Abstract
Human Immunodeficiency Virus (HIV) proviral reservoirs are cells that harbor integrated HIV proviral DNA within their nuclear genomes. These cells form a heterogeneous group, represented by peripheral blood mononuclear cells (PBMCs), tissue-resident lymphoid and monocytic cells, and glial cells of the central nervous system. The importance of studying the properties of proviral reservoirs is connected with the inaccessibility of integrated HIV proviral DNA for modern anti-retroviral therapies (ARTs) that block virus reproduction. If treatment is not effective enough or is interrupted, the proviral reservoir can reactivate. Early initiation of ART improves the prognosis of the course of HIV infection, which is explained by the reduction in the proviral reservoir pool observed in the early stages of the disease. Different HIV subtypes present differences in the number of latent reservoirs, as determined by structural and functional differences. Unique signatures of patients with HIV, such as elite controllers, have control over viral replication and can be said to have achieved a functional cure for HIV infection. Uncovering the causes of this phenomenon will bring humanity closer to curing HIV infection, potential approaches to which include stem cell transplantation, clustered regularly interspaced short palindromic repeats (CRISPR)/cas9, “Shock and kill”, “Block and lock”, and the application of broad-spectrum neutralizing antibodies (bNAbs).
1. Introduction
At present, Human Immunodeficiency Virus (HIV) remains a major global public health issue. As of the end of 2023, it was estimated that approximately 39.9 million people were living with HIV (with a range from 36.1 million to 44.6 million), and, in some regions, the trend of increasing numbers of new infections has resumed [1]. Mortality due to HIV infection and associated opportunistic infections remains significant, amounting to approximately 630,000 deaths per year globally [1]. The development and implementation of antiretroviral therapy (ART) have contributed to a reduction in new HIV infections and deaths [2,3]. However, a complete cure for HIV remains currently unattainable [4]. Proviral reservoirs represent a major obstacle to the complete eradication of HIV from the human body [5,6]. These are cells in which HIV proviral DNA has integrated into the nuclear genome. For various reasons, some proviral reservoirs exist in a latent state, with either complete or partial cessation of viral gene transcription, creating a group of latent reservoirs [7]. In this state, infected cells can evade immune surveillance by preserving HIV DNA, until viral gene transcription is reactivated [8,9]. The existence of these latent reservoirs necessitates lifelong ART and contributes to the development of chronic HIV infection [10].
Currently, the study of proviral reservoirs is one of the most pertinent areas in HIV research, as the integrity of integrated proviral DNA is critically important for the continued progression of the infection [11]. Studies have demonstrated the impact of the timing of ART initiation following diagnosis, the clinical characteristics of the patient, the HIV genetic variant, and a number of other factors on the characteristics of proviral reservoirs [12,13,14]. Based on this research, exposure to the HIV provirus may contribute to the development of new methods for treating HIV infection [15].
5. Conclusions
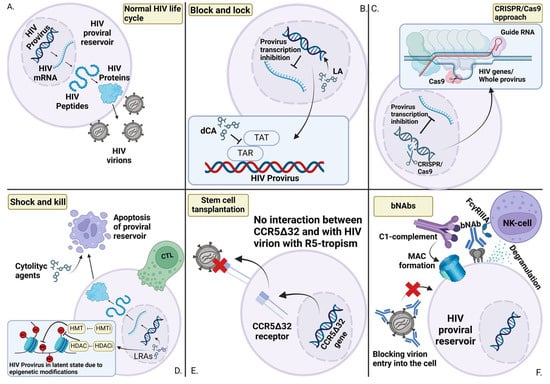
HIV proviral reservoirs remain the primary obstacle in the quest for a cure for HIV infection. For nearly 40 years, scientists and healthcare professionals worldwide have been working toward finding a cure, but no proven method for eliminating the disease has yet been discovered. Potential therapeutic approaches, such as CRISPR/Cas9, Block and Lock, and Shock and Kill, are still being explored and refined. However, none have demonstrated definitive success in clinical trials, underscoring the need for continued research into the properties of HIV proviral reservoirs.
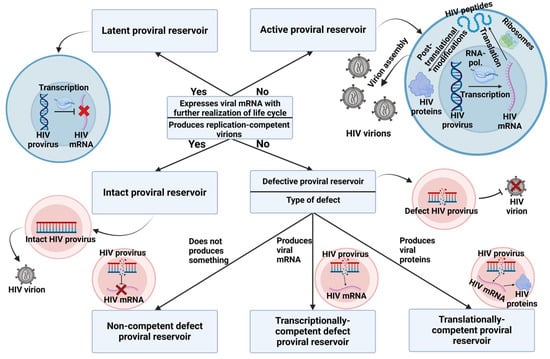
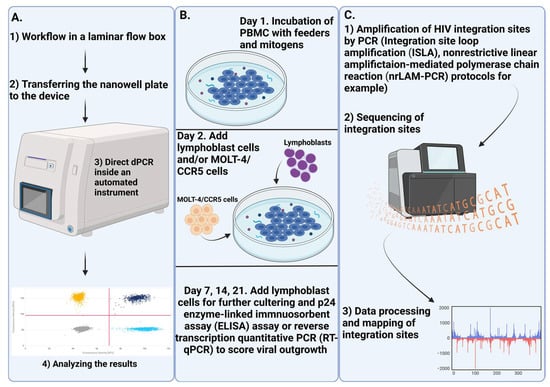
ART has shown a significant impact on the size of proviral reservoirs, with the timing of its initiation being a critical factor. There is a 12-fold difference in the amount of cell-associated HIV DNA already in the early stages of infection (Fiebig stages I and II). However, cell-associated DNA encompasses several forms of HIV DNA, not all of which lead to productive infection. Intact proviral reservoirs are likely a key component of the overall reservoir and should be prioritized for both analysis and treatment. Research findings in this area remain inconclusive, necessitating further confirmation. Currently, IPDA, NGS, and QOVA are considered the best methods for studying intact proviral reservoirs. Their varied applications enable researchers to explore different aspects of HIV molecular biology, including mapping integration sites, determining the proportion of defective and intact reservoirs, and assessing the inducibility of these reservoirs.
The genetic variant of HIV plays an important role in proviral reservoirs, with several studies showing that patients with subtype B have a larger number of reservoirs, both general and latent. These differences are linked to the structure of the long terminal repeat (LTR) region and the function of HIV proteins, which make them potential targets for intervention.
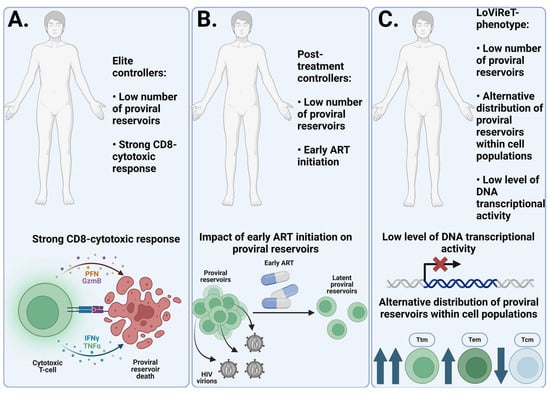
Elite and post-treatment controllers, who maintain an undetectable viral load without ART and do not progress in HIV infection, are valuable subjects for research and the identification of unique traits. Strong CD8 cytotoxic responses in elite controllers have already been shown to be a critical factor in achieving remission. Similarly, early ART initiation in post-treatment controllers highlights the benefits of this approach. Together, these findings open up promising new avenues for developing HIV treatment strategies, with the goal of achieving a stable and functional cure—a highly desirable outcome for individuals with HIV.
Author Contributions
Conceptualization, N.M.G.; writing the original manuscript and drawing design, A.I.M.; writing—review and editing K.A.E. and N.M.G. All authors have read and agreed to the published version of the manuscript.
Funding
Ministry of Science and Higher Education of the Russian Federation: Agreement No. 075-15-2019-1665.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The figures in this manuscript were created with BioRender.com (accessed on 25 November 2024).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- The Urgency of Now: AIDS at a Crossroads; Joint United Nations Programme on HIV/AIDS: Geneva, Switzerland, 2024.
