Characterization of Vaccine-Enhanced Humoral Immune Responses Against Emergent SARS-CoV-2 Variants in a Convalescent Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Recombinant RBD and Spike Ectodomain Protein Expression and Purification
2.3. Enzyme-Linked Immunosorbent Assay (ELISA) Detection of Antigen-Specific Antibodies in Serum
2.4. Spike Pseudotyped Virus Production and Neutralization Assays
2.5. Statistics
3. Results
3.1. Cohort Characteristics and Data Summary
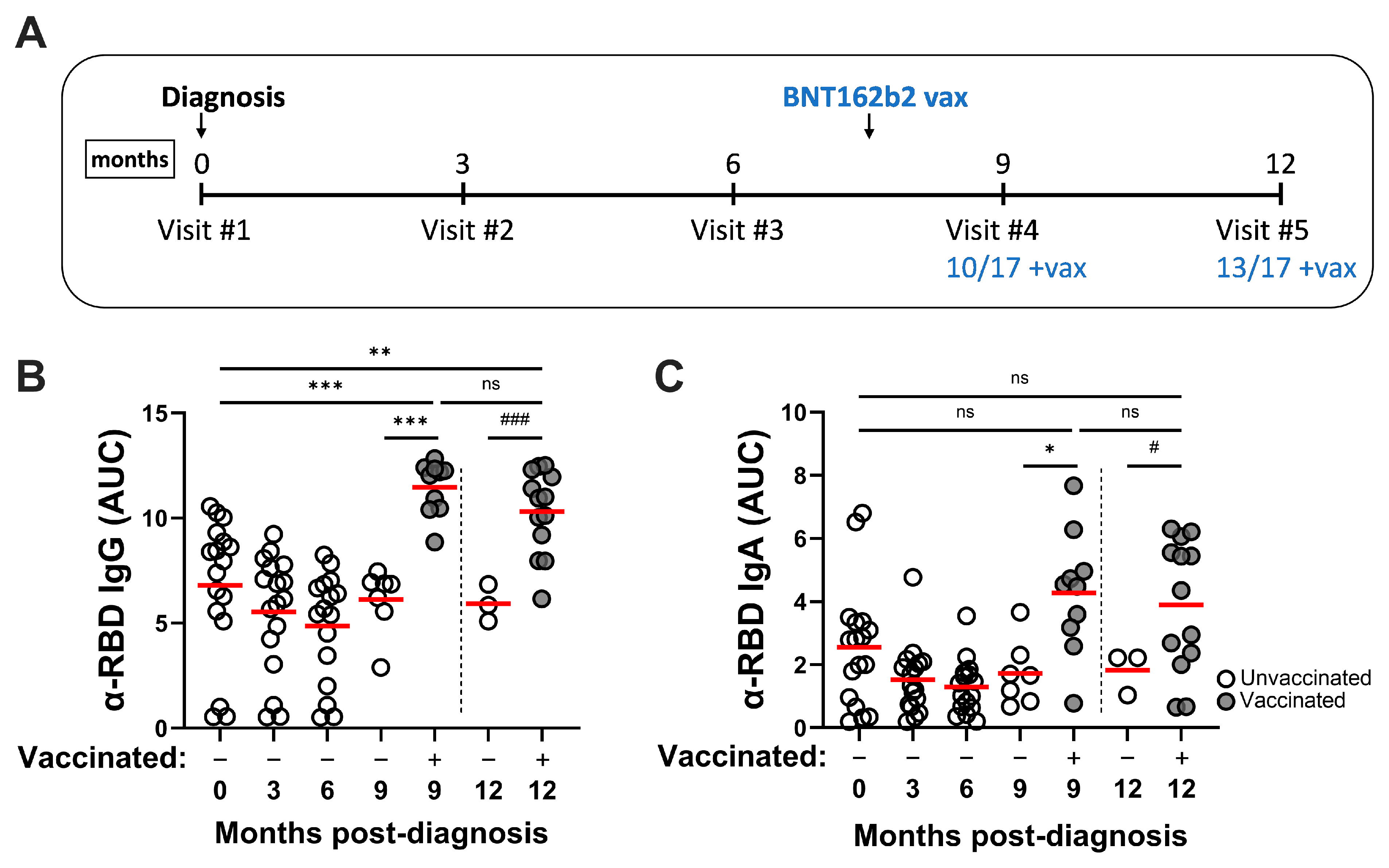
3.2. SARS-CoV-2 Infection Induces Durable RBD-Specific Humoral Responses in Serum and Vaccination Enhances Antibody Titers
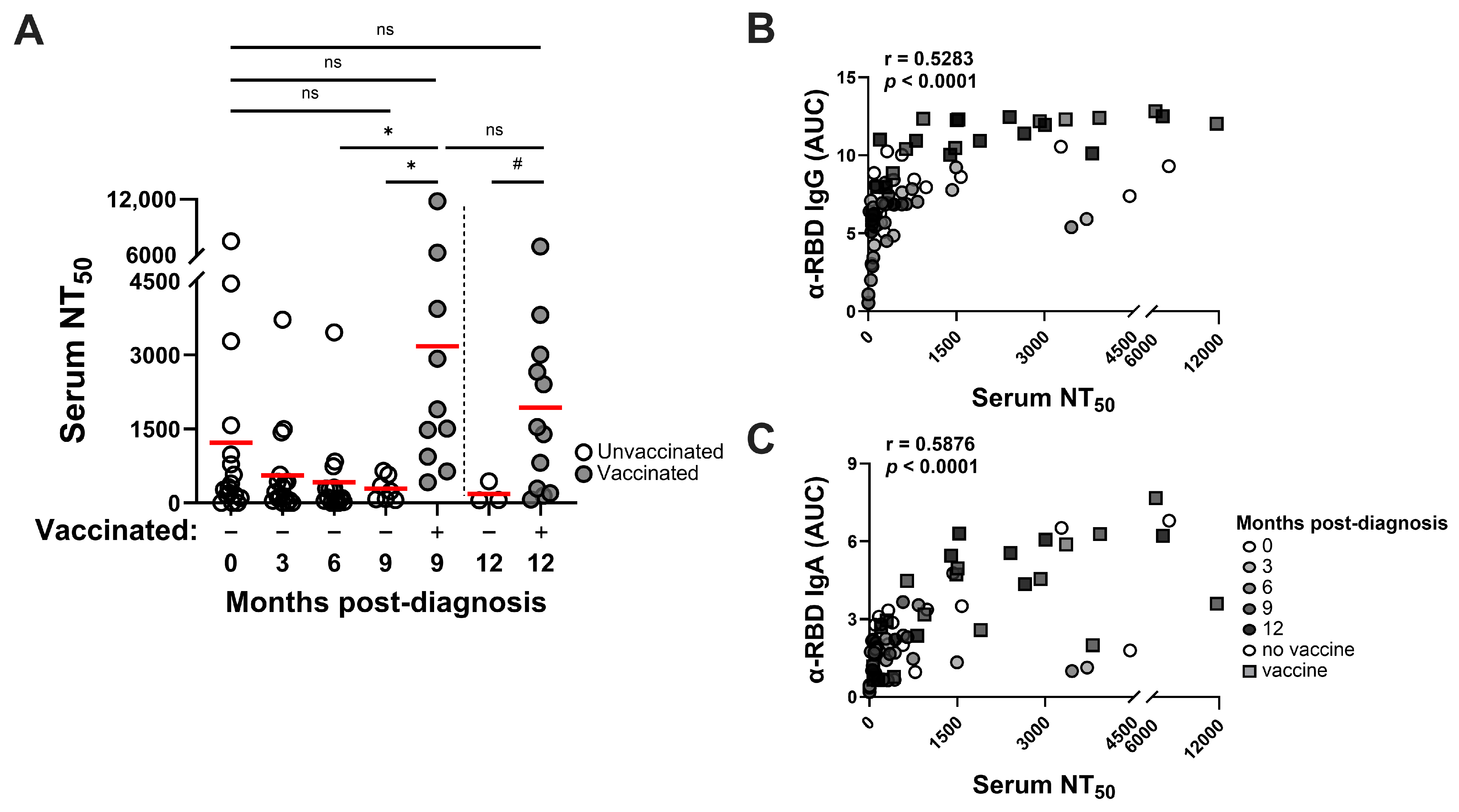
3.3. SARS-CoV-2 Infection–Induced Neutralizing Antibody Responses Are Detected Through at Least 9 Months and Vaccination Improves Neutralizing Activities
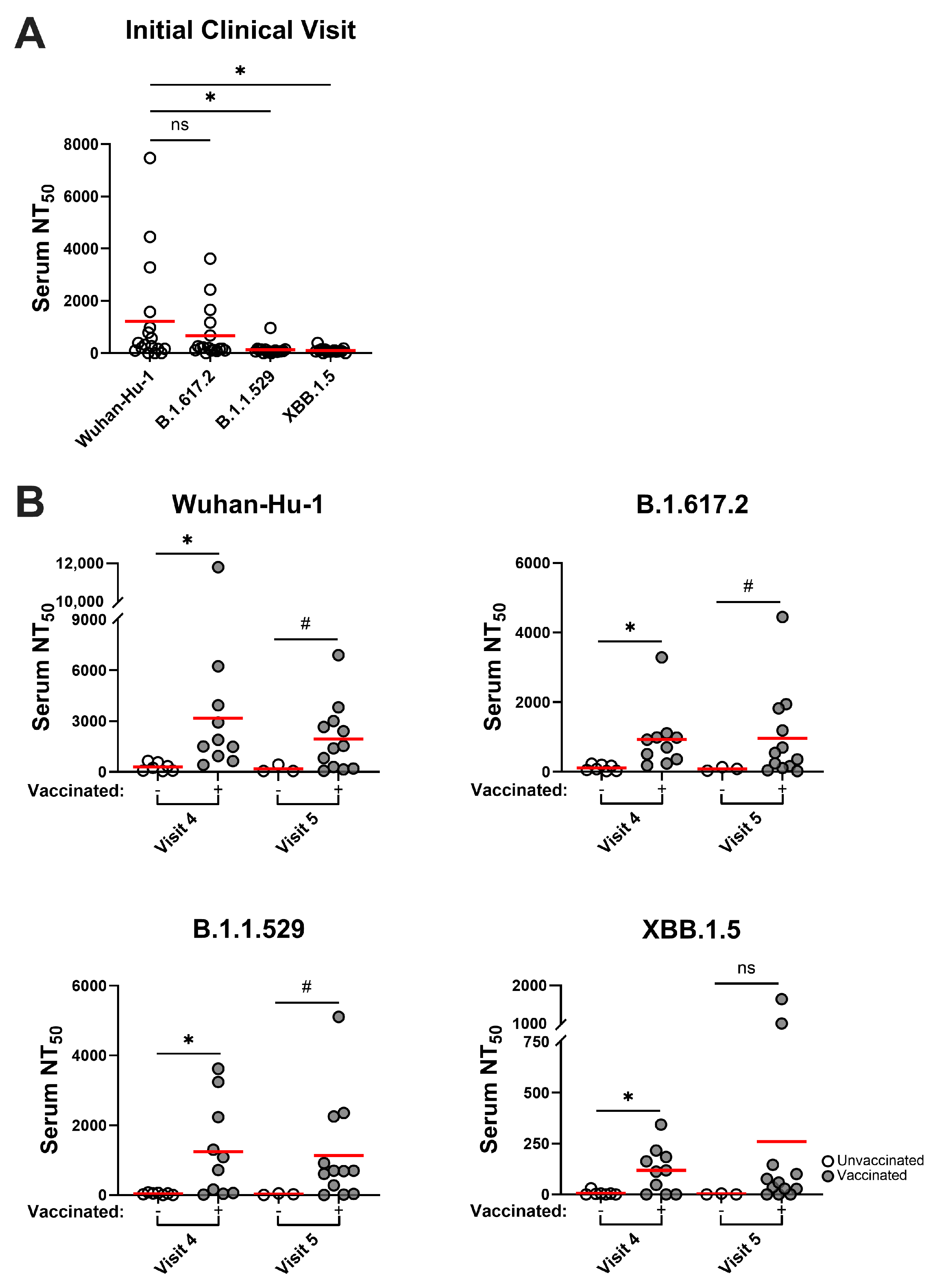
3.4. SARS-CoV-2 Variants Are Resistant to Infection-Induced Neutralizing Antibody Responses, but Hybrid Immune Sera Possess More Broadly Neutralizing Activities
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watson, O.J.; Barnsley, G.; Toor, J.; Hogan, A.B.; Winskill, P.; Ghani, A.C. Global impact of the first year of COVID-19 vaccination: A mathematical modelling study. Lancet Infect. Dis. 2022, 22, 1293–1302. [Google Scholar] [CrossRef]
- Savinkina, A.; Bilinski, A.; Fitzpatrick, M.; Paltiel, A.D.; Rizvi, Z.; Salomon, J.; Thornhill, T.; Gonsalves, G. Estimating deaths averted and cost per life saved by scaling up mRNA COVID-19 vaccination in low-income and lower-middle-income countries in the COVID-19 Omicron variant era: A modelling study. BMJ Open 2022, 12, e061752. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.C.; Hentenaar, I.T.; Morrison-Porter, A.; Solano, D.; Haddad, N.S.; Castrillon, C.; Runnstrom, M.C.; Lamothe, P.A.; Andrews, J.; Roberts, D.; et al. SARS-CoV-2-specific plasma cells are not durably established in the bone marrow long-lived compartment after mRNA vaccination. Nat. Med. 2024, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef]
