Pathogen Detection and Resistome Analysis in Healthy Shelter Dogs Using Whole Metagenome Sequencing
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Whole Metagenome Sequencing (WMS)
2.3. Data Analysis
3. Results and Discussion
3.1. Fecal Microbiome Diversity and Composition in Shelter Dogs
3.2. Fecal Pathogen Prevalence in the Shelter Dog Population
3.2.1. Known Canine Pathogens
3.2.2. Known or Potentially Zoonotic Pathogens
3.2.3. Opportunistic Pathogens (Canine and Human)
3.2.4. Other Animal Pathogens
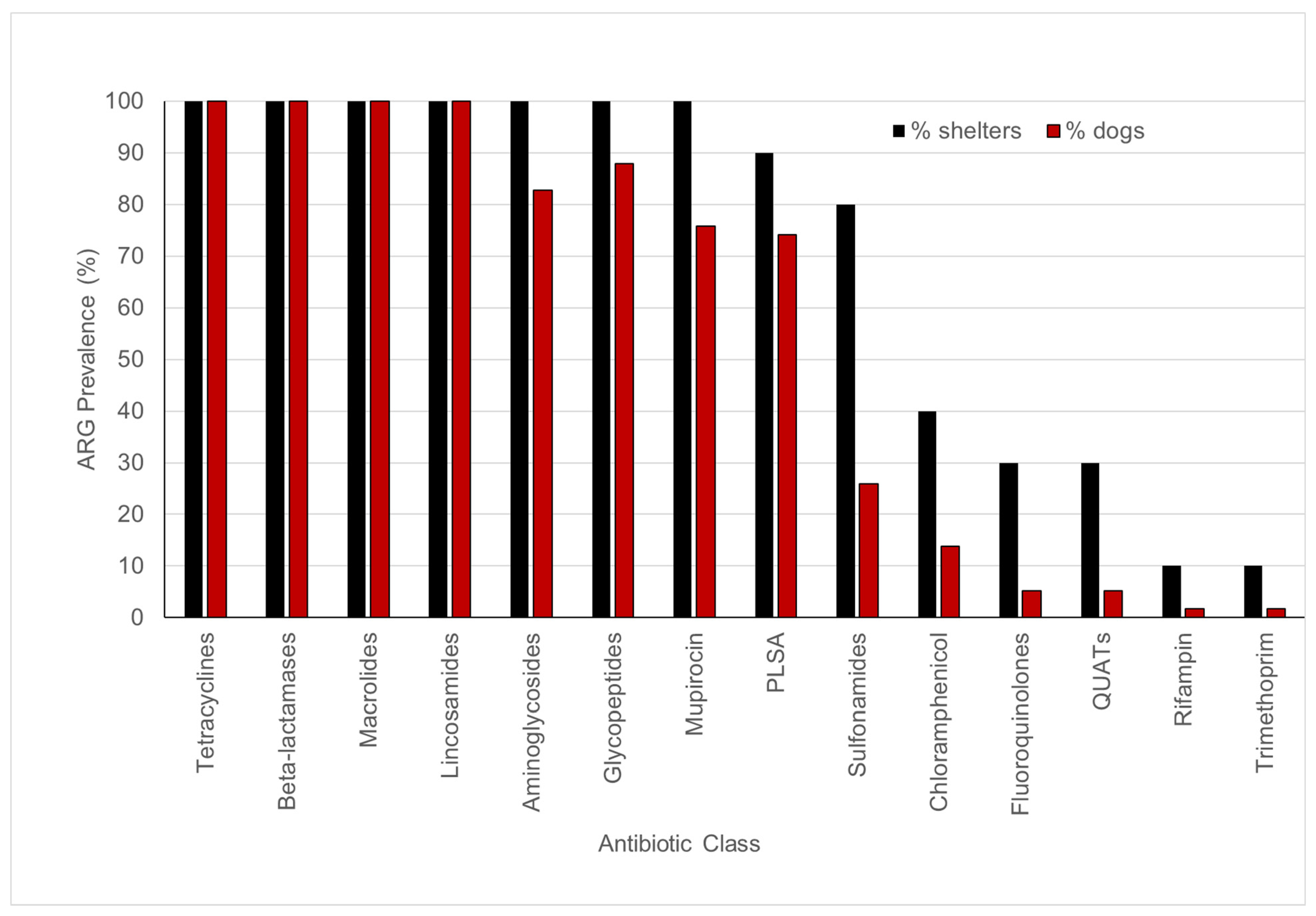
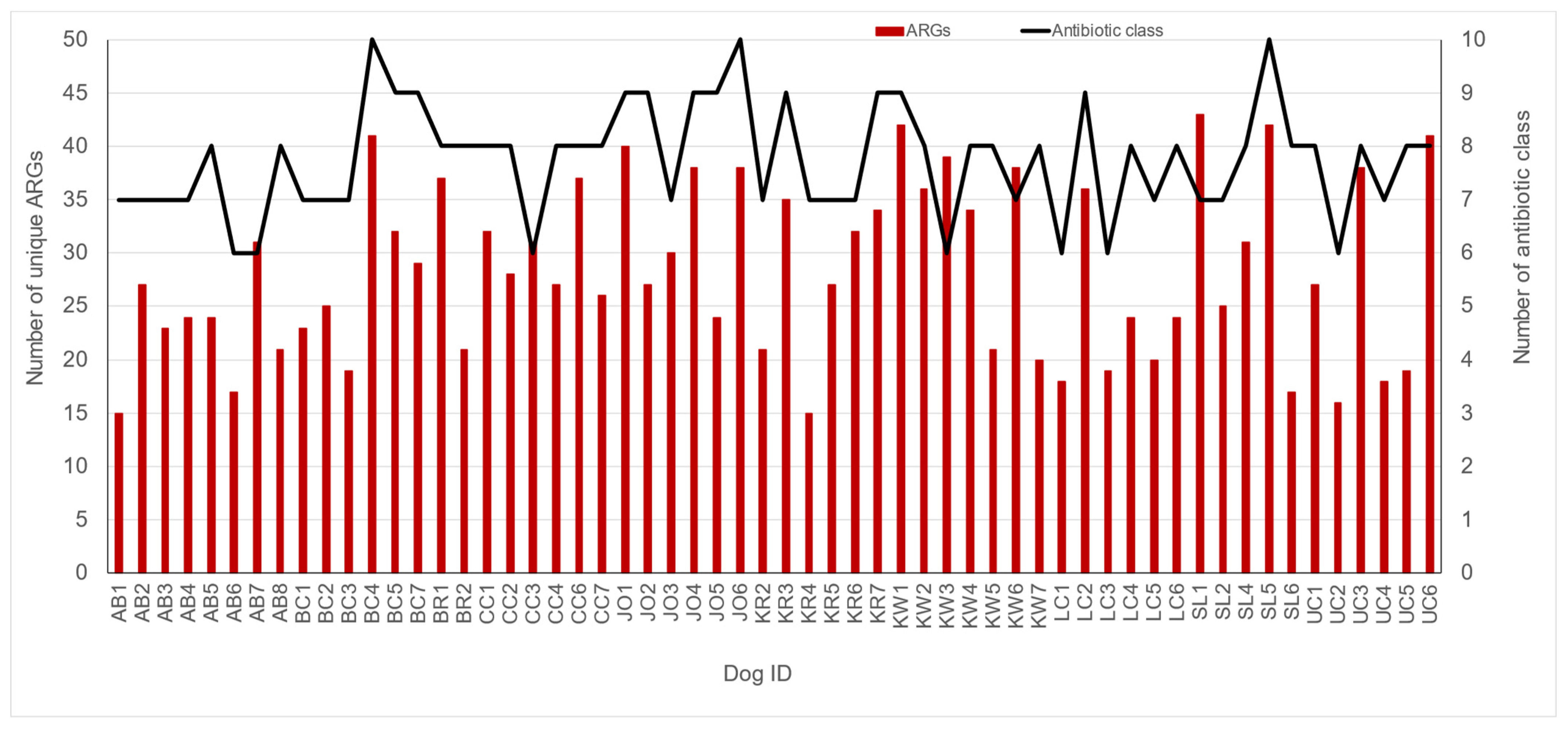
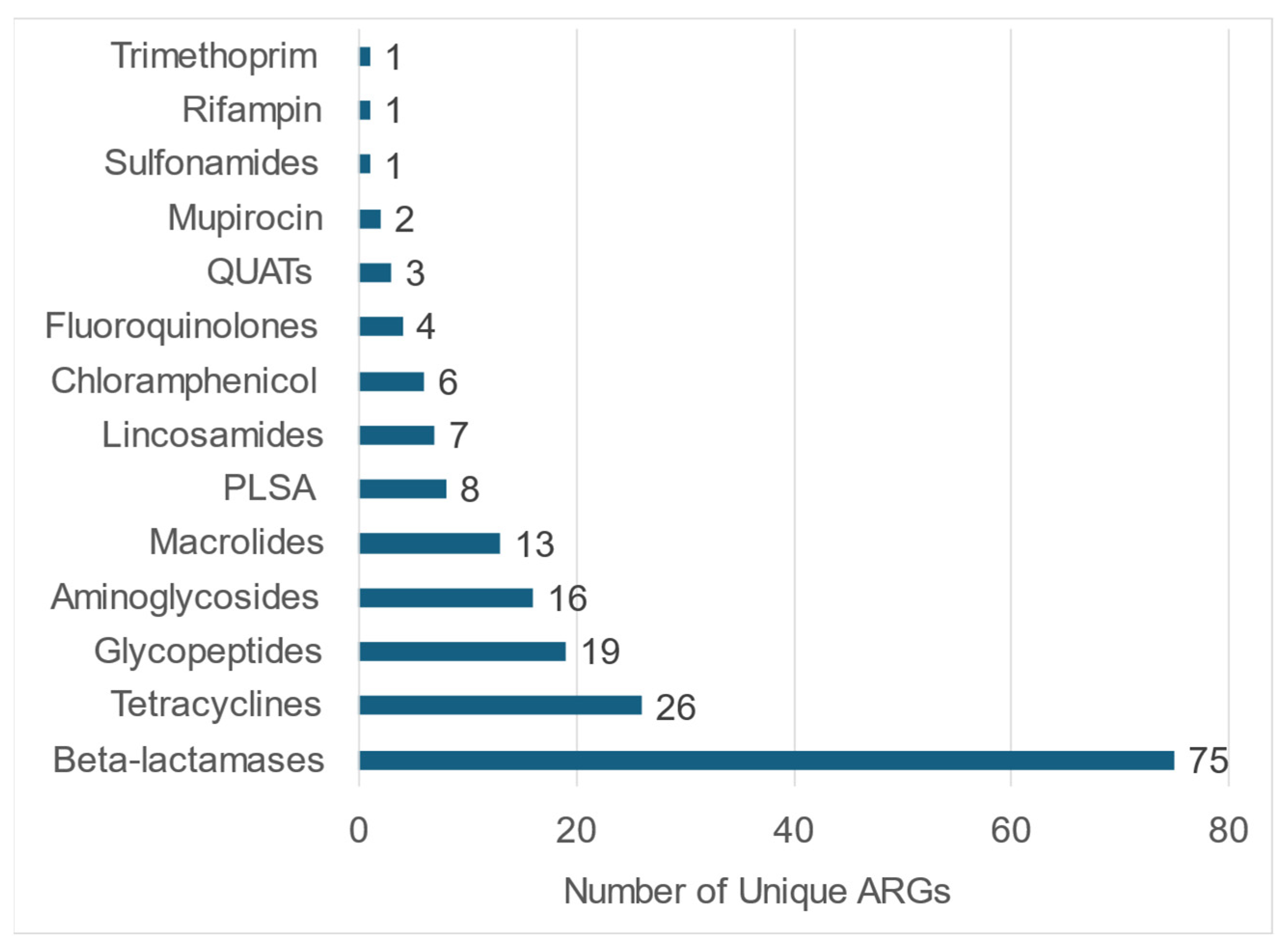