- Legarth, R.A.; Ahlström, M.G.; Kronborg, G.; Larsen, C.S.; Pedersen, C.; Pedersen, G.; Mohey, R.; Gerstoft, J. Long-Term Mortality in HIV-Infected Individuals 50 Years or Older: A Nationwide, Population-Based Cohort Study. J. Acquir. Immune Defic. Syndr. 2016, 71, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Samji, H.; Cescon, A.; Hogg, R.S.; Modur, S.P.; Althoff, K.N.; Buchacz, K.; Burchell, A.N.; Cohen, M.; Gebo, K.A.; Gill, M.J.; et al. Closing the Gap: Increases in Life Expectancy among Treated HIV-Positive Individuals in the United States and Canada. PLoS ONE 2013, 8, e81355. [Google Scholar] [CrossRef] [PubMed]
- Pitman, M.C.; Lau, J.S.Y.; McMahon, J.H. Barriers and strategies to achieve a cure for HIV. Lancet HIV 2018, 5, e317–e328. [Google Scholar] [CrossRef] [PubMed]
- Chun, T.-W.; Moir, S. HIV reservoirs as obstacles and opportunities for an HIV cure. Nat. Immunol. 2015, 16, 584–589. [Google Scholar] [CrossRef]
- Dahabieh, M.S.; Battivelli, E. Understanding HIV Latency: The Road to an HIV Cure. Annu. Rev. Med. 2015, 66, 407–421. [Google Scholar] [CrossRef]
- Cary, D.C.; Fujinaga, K. Molecular mechanisms of HIV latency. J. Clin. Investig. 2016, 126, 448–454. [Google Scholar] [CrossRef]
- Pace, M.J.; Agosto, L.; Graf, E.H. HIV reservoirs and latency models. Virology 2011, 411, 344–354. [Google Scholar] [CrossRef]
- Shukla, A.; Ramirez, N.-G.P. HIV-1 Proviral Transcription and Latency in the New Era. Viruses 2020, 12, 555. [Google Scholar] [CrossRef]
- Volberding, P.A. Antiretroviral therapy and management of HIV infection. Lancet 2010, 376, 49–62. [Google Scholar] [CrossRef]
- Ho, Y.-C.; Shan, L.; Hosmane, N.; Wang, J.; Laskey, S.; Rosenbloom, D.S.; Lai, J.; Blankson, J.; Siliciano, J. Replication-Competent Noninduced Proviruses in the Latent Reservoir Increase Barrier to HIV-1 Cure. Cell 2013, 155, 540–551. [Google Scholar] [CrossRef]
- Ananworanich, J.; Dubé, K. How does the timing of antiretroviral therapy initiation in acute infection affect HIV reservoirs? Curr. Opin. HIV AIDS 2015, 10, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Ananworanich, J.; Sacdalan, C.P.; Pinyakorn, S.; Chomont, N.; Souza, M.; Luekasemsuk, T.; Schuetz, A.; Krebs, S.J.; Dewar, R.; Jagodzinski, L.; et al. Virological and immunological characteristics of HIV-infected individuals at the earliest stage of infection. J. Virus Erad. 2016, 2, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Prodger, J.L.; Lai, J.; Reynolds, S.J.; Keruly, J.C.; Moore, R.D.; Kasule, J.; Kityamuweesi, T.; Buule, P.; Serwadda, D.; Nason, M.; et al. Reduced Frequency of Cells Latently Infected With Replication-Competent Human Immunodeficiency Virus-1 in Virally Suppressed Individuals Living in Rakai, Uganda. Clin. Infect. Dis. 2017, 65, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Cohn, L.B.; Chomont, N. The Biology of the HIV-1 Latent Reservoir and Implications for Cure Strategies. Cell Host Microbe 2020, 27, 519–530. [Google Scholar] [CrossRef]
- Churchill, M.J.; Deeks, S.G.; Margolis, D.M.; Siliciano, R.F.; Swanstrom, R. HIV reservoirs: What, where and how to target them. Nat. Rev. Microb. 2015, 14, 55–60. [Google Scholar] [CrossRef]
- Eisele, E. Redefining the Viral Reservoirs that Prevent HIV-1 Eradication. Immunity 2012, 37, 377–388. [Google Scholar] [CrossRef]
- Falcinelli, S.D.; Ceriani, C.; Margolis, D.M. New Frontiers in Measuring and Characterizing the HIV Reservoir. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Vanhamel, J.; Bruggemans, A. Establishment of latent HIV-1 reservoirs: What do we really know? J. Virus Erad. 2019, 5, 3–9. [Google Scholar] [CrossRef]
- Imamichi, H.; Smith, M.; Adelsberger, J.W.; Izumi, T.; Scrimieri, F.; Sherman, B.T.; Rehm, C.A.; Imamichi, T.; Pau, A.; Catalfamo, M.; et al. Defective HIV-1 proviruses produce viral proteins. Proc. Natl. Acad. Sci. USA 2020, 117, 3704–3710. [Google Scholar] [CrossRef]
- Besson, G.J.; Lalama, C.M.; Bosch, R.J.; Gandhi, R.T.; Bedison, M.A.; Aga, E.; Riddler, S.A.; McMahon, D.K.; Hong, F. HIV-1 DNA Decay Dynamics in Blood During More Than a Decade of Suppressive Antiretroviral Therapy. Clin. Infect. Dis. 2014, 59, 1312–1321. [Google Scholar] [CrossRef]
- Oliveira, M.F.; Pankow, A.; Vollbrecht, T.; Kumar, N.M.; Cabalero, G.; Ignacio, C.; Zhao, M.; Vitomirov, A.; Gouaux, B.; Nakawawa, M.; et al. Evaluation of Archival HIV DNA in Brain and Lymphoid Tissues. J. Virol. 2023, 97, e00543-23. [Google Scholar] [CrossRef] [PubMed]
- Fiebig, E.W.; Wright, D.J.; Rawal, B.D.; Garrett, P.E.; Schumacher, R.T.; Peddada, L.; Heldebrant, C.; Smith, R.; Conrad, A.; Kleinman, S.H. Dynamics of HIV viremia and antibody seroconversion in plasma donors: Implications for diagnosis and staging of primary HIV infection. AIDS 2003, 17, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Ananworanich, J.; Chomont, N.; Eller, L.A.; Kroon, E.; Tovanabutra, S.; Bose, M.; Nau, M.; Fletcher, J.L.K.; Tipsuk, S.; Vandergeeten, C.; et al. HIV DNA Set Point is Rapidly Established in Acute HIV Infection and Dramatically Reduced by Early ART. eBioMedicine 2016, 11, 68–72. [Google Scholar] [CrossRef]
- Kwon, K.J.; Timmons, A.E.; Sengupta, S.; Simonetti, F.R.; Zhang, H.; Hoh, R.; Deeks, S.G.; Siliciano, J.D. Different human resting memory CD4+ T cell subsets show similar low inducibility of latent HIV-1 proviruses. Sci. Transl. Med. 2020, 12, eaax6795. [Google Scholar] [CrossRef]
- Gálvez, C.; Grau-Expósito, J.; Urrea, V.; Clotet, B.; Falcó, V.; Buzón, M.J. Atlas of the HIV-1 Reservoir in Peripheral CD4 T Cells of Individuals on Successful Antiretroviral Therapy. mBio 2021, 12, e03078-21. [Google Scholar] [CrossRef]
- Buzon, M.J.; Martin-Gayo, E.; Pereyra, F.; Ouyang, Z.; Sun, H.; Li, J.Z.; Piovoso, M.; Shaw, A.; Dalmau, J.; Zangger, N.; et al. Long-Term Antiretroviral Treatment Initiated at Primary HIV-1 Infection Affects the Size, Composition, and Decay Kinetics of the Reservoir of HIV-1-Infected CD4 T Cells. J. Virol. 2014, 88, 10056–10065. [Google Scholar] [CrossRef]
- Hiener, B.; Horsburgh, B.A.; Eden, J.-S.; Barton, K.; Schlub, T.E.; Lee, E.; von Stockenstrom, S.; Odevall, L.; Milush, J.M.; Liegler, T.; et al. Identification of Genetically Intact HIV-1 Proviruses in Specific CD4 + T Cells from Effectively Treated Participants. Cell Rep. 2017, 21, 813–822. [Google Scholar] [CrossRef]