- Wang, Z.; Muecksch, F.; Schaefer-Babajew, D.; Finkin, S.; Viant, C.; Gaebler, C.; Hoffmann, H.H.; Barnes, C.O.; Cipolla, M.; Ramos, V.; et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature 2021, 595, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Pegu, A.; O’Connell, S.E.; Schmidt, S.D.; O’Dell, S.; Talana, C.A.; Lai, L.; Albert, J.; Anderson, E.; Bennett, H.; Corbett, K.S.; et al. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science 2021, 373, 1372–1377. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Yang, Z.R.; Jiang, Y.W.; Li, F.X.; Liu, D.; Lin, T.F.; Zhao, Z.Y.; Wei, C.; Jin, Q.Y.; Li, X.M.; Jia, Y.X.; et al. Efficacy of SARS-CoV-2 vaccines and the dose-response relationship with three major antibodies: A systematic review and meta-analysis of randomised controlled trials. Lancet Microbe 2023, 4, e236–e246. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guo, Y.; Iketani, S.; Nair, M.S.; Li, Z.; Mohri, H.; Wang, M.; Yu, J.; Bowen, A.D.; Chang, J.Y.; et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature 2022, 608, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Rees-Spear, C.; Muir, L.; Griffith, S.A.; Heaney, J.; Aldon, Y.; Snitselaar, J.L.; Thomas, P.; Graham, C.; Seow, J.; Lee, N.; et al. The effect of spike mutations on SARS-CoV-2 neutralization. Cell Rep. 2021, 34, 108890. [Google Scholar] [CrossRef]
- Goel, R.R.; Painter, M.M.; Lundgreen, K.A.; Apostolidis, S.A.; Baxter, A.E.; Giles, J.R.; Mathew, D.; Pattekar, A.; Reynaldi, A.; Khoury, D.S.; et al. Efficient recall of Omicron-reactive B cell memory after a third dose of SARS-CoV-2 mRNA vaccine. Cell 2022, 185, 1875–1887.e8. [Google Scholar] [CrossRef]