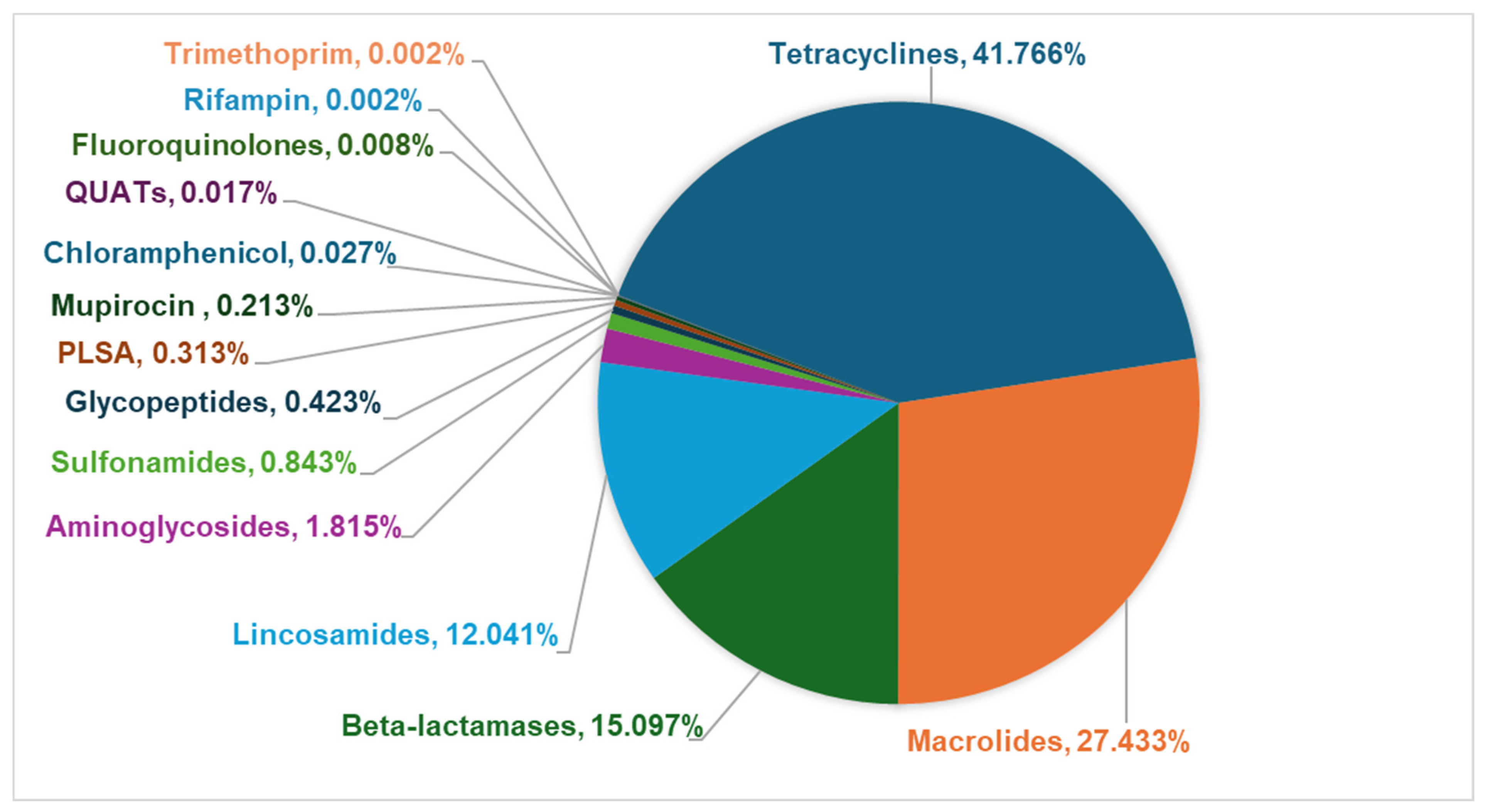
3.3. Fecal Resistome in Shelter Dog Population
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Megna, M. Pet Ownership Statistics 2024. Available online: https://www.forbes.com/advisor/pet-insurance/pet-ownership-statistics/#sources_section (accessed on 7 October 2024).