- Soriano-Sarabia, N.; Archin, N.M.; Bateson, R.; Dahl, N.P.; Crooks, A.M.; Kuruc, J.D.; Garrido, C. Peripheral Vγ9Vδ2 T Cells Are a Novel Reservoir of Latent HIV Infection. PLoS Pathog. 2015, 11, e1005201. [Google Scholar] [CrossRef]
- Sun, H.; Kim, D.; Li, X.; Kiselinova, M.; Ouyang, Z.; Vandekerckhove, L.; Shang, H.; Rosenberg, E.S.; Yu, X.G. Th1/17 Polarization of CD4 T Cells Supports HIV-1 Persistence during Antiretroviral Therapy. J. Virol. 2015, 89, 11284–11293. [Google Scholar] [CrossRef]
- Perreau, M.; Savoye, A.-L.; De Crignis, E.; Corpataux, J.-M.; Cubas, R.; Haddad, E.K.; De Leval, L.; Graziosi, C. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J. Exp. Med. 2012, 210, 143–156. [Google Scholar] [CrossRef]
- Wu, V.H.; Nordin, J.M.L.; Nguyen, S.; Joy, J.; Mampe, F.; del Rio Estrada, P.M.; Torres-Ruiz, F.; González-Navarro, M.; Luna-Villalobos, Y.A.; Ávila-Ríos, S.; et al. Profound phenotypic and epigenetic heterogeneity of the HIV-1-infected CD4+ T cell reservoir. Nat. Immunol. 2022, 24, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Alexaki, A.; Liu, Y. Cellular Reservoirs of HIV-1 and their Role in Viral Persistence. Curr. HIV Res. 2008, 6, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Veenhuis, R.T.; Abreu, C.M.; Costa, P.A.G.; Ferreira, E.A.; Ratliff, J.; Pohlenz, L.; Shirk, E.N.; Rubin, L.H.; Blankson, J.N.; Gama, L. Monocyte-derived macrophages contain persistent latent HIV reservoirs. Nat. Microbiol. 2023, 8, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Swingler, S.; Mann, A.M.; Zhou, J.; Swingler, C. Apoptotic Killing of HIV-1–Infected Macrophages Is Subverted by the Viral Envelope Glycoprotein. PLoS Pathog. 2007, 3, e134. [Google Scholar] [CrossRef]
- Vérollet, C.; Souriant, S.; Bonnaud, E.; Jolicoeur, P.; Raynaud-Messina, B.; Kinnaer, C.; Fourquaux, I.; Imle, A.; Benichou, S.; Fackler, O.T.; et al. HIV-1 reprograms the migration of macrophages. Blood 2015, 125, 1611–1622. [Google Scholar] [CrossRef]
- Ene, L.; Ruta, S.M. How much do antiretroviral drugs penetrate into the central nervous system? J. Med. Life 2011, 4, 432–439. [Google Scholar]
- Heaton, R.K.; Clifford, D.B.; Franklin, D.R.; Woods, S.P.; Ake, C.; Vaida, F.; Ellis, R.J.; Letendre, S.L.; Marcotte, T.D.; Atkinson, J.H.; et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010, 75, 2087–2096. [Google Scholar] [CrossRef]
- Ash, M.K.; Al-Harthi, L. HIV in the Brain: Identifying Viral Reservoirs and Addressing the Challenges of an HIV Cure. Vaccines 2021, 9, 867. [Google Scholar] [CrossRef]
- Lutgen, V.; Narasipura, S.D.; Barbian, H.J.; Richards, M.; Wallace, J.; Razmpour, R.; Buzhdygan, T.; Ramirez, S.H.; Prevedel, L.; Eugenin, E.A. HIV infects astrocytes in vivo and egresses from the brain to the periphery. PLoS Pathog. 2020, 16, e1008381. [Google Scholar] [CrossRef]
- Tang, Y.; Chaillon, A.; Gianella, S.; Wong, L.M.; Li, D.; Simermeyer, T.L.; Porrachia, M.; Ignacio, C.; Woodworth, B.; Zhong, D.; et al. Brain microglia serve as a persistent HIV reservoir despite durable antiretroviral therapy. J. Clin. Investig. 2023, 133, e167417. [Google Scholar] [CrossRef]
- Thompson, K.A.; Cherry, C.L.; Bell, J.E. Brain Cell Reservoirs of Latent Virus in Presymptomatic HIV-Infected Individuals. Am. J. Pathol. 2011, 179, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- King, J.E.; Eugenin, E.A.; Buckner, C.M. HIV tat and neurotoxicity. Microbes Infect. 2006, 8, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Mamik, M.K.; Hui, E.; Branton, W.G.; McKenzie, B.A.; Chisholm, J.; Cohen, E.A. HIV-1 Viral Protein R Activates NLRP3 Inflammasome in Microglia: Implications for HIV-1 Associated Neuroinflammation. J. Neuroimmune Pharmacol. 2016, 12, 233–248. [Google Scholar] [CrossRef]
- Dahmani, S.; Kaliss, N.; VanMeter, J.W.; Moore, D.J.; Ellis, R.J. Alterations of Brain Metabolites in Adults With HIV: A Systematic Meta-analysis of Magnetic Resonance Spectroscopy Studies. Neurology 2021, 97, e1085–e1096. [Google Scholar] [CrossRef]
- Alakkas, A.; Ellis, R.J.; Watson, C.W.-M.; Umlauf, A.; Heaton, R.K.; Letendre, S.; Collier, A.; Marra, C.; Clifford, D.B.; Gelman, B.; et al. White matter damage, neuroinflammation, and neuronal integrity in HAND. J. Neurovirology 2018, 25, 32–41. [Google Scholar] [CrossRef]
- Dahabieh, M.S.; Ooms, M.; Simon, V. A Doubly Fluorescent HIV-1 Reporter Shows that the Majority of Integrated HIV-1 Is Latent Shortly after Infection. J. Virol. 2013, 87, 4716–4727. [Google Scholar] [CrossRef]
- Swiggard, W.J.; Baytop, C.; Yu, J.J.; Dai, J.; Li, C.; Schretzenmair, R.; Theodosopoulos, T. Human Immunodeficiency Virus Type 1 Can Establish Latent Infection in Resting CD4+T Cells in the Absence of Activating Stimuli. J. Virol. 2005, 79, 14179–14188. [Google Scholar] [CrossRef]
- Rezaei, S.D.; Lu, H.K.; Chang, J.J.; Rhodes, A.; Lewin, S.R. The Pathway To Establishing HIV Latency Is Critical to How Latency Is Maintained and Reversed. J. Virol. 2018, 92, e02225-17. [Google Scholar] [CrossRef]
- Chavez, L.; Calvanese, V. HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T Cells. PLoS Pathog. 2015, 11, e1004955. [Google Scholar] [CrossRef]
- Meng, Y.; Zhong, J.; Lv, Y. Research progress on HIV-1 immune escape mechanisms. AIDS Rev. 2022, 24, 133–138. [Google Scholar] [CrossRef]
- Pasternak, A.O.; Berkhout, B. HIV persistence: Silence or resistance? Curr. Opin. Virol. 2023, 59, 101301. [Google Scholar] [CrossRef] [PubMed]
- Sarmati, L.; D’Ettorre, G.; Parisi, S. HIV Replication at Low Copy Number and its Correlation with the HIV Reservoir: A Clinical Perspective. Curr. HIV Res. 2015, 13, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Virgilio, M.C. The Impact of Cellular Proliferation on the HIV-1 Reservoir. Viruses 2020, 12, 127. [Google Scholar] [CrossRef]
- Chomont, N.; El-Far, M.; Ancuta, P.; Trautmann, L.; Procopio, F.A.; Yassine-Diab, B.; Boucher, G.; Boulassel, M.-R.; Ghattas, G.; Brenchley, J.M.; et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med. 2009, 15, 893–900. [Google Scholar] [CrossRef]
