Introduction of Vector-Borne Infections in Europe: Emerging and Re-Emerging Viral Pathogens with Potential Impact on One Health
Abstract
1. Introduction
2. Search Strategy and Selection Criteria
- (1)
- Pathologies with active transmission and autochthonous cases in human OR in animals transmitted by mosquitoes permanently present in Europe (Aedes albopictus and Culex spp.):
- -
- Chikungunya virus (CHIKV)
- -
- Dengue virus (DENV)
- -
- Zika virus (ZIKV)
- -
- Japanese encephalitis virus (JEV)
- (2)
- Pathologies with active transmission and autochthonous cases in human OR in animals transmitted by ticks permanently present in Europe (Hyalomma spp.):
- -
- Crimean–Congo Hemorragic virus (CCHV)
- (3)
- Pathologies without autochthonous transmission but with the presence of competent vectors in Europe:
- -
- O’nyong’nyong virus (ONNV)
- -
- Rift Valley fever virus (RVFV)
- -
- Yellow fever virus (YFV)
- (4)
- Pathologies without autochthonous transmission and without the presence of the vector on European territory:
- -
- Oropouche virus (OROV)
3. Pathologies with Active Transmission and Autochthonous Cases in Human or in Animals Transmitted by Vectors Permanently Present Across Europe
3.1. Chikungunya Virus
3.2. Dengue Virus
3.3. Zika Virus
3.4. Japanese Encephalitis Virus
3.5. Crimean–Congo Hemorrhagic Fever Virus
4. Pathologies Without Autochthonous Transmission in Humans or Animals but with Presence of Competent Vectors in Europe
4.1. O’nyong-Nyong Virus
4.2. Rift Valley Virus
4.3. Yellow Fever Virus
5. Pathologies Without Autochthonous Transmission in Humans or Animals and Without the Presence of Competent Vectors in Europeviruses Transmitted by Midges (Culicoides spp.)
Oropouche Virus
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, X.; Lu, Y.; Zhou, S.; Chen, L.; Xu, B. Impact of Climate Change on Human Infectious Diseases: Empirical Evidence and Human Adaptation. Environ. Int. 2016, 86, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Semenza, J.C.; Paz, S. Climate Change and Infectious Disease in Europe: Impact, Projection and Adaptation. Lancet Reg. Health Eur. 2021, 9, 100230. [Google Scholar] [CrossRef] [PubMed]
- Skinner, E.B.; Glidden, C.K.; MacDonald, A.J.; Mordecai, E.A. Human Footprint Is Associated with Shifts in the Assemblages of Major Vector-Borne Diseases. Nat. Sustain. 2023, 6, 652–661. [Google Scholar] [CrossRef]
- Glidden, C.K.; Nova, N.; Kain, M.P.; Lagerstrom, K.M.; Skinner, E.B.; Mandle, L.; Sokolow, S.H.; Plowright, R.K.; Dirzo, R.; De Leo, G.A.; et al. Human-Mediated Impacts on Biodiversity and the Consequences for Zoonotic Disease Spillover. Curr. Biol. 2021, 31, R1342–R1361. [Google Scholar] [CrossRef] [PubMed]
- Zarzyczny, K.M.; Rius, M.; Williams, S.T.; Fenberg, P.B. The Ecological and Evolutionary Consequences of Tropicalisation. Trends Ecol. Evol. 2024, 39, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Sokolow, S.H.; Nova, N.; Jones, I.J.; Wood, C.L.; Lafferty, K.D.; Garchitorena, A.; Hopkins, S.R.; Lund, A.J.; MacDonald, A.J.; LeBoa, C.; et al. Ecological and Socioeconomic Factors Associated with the Human Burden of Environmentally Mediated Pathogens: A Global Analysis. Lancet Planet. Health 2022, 6, e870–e879. [Google Scholar] [CrossRef]
- Climate ADAPT European Climate Risk Assessment a Comprehensive Assessment of Current and Future Climate Risks in Europe. Available online: https://climate-adapt.eea.europa.eu/en/eu-adaptation-policy/key-eu-actions/european-climate-risk-assessment (accessed on 1 December 2024).
- European Commission, Copernicus Europe’s Eyes on Earth August Climate Bulletins|Newsflash Copernicus: Summer 2024—Hottest on Record Globally and for Europe. Available online: https://climate.copernicus.eu/copernicus-summer-2024-hottest-record-globally-and-europe#:~:text=The%20average%20temperature%20for%20European%20land%20for%20summer%20(June%2DAugust,2022%20(1.34%C2%B0C) (accessed on 6 September 2024).
- Magallanes, S.; Llorente, F.; Ruiz-López, M.J.; Puente, J.M.L.; Ferraguti, M.; Gutiérrez-López, R.; Soriguer, R.; Aguilera-Sepúlveda, P.; Fernández-Delgado, R.; Jímenez-Clavero, M.Á.; et al. Warm Winters Are Associated to More Intense West Nile Virus Circulation in Southern Spain. Emerg. Microbes Infect. 2024, 13, 2348510. [Google Scholar] [CrossRef] [PubMed]
- Tjaden, N.B.; Suk, J.E.; Fischer, D.; Thomas, S.M.; Beierkuhnlein, C.; Semenza, J.C. Modelling the Effects of Global Climate Change on Chikungunya Transmission in the 21st Century. Sci. Rep. 2017, 7, 3813. [Google Scholar] [CrossRef]
- Klepac, P.; Hsieh, J.L.; Ducker, C.L.; Assoum, M.; Booth, M.; Byrne, I.; Dodson, S.; Martin, D.L.; Turner, C.M.R.; van Daalen, K.R.; et al. Climate Change, Malaria and Neglected Tropical Diseases: A Scoping Review. Trans. R. Soc. Trop. Med. Hyg. 2024, 118, 561–579. [Google Scholar] [CrossRef] [PubMed]
- Rossi, B.; Barreca, F.; Benvenuto, D.; Braccialarghe, N.; Campogiani, L.; Lodi, A.; Aguglia, C.; Cavasio, R.A.; Giacalone, M.L.; Kontogiannis, D.; et al. Human Arboviral Infections in Italy: Past, Current, and Future Challenges. Viruses 2023, 15, 368. [Google Scholar] [CrossRef]
- Simonin, Y. Circulation of West Nile Virus and Usutu Virus in Europe: Overview and Challenges. Viruses 2024, 16, 599. [Google Scholar] [CrossRef]
- Semenza, J.C.; Rocklöv, J.; Ebi, K.L. Climate Change and Cascading Risks from Infectious Disease. Infect. Dis. Ther. 2022, 11, 1371–1390. [Google Scholar] [CrossRef]
- Pörtner, H.-O.; Roberts, D.C.; Tignor, M.; Poloczanska, E.S.; Mintenbeck, K.; Alegría, A.; Craig, M.; Langsdorf, S.; Löschke, S.; Möller, V.; et al. (Eds.) Contribution of Working Group II to the Sixth Assessment Report of the; Intergovernmental Panel on Climate Change. In Climate Change 2022 Impacts Adaptation and Vulnerability; IPCC: Paris, France, 2022. [Google Scholar]
- EFSA Autorità Europea per la Sicurezza Alimentare. Malattie Trasmesse Da Vettori. Available online: https://www.efsa.europa.eu/it/topics/topic/vector-borne-diseases (accessed on 21 December 2023).
- WHO Vector-Borne Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 26 September 2024).
- European Centre for Disease Notification Rates of Confirmed Locally-Acquired Tick-Borne Encephalitis Cases per 100,000 Population, EU/EEA Countries, 2022. Notification Rates of Confirmed Locally-Acquired Tick-Borne Encephalitis Cases per 100,000 Population, EU/EEA Countries, 2023. Available online: https://www.ecdc.europa.eu/en/publications-data/notification-rates-confirmed-locally-acquired-tick-borne-encephalitis-cases (accessed on 3 December 2024).
- European Centre for Disease Surveillance of West Nile Virus Infections in Humans, Weekly Report. Available online: https://www.ecdc.europa.eu/en/west-nile-fever/surveillance-and-disease-data/disease-data-ecdc (accessed on 26 November 2024).
- European Centre for Disease. Mosquito-Borne Diseases—An Increasing Risk in Europe; European Centre for Disease: Stockholm, Sweden, 2009.