- Arunachalam, P.S.; Lai, L.; Samaha, H.; Feng, Y.; Hu, M.; Hui, H.S.; Wali, B.; Ellis, M.; Davis-Gardner, M.E.; Huerta, C.; et al. Durability of immune responses to mRNA booster vaccination against COVID-19. J. Clin. Investig. 2023, 133, e167955. [Google Scholar] [CrossRef] [PubMed]
- Qu, P.; Faraone, J.N.; Evans, J.P.; Zheng, Y.M.; Yu, L.; Ma, Q.; Carlin, C.; Lozanski, G.; Saif, L.J.; Oltz, E.M.; et al. Durability of Booster mRNA Vaccine against SARS-CoV-2 BA.2.12.1, BA.4, and BA.5 Subvariants. N. Engl. J. Med. 2022, 387, 1329–1331. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.; Ardizzone, C.M.; Khanna, M.; Trauth, A.J.; Hagensee, M.E.; Ramsay, A.J. Dynamics of Serum-Neutralizing Antibody Responses in Vaccinees through Multiple Doses of the BNT162b2 Vaccine. Vaccines 2023, 11, 1720. [Google Scholar] [CrossRef]
- Hall, V.; Foulkes, S.; Insalata, F.; Kirwan, P.; Saei, A.; Atti, A.; Wellington, E.; Khawam, J.; Munro, K.; Cole, M.; et al. Protection against SARS-CoV-2 after Covid-19 Vaccination and Previous Infection. N. Engl. J. Med. 2022, 386, 1207–1220. [Google Scholar] [CrossRef]
- Altarawneh, H.N.; Chemaitelly, H.; Ayoub, H.H.; Tang, P.; Hasan, M.R.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Coyle, P.; Al-Kanaani, Z.; et al. Effects of Previous Infection and Vaccination on Symptomatic Omicron Infections. N. Engl. J. Med. 2022, 387, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tong, P.; Whiteman, N.; Sanjari Moghaddam, A.; Zarghami, M.; Zuiani, A.; Habibi, S.; Gautam, A.; Keerti; Bi, C.; et al. Immune recall improves antibody durability and breadth to SARS-CoV-2 variants. Sci. Immunol. 2022, 7, eabp8328. [Google Scholar] [CrossRef]