- TheHumaneSociety. Pets by the Numbers. Available online: https://humanepro.org/page/pets-by-the-numbers (accessed on 7 October 2024).
- ShelterAnimalsCount. 2023 Annual Analysis Report. Available online: https://www.shelteranimalscount.org/wp-content/uploads/2024/01/Full-Year-2023-Report.pdf (accessed on 10 October 2024).
- Parsons, B.N.; Williams, N.J.; Pinchbeck, G.L.; Christley, R.M.; Hart, C.A.; Gaskell, R.M.; Dawson, S. Prevalence and shedding patterns of Campylobacter spp. in longitudinal studies of kennelled dogs. Vet. J. 2011, 190, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Acke, E.; Whyte, P.; Jones, B.R.; McGill, K.; Collins, J.D.; Fanning, S. Prevalence of thermophilic Campylobacter species in cats and dogs in two animal shelters in Ireland. Vet. Rec. 2006, 158, 51–54. [Google Scholar] [CrossRef]
- Leahy, A.M.; Cummings, K.J.; Rodriguez-Rivera, L.D.; Hamer, S.A.; Lawhon, S.D. Faecal Campylobacter shedding among dogs in animal shelters across Texas. Zoonoses Public. Health 2017, 64, 623–627. [Google Scholar] [CrossRef] [PubMed]
- LaLonde-Paul, D.; Cummings, K.J.; Rodriguez-Rivera, L.D.; Wu, J.; Lawhon, S.D. Ciprofloxacin resistance among Campylobacter jejuni isolates obtained from shelter dogs in Texas. Zoonoses Public. Health 2019, 66, 337–342. [Google Scholar] [CrossRef]
- Spangler, D.; Kish, D.; Beigel, B.; Morgan, J.; Gruszynski, K.; Naikare, H.; Nahar, V.K.; Coarsey, M.D.; Verma, A. Leptospiral shedding and seropositivity in shelter dogs in the Cumberland Gap Region of Southeastern Appalachia. PLoS ONE 2020, 15, e0228038. [Google Scholar] [CrossRef]
- Verma, A.; Carney, K.; Taylor, M.; Amsler, K.; Morgan, J.; Gruszynski, K.; Erol, E.; Carter, C.; Locke, S.; Callipare, A.; et al. Occurrence of potentially zoonotic and cephalosporin resistant enteric bacteria among shelter dogs in the Central and South-Central Appalachia. BMC Vet. Res. 2021, 17, 313. [Google Scholar] [CrossRef]
- Gingrich, E.N.; Kurt, T.; Hyatt, D.R.; Lappin, M.R.; Ruch-Gallie, R. Prevalence of methicillin-resistant staphylococci in northern Colorado shelter animals. J. Vet. Diagn. Investig. 2011, 23, 947–950. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.Y.; Cummings, K.J.; Hodo, C.L.; Hamer, S.A. A repeated cross-sectional study of intestinal parasites in Texas shelter dogs using fecal flotation and saline sedimentation. Parasitol. Res. 2023, 122, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.E.; Lineberry, M.W. Trypanosoma cruzi and Other Vector-Borne Infections in Shelter Dogs in Two Counties of Oklahoma, United States. Vector Borne Zoonotic Dis. 2022, 22, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Patterson, G.; Tanhauser, M.; Schmidt, P.; Spangler, D.; Faulkner, C.; Faulkner, V.; Kish, D.; Gruszynski, K.; Naikare, H.; Coarsey, M.D.; et al. Serosurvey of arthropod-borne diseases among shelter dogs in the Cumberland Gap Region of the United States. BMC Vet. Res. 2020, 16, 221. [Google Scholar] [CrossRef]
- Backus, L.; Foley, J.; Chung, C.; Virata, S.; Zazueta, O.E.; Lopez-Perez, A. Tick-borne pathogens detected in sheltered dogs during an epidemic of Rocky Mountain spotted fever, a One Health challenge. J. Am. Vet. Med. Assoc. 2022, 261, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.; Ellis, A.; Liang, R.; Lim, A.; Newbury, S. Prolonged persistence of canine distemper virus RNA, and virus isolation in naturally infected shelter dogs. PLoS ONE 2023, 18, e0280186. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Song, L.; Breitwieser, F.P.; Salzberg, S.L. Centrifuge: Rapid and sensitive classification of metagenomic sequences. Genome Res. 2016, 26, 1721–1729. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Dowd, S.E.; Suchodolski, J.S.; Middelbos, I.S.; Vester, B.M.; Barry, K.A.; Nelson, K.E.; Torralba, M.; Henrissat, B.; Coutinho, P.M.; et al. Phylogenetic and gene-centric metagenomics of the canine intestinal microbiome reveals similarities with humans and mice. ISME J. 2011, 5, 639–649. [Google Scholar] [CrossRef]
- Suchodolski, J.S. Analysis of the gut microbiome in dogs and cats. Vet. Clin. Pathol. 2022, 50, 6–17. [Google Scholar] [CrossRef]