- de Lima Cavalcanti, T.Y.V.; Pereira, M.R.; de Paula, S.O.; Franca, R.F.d.O. A Review on Chikungunya Virus Epidemiology, Pathogenesis and Current Vaccine Development. Viruses 2022, 14, 969. [Google Scholar] [CrossRef] [PubMed]
- Bartholomeeusen, K.; Daniel, M.; LaBeaud, D.A.; Gasque, P.; Peeling, R.W.; Stephenson, K.E.; Ng, L.F.P.; Ariën, K.K. Chikungunya Fever. Nat. Rev. Dis. Primer 2023, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Vairo, F.; Haider, N.; Kock, R.; Ntoumi, F.; Ippolito, G.; Zumla, A. Chikungunya: Epidemiology, Pathogenesis, Clinical Features, Management, and Prevention. Infect. Dis. Clin. N. Am. 2019, 33, 1003–1025. [Google Scholar] [CrossRef] [PubMed]
- Longbottom, J.; Walekhwa, A.W.; Mwingira, V.; Kijanga, O.; Mramba, F.; Lord, J.S. Aedes Albopictus Invasion across Africa: The Time Is Now for Cross-Country Collaboration and Control. Lancet Glob. Health 2023, 11, e623–e628. [Google Scholar] [CrossRef] [PubMed]
- Contopoulos-Ioannidis, D.; Newman-Lindsay, S.; Chow, C.; LaBeaud, A.D. Mother-to-Child Transmission of Chikungunya Virus: A Systematic Review and Meta-Analysis. PLoS Neglected Trop. Dis. 2018, 12, e0006510. [Google Scholar] [CrossRef] [PubMed]
- Yergolkar, P.; Tandale, B.; Arankalle, V.; Sathe, P.; Gandhe, S.; Gokhle, M.; Jacob, G.; Hundekar, S.; Mishra, A.; AB, S. Chikungunya Outbreaks Caused by African Genotype, India. Emerg. Infect. Dis. 2006, 12, 1580–1583. [Google Scholar] [CrossRef]
- Charrel, R.N.; De Lamballerie, X.; Raoult, D. Chikungunya Outbreaks—The Globalization of Vectorborne Diseases. N. Engl. J. Med. 2007, 356, 769–771. [Google Scholar] [CrossRef] [PubMed]
- Powers, A.M.; Brault, A.C.; Tesh, R.B.; Weaver, S.C. Re-Emergence of Chikungunya and o’nyong-Nyong Viruses: Evidence for Distinct Geographical Lineages and Distant Evolutionary Relationships. Microbiology 2000, 81, 471–479. [Google Scholar] [CrossRef]
- Rezza, G.; Nicoletti, L.; Angelini, R.; Romi, R.; Finarelli, A.; Panning, M.; Cordioli, P.; Fortuna, C.; Boros, S.; Magurano, F.; et al. Infection with Chikungunya Virus in Italy: An Outbreak in a Temperate Region. Lancet 2007, 370, 1840–1846. [Google Scholar] [CrossRef] [PubMed]
- AbuBakar, S.; Sam, I.-C.; Wong, P.-F.; MatRahim, N.; Hooi, P.-S.; Roslan, N. Reemergence of Endemic Chikungunya, Malaysia. Emerg. Infect. Dis. 2007, 13, 147–149. [Google Scholar] [CrossRef]
- Dommar, C.J.; López, L.; Paul, R.; Rodó, X. The 2013 Chikungunya Outbreak in the Caribbean Was Structured by the Network of Cultural Relationships among Islands. R. Soc. Open Sci. 2023, 10, 230909. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Ixchiq Chikungunya Vaccine (Live); European Medicines Agency: London, UK, 2006.
- CDC. Chikungunya Vaccine Information for Healthcare Providers; CDC: Atlanta, GA, USA, 2024.
- Peinado, R.D.S.; Eberle, R.J.; Pacca, C.C.; Arni, R.K.; Coronado, M.A. Review of -Omics Studies on Mosquito-Borne Viruses of the Flavivirus Genus. Virus Res. 2022, 307, 198610. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.B.; Yang, Z.-S.; Lin, C.-Y.; Hsu, M.-C.; Urbina, A.N.; Assavalapsakul, W.; Wang, W.-H.; Chen, Y.-H.; Wang, S.-F. Dengue Overview: An Updated Systemic Review. J. Infect. Public Health 2023, 16, 1625–1642. [Google Scholar] [CrossRef]
- Fortuna, C.; Severini, F.; Marsili, G.; Toma, L.; Amendola, A.; Venturi, G.; Argentini, C.; Casale, F.; Bernardini, I.; Boccolini, D.; et al. Assessing the Risk of Dengue Virus Local Transmission: Study on Vector Competence of Italian Aedes Albopictus. Viruses 2024, 16, 176. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.C.; Cordellier, R.; Hervy, J.P.; Digoutte, J.P.; Monteny, N. Isolement de 96 souches de virus dengue 2 à partir de moustiques capturés en cote-d’ivoire et Haute-Volta. Ann. Inst. Pasteur Virol. 1983, 134, 233–244. [Google Scholar] [CrossRef]
- Diallo, D.; Diouf, B.; Gaye, A.; NDiaye, E.H.; Sene, N.M.; Dia, I.; Diallo, M. Dengue Vectors in Africa: A Review. Heliyon 2022, 8, e09459. [Google Scholar] [CrossRef]
- Powell, J.R.; Gloria-Soria, A.; Kotsakiozi, P. Recent History of Aedes Aegypti: Vector Genomics and Epidemiology Records. Bioscience 2018, 68, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Seixas, G.; Salgueiro, P.; Bronzato-Badial, A.; Gonçalves, Y.; Reyes-Lugo, M.; Gordicho, V.; Ribolla, P.; Viveiros, B.; Silva, A.C.; Pinto, J.; et al. Origin and Expansion of the Mosquito Aedes Aegypti in Madeira Island (Portugal). Sci. Rep. 2019, 9, 2241. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Bai, Z.; Zhou, H.; Tu, Z.; Fang, M.; Tang, B.; Liu, J.; Liu, L.; Liu, J.; Chen, W. Molecular Epidemiology of Dengue Viruses in Southern China from 1978 to 2006. Virol. J. 2011, 8, 322. [Google Scholar] [CrossRef] [PubMed]
- Brathwaite Dick, O.; San Martín, J.L.; Montoya, R.H.; del Diego, J.; Zambrano, B.; Dayan, G.H. The History of Dengue Outbreaks in the Americas. Am. J. Trop. Med. Hyg. 2012, 87, 584–593. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Disease Outbreak News; Dengue—Global Situation. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON518 (accessed on 30 May 2024).
- European Centre for Disease Local Transmission of Dengue Virus in Mainland EU/EEA, 2010-Present. Available online: https://www.ecdc.europa.eu/en/all-topics-z/dengue/surveillance-and-disease-data/autochthonous-transmission-dengue-virus-eueea (accessed on 6 December 2024).
- Istituto Superiore di Sanità (ISS). EpiCentro Sistema Nazionale Di Sorveglianza Delle Arbovirosi; ISS: Roma, Italy, 2024. [Google Scholar]
- Naddaf, M. Dengue Is Spreading in Europe: How Worried Should We Be? Nature 2023. Available online: https://doi.org/10.1038/d41586-023-03407-6 (accessed on 31 January 2023). [CrossRef] [PubMed]
- Sacco, C.; Liverani, A.; Venturi, G.; Gavaudan, S.; Riccardo, F.; Salvoni, G.; Fortuna, C.; Marinelli, K.; Marsili, G.; Pesaresi, A.; et al. Autochthonous Dengue Outbreak in Marche Region, Central Italy, August to October 2024. Euro Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 2024, 29, 2400713. [Google Scholar] [CrossRef]
- Bellissimo-Rodrigues, F.; Dal Fabbro, A.L. Dengue Fever: Working towards Global Control Considering Biological, Social, and Planetary Determinants. Lancet Infect. Dis. 2023, 23, e506. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Dengue Worldwide Overview. Available online: https://www.ecdc.europa.eu/en/dengue-monthly (accessed on 1 December 2024).