- Mendoza, P.; Jackson, J.R.; Oliveira, T.Y.; Gaebler, C.; Ramos, V.; Caskey, M.; Jankovic, M.; Nussenzweig, M.C. Antigen-responsive CD4+ T cell clones contribute to the HIV-1 latent reservoir. J. Exp. Med. 2020, 217, e20200051. [Google Scholar] [CrossRef]
- Maldarelli, F.; Wu, X.; Su, L.; Simonetti, F.R.; Shao, W.; Hill, S.; Spindler, J.; Ferris, A.L.; Mellors, J.W.; Kearney, M.F.; et al. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 2014, 345, 179–183. [Google Scholar] [CrossRef]
- Simonetti, F.R.; White, J.A.; Tumiotto, C.; Ritter, K.D.; Cai, M.; Gandhi, R.T.; Deeks, S.G.; Howell, B.J.; Montaner, L.J.; Blankson, J.N.; et al. Intact proviral DNA assay analysis of large cohorts of people with HIV provides a benchmark for the frequency and composition of persistent proviral DNA. Proc. Natl. Acad. Sci. USA 2020, 117, 18692–18700. [Google Scholar] [CrossRef]
- Li, J.Z.; Melberg, M.; Kittilson, A.; Abdel-Mohsen, M.; Li, Y.; Aga, E.; Bosch, R.J.; Wonderlich, E.R.; Kinslow, J.; Giron, L.B.; et al. Predictors of HIV rebound differ by timing of antiretroviral therapy initiation. JCI Insight 2024, 9, e173864. [Google Scholar] [CrossRef]
- Bruner, K.M.; Murray, A.J.; Pollack, R.A.; Soliman, M.G.; Laskey, S.B.; Capoferri, A.A.; Lai, J.; Strain, M.C.; Lada, S.M.; Hoh, R.; et al. Defective proviruses rapidly accumulate during acute HIV-1 infection. Nat. Med. 2016, 22, 1043–1049. [Google Scholar] [CrossRef]
- Baxter, A.E.; O’Doherty, U.; Kaufmann, D.E. Beyond the replication-competent HIV reservoir: Transcription and translation-competent reservoirs. Retrovirology 2018, 15, 18. [Google Scholar] [CrossRef]
- Imamichi, H.; Dewar, R.L.; Adelsberger, J.W.; Rehm, C.A.; O’Doherty, U.; Paxinos, E.E.; Fauci, A.S. Defective HIV-1 proviruses produce novel protein-coding RNA species in HIV-infected patients on combination antiretroviral therapy. Proc. Natl. Acad. Sci. USA 2016, 113, 8783–8788. [Google Scholar] [CrossRef] [PubMed]
- El-Far, M.; Halwani, R.; Said, E.; Trautmann, L.; Doroudchi, M.; Janbazian, L.; Fonseca, S.; van Grevenynghe, J.; Yassine-Diab, B.; Sékaly, R.-P. T-cell exhaustion in HIV infection. Curr. HIV/AIDS Rep. 2008, 5, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Avettand-Fènoël, V.; Hocqueloux, L.; Ghosn, J.; Cheret, A.; Frange, P.; Melard, A.; Viard, J.-P.; Rouzioux, C. Total HIV-1 DNA, a Marker of Viral Reservoir Dynamics with Clinical Implications. Clin. Microbiol. Rev. 2016, 29, 859–880. [Google Scholar] [CrossRef]
- Williams, J.P.; Hurst, J.; Stöhr, W.; Robinson, N.; Brown, H.; Fisher, M.; Kinloch, S.; Cooper, D.; Schechter, M.; Tambussi, G.; et al. HIV-1 DNA predicts disease progression and post-treatment virological control. eLife 2014, 3, e03821. [Google Scholar] [CrossRef]
- Goujard, C.; Bonarek, M.; Meyer, L.; Bonnet, F.; Chaix, M.-L.; Deveau, C.; Sinet, M.; Galimand, J.; Delfraissy, J.-F.; Venet, A.; et al. CD4 Cell Count and HIV DNA Level Are Independent Predictors of Disease Progression after Primary HIV Type 1 Infection in Untreated Patients. Clin. Infect. Dis. 2006, 42, 709–715. [Google Scholar] [CrossRef]
- Rouzioux, C.; Hubert, J.; Burgard, M.; Deveau, C.; Goujard, C.; Bary, M.; Séréni, D.; Viard, J.; Delfraissy, J. Early Levels of HIV-1 DNA in Peripheral Blood Mononuclear Cells Are Predictive of Disease Progression Independently of HIV-1 RNA Levels and CD4+T Cell Counts. J. Infect. Dis. 2005, 192, 46–55. [Google Scholar] [CrossRef]
- Tsiara, C.G.; Nikolopoulos, G.K.; Bagos, P.G.; Goujard, C.; Katzenstein, T.L.; Minga, A.K.; Rouzioux, C. Impact of HIV Type 1 DNA Levels on Spontaneous Disease Progression: A Meta-Analysis. AIDS Res. Hum. Retroviruses 2012, 28, 366–373. [Google Scholar] [CrossRef]
- Ananworanich, J.; Schuetz, A.; Vandergeeten, C.; Sereti, I.; de Souza, M.; Rerknimitr, R.; Dewar, R.; Marovich, M.; van Griensven, F.; Sekaly, R.; et al. Impact of Multi-Targeted Antiretroviral Treatment on Gut T Cell Depletion and HIV Reservoir Seeding during Acute HIV Infection. PLoS ONE 2012, 7, e33948. [Google Scholar] [CrossRef]
- Hoen, B.; Cooper, D.A.; Lampe, F.C.; Perrin, L.; Clumeck, N.; Phillips, A.N.; Goh, L.-E.; Lindback, S.; Sereni, D.; Gazzard, B.; et al. Predictors of Virological Outcome and Safety in Primary HIV Type 1-Infected Patients Initiating Quadruple Antiretroviral Therapy: QUEST GW PROB3005. Clin. Infect. Dis. 2007, 45, 381–390. [Google Scholar] [CrossRef]
- Koelsch, K.; Liu, L.; Haubrich, R.; May, S.; Havlir, D.; Günthard, H.; Ignacio, C.; Campos-Soto, P.; Little, S.; Shafer, R.; et al. Dynamics of Total, Linear Nonintegrated, and Integrated HIV-1 DNA In Vivo and In Vitro. J. Infect. Dis. 2008, 197, 411–419. [Google Scholar] [CrossRef]
- Leyre, L.; Kroon, E.; Vandergeeten, C.; Sacdalan, C.; Colby, D.J.; Buranapraditkun, S.; Schuetz, A.; Chomchey, N.; de Souza, M.; Bakeman, W.; et al. Abundant HIV-infected cells in blood and tissues are rapidly cleared upon ART initiation during acute HIV infection. Sci. Transl. Med. 2020, 12, eaav3491. [Google Scholar] [CrossRef] [PubMed]
- Leite, T.F.; Delatorre, E.; Côrtes, F.H.; Ferreira, A.C.G.; Cardoso, S.W.; Grinsztejn, B.; de Andrade, M.M.; Veloso, V.G.; Morgado, M.G. Reduction of HIV-1 Reservoir Size and Diversity After 1 Year of cART Among Brazilian Individuals Starting Treatment During Early Stages of Acute Infection. Front. Microbiol. 2019, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Groot, F.; van Capel, T.M.M.; Schuitemaker, J.H.N.; de Jong, E.C. Differential susceptibility of naïve, central memory and effector memory T cells to dendritic cell-mediated HIV-1 transmission. Retrovirology 2006, 3, 52. [Google Scholar] [CrossRef]
- Peluso, M.J.; Bacchetti, P.; Ritter, K.D.; Beg, S.; Lai, J.; Martin, J.N.; Hunt, P.W.; Henrich, T.J.; Siliciano, J.D.; Siliciano, R.F.; et al. Differential decay of intact and defective proviral DNA in HIV-1–infected individuals on suppressive antiretroviral therapy. JCI Insight 2020, 5, e132997. [Google Scholar] [CrossRef]
- Gottlieb, G.S.; Raugi, D.N.; Smith, R.A. 90-90-90 for HIV-2? Ending the HIV-2 epidemic by enhancing care and clinical management of patients infected with HIV-2. Lancet HIV 2018, 5, e390–e399. [Google Scholar] [CrossRef]
- Ren, J.; Bird, L.E.; Chamberlain, P.P.; Stewart-Jones, G.B.; Stuart, D.I.; Stammers, D.K. Structure of HIV-2 reverse transcriptase at 2.35-A resolution and the mechanism of resistance to non-nucleoside inhibitors. Proc. Natl. Acad. Sci. USA 2002, 99, 14410–14415. [Google Scholar] [CrossRef]
