.B.; Funding acquisition: V.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Regional Prevention Plan (PRP) 2020–2025 of the Veneto Region, year 2024 - D.D.R. n. 25 16/05/2024.
Institutional Review Board Statement
Hospital Discharge Records (HDRs) were obtained from the administrative databases of the Veneto Region, and the disclosure and utilization of such records. For educational and scientific purposes do not necessitate approval from ethical committees. On 24 January 2023, the Veneto Region implemented the Code of Conduct for the use of health data for educational and scientific publication purposes (Official Bulletin of the Region, “Bollettino Ufficiale della Regione” no. 10), as established by the European Committee (European Regulation 2016/679). This implementation received approval from the Italian Personal Data Protection Authority on 14 January 2021. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards [30].
Informed Consent Statement
Patient consent was waived because the HDR data are exclusively utilized and published in aggregate form, without any reference to patients’ personal information, in accordance with current Italian privacy legislation. Prior to the authors gaining access to the data, all personal data that could potentially lead to identification were substituted with anonymous codes, in accordance with current privacy regulations (Legislative Decree no. 196 of 30 June 2003).
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author.
Acknowledgments
We are very grateful to all the junior and senior medical staff for their help in collecting data and their ongoing support for this work.
Conflicts of Interest
Vincenzo Baldo received grants from MSD, GSK, Pfizer, Seqirus, Moderna, Janssen, and Sanofi for taking part in advisory boards, expert meetings, and for acting as a speaker and/or organizer of meetings/congresses. Carlo Giaquinto declares to have participated as a speaker at sponsored lectures/symposia and as a scientific expert at advisory boards organized by different pharmaceutical companies, including Sanofi, Janssen, Merck/MSD, Pfizer, and Gilead. Carlo Giaquinto received research support from the European Community (EC), the Italian Agency of Drugs (AIFA), and the Italian Ministry for University and Research (MIUR). Other authors have no conflicts of interest to declare.
References
- Zaoutis, T.E.; Argon, J.; Chu, J.; Berlin, J.A.; Walsh, T.J.; Feudtner, C. The Epidemiology and Attributable Outcomes of Candidemia in Adults and Children Hospitalized in the United States: A Propensity Analysis. Clin. Infect. Dis. 2005, 41, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Erjavec, Z.; Kluin-Nelemans, H.; Verweij, P.E. Trends in invasive fungal infections, with emphasis on invasive aspergillosis. Clin. Microbiol. Infect. 2009, 15, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Nosari, A.; Caira, M.; Pioltelli, M.; Fanci, R.; Bonini, A.; Cattaneo, C.; Castagnola, C.; Capalbo, S.; De Fabritiis, P.; Mettivier, V.; et al. Hema e-Chart Registry of invasive fungal infections in haematological patients: Improved outcome in recent years in mould infections. Clin. Microbiol. Infect. 2013, 19, 757–762. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Suleyman, G.; Alangaden, G.J. Nosocomial Fungal Infections: Epidemiology, Infection Control, and Prevention. Infect. Dis. Clin. N. Am. 2021, 35, 1027–1053. [Google Scholar] [CrossRef]
- Tragiannidis, A.; Fegeler, W.; Rellensmann, G.; Debus, V.; Müller, V.; Hoernig-Franz, I.; Siam, K.; Pana, Z.-D.; Jürgens, H.; Groll, A. Candidaemia in a European Paediatric University Hospital: A 10-year observational study. Clin. Microbiol. Infect. 2012, 18, E27–E30. [Google Scholar] [CrossRef]
- Lehrnbecher, T.; Schöning, S.; Poyer, F.; Georg, J.; Becker, A.; Gordon, K.; Attarbaschi, A.; Groll, A.H. Incidence and Outcome of Invasive Fungal Diseases in Children With Hematological Malignancies and/or Allogeneic Hematopoietic Stem Cell Transplantation: Results of a Prospective Multicenter Study. Front. Microbiol. 2019, 10, 681. [Google Scholar] [CrossRef]
- Registries, C.o.Z.a.F.; Pana, Z.D.; Seidel, D.; Skiada, A.; Groll, A.H.; Petrikkos, G.; Cornely, O.A.; Roilides, E. Invasive mucormycosis in children: An epidemiologic study in European and non-European countries based on two registries. BMC Infect. Dis. 2016, 16, 1–9. [Google Scholar] [CrossRef]
- Vasileiou, E.; Paisiou, A.; Tsipou, C.; Pourtsidis, A.; Galani, V.; Katzilakis, N.; Antoniadi, K.; Papakonstantinou, E.; Ioannidou, E.; Stiakaki, E.; et al. Candidemia in Children with Malignancies: Report from the Infection Working Group of the Hellenic Society of Pediatric Hematology-Oncology. J. Fungi 2020, 6, 276. [Google Scholar] [CrossRef]
- Warris, A.; Lehrnbecher, T.; Roilides, E.; Castagnola, E.; Bruggemann, R.J.M.; Groll, A.H. ESCMID-ECMM guideline: Diagnosis and management of invasive aspergillosis in neonates and children. Clin. Microbiol. Infect. 2019, 25, 1096–1113. [Google Scholar] [CrossRef]
- Bonifaz, A.; Rojas, R.; Tirado-Sánchez, A.; Chávez-López, D.; Mena, C.; Calderón, L.; María, P.-O.R. Superficial Mycoses Associated with Diaper Dermatitis. Mycopathologia 2016, 181, 671–679. [Google Scholar] [CrossRef]
- Ray, A.; Singh, B.S.; Kar, B.R. Clinicomycological Profile of Pediatric Dermatophytoses. Indian Dermatol. Online J. 2022, 13, 361–365. [Google Scholar] [CrossRef]
- Pana, Z.D.; Roilides, E.; Warris, A.; Groll, A.H.; Zaoutis, T. Epidemiology of Invasive Fungal Disease in Children. J. Pediatr. Infect. Dis. Soc. 2017, 6, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Demographic Statistics Veneto Region (Population Density, Population, Median Age, Households, Foreigners). Available online: https://ugeo.urbistat.com/AdminStat/it/it/demografia/dati-sintesi/veneto/5/2 (accessed on 18 June 2024).