- World Health Organization. Dengue Vaccines: WHO Position Paper—Wkly Epidemiol Rec 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Petri, E.; Biswal, S.; Lloyd, E.; Tricou, V.; Folschweiller, N. Early Onset of Protection of the TAK-003 Dengue Vaccine: Data from the DEN-301 Clinical Trial. Vaccine 2024, 42, 126309. [Google Scholar] [CrossRef]
- Tricou, V.; Yu, D.; Reynales, H.; Biswal, S.; Saez-Llorens, X.; Sirivichayakul, C.; Lopez, P.; Borja-Tabora, C.; Bravo, L.; Kosalaraksa, P.; et al. Long-Term Efficacy and Safety of a Tetravalent Dengue Vaccine (TAK-003): 4·5-Year Results from a Phase 3, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Glob. Health 2024, 12, e257–e270. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Gubler, D.J. Zika Virus. Clin. Microbiol. Rev. 2016, 29, 487–524. [Google Scholar] [CrossRef] [PubMed]
- Halani, S.; Tombindo, P.E.; O’Reilly, R.; Miranda, R.N.; Erdman, L.K.; Whitehead, C.; Bielecki, J.M.; Ramsay, L.; Ximenes, R.; Boyle, J.; et al. Clinical Manifestations and Health Outcomes Associated with Zika Virus Infections in Adults: A Systematic Review. PLoS Negl. Trop. Dis. 2021, 15, e0009516. [Google Scholar] [CrossRef]
- Tajik, S.; Farahani, A.V.; Ardekani, O.S.; Seyedi, S.; Tayebi, Z.; Kami, M.; Aghaei, F.; Hosseini, T.M.; Nia, M.M.K.; Soheili, R.; et al. Zika Virus Tropism and Pathogenesis: Understanding Clinical Impacts and Transmission Dynamics. Virol. J. 2024, 21, 271. [Google Scholar] [CrossRef]
- Epelboin, Y.; Talaga, S.; Epelboin, L.; Dusfour, I. Zika Virus: An Updated Review of Competent or Naturally Infected Mosquitoes. PLoS Negl. Trop. Dis. 2017, 11, e0005933. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.J.; Carlson, C.J.; Mordecai, E.A.; Johnson, L.R. Global Expansion and Redistribution of Aedes-Borne Virus Transmission Risk with Climate Change. PLoS Negl. Trop. Dis. 2019, 13, e0007213. [Google Scholar] [CrossRef]
- McKenzie, B.A.; Wilson, A.E.; Zohdy, S. Aedes Albopictus Is a Competent Vector of Zika Virus: A Meta-Analysis. PLoS ONE 2019, 14, e0216794. [Google Scholar] [CrossRef] [PubMed]
- Grard, G.; Caron, M.; Mombo, I.M.; Nkoghe, D.; Mboui Ondo, S.; Jiolle, D.; Fontenille, D.; Paupy, C.; Leroy, E.M. Zika Virus in Gabon (Central Africa)—2007: A New Threat from Aedes Albopictus? PLoS Negl. Trop. Dis. 2014, 8, e2681. [Google Scholar] [CrossRef]
- Brady, O.J.; Hay, S.I. The First Local Cases of Zika Virus in Europe. Lancet 2019, 394, 1991–1992. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.R.; Jamieson, D.J.; Powers, A.M.; Honein, M.A. Zika Virus. N. Engl. J. Med. 2016, 374, 1552–1563. [Google Scholar] [CrossRef]
- Fagbami, A.H. Zika Virus Infections in Nigeria: Virological and Seroepidemiological Investigations in Oyo State. J. Hyg. 1979, 83, 213–219. [Google Scholar] [CrossRef]
- Olson, J.G.; Ksiazek, T.G.; Suhandiman; Triwibowo. Zika Virus, a Cause of Fever in Central Java, Indonesia. Trans. R. Soc. Trop. Med. Hyg. 1981, 75, 389–393. [Google Scholar] [CrossRef]
- Musso, D.; Ko, A.I.; Baud, D. Zika Virus Infection—After the Pandemic. N. Engl. J. Med. 2019, 381, 1444–1457. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.R.; Chen, T.-H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika Virus Outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Gatherer, D.; Kohl, A. Zika Virus: A Previously Slow Pandemic Spreads Rapidly through the Americas. J. Gen. Virol. 2016, 97, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Zanluca, C.; de Melo, V.C.A.; Mosimann, A.L.P.; Santos, G.I.V.D.; Santos, C.N.D.D.; Luz, K. First Report of Autochthonous Transmission of Zika Virus in Brazil. Mem. Inst. Oswaldo Cruz 2015, 110, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Gulland, A. Zika Virus Is a Global Public Health Emergency, Declares WHO. BMJ 2016, 352, i657. [Google Scholar] [CrossRef] [PubMed]
- Giron, S.; Franke, F.; Decoppet, A.; Cadiou, B.; Travaglini, T.; Thirion, L.; Durand, G.; Jeannin, C.; L’Ambert, G.; Grard, G.; et al. Vector-Borne Transmission of Zika Virus in Europe, Southern France, August 2019. Euro Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 2019, 24, 1900655. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guidelines for the Prevention of Sexual Transmission of Zika Virus; WHO Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2020; ISBN 978-92-4-155048-2. [Google Scholar]
- Zhu, Y.; He, Z.; Qi, Z. Virus-Host Interactions in Early Japanese Encephalitis Virus Infection. Virus Res. 2023, 331, 199120. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.B.; Vrati, S.; Kalia, M. Pathobiology of Japanese Encephalitis Virus Infection. Mol. Asp. Med. 2021, 81, 100994. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.R.S.; Strathe, E.; Etcheverry, L.; Cohnstaedt, L.W.; McVey, D.S.; Piaggio, J.; Cernicchiaro, N. Assessment of Data on Vector and Host Competence for Japanese Encephalitis Virus: A Systematic Review of the Literature. Prev. Vet. Med. 2018, 154, 71–89. [Google Scholar] [CrossRef]
- Levesque, Z.A.; Walsh, M.G.; Webb, C.E.; Zadoks, R.N.; Brookes, V.J. A Scoping Review of Evidence of Naturally Occurring Japanese Encephalitis Infection in Vertebrate Animals Other than Humans, Ardeid Birds and Pigs. PLoS Negl. Trop. Dis. 2024, 18, e0012510. [Google Scholar] [CrossRef]
- Gresser, I.; Hardy, J.L.; Hu, S.M.; Scherer, W.F. Factors Influencing Transmission of Japanese B Encephalitis Virus by a Colonized Strain of Culex Tritaeniorhynchus Giles, from Infected Pigs and Chicks to Susceptible Pigs and Birds. Am. J. Trop. Med. Hyg. 1958, 7, 365–373. [Google Scholar] [CrossRef]
- Tuno, N.; Tsuda, Y.; Takagi, M. How Zoophilic Japanese Encephalitis Vector Mosquitoes Feed on Humans. J. Med. Entomol. 2017, 54, 8–13. [Google Scholar] [CrossRef]
- Mansfield, K.L.; Hernández-Triana, L.M.; Banyard, A.C.; Fooks, A.R.; Johnson, N. Japanese Encephalitis Virus Infection, Diagnosis and Control in Domestic Animals. Vet. Microbiol. 2017, 201, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.S.; Gubler, D.J.; Petersen, L.R. Emerging Flaviviruses: The Spread and Resurgence of Japanese Encephalitis, West Nile and Dengue Viruses. Nat. Med. 2004, 10, S98–S109. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Singh, R.; Mani, V.E.; Poddar, B. Japanese B Encephalitis. Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 2021, 25, S171–S174. [Google Scholar] [CrossRef]
- Hernandez-Valencia, J.C.; Muñoz-Laiton, P.; Gómez, G.F.; Correa, M.M. A Systematic Review on the Viruses of Anopheles Mosquitoes: The Potential Importance for Public Health. Trop. Med. Infect. Dis. 2023, 8, 459. [Google Scholar] [CrossRef]
- Mulvey, P.; Duong, V.; Boyer, S.; Burgess, G.; Williams, D.T.; Dussart, P.; Horwood, P.F. The Ecology and Evolution of Japanese Encephalitis Virus. Pathogens 2021, 10, 1534. [Google Scholar] [CrossRef]
- Bae, W.; Kim, J.H.; Kim, J.; Lee, J.; Hwang, E.S. Changes of Epidemiological Characteristics of Japanese Encephalitis Viral Infection and Birds as a Potential Viral Transmitter in Korea. J. Korean Med. Sci. 2018, 33, e70. [Google Scholar] [CrossRef] [PubMed]
- Platonov, A.; Rossi, G.; Karan, L.; Mironov, K.; Busani, L.; Rezza, G. Does the Japanese Encephalitis Virus (JEV) Represent a Threat for Human Health in Europe? Detection of JEV RNA Sequences in Birds Collected in Italy. Euro Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 2012, 17, 20241. [Google Scholar] [CrossRef]
- Ravanini, P.; Huhtamo, E.; Ilaria, V.; Crobu, M.G.; Nicosia, A.M.; Servino, L.; Rivasi, F.; Allegrini, S.; Miglio, U.; Magri, A.; et al. Japanese Encephalitis Virus RNA Detected in Culex Pipiens Mosquitoes in Italy. Euro Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 2012, 17, 20221. [Google Scholar] [CrossRef] [PubMed]
- Furuya-Kanamori, L.; Gyawali, N.; Mills, D.J.; Hugo, L.E.; Devine, G.J.; Lau, C.L. The Emergence of Japanese Encephalitis in Australia and the Implications for a Vaccination Strategy. Trop. Med. Infect. Dis. 2022, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Simon-Loriere, E.; Faye, O.; Prot, M.; Casademont, I.; Fall, G.; Fernandez-Garcia, M.D.; Diagne, M.M.; Kipela, J.-M.; Fall, I.S.; Holmes, E.C.; et al. Autochthonous Japanese Encephalitis with Yellow Fever Coinfection in Africa. N. Engl. J. Med. 2017, 376, 1483–1485. [Google Scholar] [CrossRef]
- Turtle, L.; Solomon, T. Japanese Encephalitis—The Prospects for New Treatments. Nat. Rev. Neurol. 2018, 14, 298–313. [Google Scholar] [CrossRef]
- Hills, S.L.; Walter, E.B.; Atmar, R.L.; Fischer, M.; ACIP Japanese Encephalitis Vaccine Work Group. Japanese Encephalitis Vaccine: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm. Rep. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2019, 68, 1–33. [Google Scholar] [CrossRef]
- World Health Organization. Japanese Encephalitis Vaccines: WHO Position Paper; WHO TEAM Immunization, Vaccines and Biologicals (IVB): Geneva, Switzerland, 2015; p. 19. [Google Scholar]
- Bente, D.A.; Forrester, N.L.; Watts, D.M.; McAuley, A.J.; Whitehouse, C.A.; Bray, M. Crimean-Congo Hemorrhagic Fever: History, Epidemiology, Pathogenesis, Clinical Syndrome and Genetic Diversity. Antivir. Res. 2013, 100, 159–189. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Dhar, P.S.; Rahman, M.M. Recent Concern about the Outbreak of Crimean-Congo Hemorrhagic Fever: Virology, Etiology, Pathogenesis, Symptoms, Transmission, Diagnostics, and Treatment. Int. J. Surg. 2023, 109, 3215–3216. [Google Scholar] [CrossRef]
- European Centre for Disease, Prevention and Control (ECDC), European Food Safety Authority (EFSA). Distribution Maps of Ticks. Available online: https://www.ecdc.europa.eu/en/disease-vectors/surveillance-and-disease-data/tick-maps (accessed on 11 June 2024).