- Poveda, E.; Rodes, B.; Toro, C.; Soriano, V. Are fusion inhibitors active against all HIV variants? AIDS Res. Hum. Retroviruses 2004, 20, 347–348. [Google Scholar] [CrossRef]
- Martinez-Steele, E.; Awasana, A.A.; Corrah, T.; Sabally, S.; van der Sande, M.; Jaye, A.; Togun, T.; Sarge-Njie, R.; McConkey, S.J.; Whittle, H.; et al. Is HIV-2 induced AIDS different from HIV-1-associated AIDS? Data from a West African clinic. AIDS 2007, 21, 317–324. [Google Scholar] [CrossRef]
- Andersson, S.; Norrgren, H.; da Silva, Z.; Biague, A.; Bamba, S.; Kwok, S.; Christopherson, C.; Biberfeld, G.; Albert, J. Plasma viral load in HIV-1 and HIV-2 singly and dually infected individuals in Guinea-Bissau, west Africa: Significantly lower plasma virus set point in HIV-2 infection than in HIV-1 infection. Arch. Intern. Med. 2000, 160, 3286–3293. [Google Scholar] [CrossRef]
- Koofhethile, C.K.; Gao, C.; Chang, C.; Lian, X.; Shapiro, R.; Yu, X.G.; Lichterfeld, M.; Kanki, P.J. The HIV-2 proviral landscape is dominated by defective proviruses. AIDS 2024, 38, 309–316. [Google Scholar] [CrossRef]
- Godinho-Santos, A.; Foxall, R.B.; Antão, A.V.; Tavares, B.; Ferreira, T.; Serra-Caetano, A.; Matoso, P.; Sousa, A.E. Follicular Helper T Cells Are Major Human Immunodeficiency Virus-2 Reservoirs and Support Productive Infection. J. Infect. Dis. 2020, 221, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.S.; Sobieszczyk, M.E.; McCutchan, F.E. The Challenge of HIV-1 Subtype Diversity. N. Engl. J. Med. 2008, 358, 1590–1602. [Google Scholar] [CrossRef] [PubMed]
- Kanki, P.; Hamel, D.; Sankalé, J.; Hsieh, C.; Thior, I.; Barin, F.; Woodcock, S.; Guèye-Ndiaye, A.; Zhang, E.; Montano, M.; et al. Human Immunodeficiency Virus Type 1 Subtypes Differ in Disease Progression. J. Infect. Dis. 1999, 179, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Amornkul, P.N.; Karita, E.; Kamali, A.; Rida, W.N.; Sanders, E.J.; Lakhi, S.; Price, M.A.; Kilembe, W.; Cormier, E.; Anzala, O.; et al. Disease progression by infecting HIV-1 subtype in a seroconverter cohort in sub-Saharan Africa. AIDS 2013, 27, 2775–2786. [Google Scholar] [CrossRef]
- Ssemwanga, D.; Nsubuga, R.N.; Mayanja, B.N.; Lyagoba, F.; Magambo, B.; Yirrell, D.; Van der Paal, L.; Grosskurth, H. Effect of HIV-1 Subtypes on Disease Progression in Rural Uganda: A Prospective Clinical Cohort Study. PLoS ONE 2013, 8, e71768. [Google Scholar] [CrossRef]
- Omondi, F.H.; Chandrarathna, S.; Mujib, S.; Brumme, C.J.; Jin, S.W.; Sudderuddin, H.; Miller, R.L.; Rahimi, A.; Laeyendecker, O.; Bonner, P.; et al. HIV Subtype and Nef-Mediated Immune Evasion Function Correlate with Viral Reservoir Size in Early-Treated Individuals. J. Virol. 2019, 93, e01832-18. [Google Scholar] [CrossRef]
- Bachmann, N.; von Siebenthal, C.; Vongrad, V.; Turk, T.; Neumann, K.; Beerenwinkel, N.; Bogojeska, J.; Fellay, J.; Roth, V.; Kok, Y.L.; et al. Determinants of HIV-1 reservoir size and long-term dynamics during suppressive ART. Nat. Commun. 2019, 10, 3193. [Google Scholar] [CrossRef]
- Jeeninga, R.E.; Hoogenkamp, M.; Armand-Ugon, M.; de Baar, M.; Verhoef, K. Functional Differences between the Long Terminal Repeat Transcriptional Promoters of Human Immunodeficiency Virus Type 1 Subtypes A through G. J. Virol. 2000, 74, 3740–3751. [Google Scholar] [CrossRef]
- Mann, J.K.; Byakwaga, H.; Kuang, X.T.; Le, A.Q.; Brumme, C.J.; Mwimanzi, P.; Omarjee, S.; Martin, E.; Lee, G.Q.; Baraki, B.; et al. Ability of HIV-1 Nef to downregulate CD4 and HLA class I differs among viral subtypes. Retrovirology 2013, 10, 100. [Google Scholar] [CrossRef]
- Lisovsky, I.; Schader, S.M.; Sloan, R.D.; Oliveira, M.; Coutsinos, D.; Bernard, N.F. HIV-1 Subtype Variability in Vif Derived from Molecular Clones Affects APOBEC3G-Mediated Host Restriction. Intervirology 2013, 56, 258–264. [Google Scholar] [CrossRef]
- Langer, S.; Hammer, C.; Hopfensperger, K.; Klein, L.; Hotter, D.; De Jesus, P.D.; Herbert, K.M.; Pache, L.; Smith, N.; van der Merwe, J.A.; et al. HIV-1 Vpu is a potent transcriptional suppressor of NF-κB-elicited antiviral immune responses. eLife 2019, 8, e41930. [Google Scholar] [CrossRef] [PubMed]
- Slyker, J.A.; Guthrie, B.; Pankau, M.; Tapia, K.; Wamalwa, D.; Benki-Nugent, S.; Ngugi, E.; Huang, M.L.; Njuguna, I.; Langat, A.; et al. Association Between Cytomegalovirus and Epstein-Barr Virus Viremia And Human Immunodeficiency Virus DNA Levels in the Reservoir of Kenyan Infants Receiving Antiretroviral Therapy. J. Infect. Dis. 2021, 223, 1923–1927. [Google Scholar] [CrossRef] [PubMed]
- Platt, L.; Easterbrook, P.; Gower, E.; McDonald, B.; Sabin, K.; McGowan, C.; Yanny, I.; Razavi, H.; Vickerman, P. Prevalence and burden of HCV co-infection in people living with HIV: A global systematic review and meta-analysis. Lancet Infect Dis. 2016, 16, 797–808. [Google Scholar] [CrossRef]
- López-Huertas, M.R.; Palladino, C.; Garrido-Arquero, M.; Esteban-Cartelle, B.; Sánchez-Carrillo, M.; Martínez-Román, P.; Martín-Carbonero, L.; Ryan, P.; Domínguez-Domínguez, L.; Santos, I.L.; et al. HCV-coinfection is related to an increased HIV-1 reservoir size in cART-treated HIV patients: A cross-sectional study. Sci. Rep. 2019, 9, 5606. [Google Scholar] [CrossRef]
- López-Cortés, L.F.; Trujillo-Rodríguez, M.; Báez-Palomo, A.; Benmarzouk-Hidalgo, O.J.; Dominguez-Molina, B.; Milanés-Guisado, Y.; Espinosa, N.; Viciana, P.; Gutiérrez-Valencia, A. Eradication of Hepatitis C Virus (HCV) Reduces Immune Activation, Microbial Translocation, and the HIV DNA Level in HIV/HCV-Coinfected Patients. J. Infect. Dis. 2018, 218, 624–632. [Google Scholar] [CrossRef]
- Martínez-Román, P.; Crespo-Bermejo, C.; Valle-Millares, D.; Lara-Aguilar, V.; Arca-Lafuente, S.; Martín-Carbonero, L.; Ryan, P.; de Los Santos, I.; López-Huertas, M.R.; Palladino, C.; et al. Dynamics of HIV Reservoir and HIV-1 Viral Splicing in HCV-Exposed Individuals after Elimination with DAAs or Spontaneous Clearance. J. Clin. Med. 2022, 11, 3579. [Google Scholar] [CrossRef]