- Suryawanshi, R.K.; Chen, I.P.; Ma, T.; Syed, A.M.; Brazer, N.; Saldhi, P.; Simoneau, C.R.; Ciling, A.; Khalid, M.M.; Sreekumar, B.; et al. Limited cross-variant immunity from SARS-CoV-2 Omicron without vaccination. Nature 2022, 607, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.S.; Ash, N.; Alroy-Preis, S.; Huppert, A.; Milo, R. Protection and Waning of Natural and Hybrid Immunity to SARS-CoV-2. N. Engl. J. Med. 2022, 386, 2201–2212. [Google Scholar] [CrossRef] [PubMed]
- Wratil, P.R.; Stern, M.; Priller, A.; Willmann, A.; Almanzar, G.; Vogel, E.; Feuerherd, M.; Cheng, C.C.; Yazici, S.; Christa, C.; et al. Three exposures to the spike protein of SARS-CoV-2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern. Nat. Med. 2022, 28, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Bobrovitz, N.; Ware, H.; Ma, X.; Li, Z.; Hosseini, R.; Cao, C.; Selemon, A.; Whelan, M.; Premji, Z.; Issa, H.; et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: A systematic review and meta-regression. Lancet Infect. Dis. 2023, 23, 556–567. [Google Scholar] [CrossRef]
- de Gier, B.; Huiberts, A.J.; Hoeve, C.E.; den Hartog, G.; van Werkhoven, H.; van Binnendijk, R.; Hahné, S.J.M.; de Melker, H.E.; van den Hof, S.; Knol, M.J. Effects of COVID-19 vaccination and previous infection on Omicron SARS-CoV-2 infection and relation with serology. Nat. Commun. 2023, 14, 4793. [Google Scholar] [CrossRef] [PubMed]
- Sterlin, D.; Mathian, A.; Miyara, M.; Mohr, A.; Anna, F.; Claer, L.; Quentric, P.; Fadlallah, J.; Devilliers, H.; Ghillani, P.; et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021, 13, eabd2223. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, D.; Bennett, S.J.; Sheehan, J.; Trauth, A.J.; Tso, F.Y.; West, J.T.; Hagensee, M.E.; Ramsay, A.J.; Wood, C. Longitudinal Variations in Antibody Responses against SARS-CoV-2 Spike Epitopes upon Serial Vaccinations. Int. J. Mol. Sci. 2023, 24, 7292. [Google Scholar] [CrossRef]