- Gargili, A.; Estrada-Peña, A.; Spengler, J.R.; Lukashev, A.; Nuttall, P.A.; Bente, D.A. The Role of Ticks in the Maintenance and Transmission of Crimean-Congo Hemorrhagic Fever Virus: A Review of Published Field and Laboratory Studies. Antivir. Res. 2017, 144, 93–119. [Google Scholar] [CrossRef]
- Gargili, A.; Midilli, K.; Ergonul, O.; Ergin, S.; Alp, H.G.; Vatansever, Z.; Iyisan, S.; Cerit, C.; Yilmaz, G.; Altas, K.; et al. Crimean-Congo Hemorrhagic Fever in European Part of Turkey: Genetic Analysis of the Virus Strains from Ticks and a Seroepidemiological Study in Humans. Vector Borne Zoonotic Dis. 2011, 11, 747–752. [Google Scholar] [CrossRef]
- Hawman, D.W.; Feldmann, H. Crimean-Congo Haemorrhagic Fever Virus. Nat. Rev. Microbiol. 2023, 21, 463–477. [Google Scholar] [CrossRef]
- Maltezou, H.C.; Papa, A. Crimean-Congo Hemorrhagic Fever: Risk for Emergence of New Endemic Foci in Europe? Travel Med. Infect. Dis. 2010, 8, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, G.R.; Buzgan, T.; Irmak, H.; Safran, A.; Uzun, R.; Cevik, M.A.; Torunoglu, M.A. The Epidemiology of Crimean-Congo Hemorrhagic Fever in Turkey, 2002-2007. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2009, 13, 380–386. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease. Cases of Crimean–Congo Haemorrhagic Fever Infected in the EU/EEA, 2013–Present; European Centre for Disease: Stockholm, Sweden, 2024.
- Fanelli, A.; Buonavoglia, D.; Lanave, G.; Monaco, F.; Quaranta, V.; Catanzariti, R.; Ruiz-Fons, F.; Buonavoglia, C. First Serological Evidence of Crimean–Congo Haemorrhagic Fever Virus in Transhumant Bovines in Italy. Transbound. Emerg. Dis. 2022, 69, 4022–4027. [Google Scholar] [CrossRef] [PubMed]
- Grech-Angelini, S.; Lancelot, R.; Ferraris, O.; Peyrefitte, C.N.; Vachiery, N.; Pédarrieu, A.; Peyraud, A.; Rodrigues, V.; Bastron, D.; Libeau, G.; et al. Crimean-Congo Hemorrhagic Fever Virus Antibodies among Livestock on Corsica, France, 2014–2016. Emerg. Infect. Dis. 2020, 26, 1041–1044. [Google Scholar] [CrossRef] [PubMed]
- Deézsi-Magyar, N.; Dénes, B.; Novák, B.; Zsidei, G.; Déri, D.; Henczkó, J.; Pályi, B.; Kis, Z. First Broad-Range Serological Survey of Crimean–Congo Hemorrhagic Fever among Hungarian Livestock. Viruses 2024, 16, 875. [Google Scholar] [CrossRef]
- Mesquita, J.R.; Cruz, R.; Esteves, F.; Santos, C.; Pousa, H.; Coelho, C.; Mega, A.C.; Nóbrega, C.; Vala, H.; Peyrefitte, C.N.; et al. Crimean-Congo Hemorrhagic Fever Virus Circulating among Sheep of Portugal: A Nationwide Serosurvey Assessment. Trop. Anim. Health Prod. 2022, 54, 237. [Google Scholar] [CrossRef]
- Mancuso, E.; Toma, L.; Pascucci, I.; d’Alessio, S.G.; Marini, V.; Quaglia, M.; Riello, S.; Ferri, A.; Spina, F.; Serra, L.; et al. Direct and Indirect Role of Migratory Birds in Spreading CCHFV and WNV: A Multidisciplinary Study on Three Stop-Over Islands in Italy. Pathogens 2022, 11, 1056. [Google Scholar] [CrossRef]
- World Health Organization. Crimean-Congo Haemorrhagic Fever. Available online: https://www.who.int/news-room/fact-sheets/detail/crimean-congo-haemorrhagic-fever (accessed on 23 May 2022).
- Ahata, B.; Akçapınar, G.B. CCHFV Vaccine Development, Current Challenges, Limitations, and Future Directions. Front. Immunol. 2023, 14, 1238882. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Manjunathachar, H.V.; Ghosh, S. A Review on Hyalomma Species Infestations on Human and Animals and Progress on Management Strategies. Heliyon 2020, 6, e05675. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.G.; Weaver, G.; Raabe, V.; State of the Clinical Science Working Group of the National Emerging Pathogens Training; Education Center’s Special Pathogens Research Network2; State of the Clinical Science Working Group of the National Emerging Pathogens Training Education Center’s Special Pathogens Research Network. Crimean-Congo Hemorrhagic Fever Virus for Clinicians-Epidemiology, Clinical Manifestations, and Prevention. Emerg. Infect. Dis. 2024, 30, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Gozel, M.G.; Dokmetas, I.; Oztop, A.Y.; Engin, A.; Elaldi, N.; Bakir, M. Recommended Precaution Procedures Protect Healthcare Workers from Crimean-Congo Hemorrhagic Fever Virus. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2013, 17, e1046–e1050. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tong Jia Ming, S.; Tan Yi Jun, K.; Carissimo, G. Pathogenicity and Virulence of O’nyong-Nyong Virus: A Less Studied Togaviridae with Pandemic Potential. Virulence 2024, 15, 2355201. [Google Scholar] [CrossRef]
- Rezza, G.; Chen, R.; Weaver, S.C. O’nyong-Nyong Fever: A Neglected Mosquito-Borne Viral Disease. Pathog. Glob. Health 2017, 111, 271–275. [Google Scholar] [CrossRef]
- Torres-Ruesta, A.; Teo, T.-H.; Chan, Y.-H.; Amrun, S.N.; Yeo, N.K.-W.; Lee, C.Y.-P.; Nguee, S.Y.-T.; Tay, M.Z.; Nosten, F.; Fong, S.-W.; et al. Malaria Abrogates O’nyong-Nyong Virus Pathologies by Restricting Virus Infection in Nonimmune Cells. Life Sci. Alliance 2022, 5, e202101272. [Google Scholar] [CrossRef]
- Teo, T.-H.; Lum, F.-M.; Ghaffar, K.; Chan, Y.-H.; Amrun, S.N.; Tan, J.J.L.; Lee, C.Y.P.; Chua, T.-K.; Carissimo, G.; Lee, W.W.L.; et al. Plasmodium Co-Infection Protects against Chikungunya Virus-Induced Pathologies. Nat. Commun. 2018, 9, 3905. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Anopheles Maculipennis s.l.—Current Known Distribution: March 2022. Available online: https://www.ecdc.europa.eu/en/publications-data/anopheles-maculipennis-sl-current-known-distribution-march-2022 (accessed on 12 April 2022).
- European Centre for Disease. Prevention and Control Aedes Aegypti—Current Known Distribution: May 2024. Available online: https://www.ecdc.europa.eu/en/publications-data/aedes-aegypti-current-known-distribution-may-2024 (accessed on 11 June 2024).