- Álvarez, B.; Navarrete-Muñoz, M.A.; Briz, V.; Olmedillas-López, S.; Nistal, S.; Cabello, A.; Prieto, L.; Górgolas, M.; García-Arranz, M.; Benito, J.M.; et al. HIV-reservoir size is not affected either by HCV coinfection or by direct acting antivirals (DAAs) therapy. Sci. Rep. 2022, 12, 5095. [Google Scholar] [CrossRef]
- Getahun, H.; Gunneberg, C.; Granich, R.; Nunn, P. HIV infection-associated tuberculosis: The epidemiology and the response. Clin. Infect. Dis. 2010, 50, 201–207. [Google Scholar] [CrossRef]
- Xun, J.; Qi, T.; Zou, L.; Tang, Q.; Shen, Y.; Yang, J.; Xie, L.; Ji, Y.; Zhang, R.; Liu, L.; et al. Mycobacterium tuberculosis co-infection is associated with increased surrogate marker of the HIV reservoir. AIDS Res. Ther. 2020, 17, 63. [Google Scholar] [CrossRef]
- Naidoo, K.; Rampersad, S.; Karim, S.A. Improving survival with tuberculosis & HIV treatment integration: A mini-review. Indian J. Med. Res. 2019, 150, 131–138. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, H.; Wu, Y.; Dou, Z.; Zhang, Y.; Kleinman, N.; Bulterys, M.; Wu, Z.; Ma, Y.; Zhao, D.; et al. HIV; hepatitis B virus, and hepatitis C virus co-infection in patients in the China National Free Antiretroviral Treatment Program, 2010–2012: A retrospective observational cohort study. Lancet Infect. Dis. 2014, 14, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Deeks, S.G. Human Immunodeficiency Virus Controllers: Mechanisms of Durable Virus Control in the Absence of Antiretroviral Therapy. Immunity 2007, 27, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, B.D.; Blazkova, J.; Justement, J.S.; Shi, V.; Rai, M.A.; Manning, M.R.; Praiss, L.; Gittens, K.; Wender, P.A.; Patro, S.; et al. Comprehensive analysis of HIV reservoirs in elite controllers. J. Clin. Investig. 2023, 133, e165446. [Google Scholar] [CrossRef]
- Kwaa, A.K.R.; Garliss, C.C.; Ritter, K.D.; Laird, G.M. Elite suppressors have low frequencies of intact HIV-1 proviral DNA. AIDS 2020, 34, 641–643. [Google Scholar] [CrossRef]
- Sáez-Cirión, A.; Bacchus, C.; Hocqueloux, L.; Avettand-Fenoel, V.; Girault, I.; Lecuroux, C.; Potard, V.; Versmisse, P.; Melard, A.; Prazuck, T.; et al. Post-Treatment HIV-1 Controllers with a Long-Term Virological Remission after the Interruption of Early Initiated Antiretroviral Therapy ANRS VISCONTI Study. PLoS Pathog. 2013, 9, e1003211. [Google Scholar] [CrossRef]
- Gálvez, C.; Urrea, V.; Garcia-Guerrero, M.D.C.; Bernal, S.; Benet, S.; Mothe, B.; Bailón, L.; Dalmau, J.; Martinez, A.; Nieto, A.; et al. Altered T-cell subset distribution in the viral reservoir in HIV-1-infected individuals with extremely low proviral DNA (LoViReTs). J. Intern. Med. 2022, 292, 308–320. [Google Scholar] [CrossRef]
- Bruner, K.M.; Wang, Z.; Simonetti, F.R.; Bender, A.M.; Kwon, K.J.; Sengupta, S.; Fray, E.J.; Beg, S.A.; Antar, A.A.R.; Jenike, K.M.; et al. A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature 2019, 566, 120–125. [Google Scholar] [CrossRef]
- Delporte, M.; van Snippenberg, W.; Blomme, E.E.; Rutsaert, S.; Verschoore, M.; De Smet, E.; De Scheerder, M.-A.; Gerlo, S.; Vandekerckhove, L. Integrative assessment of total and intact HIV-1 reservoir by a five-region multiplexed Rainbow digital PCR assay. bioRxiv 2023, 553846. [Google Scholar] [CrossRef]
- Rutsaert, S.; Bosman, K.; Trypsteen, W.; Nijhuis, M. Digital PCR as a tool to measure HIV persistence. Retrovirology 2018, 15, 16. [Google Scholar] [CrossRef]
- Bosman, K.J.; Nijhuis, M.; van Ham, P.M.; Wensing, A.M.J.; Vervisch, K.; De Spiegelaere, W. Comparison of digital PCR platforms and semi-nested qPCR as a tool to determine the size of the HIV reservoir. Sci. Rep. 2015, 5, 1381. [Google Scholar] [CrossRef]
- Einkauf, K.B.; Lee, G.Q.; Gao, C.; Sharaf, R.; Sun, X.; Hua, S.; Chen, S.M.Y.; Jiang, C.; Lian, X.; Chowdhury, F.Z.; et al. Intact HIV-1 proviruses accumulate at distinct chromosomal positions during prolonged antiretroviral therapy. J. Clin. Investig. 2019, 129, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Enick, P.N.; Brooker, J.P.; Tumiotto, C.M.; Staines, B.T.; Eron, J.J.; McMahon, D.K.; Gandhi, R.T.; Mellors, J.W. Comparison of methods to quantify inducible HIV-1 outgrowth. J. Virus Erad. 2021, 7, 100043. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.A.; McLaughlin, S.; Garg, K.; Cheung, C.Y.; Larsen, B.B.; Styrchak, S.; Huang, H.C.; Edlefsen, P.T.; Mullins, J.I.; Frenkel, L.M. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science 2014, 345, 570–573. [Google Scholar] [CrossRef]
- Paruzynski, A.; Arens, A.; Gabriel, R.; Bartholomae, C.C.; Scholz, S.; Wang, W.; Wolf, S.; Glimm, H.; Schmidt, M.; von Kalle, C. Genome-wide high-throughput integrome analyses by nrLAM-PCR and next-generation sequencing. Nat. Protoc. 2010, 5, 1379–1395. [Google Scholar] [CrossRef]
- Gandhi, R.T.; Landovitz, R.J.; Sax, P.E.; Smith, D.M.; Springer, S.A.; Günthard, H.F.; Thompson, M.A.; Bedimo, R.J.; Benson, C.A.; Buchbinder, S.P.; et al. Antiretroviral Drugs for Treatment and Prevention of HIV in Adults: 2024 Recommendations of the International Antiviral Society-USA Panel. JAMA 2024. [Google Scholar] [CrossRef]
- Markowitz, M.; Evering, T.H.; Garmon, D.; Caskey, M.; La Mar, M.; Rodriguez, K.; Sahi, V.; Palmer, S.; Prada, N. A Randomized Open-Label Study of 3- Versus 5-Drug Combination Antiretroviral Therapy in Newly HIV-1–Infected Individuals. J. Acquir. Immune Defic. Syndr. 2014, 66, 140–147. [Google Scholar] [CrossRef]
- Lombardi, F.; Belmonti, S.; Borghetti, A.; Fabbiani, M.; Marchetti, S.; Tamburrini, E.; Cauda, R.; di Giambenedetto, S. Evolution of cellular HIV DNA levels in virologically suppressed patients switching to dolutegravir/lamivudine versus maintaining a triple regimen: A prospective, longitudinal, matched, controlled study. J. Antimicrob. Chemother. 2020, 75, 1599–1603. [Google Scholar] [CrossRef]
- Salgado, M.; Gálvez, C.; Nijhuis, M.; Kwon, M.; Cardozo-Ojeda, E.F.; Badiola, J.; Gorman, M.J.; Huyveneers, L.E.P.; Urrea, V.; Bandera, A.; et al. Dynamics of virological and immunological markers of HIV persistence after allogeneic haematopoietic stem-cell transplantation in the IciStem cohort: A prospective observational cohort study. Lancet HIV 2024, 11, e389–e405. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, N.; Berkhout, B. CRISPR-Cas based antiviral strategies against HIV-1. Virus Res. 2018, 244, 321–332. [Google Scholar] [CrossRef]