- Pilz, S.; Theiler-Schwetz, V.; Trummer, C.; Krause, R.; Ioannidis, J.P.A. SARS-CoV-2 reinfections: Overview of efficacy and duration of natural and hybrid immunity. Environ. Res. 2022, 209, 112911. [Google Scholar] [CrossRef]
- Ruxton, G.D. The unequal variance t-test is an underused alternative to Student’s t-test and the Mann-Whitney U-test. Behav. Ecol. 2006, 17, 688–690. [Google Scholar] [CrossRef]
- Fagerland, M.W. t-tests, non-parametric tests, and large studies-a paradox of statistical practice? BMC Med. Res. Methodol. 2012, 12, 78. [Google Scholar] [CrossRef]
- Mao, T.; Israelow, B.; Pena-Hernandez, M.A.; Suberi, A.; Zhou, L.; Luyten, S.; Reschke, M.; Dong, H.; Homer, R.J.; Saltzman, W.M.; et al. Unadjuvanted intranasal spike vaccine elicits protective mucosal immunity against sarbecoviruses. Science 2022, 378, eabo2523. [Google Scholar] [CrossRef] [PubMed]
- Sette, A.; Sidney, J.; Crotty, S. T Cell Responses to SARS-CoV-2. Annu. Rev. Immunol. 2023, 41, 343–373. [Google Scholar] [CrossRef] [PubMed]
- Tarke, A.; Sidney, J.; Methot, N.; Yu, E.D.; Zhang, Y.; Dan, J.M.; Goodwin, B.; Rubiro, P.; Sutherland, A.; Wang, E.; et al. Impact of SARS-CoV-2 variants on the total CD4+ and CD8+ T cell reactivity in infected or vaccinated individuals. Cell Rep. Med. 2021, 2, 100355. [Google Scholar] [CrossRef] [PubMed]
- McMahan, K.; Wegmann, F.; Aid, M.; Sciacca, M.; Liu, J.; Hachmann, N.P.; Miller, J.; Jacob-Dolan, C.; Powers, O.; Hope, D.; et al. Mucosal boosting enhances vaccine protection against SARS-CoV-2 in macaques. Nature 2024, 626, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Yisimayi, A.; Song, W.; Wang, J.; Jian, F.; Yu, Y.; Chen, X.; Xu, Y.; Yang, S.; Niu, X.; Xiao, T.; et al. Repeated Omicron exposures override ancestral SARS-CoV-2 immune imprinting. Nature 2024, 625, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Irrgang, P.; Gerling, J.; Kocher, K.; Lapuente, D.; Steininger, P.; Habenicht, K.; Wytopil, M.; Beileke, S.; Schafer, S.; Zhong, J.; et al. Class switch toward noninflammatory, spike-specific IgG4 antibodies after repeated SARS-CoV-2 mRNA vaccination. Sci. Immunol. 2023, 8, eade2798. [Google Scholar] [CrossRef] [PubMed]