- Vanlandingham, D.L.; Hong, C.; Klingler, K.; Tsetsarkin, K.; McElroy, K.L.; Powers, A.M.; Lehane, M.J.; Higgs, S. Differential Infectivities of o’nyong-Nyong and Chikungunya Virus Isolates in Anopheles Gambiae and Aedes Aegypti Mosquitoes. Am. J. Trop. Med. Hyg. 2005, 72, 616–621. [Google Scholar] [CrossRef]
- Pezzi, L.; LaBeaud, A.D.; Reusken, C.B.; Drexler, J.F.; Vasilakis, N.; Diallo, M.; Simon, F.; Jaenisch, T.; Gallian, P.; Sall, A.; et al. GloPID-R Report on Chikungunya, o’nyong-Nyong and Mayaro Virus, Part 2: Epidemiological Distribution of o’nyong-Nyong Virus. Antivir. Res. 2019, 172, 104611. [Google Scholar] [CrossRef] [PubMed]
- Zumla, A. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Lancet Infect. Dis. 2010, 10, 303–304. [Google Scholar] [CrossRef]
- Tappe, D.; Kapaun, A.; Emmerich, P.; Campos, R.D.M.; Cadar, D.; Günther, S.; Schmidt-Chanasit, J. O’nyong-Nyong Virus Infection Imported to Europe from Kenya by a Traveler. Emerg. Infect. Dis. 2014, 20, 1766–1767. [Google Scholar] [CrossRef]
- Fox, J.M.; Long, F.; Edeling, M.A.; Lin, H.; van Duijl-Richter, M.K.S.; Fong, R.H.; Kahle, K.M.; Smit, J.M.; Jin, J.; Simmons, G.; et al. Broadly Neutralizing Alphavirus Antibodies Bind an Epitope on E2 and Inhibit Entry and Egress. Cell 2015, 163, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.M.; Huang, L.; Tahan, S.; Powell, L.A.; Crowe, J.E.; Wang, D.; Diamond, M.S. A Cross-Reactive Antibody Protects against Ross River Virus Musculoskeletal Disease despite Rapid Neutralization Escape in Mice. PLoS Pathog. 2020, 16, e1008743. [Google Scholar] [CrossRef]
- Meertens, L.; Hafirassou, M.L.; Couderc, T.; Bonnet-Madin, L.; Kril, V.; Kümmerer, B.M.; Labeau, A.; Brugier, A.; Simon-Loriere, E.; Burlaud-Gaillard, J.; et al. FHL1 Is a Major Host Factor for Chikungunya Virus Infection. Nature 2019, 574, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Depner, K.; Drewe, J.A.; Garin-Bastuji, B.; Rojas, J.L.G.; Schmidt, C.G.; Michel, V.; et al. Rift Valley Fever—Epidemiological Update and Risk of Introduction into Europe. EFSA J. 2020, 18, e06041. [Google Scholar] [CrossRef]
- Pepin, M.; Bouloy, M.; Bird, B.H.; Kemp, A.; Paweska, J. Rift Valley Fever Virus (Bunyaviridae: Phlebovirus): An Update on Pathogenesis, Molecular Epidemiology, Vectors, Diagnostics and Prevention. Vet. Res. 2010, 41, 61. [Google Scholar] [CrossRef]
- Kwaśnik, M.; Rożek, W.; Rola, J. Rift Valley Fever—A Growing Threat To Humans and Animals. J. Vet. Res. 2021, 65, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Grossi-Soyster, E.N.; Lee, J.; King, C.H.; LaBeaud, A.D. The Influence of Raw Milk Exposures on Rift Valley Fever Virus Transmission. PLoS Negl. Trop. Dis. 2019, 13, e0007258. [Google Scholar] [CrossRef] [PubMed]
- Otte, M.J.; Nugent, R.; McLeod, A. Transboundary Animal Diseases: Assessment of Socio-Economic Impacts and Institutional Responses; FAO, Food and Agriculture Organization: Roma, Italy, 2024. [Google Scholar]
- 11th Meeting of the Global Steering Committee of the Global Framework for the Progressive Control of Transboundary Animal Diseases (GF-TADs)—Activity Report—November 2018 to October 2020; O.I.E (World Organisation for Animal Health): Paris, France, 2022.
- Ikegami, T. Molecular Biology and Genetic Diversity of Rift Valley Fever Virus. Antivir. Res. 2012, 95, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Nanyingi, M.O.; Munyua, P.; Kiama, S.G.; Muchemi, G.M.; Thumbi, S.M.; Bitek, A.O.; Bett, B.; Muriithi, R.M.; Njenga, M.K. A Systematic Review of Rift Valley Fever Epidemiology 1931–2014. Infect. Ecol. Epidemiol. 2015, 5, 28024. [Google Scholar] [CrossRef] [PubMed]
- Clemmons, E.A.; Alfson, K.J.; Dutton, J.W. Transboundary Animal Diseases, an Overview of 17 Diseases with Potential for Global Spread and Serious Consequences. Animals 2021, 11, 2039. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Facts About Rift Valley Fever. Available online: https://www.ecdc.europa.eu/en/rift-valley-fever/facts (accessed on 8 February 2024).
- EFSA Panel on Animal Health and Welfare (AHAW); Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Depner, K.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Gortázar Schmidt, C.; et al. Rift Valley Fever: Risk of Persistence, Spread and Impact in Mayotte (France). EFSA J. 2020, 18, e06093. [Google Scholar] [CrossRef]
- Gür, S.; Kale, M.; Erol, N.; Yapici, O.; Mamak, N.; Yavru, S. The First Serological Evidence for Rift Valley Fever Infection in the Camel, Goitered Gazelle and Anatolian Water Buffaloes in Turkey. Trop. Anim. Health Prod. 2017, 49, 1531–1535. [Google Scholar] [CrossRef]
- Selmi, R.; Mamlouk, A.; Ben Said, M.; Ben Yahia, H.; Abdelaali, H.; Ben Chehida, F.; Daaloul-Jedidi, M.; Gritli, A.; Messadi, L. First Serological Evidence of the Rift Valley Fever Phlebovirus in Tunisian Camels. Acta Trop. 2020, 207, 105462. [Google Scholar] [CrossRef] [PubMed]
- Hanafi, H.A.; Fryauff, D.J.; Saad, M.D.; Soliman, A.K.; Mohareb, E.W.; Medhat, I.; Zayed, A.B.; Szumlas, D.E.; Earhart, K.C. Virus Isolations and High Population Density Implicate Culex Antennatus (Becker) (Diptera: Culicidae) as a Vector of Rift Valley Fever Virus during an Outbreak in the Nile Delta of Egypt. Acta Trop. 2011, 119, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, M.K.; Hachid, A.; Derrar, F.; Messahel, N.E.; Bia, T.; Mockbel, Y.; Khardine, A.F.; Degui, D.; Bellout, L.; Benaissa, M.H.; et al. Serological Evidence of Rift Valley Fever Viral Infection among Camels Imported into Southern Algeria. Comp. Immunol. Microbiol. Infect. Dis. 2023, 100, 102035. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global Trends in Emerging Infectious Diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.D.T.; Higgs, S. Yellow Fever: A Disease That Has yet to Be Conquered. Annu. Rev. Entomol. 2007, 52, 209–229. [Google Scholar] [CrossRef]
- Damasceno-Caldeira, R.; Nunes-Neto, J.P.; Aragão, C.F.; Freitas, M.N.O.; Ferreira, M.S.; de Castro, P.H.G.; Dias, D.D.; Araújo, P.A.d.S.; Brandão, R.C.F.; Nunes, B.T.D.; et al. Vector Competence of Aedes Albopictus for Yellow Fever Virus: Risk of Reemergence of Urban Yellow Fever in Brazil. Viruses 2023, 15, 1019. [Google Scholar] [CrossRef] [PubMed]
- Amraoui, F.; Pain, A.; Piorkowski, G.; Vazeille, M.; Couto-Lima, D.; de Lamballerie, X.; Lourenço-de-Oliveira, R.; Failloux, A.-B. Experimental Adaptation of the Yellow Fever Virus to the Mosquito Aedes Albopictus and Potential Risk of Urban Epidemics in Brazil, South America. Sci. Rep. 2018, 8, 14337. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.E.; Holmes, E.C.; Barrett, A.D.T. Out of Africa: A Molecular Perspective on the Introduction of Yellow Fever Virus into the Americas. PLoS Pathog. 2007, 3, e75. [Google Scholar] [CrossRef]
- Gianchecchi, E.; Cianchi, V.; Torelli, A.; Montomoli, E. Yellow Fever: Origin, Epidemiology, Preventive Strategies and Future Prospects. Vaccines 2022, 10, 372. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Barrett, A.D.T. Transmission Cycles, Host Range, Evolution and Emergence of Arboviral Disease. Nat. Rev. Microbiol. 2004, 2, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Li, S.L.; Acosta, A.L.; Hill, S.C.; Brady, O.J.; De Almeida, M.A.B.; Cardoso, J.D.C.; Hamlet, A.; Mucci, L.F.; Telles De Deus, J.; Iani, F.C.M.; et al. Mapping Environmental Suitability of Haemagogus and Sabethes Spp. Mosquitoes to Understand Sylvatic Transmission Risk of Yellow Fever Virus in Brazil. PLoS Negl. Trop. Dis. 2022, 16, e0010019. [Google Scholar] [CrossRef]
- Vasilakis, N.; Cardosa, J.; Hanley, K.A.; Holmes, E.C.; Weaver, S.C. Fever from the Forest: Prospects for the Continued Emergence of Sylvatic Dengue Virus and Its Impact on Public Health. Nat. Rev. Microbiol. 2011, 9, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Soto, A.; Torres, M.C.; Lima De Mendonça, M.C.; Mares-Guia, M.A.; Dos Santos Rodrigues, C.D.; Fabri, A.A.; Dos Santos, C.C.; Machado Araújo, E.S.; Fischer, C.; Ribeiro Nogueira, R.M.; et al. Evidence for Multiple Sylvatic Transmission Cycles during the 2016–2017 Yellow Fever Virus Outbreak, Brazil. Clin. Microbiol. Infect. 2018, 24, 1019.e1–1019.e4. [Google Scholar] [CrossRef]
- Iannetta, M.; Di Caro, A.; Nicastri, E.; Vairo, F.; Masanja, H.; Kobinger, G.; Mirazimi, A.; Ntoumi, F.; Zumla, A.; Ippolito, G. Viral Hemorrhagic Fevers Other than Ebola and Lassa. Infect. Dis. Clin. N. Am. 2019, 33, 977–1002. [Google Scholar] [CrossRef]
- Gossner, C.M.; Haussig, J.M.; De Bellegarde De Saint Lary, C.; Kaasik Aaslav, K.; Schlagenhauf, P.; Sudre, B. Increased Risk of Yellow Fever Infections among Unvaccinated European Travellers Due to Ongoing Outbreak in Brazil, July 2017 to March 2018. Eurosurveillance 2018, 23, 18-00106. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Yellow Fever—Annual Epidemiological Report for 2019; ECDC: Stockholm, Sweden, 2021.