- Jager, M.C.; Tomlinson, J.E.; Lopez-Astacio, R.A.; Parrish, C.R.; Van De Walle, G.R. Small but mighty: Old and new parvoviruses of veterinary significance. Virol. J. 2021, 18, 210. [Google Scholar] [CrossRef]
- Horecka, K.; Porter, S.; Amirian, E.S.; Jefferson, E. A Decade of Treatment of Canine Parvovirus in an Animal Shelter: A Retrospective Study. Animals 2020, 10, 939. [Google Scholar] [CrossRef]
- Lavan, R.; Knesl, O. Prevalence of canine infectious respiratory pathogens in asymptomatic dogs presented at US animal shelters. J. Small Anim. Pract. 2015, 56, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Acke, E. Campylobacteriosis in dogs and cats: A review. N. Z. Vet. J. 2018, 66, 221–228. [Google Scholar] [CrossRef]
- Procter, T.D.; Pearl, D.L.; Finley, R.L.; Leonard, E.K.; Janecko, N.; Reid-Smith, R.J.; Weese, J.S.; Peregrine, A.S.; Sargeant, J.M. A cross-sectional study examining Campylobacter and other zoonotic enteric pathogens in dogs that frequent dog parks in three cities in south-western Ontario and risk factors for shedding of Campylobacter spp. Zoonoses Public. Health 2014, 61, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Same, R.G.; Tamma, P.D. Campylobacter Infections in Children. Pediatr. Rev. 2018, 39, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Igwaran, A.; Okoh, A.I. Human campylobacteriosis: A public health concern of global importance. Heliyon 2019, 5, e02814. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Campylobacter Infection (Campylobacteriosis). Available online: https://www.cdc.gov/campylobacter/about/index.html (accessed on 8 October 2024).
- Couturier, B.A.; Hale, D.C.; Couturier, M.R. Association of Campylobacter upsaliensis with Persistent Bloody Diarrhea. J. Clin. Microbiol. 2012, 50, 3792–3794. [Google Scholar] [CrossRef]
- Damborg, P.; Olsen, K.E.P.; Møller Nielsen, E.; Guardabassi, L. Occurrence of Campylobacter jejuni in Pets Living with Human Patients Infected with C. jejuni. J. Clin. Microbiol. 2004, 42, 1363–1364. [Google Scholar] [CrossRef] [PubMed]
- Gras, L.M.; Smid, J.H.; Wagenaar, J.A.; Koene, M.G.J.; Havelaar, A.H.; Friesema, I.H.M.; French, N.P.; Flemming, C.; Galson, J.D.; Graziani, C.; et al. Increased risk for Campylobacter jejuni and C. coli infection of pet origin in dog owners and evidence for genetic association between strains causing infection in humans and their pets. Epidemiol. Infect. 2013, 141, 2526–2535. [Google Scholar] [CrossRef]
- Steneroden, K.K.; Hill, A.E.; Salman, M.D. Zoonotic disease awareness in animal shelter workers and volunteers and the effect of training. Zoonoses Public. Health 2011, 58, 449–453. [Google Scholar] [CrossRef]
- Fitzgerald, C. Campylobacter. Clin. Lab. Med. 2015, 35, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Leahy, A.M.; Cummings, K.J.; Rodriguez-Rivera, L.D.; Rankin, S.C.; Hamer, S.A. Evaluation of Faecal Salmonella Shedding Among Dogs at Seven Animal Shelters across Texas. Zoonoses Public. Health 2016, 63, 515–521. [Google Scholar] [CrossRef]
- Reimschuessel, R.; Grabenstein, M.; Guag, J.; Nemser, S.M.; Song, K.; Qiu, J.; Clothier, K.A.; Byrne, B.A.; Marks, S.L.; Cadmus, K.; et al. Multilaboratory Survey To Evaluate Salmonella Prevalence in Diarrheic and Nondiarrheic Dogs and Cats in the United States between 2012 and 2014. J. Clin. Microbiol. 2017, 55, 1350–1368. [Google Scholar] [CrossRef] [PubMed]
- Nichols, M.; Stapleton, G.S.; Rotstein, D.S.; Gollarza, L.; Adams, J.; Caidi, H.; Chen, J.; Hodges, A.; Glover, M.; Peloquin, S.; et al. Outbreak of multidrug-resistant Salmonella infections in people linked to pig ear pet treats, United States, 2015–2019: Results of a multistate investigation. Lancet Reg. Health-Am. 2024, 34, 100769. [Google Scholar] [CrossRef] [PubMed]
- Morse, E.V.; Duncan, M.A.; Estep, D.A.; Riggs, W.A.; Blackburn, B.O. Canine salmonellosis: A review and report of dog to child transmission of Salmonella enteritidis. Am. J. Public. Health 1976, 66, 82–83. [Google Scholar] [CrossRef] [PubMed]