- Buhre, J.S.; Pongracz, T.; Kunsting, I.; Lixenfeld, A.S.; Wang, W.; Nouta, J.; Lehrian, S.; Schmelter, F.; Lunding, H.B.; Duhring, L.; et al. mRNA vaccines against SARS-CoV-2 induce comparably low long-term IgG Fc galactosylation and sialylation levels but increasing long-term IgG4 responses compared to an adenovirus-based vaccine. Front. Immunol. 2022, 13, 1020844. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Ioannidis, J.P.A. Does natural and hybrid immunity obviate the need for frequent vaccine boosters against SARS-CoV-2 in the endemic phase? Eur. J. Clin. Investig. 2023, 53, e13906. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.J.; Pade, C.; Gibbons, J.M.; Otter, A.D.; Lin, K.M.; Munoz Sandoval, D.; Pieper, F.P.; Butler, D.K.; Liu, S.; Joy, G.; et al. Immune boosting by B.1.1.529 (Omicron) depends on previous SARS-CoV-2 exposure. Science 2022, 377, eabq1841. [Google Scholar] [CrossRef]
- Park, Y.J.; Pinto, D.; Walls, A.C.; Liu, Z.; De Marco, A.; Benigni, F.; Zatta, F.; Silacci-Fregni, C.; Bassi, J.; Sprouse, K.R.; et al. Imprinted antibody responses against SARS-CoV-2 Omicron sublineages. Science 2022, 378, 619–627. [Google Scholar] [CrossRef] [PubMed]
| Demographics (N = 17) | ||||||||||
| Age|Mean (SD) | 53.8 (12.8) | |||||||||
| Sex|Male (Female) | 5 (12) | |||||||||
| Race/Ethnicity (N) | AA (2), H (2), W (13) | |||||||||
| Antibody | 0 mo | 3 mo | 6 mo | 9 mo | 9 mo + vax | 12 mo | 12 mo + vax | |||
| Spike RBD IgG Response | N (%) | |||||||||
| Below LOD | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Low | 3 (17.64%) | 4 (23.52%) | 4 (23.52%) | 1 (14.28%) | 0 | 0 | 0 | |||