- De Freitas, C.S.; Higa, L.M.; Sacramento, C.Q.; Ferreira, A.C.; Reis, P.A.; Delvecchio, R.; Monteiro, F.L.; Barbosa-Lima, G.; James Westgarth, H.; Vieira, Y.R.; et al. Yellow Fever Virus Is Susceptible to Sofosbuvir Both in Vitro and in Vivo. PLoS Negl. Trop. Dis. 2019, 13, e0007072. [Google Scholar] [CrossRef] [PubMed]
- Mendes, É.A.; de Pilger, D.R.B.; Santos Nastri, A.C.d.S.; Malta, F.d.M.; Pascoalino, B.D.S.; Carneiro D’Albuquerque, L.A.; Balan, A.; de Freitas, L.H.G.; Durigon, E.L.; Carrilho, F.J.; et al. Sofosbuvir Inhibits Yellow Fever Virus in Vitro and in Patients with Acute Liver Failure. Ann. Hepatol. 2019, 18, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Rezende, I.M.; Mendonça, D.C.; Costa, T.A.; Oliveria, G.F.G.D.; Arruda, M.S.; Gonçalves, A.P.; Alves, P.A.; Calzavara-Silva, C.E.; Martins-Filho, O.A.; Teixeira-Carvalho, A.; et al. Sofosbuvir Off-Label Treatment of Yellow Fever Patients During an Outbreak in Brazil, 2018: A Cohort Study. Open Forum Infect. Dis. 2024, 11, ofae312. [Google Scholar] [CrossRef]
- Wieten, R.W.; Goorhuis, A.; Jonker, E.F.F.; De Bree, G.J.; De Visser, A.W.; Van Genderen, P.J.J.; Remmerswaal, E.B.M.; Ten Berge, I.J.M.; Visser, L.G.; Grobusch, M.P.; et al. 17D Yellow Fever Vaccine Elicits Comparable Long-Term Immune Responses in Healthy Individuals and Immune-Compromised Patients. J. Infect. 2016, 72, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Javelle, E.; Gautret, P.; Raoult, D. Towards the Risk of Yellow Fever Transmission in Europe. Clin. Microbiol. Infect. 2019, 25, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Laporta, G.Z.; Potter, A.M.; Oliveira, J.F.A.; Bourke, B.P.; Pecor, D.B.; Linton, Y.-M. Global Distribution of Aedes Aegypti and Aedes Albopictus in a Climate Change Scenario of Regional Rivalry. Insects 2023, 14, 49. [Google Scholar] [CrossRef]
- Kuhn, J.H.; Abe, J.; Adkins, S.; Alkhovsky, S.V.; Avšič-Županc, T.; Ayllón, M.A.; Bahl, J.; Balkema-Buschmann, A.; Ballinger, M.J.; Kumar Baranwal, V.; et al. Annual (2023) Taxonomic Update of RNA-Directed RNA Polymerase-Encoding Negative-Sense RNA Viruses (Realm Riboviria: Kingdom Orthornavirae: Phylum Negarnaviricota). J. Gen. Virol. 2023, 104, 001864. [Google Scholar] [CrossRef] [PubMed]
- Files, M.A.; Hansen, C.A.; Herrera, V.C.; Schindewolf, C.; Barrett, A.D.T.; Beasley, D.W.C.; Bourne, N.; Milligan, G.N. Baseline Mapping of Oropouche Virology, Epidemiology, Therapeutics, and Vaccine Research and Development. NPJ Vaccines 2022, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Travassos da Rosa, J.F.; de Souza, W.M.; de Pinheiro, F.P.; Figueiredo, M.L.; Cardoso, J.F.; Acrani, G.O.; Nunes, M.R.T. Oropouche Virus: Clinical, Epidemiological, and Molecular Aspects of a Neglected Orthobunyavirus. Am. J. Trop. Med. Hyg. 2017, 96, 1019–1030. [Google Scholar] [CrossRef]
- Sakkas, H.; Bozidis, P.; Franks, A.; Papadopoulou, C. Oropouche Fever: A Review. Viruses 2018, 10, 175. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wu, Z.; Feng, S.; Lu, K.; Zhu, W.; Sun, H.; Niu, G. Oropouche Virus: A Neglected Global Arboviral Threat. Virus Res. 2024, 341, 199318. [Google Scholar] [CrossRef]
- Feitoza, L.H.M.; de Carvalho, L.P.C.; da Silva, L.R.; Meireles, A.C.A.; Rios, F.G.F.; Silva, G.S.; de Paulo, P.F.M.; Pessoa, F.A.C.; de Medeiros, J.F.; Julião, G.R. Influence of Meteorological and Seasonal Parameters on the Activity of Culicoides Paraensis (Diptera: Ceratopogonidae), an Annoying Anthropophilic Biting Midge and Putative Vector of Oropouche Virus in Rondônia, Brazilian Amazon. Acta Trop. 2023, 243, 106928. [Google Scholar] [CrossRef] [PubMed]
- Wesselmann, K.M.; Postigo-Hidalgo, I.; Pezzi, L.; de Oliveira-Filho, E.F.; Fischer, C.; de Lamballerie, X.; Drexler, J.F. Emergence of Oropouche Fever in Latin America: A Narrative Review. Lancet Infect. Dis. 2024, 24, e439–e452. [Google Scholar] [CrossRef] [PubMed]
- de Mendonça, S.F.; Rocha, M.N.; Ferreira, F.V.; Leite, T.H.J.F.; Amadou, S.C.G.; Sucupira, P.H.F.; Marques, J.T.; Ferreira, A.G.A.; Moreira, L.A. Evaluation of Aedes Aegypti, Aedes Albopictus, and Culex Quinquefasciatus Mosquitoes Competence to Oropouche Virus Infection. Viruses 2021, 13, 755. [Google Scholar] [CrossRef] [PubMed]
- The Lancet Infectious Diseases Oropouche Fever, the Mysterious Threat. Lancet Infect. Dis. 2024, 24, 935. [CrossRef] [PubMed]
- Gutierrez, B.; Wise, E.L.; Pullan, S.T.; Logue, C.H.; Bowden, T.A.; Escalera-Zamudio, M.; Trueba, G.; Nunes, M.R.T.; Faria, N.R.; Pybus, O.G. Evolutionary Dynamics of Oropouche Virus in South America. J. Virol. 2020, 94, e01127-19. [Google Scholar] [CrossRef]
- Bonifay, T.; Le Turnier, P.; Epelboin, Y.; Carvalho, L.; De Thoisy, B.; Djossou, F.; Duchemin, J.-B.; Dussart, P.; Enfissi, A.; Lavergne, A.; et al. Review on Main Arboviruses Circulating on French Guiana, An Ultra-Peripheric European Region in South America. Viruses 2023, 15, 1268. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Oropouche Virus Disease—Cuba. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON521 (accessed on 22 October 2024).
- European Centre for Disease Prevention and Control. Threat Assessment Brief: Oropouche Virus Disease Cases Imported to the European Union. Available online: https://www.ecdc.europa.eu/en/publications-data/threat-assessment-brief-oropouche-virus-disease-cases-imported-european-union (accessed on 9 August 2024).