- Ekman, E.; Fredriksson, M.; Trowald-Wigh, G. Helicobacter spp. in the saliva, stomach, duodenum and faeces of colony dogs. Vet. J. 2013, 195, 127–129. [Google Scholar] [CrossRef]
- Ashaolu, J.O.; Tsai, Y.J.; Liu, C.C.; Ji, D.D. Prevalence, diversity and public health implications of Helicobacter species in pet and stray dogs. One Health 2022, 15, 100430. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, S.; Ojeda, J.; Martínez, O.A.; Vidal-Veuthey, B.; Collado, L. Exploring the role of healthy dogs as hosts of enterohepatic Helicobacter species using cultivation-dependent and -independent approaches. Zoonoses Public. Health 2021, 68, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.G.; Drolet, R.; Higgins, R.; Messier, S.; Yan, L.; Coleman, B.E.; Paster, B.J.; Dewhirst, F.E. Helicobacter canis isolated from a dog liver with multifocal necrotizing hepatitis. J. Clin. Microbiol. 1996, 34, 2479–2482. [Google Scholar] [CrossRef]
- Lardinois, B.; Belkhir, L.; Verroken, A. Helicobacter canis: A Review of Microbiological and Clinical Features. Front. Microbiol. 2022, 12, 814944. [Google Scholar] [CrossRef]
- van Wezel, E.M.; van der Beek, E.S.J.; Siebrecht, M.A.N.; Stel, A.J.; Wouthuyzen-Bakker, M.; Meessen, N.E.L. Pet-related bacterial zoonotic infections: Three cases of severe infections in the immunocompromised host. IDCases 2022, 30, e01623. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.E.; Sidak-Loftis, L.C.; Sahl, J.W.; Vazquez, A.J.; Wiggins, K.B.; Gillece, J.D.; Hicks, N.D.; Schupp, J.M.; Busch, J.D.; Keim, P.; et al. More than 50% of Clostridium difficile Isolates from Pet Dogs in Flagstaff, USA, Carry Toxigenic Genotypes. PLoS ONE 2016, 11, e0164504. [Google Scholar] [CrossRef]
- Buogo, C.; Burnens, A.P.; Perrin, J.; Nicolet, J. Presence of Campylobacter spp., Clostridium difficile, C. perfringens and salmonellae in litters of puppies and in adult dogs in a shelter. Schweiz. Arch. Tierheilkd. 1995, 137, 165–171. [Google Scholar] [PubMed]
- Perrin, J.; Buogo, C.; Gallusser, A.; Burnens, A.P.; Nicolet, J. Intestinal carriage of Clostridium difficile in neonate dogs. Zentralbl Vet. B 1993, 40, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Struble, A.L.; Tang, Y.J.; Kass, P.H.; Gumerlock, P.H.; Madewell, B.R.; Silva, J. Fecal Shedding of Clostridium Difficile in Dogs: A Period Prevalence Survey in a Veterinary Medical Teaching Hospital. J. Vet. Diagn. Investig. 1994, 6, 342–347. [Google Scholar] [CrossRef]
- Clooten, J.; Kruth, S.; Arroyo, L.; Weese, J.S. Prevalence and risk factors for Clostridium difficile colonization in dogs and cats hospitalized in an intensive care unit. Vet. Microbiol. 2008, 129, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Sharma, R.K.; Borah, P.; Rajkhowa, S.; Hussain, I.; Barkalita, L.M.; Hasin, D.; Choudhury, M.; Rupnik, M.; Deka, N.K.; et al. Isolation and characterization of Clostridium difficile from pet dogs in Assam, India. Anaerobe 2015, 36, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Usui, M.; Suzuki, K.; Oka, K.; Miyamoto, K.; Takahashi, M.; Inamatsu, T.; Kamiya, S.; Tamura, Y. Distribution and characterization of Clostridium difficile isolated from dogs in Japan. Anaerobe 2016, 37, 58–61. [Google Scholar] [CrossRef]
- Weese, J.S. Clostridium (Clostridioides) difficile in animals. J. Vet. Diagn. Investig. 2020, 32, 213–221. [Google Scholar] [CrossRef]
- Hernandez, B.G.; Vinithakumari, A.A.; Sponseller, B.; Tangudu, C.; Mooyottu, S. Prevalence, Colonization, Epidemiology, and Public Health Significance of Clostridioides difficile in Companion Animals. Front. Vet. Sci. 2020, 7, 512551. [Google Scholar] [CrossRef]
- Unterer, S.; Busch, K. Acute Hemorrhagic Diarrhea Syndrome in Dogs. Vet. Clin. N. Am. Small Anim. Pract. 2021, 51, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Marks, S.L.; Rankin, S.C.; Byrne, B.A.; Weese, J.S. Enteropathogenic Bacteria in Dogs and Cats: Diagnosis, Epidemiology, Treatment, and Control. J. Vet. Intern. Med. 2011, 25, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Barsanti, J.A. Botulism, Chapter 40, 4th ed.; Elsevier Saunders: Philadelphia, PA, USA, 2012. [Google Scholar]