| Medium | 6 (35.29%) | 7 (41.17%) | 9 (52.94%) | 6 (85.71%) | 0 | 3 (100%) | 1 (7.69%) | |||
| High | 8 (47.05%) | 6 (35.29%) | 4 (23.52%) | 0 | 10 (100%) | 0 | 12 (32.31%) | |||
| Spike RBD IgA Response | ||||||||||
| Below LOD | 3 (17.64%) | 3 (17.64%) | 3 (17.64%) | 0 | 0 | 0 | 0 | |||
| Low | 9 (52.94%) | 12 (70.58%) | 12 (70.58%) | 6 (85.71%) | 2 (20.0%) | 3 (100%) | 5 (38.46%) | |||
| Medium | 3 (17.64%) | 2 (11.76%) | 2 (11.76%) | 1 (17.28%) | 6 (60.0%) | 0 | 6 (46.15%) | |||
| High | 2 (11.76%) | 0 | 0 | 0 | 2 (20.0%) | 0 | 2 (15.38%) | |||
| Neutralization | 1218.6 (2030.33) | 556.21 (932.04) | 416.15 (849.79) | 288.27 (243.59) | 3179.55 (3507.97) | 183.95 (221.62) | 1936.49 (2003.1) | |||
| Mean serum | ||||||||||
| NT50 (SD) | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheehan, J.; Trauth, A.J.; Hagensee, M.E.; Ramsay, A.J. Characterization of Vaccine-Enhanced Humoral Immune Responses Against Emergent SARS-CoV-2 Variants in a Convalescent Cohort. Pathogens 2025, 14, 44. https://doi.org/10.3390/pathogens14010044
Sheehan J, Trauth AJ, Hagensee ME, Ramsay AJ. Characterization of Vaccine-Enhanced Humoral Immune Responses Against Emergent SARS-CoV-2 Variants in a Convalescent Cohort. Pathogens. 2025; 14(1):44. https://doi.org/10.3390/pathogens14010044
Chicago/Turabian StyleSheehan, Jared, Amber J. Trauth, Michael E. Hagensee, and Alistair J. Ramsay. 2025. "Characterization of Vaccine-Enhanced Humoral Immune Responses Against Emergent SARS-CoV-2 Variants in a Convalescent Cohort" Pathogens 14, no. 1: 44. https://doi.org/10.3390/pathogens14010044
APA StyleSheehan, J., Trauth, A. J., Hagensee, M. E., & Ramsay, A. J. (2025). Characterization of Vaccine-Enhanced Humoral Immune Responses Against Emergent SARS-CoV-2 Variants in a Convalescent Cohort. Pathogens, 14(1), 44. https://doi.org/10.3390/pathogens14010044