- Ferre, F.; de Belvis, A.G.; Valerio, L.; Longhi, S.; Lazzari, A.; Fattore, G.; Ricciardi, W.; Maresso, A. Italy: Health system review. Health Syst. Transit. 2014, 16, 1–168. [Google Scholar] [PubMed]
- Regione del Veneto. Decreto no. 118, “Aggiornamento Della Disciplina del Flusso Informativo ‘Scheda di Dimissione Ospedaliera’ e dei Tracciati Record”. Available online: https://salute.regione.veneto.it/c/document_library/get_file?p_l_id=8278039&folderId=1026448&name=DLFE-31626.pdf (accessed on 18 June 2024).
- Istat (Istituto Nazionale di Statistica). Available online: https://salute.regione.veneto.it/c/document_library/get_file?p_l_id=8278039&folderId=1026448&name=DLFE-31626.pdf (accessed on 18 June 2024).
- Regione del Veneto. Allegato A. Tariffe DRG. Available online: https://salute.regione.veneto.it/c/document_library/get_file?p_l_id=8278039&folderId=1026448&name=DLFE-32075.xls (accessed on 18 June 2024).
- Kim, H.J.; Fay, M.P.; Feuer, E.J.; Midthune, D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000, 19, 335–351, Erratum in: Stat. Med. 2001, 20, 655. [Google Scholar] [CrossRef]
- Kaur, H.; Chakrabarti, A. Strategies to Reduce Mortality in Adult and Neonatal Candidemia in Developing Countries. J. Fungi 2017, 3, 41. [Google Scholar] [CrossRef]
- Cook, A.; Ferreras-Antolin, L.; Adhisivam, B.; Ballot, D.A.; Berkley, J.; Bernaschi, P.; Carvalheiro, C.G.; Chaikittisuk, N.; Chen, Y.; Chibabhai, V.; et al. Neonatal invasive candidiasis in low- and middle-income countries: Data from the NeoOBS study. Med. Mycol. 2023, 61. [Google Scholar] [CrossRef]
- Zilberberg, M.D.; Shorr, A.F.; Kollef, M.H. Secular Trends in Candidemia-Related Hospitalization in the United States, 2000–2005. Infect. Control. Hosp. Epidemiol. 2008, 29, 978–980. [Google Scholar] [CrossRef]
- Marchetti, O.; Bille, J.; Fluckiger, U.; Eggimann, P.; Ruef, C.; Garbino, J.; Calandra, T.; Glauser, M.; Tauber, M.G.; Pittet, D.; et al. Epidemiology of Candidemia in Swiss Tertiary Care Hospitals: Secular Trends, 1991–2000. Clin. Infect. Dis. 2004, 38, 311–320. [Google Scholar] [CrossRef]
- Vallabhaneni, S.; Benedict, K.; Derado, G.; Mody, R.K. Trends in Hospitalizations Related to Invasive Aspergillosis and Mucormycosis in the United States, 2000–2013. Open Forum Infect. Dis. 2017, 4, ofw268. [Google Scholar] [CrossRef]
- Zaoutis, T.E.; Heydon, K.; Chu, J.H.; Walsh, T.J.; Steinbach, W.J. Epidemiology, Outcomes, and Costs of Invasive Aspergillosis in Immunocompromised Children in the United States, 2000. Pediatrics 2006, 117, e711–e716. [Google Scholar] [CrossRef]
- Muthu, V.; Agarwal, R.; Rudramurthy, S.M.; Thangaraju, D.; Shevkani, M.R.; Patel, A.K.; Shastri, P.S.; Tayade, A.; Bhandari, S.; Gella, V.; et al. Prevalence of co-existent COVID-19-associated pulmonary aspergillosis (CAPA) and its impact on early mortality in patients with COVID-19-associated pulmonary mucormycosis (CAPM). Mycoses 2024, 67, e13745. [Google Scholar] [CrossRef] [PubMed]
- Alhumaid, S.; Alabdulqader, M.; Al Dossary, N.; Al Alawi, Z.; Alnaim, A.A.; Al Mutared, K.M.; Al Noaim, K.; Al Ghamdi, M.A.; Albahrani, S.J.; Alahmari, A.A.; et al. Global Coinfections with Bacteria, Fungi, and Respiratory Viruses in Children with SARS-CoV-2: A Systematic Review and Meta-Analysis. Trop. Med. Infect. Dis. 2022, 7, 380. [Google Scholar] [CrossRef] [PubMed]
- Osman, H.; Shaik, A.N.; Nguyen, P.L.; Cantor, Z.; Kaafarani, M.; Soubani, A.O. The Clinical Significance of Aspergillus Detected in Lower-Respiratory-Tract Samples of Critically Ill COVID-19-Positive Patients. Adv. Respir. Med. 2023, 91, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, H.; Rutala, W.A.; Sickbert-Bennett, E.E.; Weber, D.J. Review of Fungal Outbreaks and Infection Prevention in Healthcare Settings During Construction and Renovation. Clin. Infect. Dis. 2015, 61, 433–444. [Google Scholar] [CrossRef]
- World Medical Association. WMA Declaration of Helsinki-Ethical Principles for Medical Research Involving Human Subjects. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 5 December 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).