- Lamoureux, A.; Pouzot-Nevoret, C.; Escriou, C. A case of type B botulism in a pregnant bitch. J. Small Anim. Pract. 2015, 56, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, S.R.F.; Neves, A.K.d.R.; Silva, T.P.R.; Oliveira, A.A.d.F. Botulismo em cão sem raça definida-relato de caso. Med. Veterinária 2020, 13, 325–328. [Google Scholar] [CrossRef]
- Bruchim, Y.; Steinman, A.; Markovitz, M.; Baneth, G.; Elad, D.; Shpigel, N.Y. Toxicological, bacteriological and serological diagnosis of botulism in a dog. Vet. Rec. 2006, 158, 768–769. [Google Scholar] [CrossRef]
- Farrow, B.R.; Murrell, W.G.; Revington, M.L.; Stewart, B.J.; Zuber, R.M. Type C botulism in young dogs. Aust. Vet. J. 1983, 60, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Lonati, D.; Schicchi, A.; Crevani, M.; Buscaglia, E.; Scaravaggi, G.; Maida, F.; Cirronis, M.; Petrolini, V.M.; Locatelli, C.A. Foodborne Botulism: Clinical Diagnosis and Medical Treatment. Toxins 2020, 12, 509. [Google Scholar] [CrossRef] [PubMed]
- Oxberry, S.L.; Hampson, D.J. Colonisation of pet shop puppies with Brachyspira pilosicoli. Vet. Microbiol. 2003, 93, 167–174. [Google Scholar] [CrossRef]
- Hampson, D.J. The Spirochete Brachyspira pilosicoli, Enteric Pathogen of Animals and Humans. Clin. Microbiol. Rev. 2018, 31. [Google Scholar] [CrossRef]
- Hidalgo, A.; Rubio, P.; Osorio, J.; Carvajal, A. Prevalence of Brachyspira pilosicoli and “Brachyspira canis” in dogs and their association with diarrhoea. Vet. Microbiol. 2010, 146, 356–360. [Google Scholar] [CrossRef]
- Duhamel, G.E.; Trott, D.J.; Muniappa, N.; Mathiesen, M.R.; Tarasiuk, K.; Lee, J.I.; Hampson, D.J. Canine Intestinal Spirochetes Consist of Serpulina pilosicoli and a Newly Identified Group Provisionally Designated “ Serpulina canis ” sp. nov. J. Clin. Microbiol. 1998, 36, 2264–2270. [Google Scholar] [CrossRef]
- Hampson, D.J.; Oxberry, S.L.; La, T. Potential for Zoonotic Transmission of Brachyspira pilosicoli. Emerg. Infect. Dis. 2006, 12, 869–870. [Google Scholar] [CrossRef]
- Barker, E.N.; O’Halloran, C.; Gunn-Moore, D.A. Review canine tuberculosis—An emerging concern. Vet. J. 2024, 305, 106111. [Google Scholar] [CrossRef] [PubMed]
- O’Halloran, C.; Barker, E.N.; Hope, J.C.; Gunn-Moore, D.A. Canine tuberculosis: A review of 18 new and 565 previously reported confirmed cases. Vet. J. 2024, 304, 106089. [Google Scholar] [CrossRef] [PubMed]
- Foster, E.S.; Scavelli, T.D.; Greenlee, P.G.; Gilbertson, S.R. Cutaneous lesion caused by Mycobacterium tuberculosis in a dog. J. Am. Vet. Med. Assoc. 1986, 188, 1188–1190. [Google Scholar] [PubMed]
- Hackendahl, N.C.; Mawby, D.I.; Bemis, D.A.; Beazley, S.L. Putative transmission of Mycobacterium tuberculosis infection from a human to a dog. J. Am. Vet. Med. Assoc. 2004, 225, 1573–1577. [Google Scholar] [CrossRef]
- Sykes, J.E.; Cannon, A.B.; Norris, A.J.; Byrne, B.A.; Affolter, T.; O’Malley, M.A.; Wisner, E.R. Mycobacterium tuberculosis complex infection in a dog. J. Vet. Intern. Med. 2007, 21, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Foreman, O.; Sykes, J.; Ball, L.; Yang, N.; De Cock, H. Disseminated Infection with Balamuthia mandrillaris in a Dog. Vet. Pathol. 2004, 41, 506–510. [Google Scholar] [CrossRef]
- Chien, R.C.-C.; Telford, C.R.; Roy, S.; Ali, I.K.M.; Shieh, W.-J.; Confer, A.W. Canine amoebic meningoencephalitis due to Balamuthia mandrillaris. Vet. Parasitol. Reg. Stud. Rep. 2018, 13, 156–159. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Balamuthia amebic encephalitis--California, 1999–2007. MMWR Morb. Mortal. Wkly. Rep. 2008, 57, 768–771. [Google Scholar]
- Dubey, J.P.; Murata, F.H.A.; Cerqueira-Cézar, C.K.; Kwok, O.C.H.; Yang, Y.; Su, C. Toxoplasma gondii infections in dogs: 2009–2020. Vet. Parasitol. 2020, 287, 109223. [Google Scholar] [CrossRef] [PubMed]
- Dini, F.M.; Stancampiano, L.; Poglayen, G.; Galuppi, R. Risk factors for Toxoplasma gondii infection in dogs: A serological survey. Acta Vet. Scand. 2024, 66, 14. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Trichuriasis. Available online: https://www.cdc.gov/dpdx/trichuriasis/index.html#print (accessed on 10 October 2024).