- Kim, Y.; Anderson, J.L.; Lewin, S.R. Getting the “Kill” into “Shock and Kill”: Strategies to Eliminate Latent HIV. Cell Host Microbe 2018, 23, 14–26. [Google Scholar] [CrossRef]
- Vansant, G.; Bruggemans, A.; Janssens, J. Block-And-Lock Strategies to Cure HIV Infection. Viruses 2020, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Emu, B.; Fessel, J.; Schrader, S.; Kumar, P.; Richmond, G.; Win, S.; Weinheimer, S.; Marsolais, C.; Lewis, S. Phase 3 Study of Ibalizumab for Multidrug-Resistant HIV-1. N. Engl. J. Med. 2018, 379, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Dubé, K.; Kanazawa, J.; Dee, L.; Taylor, J.; Sauceda, J.A.; Gianella, S.; Smith, D.; Deeks, S.G.; Peluso, M.J. Considerations for designing and implementing combination HIV cure trials: Findings from a qualitative in-depth interview study in the United States. AIDS Res. Ther. 2021, 18, 75. [Google Scholar] [CrossRef]
- Hiner, C.R.; Mueller, A.L.; Su, H.; Goldstein, H. Interventions during Early Infection: Opening a Window for an HIV Cure? Viruses 2024, 16, 1588. [Google Scholar] [CrossRef]
- Barrangou, R. CRISPR-Cas Systems: Prokaryotes Upgrade to Adaptive Immunity. Mol. Cell 2014, 54, 234–244. [Google Scholar] [CrossRef]
- Fan, M.; Berkhout, B. A combinatorial CRISPR-Cas12a attack on HIV DNA. Mol. Ther. Methods Clin. Dev. 2022, 25, 43–51. [Google Scholar] [CrossRef]
- Nguyen, H.; Wilson, H.; Jayakumar, S.; Kulkarni, V. Efficient Inhibition of HIV Using CRISPR/Cas13d Nuclease System. Viruses 2021, 13, 1850. [Google Scholar] [CrossRef]
- Zhu, W.; Lei, R.; Le Duff, Y.; Li, J.; Guo, F.; Wainberg, M.A. The CRISPR/Cas9 system inactivates latent HIV-1 proviral DNA. Retrovirology 2015, 12, 22. [Google Scholar] [CrossRef]
- Gao, Z.; Fan, M.; Das, A.T.; Herrera-Carrillo, E. Extinction of all infectious HIV in cell culture by the CRISPR-Cas12a system with only a single crRNA. Nucleic Acids Res. 2020, 48, 5527–5539. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, N.; Berkhout, B. A Combinatorial CRISPR-Cas9 Attack on HIV-1 DNA Extinguishes All Infectious Provirus in Infected T Cell Cultures. Cell Rep. 2016, 17, 2819–2826. [Google Scholar] [CrossRef]
- Ophinni, Y.; Inoue, M.; Kotaki, T. CRISPR/Cas9 system targeting regulatory genes of HIV-1 inhibits viral replication in infected T-cell cultures. Sci. Rep. 2018, 8, 7784. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Guo, D. Application of CRISPR/Cas9-Based Gene Editing in HIV-1/AIDS Therapy. Front. Cell Infect. Microbiol. 2019, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Pan, Q.; Gendron, P.; Zhu, W.; Guo, F.; Cen, S.; Wainberg, M. CRISPR/Cas9-Derived Mutations Both Inhibit HIV-1 Replication and Accelerate Viral Escape. Cell. Rep. 2016, 15, 481–489. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, N.; Berkhout, B. CRISPR-Cas9 Can Inhibit HIV-1 Replication but NHEJ Repair Facilitates Virus Escape. Mol. Ther. 2016, 24, 522–526. [Google Scholar] [CrossRef]
- Lattanzi, A.; Meneghini, V.; Pavani, G.; Amor, F.; Ramadier, S.; Felix, T.; Antoniani, C.; Masson, C.; Alibeu, O.; Lee, C.; et al. Optimization of CRISPR/Cas9 delivery to human hematopoietic stem and progenitor cells for therapeutic genomic rearrangements. Mol. Ther. 2019, 27, 137–150. [Google Scholar] [CrossRef]
- Han, X.; Liu, Z.; Ma, Y.; Zhang, K.; Qin, L. Cas9 ribonucleoprotein delivery via microfluidic cell-deformation chip for human T-cell genome editing and immunotherapy. Adv. Biosyst. 2017, 1, e1600007. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, P.; Feng, Q.; Wang, N.; Chen, Z.; Huang, Y.; Zheng, W.; Jiang, X. Lipid nanoparticle-mediated efficient delivery of CRISPR/Cas9 for tumor therapy. NPG Asia Mater. 2017, 9, e441. [Google Scholar] [CrossRef]
- Ju, E.; Li, T.; Ramos da Silva, S.; Gao, S.-J. Gold nanocluster-mediated efficient delivery of Cas9 protein through pH-induced assembly-disassembly for inactivation of virus oncogenes. ACS Appl. Mater. Interfaces 2019, 11, 34717–34724. [Google Scholar] [CrossRef]
- Zhang, S.; Shen, J.; Li, D.; Cheng, Y. Strategies in the delivery of Cas9 ribonucleoprotein for CRISPR/Cas9 genome editing. Theranostics 2021, 11, 614–648. [Google Scholar] [CrossRef]
- Liang, Z.; Chen, K.; Li, T.; Zhang, Y.; Wang, Y.; Zhao, Q.; Liu, J.; Zhang, H.; Liu, C.; Ran, Y.; et al. Efficient DNA-free genome editing of bread wheat using CRISPR/Cas9 ribonucleoprotein complexes. Nat. Commun. 2017, 8, 14261. [Google Scholar] [CrossRef]
- Zuris, J.A.; Thompson, D.B.; Shu, Y.; Guilinger, J.P.; Bessen, J.L.; Hu, J.H.; Maeder, M.L.; Joung, J.K.; Chen, Z.Y.; Liu, D.R. Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat. Biotechnol. 2015, 33, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Deeks, S.G. Shock and kill. Nature 2012, 487, 439–440. [Google Scholar] [CrossRef] [PubMed]
- Archin, N.M.; Kirchherr, J.L.; Sung, J.A.M.; Clutton, G.; Sholtis, K.; Xu, Y.; Allard, B.; Stuelke, E.; Kashuba, A.D.; Kuruc, J.D.; et al. Interval dosing with the HDAC inhibitor vorinostat effectively reverses HIV latency. J. Clin. Investig. 2017, 127, 3126–3135. [Google Scholar] [CrossRef]
- Elliott, J.H.; Wightman, F.; Solomon, A.; Ghneim, K.; Ahlers, J.; Cameron, M.J.; Smith, M.Z.; Spelman, T.; McMahon, J.; Velayudham, P.; et al. Activation of HIV Transcription with Short-Course Vorinostat in HIV-Infected Patients on Suppressive Antiretroviral Therapy. PLoS Pathog. 2014, 10, e1004473. [Google Scholar] [CrossRef]
- Bouchat, S.; Gatot, J.-S.; Kabeya, K.; Cardona, C.; Colin, L.; Herbein, G.; De Wit, S.; Clumeck, N.; Lambotte, O.; Rouzioux, C.; et al. Histone methyltransferase inhibitors induce HIV-1 recovery in resting CD4+ T cells from HIV-1-infected HAART-treated patients. AIDS 2012, 26, 1473–1482. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Z.; Reszka-Blanco, N.; Li, F.; Chi, L.; Ma, J.; Jeffrey, J.; Cheng, L. Specific ActivationIn Vivoof HIV-1 by a Bromodomain Inhibitor from Monocytic Cells in Humanized Mice under Antiretroviral Therapy. J. Virol. 2019, 93, e00233-19. [Google Scholar] [CrossRef]