- Capobianchi, M.R.; Castilletti, C.; Gobbi, F.G. Potential Risks of Oropouche Virus Importation into Europe. J. Travel Med. 2024, 31, taae109. [Google Scholar] [CrossRef] [PubMed]
- Castilletti, C.; Mori, A.; Matucci, A.; Ronzoni, N.; Van Duffel, L.; Rossini, G.; Sponga, P.; D’Errico, M.L.; Rodari, P.; Cristini, F.; et al. Oropouche Fever Cases Diagnosed in Italy in Two Epidemiologically Non-Related Travellers from Cuba, Late May to Early June 2024. Eurosurveillance 2024, 29, 2400362. [Google Scholar] [CrossRef]
- Schaffner, F.; Mathis, A. Dengue and Dengue Vectors in the WHO European Region: Past, Present, and Scenarios for the Future. Lancet Infect. Dis. 2014, 14, 1271–1280. [Google Scholar] [CrossRef]
- Medlock, J.M.; Leach, S.A. Effect of Climate Change on Vector-Borne Disease Risk in the UK. Lancet Infect. Dis. 2015, 15, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, S.H.; Cornejo Pontelli, M.; Mishra, N.; Zhou, C.; de Paula Souza, J.; Mendes Viana, R.M.; Lipkin, W.I.; Knipe, D.M.; Arruda, E.; Whelan, S.P.J. Vesicular Stomatitis Virus Chimeras Expressing the Oropouche Virus Glycoproteins Elicit Protective Immune Responses in Mice. mBio 2021, 12, e0046321. [Google Scholar] [CrossRef]
- Sutherst, R.W. Global Change and Human Vulnerability to Vector-Borne Diseases. Clin. Microbiol. Rev. 2004, 17, 136–173. [Google Scholar] [CrossRef] [PubMed]
- Findlater, A.; Bogoch, I.I. Human Mobility and the Global Spread of Infectious Diseases: A Focus on Air Travel. Trends Parasitol. 2018, 34, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, A.L.; van den Hurk, A.F.; Meyer, D.B.; Ritchie, S.A. Searching for the proverbial needle in a haystack: Advances in mosquito-borne arbovirus surveillance. Parasites Vectors 2018, 29, 320. [Google Scholar] [CrossRef]
- Blanco, S.; Marín, Á.L.; Frutos, M.C.; Barahona, N.Y.; Rivarola, M.E.; Carrizo, L.H.; Spinsanti, L.; Gallego, S.V. Haemovigilance survey and screening strategy for arthropod-borne viruses in blood donors from Argentina. J. Med. Virol. 2024, 96, e29476. [Google Scholar] [CrossRef]

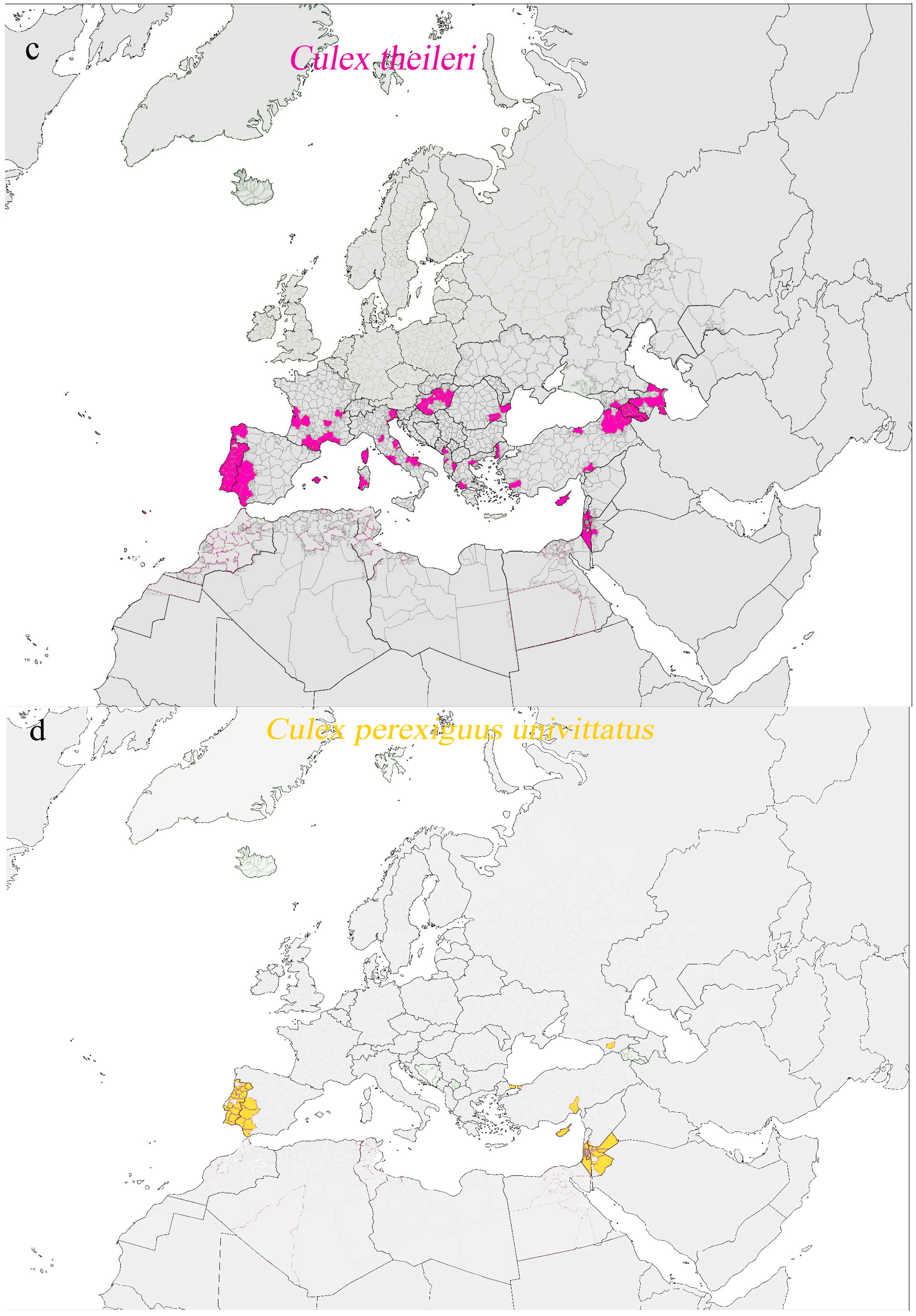
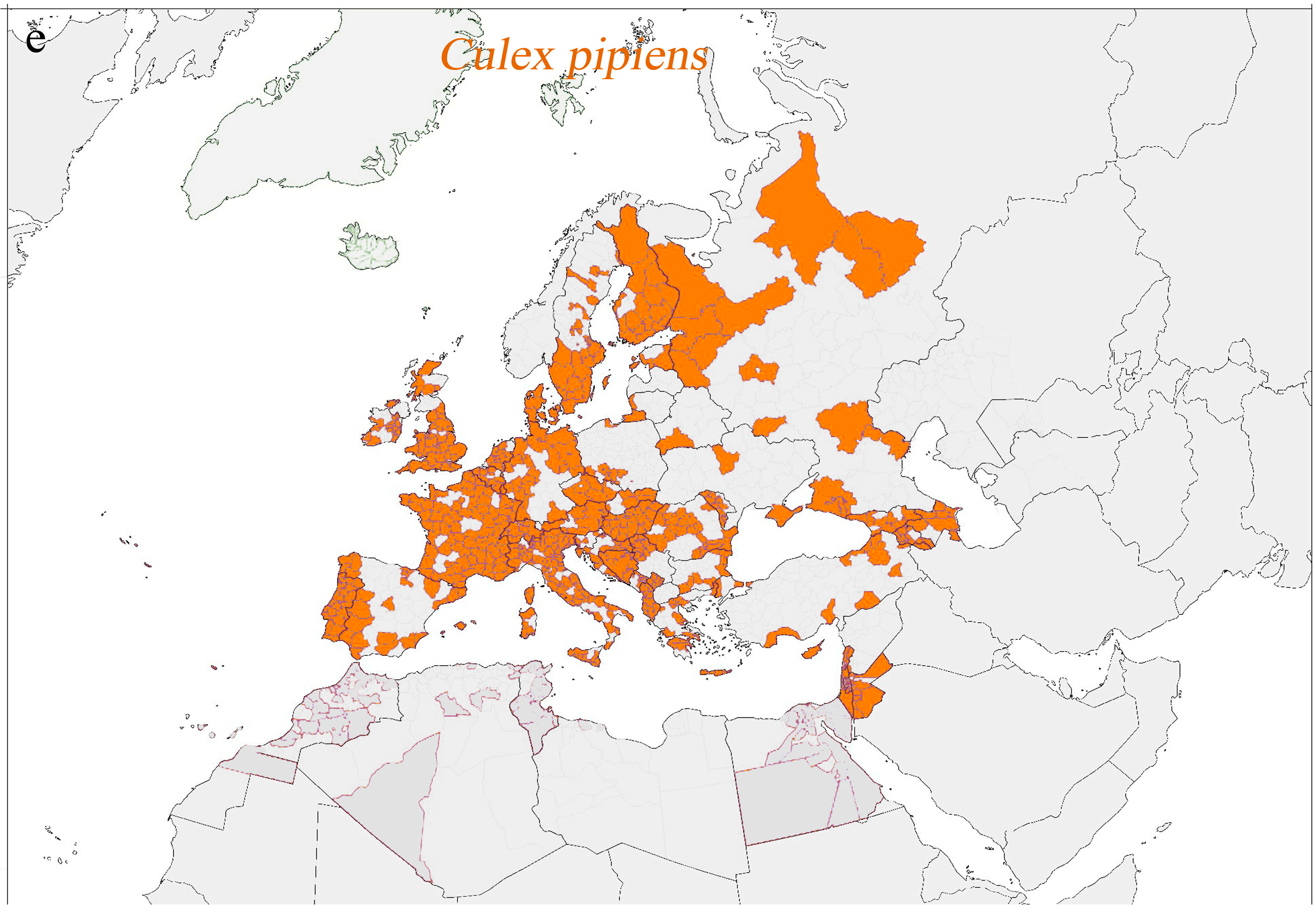

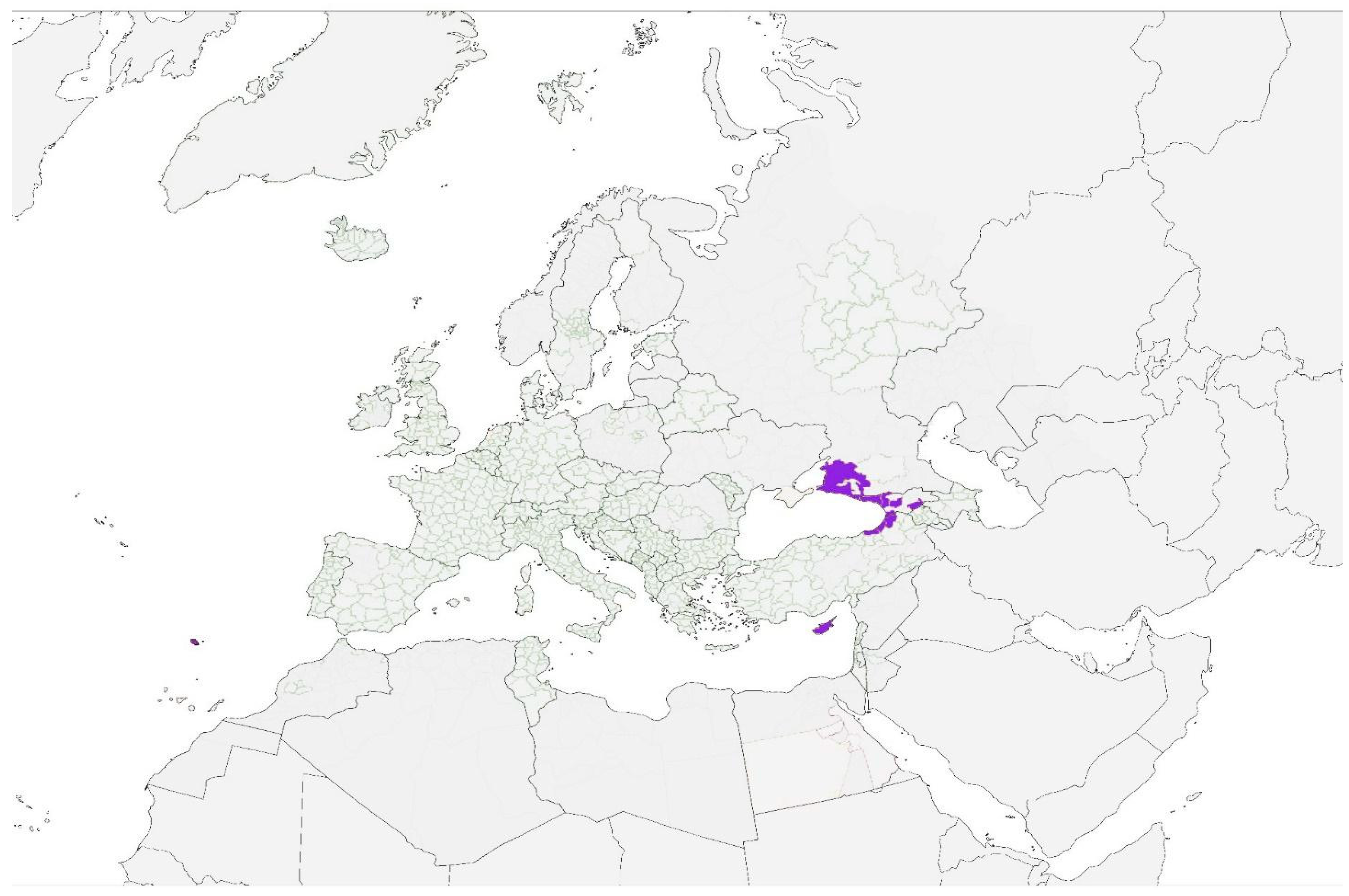
| Pathologies with Active Transmission and Autochthonous Cases in Human or in Animals Transmitted by Vectors Permanently Present Across Europe | ||||
|---|---|---|---|---|
| DISEASE | VIRUS | CASES IN EUROPE | VECTORS | VECTOR DIFFUSION |
| Chikungunya fever | Chikungunya virus | In human: Southern France (2010, 2014), Italy (2007, 2017) | Ae. albopictus Ae. aegypti | Ae. a lbopictus: widely diffused Ae. a egypti: Cyprus, Eastern Black Sea, Madeira |
| Dengue fever | Dengue virus | In human: since 2010, constant circulation in the Mediterranean basin | Ae. albopictus Ae. aegypti | Ae. a lbopictus: widely diffused Ae. a egypti: Cyprus, Eastern Black Sea, Madeira |
| Zika fever | Zika virus | In human: Southern France (2019) | Ae. albopictus Ae. aegypti | Ae. a lbopictus: widely diffused Ae. a egypti: Cyprus, Eastern Black Sea, Madeira |