- Areekul, P.; Putaporntip, C.; Pattanawong, U.; Sitthicharoenchai, P.; Jongwutiwes, S. Trichuris vulpis and T. trichiura infections among schoolchildren of a rural community in northwestern Thailand: The possible role of dogs in disease transmission. Asian Biomed. 2010, 4, 49–60. [Google Scholar] [CrossRef]
- Else, K.J.; Keiser, J.; Holland, C.V.; Grencis, R.K.; Sattelle, D.B.; Fujiwara, R.T.; Bueno, L.L.; Asaolu, S.O.; Sowemimo, O.A.; Cooper, P.J. Whipworm and roundworm infections. Nat. Rev. Dis. Primers 2020, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Waldman, E.A.; Moreira, R.C.; Saez, S.G.; Souza, D.F.C.; Carmona, R.D.C.C.; Takimoto, S.; Cortes, V.D.A. Human enterovirus infection in stray dogs. Some aspects of interest to public health. Rev. Inst. Med. Trop. São Paulo 1996, 38, 157–161. [Google Scholar] [CrossRef]
- Fieldhouse, J.K.; Wang, X.; Mallinson, K.A.; Tsao, R.W.; Gray, G.C. A systematic review of evidence that enteroviruses may be zoonotic. Emerg. Microbes Infect. 2018, 7, 164. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.; Chiu, C. The Role of Metagenomics and Next-Generation Sequencing in Infectious Disease Diagnosis. Clin. Chem. 2021, 68, 115–124. [Google Scholar] [CrossRef]
- Simner, P.J.; Miller, S.; Carroll, K.C. Understanding the Promises and Hurdles of Metagenomic Next-Generation Sequencing as a Diagnostic Tool for Infectious Diseases. Clin. Infect. Dis. 2018, 66, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Anthony, W.E.; Burnham, C.-A.D.; Dantas, G.; Kwon, J.H. The Gut Microbiome as a Reservoir for Antimicrobial Resistance. J. Infect. Dis. 2021, 223, S209–S213. [Google Scholar] [CrossRef] [PubMed]
- Røken, M.; Forfang, K.; Wasteson, Y.; Haaland, A.H.; Eiken, H.G.; Hagen, S.B.; Bjelland, A.M. Antimicrobial resistance—Do we share more than companionship with our dogs? J. Appl. Microbiol. 2022, 133, 1027–1039. [Google Scholar] [CrossRef]
- Stege, P.B.; Hordijk, J.; Sandholt, A.K.S.; Zomer, A.L.; Viveen, M.C.; Rogers, M.R.C.; Salomons, M.; Wagenaar, J.A.; Mughini-Gras, L.; Willems, R.J.L.; et al. Gut Colonization by ESBL-Producing Escherichia coli in Dogs Is Associated with a Distinct Microbiome and Resistome Composition. Microbiol. Spectr. 2023, 11, e00063-23. [Google Scholar] [CrossRef]
- Zhao, R.; Hao, J.; Yang, J.; Tong, C.; Xie, L.; Xiao, D.; Zeng, Z.; Xiong, W. The co-occurrence of antibiotic resistance genes between dogs and their owners in families. iMeta 2022, 1, e21. [Google Scholar] [CrossRef]
- Maddock, K.J.; Burbick, C.R.; Cole, S.D.; Daniels, J.B.; Lecuyer, T.E.; Li, X.-Z.; Loy, J.D.; Sanchez, S.; Stenger, B.L.S.; Diaz-Campos, D. A One Health perspective on the use of genotypic methods for antimicrobial resistance prediction. J. Am. Vet. Med. Assoc. 2024, 262, 303–312. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shringi, S.; Shah, D.H.; Carney, K.; Verma, A. Pathogen Detection and Resistome Analysis in Healthy Shelter Dogs Using Whole Metagenome Sequencing. Pathogens 2025, 14, 33. https://doi.org/10.3390/pathogens14010033
Shringi S, Shah DH, Carney K, Verma A. Pathogen Detection and Resistome Analysis in Healthy Shelter Dogs Using Whole Metagenome Sequencing. Pathogens. 2025; 14(1):33. https://doi.org/10.3390/pathogens14010033
Chicago/Turabian StyleShringi, Smriti, Devendra H. Shah, Kimberly Carney, and Ashutosh Verma. 2025. "Pathogen Detection and Resistome Analysis in Healthy Shelter Dogs Using Whole Metagenome Sequencing" Pathogens 14, no. 1: 33. https://doi.org/10.3390/pathogens14010033
APA StyleShringi, S., Shah, D. H., Carney, K., & Verma, A. (2025). Pathogen Detection and Resistome Analysis in Healthy Shelter Dogs Using Whole Metagenome Sequencing. Pathogens, 14(1), 33. https://doi.org/10.3390/pathogens14010033