- Gutiérrez, C.; Serrano-Villar, S.; Madrid-Elena, N.; Pérez-Elías, M.J.; Martín, M.E.; Barbas, C.; Ruipérez, J.; Muñoz, E.; Muñoz-Fernández, M.A.; Castor, T. Bryostatin-1 for latent virus reactivation in HIV-infected patients on antiretroviral therapy. AIDS 2019, 30, 1385–1392. [Google Scholar] [CrossRef]
- Vibholm, L.; Schleimann, M.H.; Højen, J.F.; Benfield, T.; Offersen, R.; Rasmussen, K.; Olesen, R.; Dige, A.; Agnholt, J.; Grau, J.; et al. Short-Course Toll-Like Receptor 9 Agonist Treatment Impacts Innate Immunity and Plasma Viremia in Individuals With Human Immunodeficiency Virus Infection. Clin. Infect. Dis. 2017, 64, 1686–1695. [Google Scholar] [CrossRef]
- Jones, R.B.; Mueller, S.; O’Connor, R.; Rimpel, K.; Sloan, D.D.; Karel, D.; Wong, H.C.; Jeng, E.K.; Thomas, A.S.; Whitney, J.B.; et al. A Subset of Latency-Reversing Agents Expose HIV-Infected Resting CD4+ T-Cells to Recognition by Cytotoxic T-Lymphocytes. PLoS Pathog. 2016, 15, e1005545. [Google Scholar] [CrossRef]
- Bullen, C.K.; Laird, G.M.; Durand, C.M.; Siliciano, J.D.; Siliciano, R.F. New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nat. Med. 2014, 20, 425–429. [Google Scholar] [CrossRef]
- Cillo, A.R.; Sobolewski, M.D.; Bosch, R.J.; Fyne, E.; Piatak MJr Coffin, J.M.; Mellors, J.W. Quantification of HIV-1 latency reversal in resting CD4+ T cells from patients on suppressive antiretroviral therapy. Proc. Natl. Acad. Sci. USA 2014, 111, 7078–8083. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Wu, F.; McMyn, N.; Song, B.; Walker-Sperling, V.E.; Varriale, J.; Zhang, H.; Barouch, D.H.; Siliciano, J.D.; Li, W.; et al. Genome-wide CRISPR screens identify combinations of candidate latency reversing agents for targeting the latent HIV-1 reservoir. Sci. Transl. Med. 2022, 14, eabh3351. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Deng, K.; Shroff, N.; Durand, C.; Rabi, S.; Yang, H.-C.; Zhang, H.; Margolick, J.; Blankson, J. Stimulation of HIV-1-Specific Cytolytic T Lymphocytes Facilitates Elimination of Latent Viral Reservoir after Virus Reactivation. Immunity 2012, 36, 491–501. [Google Scholar] [CrossRef]
- Chandrasekar, A.P.; Cummins, N.W.; Natesampillai, S.; Misra, A.; Alto, A.; Laird, G. The BCL-2 Inhibitor Venetoclax Augments Immune Effector Function Mediated by Fas Ligand, TRAIL, and Perforin/Granzyme B, Resulting in Reduced Plasma Viremia and Decreased HIV Reservoir Size during Acute HIV Infection in a Humanized Mouse Model. J. Virol. 2022, 96, e0173022. [Google Scholar] [CrossRef]
- Chugh, P.; Bradel-Tretheway, B.; Monteiro-Filho, C.M.R.; Planelles, V.; Maggirwar, S.B.; Dewhurst, S. Akt inhibitors as an HIV-1 infected macrophage-specific anti-viral therapy. Retrovirology 2008, 5, 11. [Google Scholar] [CrossRef]
- Campbell, G.R. DIABLO/SMAC mimetics selectively kill HIV-1-infected resting memory CD4+ T cells: A potential role in a cure strategy for HIV-1 infection. Autophagy 2019, 15, 744–746. [Google Scholar] [CrossRef]
- Li, P.; Kaiser, P.; Lampiris, H.W.; Kim, P.; Yukl, S.A.; Havlir, D.V.; Greene, W.C. Stimulating the RIG-I pathway to kill cells in the latent HIV reservoir following viral reactivation. Nat. Med. 2016, 22, 807–811. [Google Scholar] [CrossRef]
- Darcis, G.; Van Driessche, B.; Van Lint, C. HIV Latency: Should We Shock or Lock? Trends Immunol. 2017, 38, 217–228. [Google Scholar] [CrossRef]
- Kessing, C.F.; Nixon, C.C.; Li, C.; Tsai, P.; Takata, H.; Mousseau, G.; Ho, P.T.; Honeycutt, J.B.; Fallahi, M.; Trautmann, L.; et al. In Vivo Suppression of HIV Rebound by Didehydro-Cortistatin A, a “Block-and-Lock” Strategy for HIV-1 Treatment. Cell. Rep. 2017, 21, 600–611. [Google Scholar] [CrossRef]
- Bruggemans, A.; Vansant, G.; Balakrishnan, M.; Mitchell, M.L.; Cai, R.; Christ, F.; Debyser, Z. GS-9822, a Preclinical LEDGIN Candidate, Displays a Block-and-Lock Phenotype in Cell Culture. Antimicrob. Agents Chemother. 2021, 65, e02328-20. [Google Scholar] [CrossRef]
- Jean, M.J.; Hayashi, T.; Huang, H.; Brennan, J.; Simpson, S.; Purmal, A.; Gurova, K.; Keefer, M.C.; Kobie, J.J.; Santoso, N.G.; et al. Curaxin CBL0100 Blocks HIV-1 Replication and Reactivation through Inhibition of Viral Transcriptional Elongation. Front. Microbiol. 2017, 8, 2007. [Google Scholar] [CrossRef] [PubMed]
- Anderson, I.; Low, J.S.; Weston, S.; Weinberger, M.; Zhyvoloup, A.; Labokha, A.A.; Corazza, G.; Kitson, R.A.; Moody, C.J.; Marcello, A. Heat shock protein 90 controls HIV-1 reactivation from latency. Proc. Natl. Acad. Sci. USA 2014, 111, E1528–E1537. [Google Scholar] [CrossRef] [PubMed]
- Gavegnano, C.; Brehm, J.H.; Dupuy, F.P.; Talla, A.; Ribeiro, S.P.; Kulpa, D.A.; Cameron, C.; Santos, S.; Hurwitz, S.J.; Marconi, V.C.; et al. Novel mechanisms to inhibit HIV reservoir seeding using Jak inhibitors. PLoS Pathog. 2017, 13, e1006740. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, R.L.; Ajibola, G.; Maswabi, K.; Hughes, M.; Nelson, B.S.; Niesar, A.; Pretorius Holme, M.; Powis, K.M.; Sakoi, M.; Batlang, O.; et al. Broadly neutralizing antibody treatment maintained HIV suppression in children with favorable reservoir characteristics in Botswana. Sci. Transl. Med. 2023, 15, eadh0004. [Google Scholar] [CrossRef]
- Ananworanich, J.; McSteen, B.; Robb, M.L. Broadly neutralizing antibody and the HIV reservoir in acute HIV infection: A strategy toward HIV remission? Curr. Opin. HIV AIDS 2015, 10, 198–206. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, W.; Sun, M.; Li, T. Broadly neutralizing antibodies for HIV-1: Efficacies, challenges and opportunities. Emerg. Microbes Infect. 2020, 9, 194–206. [Google Scholar] [CrossRef]
- Chun, T.W.; Murray, D.; Justement, J.S.; Blazkova, J.; Hallahan, C.W.; Fankuchen, O.; Gittens, K.; Benko, E.; Kovacs, C.; Moir, S.; et al. Broadly neutralizing antibodies suppress HIV in the persistent viral reservoir. Proc. Natl. Acad. Sci. USA 2014, 111, 13151–13156. [Google Scholar] [CrossRef]
- Bruel, T.; Guivel-Benhassine, F.; Amraoui, S.; Malbec, M.; Richard, L.; Bourdic, K.; Donahue, D.A.; Lorin, V.; Casartelli, N.; Noël, N.; et al. Elimination of HIV-1-infected cells by broadly neutralizing antibodies. Nat. Commun. 2016, 7, 10844. [Google Scholar] [CrossRef]
- Research Toward a Cure Trials—Treatment Action Group. Available online: https://www.treatmentactiongroup.org/cure/trials/ (accessed on 23 October 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).