| Japanese encephalitis | Japanese encephalitis virus | In animal: birds in Italy (2012) | Culex tritaeniorhynchus (also Culex annulirostris, Culex annulus, Culex fuscocephala, Culex gelidus, Culex sitiens, and Culex vishnui) | Culex tritaeniorhynchus: Albania, Greece, Turkey, and the Middle East Mediterranean |
| Crimean–Congo hemorrhagic fever | Crimean–Congo hemorrhagic fever virus | In human: Turkey, Spain, Bulgaria, Russia In animals: seropositive cases in bovines in Italy | Hyalomma marginatum (also Hyalomma aegyptium, Rhipicephalus bursa, and Rhipicephalus (Boophilus) annulatus)) | Hyalomma marginatum: Spain, Southern France, Italy, Switzerland, the Balkan region, countries bordering the Black Sea, Russia |
| Pathologies Without Autochthonous Transmission in Humans or Animals but with Presence of Competent Vectors in Europe | ||||
| DISEASE | VIRUS | CASES IN EUROPE | VECTORS | VECTOR DIFFUSION |
| O’nyong-nyong fever | O’nyong-nyong virus | Sporadic imported cases reported in Europe | Anopheles gambiae, A. funestus, and A. stephensi. | Only Anopheles maculipennis is widely diffused (theoretically competent). |
| Rift Valley fever | Rift Valley virus | In human: only seropositive cases in Turkey In animals: seropositive cases in Turkey | Aedes spp., Culex spp. (also Anopheles, Coquillettidia, Culiseta, Eretmapodites, and Mansoni) | Widely diffused |
| Yellow fever | Yellow fever virus | In human: 20 travel-associated cases (1999–2018) | Ae. Albopictus and Ae. aegypti | Ae. a lbopictus: widely diffused Ae. a egypti: Cyprus, Eastern Black Sea, Madeira |
| Pathologies Without Autochthonous Transmission in Humans or Animals and Without Presence of Competent Vectors in Europe | ||||
| DISEASE | VIRUS | CASES IN EUROPE | VECTORS | VECTOR DIFFUSION |
| Oropouche fever | Oropouche virus | In human: 19 recent travel-associated cases | Culicoides paraensis | No diffusion in Europe |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Logiudice, J.; Alberti, M.; Ciccarone, A.; Rossi, B.; Tiecco, G.; De Francesco, M.A.; Quiros-Roldan, E. Introduction of Vector-Borne Infections in Europe: Emerging and Re-Emerging Viral Pathogens with Potential Impact on One Health. Pathogens 2025, 14, 63. https://doi.org/10.3390/pathogens14010063
Logiudice J, Alberti M, Ciccarone A, Rossi B, Tiecco G, De Francesco MA, Quiros-Roldan E. Introduction of Vector-Borne Infections in Europe: Emerging and Re-Emerging Viral Pathogens with Potential Impact on One Health. Pathogens. 2025; 14(1):63. https://doi.org/10.3390/pathogens14010063
Chicago/Turabian StyleLogiudice, Jacopo, Maria Alberti, Andrea Ciccarone, Benedetta Rossi, Giorgio Tiecco, Maria Antonia De Francesco, and Eugenia Quiros-Roldan. 2025. "Introduction of Vector-Borne Infections in Europe: Emerging and Re-Emerging Viral Pathogens with Potential Impact on One Health" Pathogens 14, no. 1: 63. https://doi.org/10.3390/pathogens14010063
APA StyleLogiudice, J., Alberti, M., Ciccarone, A., Rossi, B., Tiecco, G., De Francesco, M. A., & Quiros-Roldan, E. (2025). Introduction of Vector-Borne Infections in Europe: Emerging and Re-Emerging Viral Pathogens with Potential Impact on One Health. Pathogens, 14(1), 63. https://doi.org/10.3390/pathogens14010063








