Molecular Confirmation, Epidemiology, and Pathophysiology of Ehrlichia canis Prevalence in Eastern India
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Area and Ethical Approach
2.2. Epidemiological Risk Factors
2.3. Haemato-Biochemical Examinations
2.4. Molecular Confirmation through PCR, Sequencing, and Phylogenetic Study
2.5. Ultrasonography
2.6. Identification of Ticks through Scanning Electron Microscopy (SEM)
2.7. Pathology
3. Results
3.1. Epidemiological Risk Factors
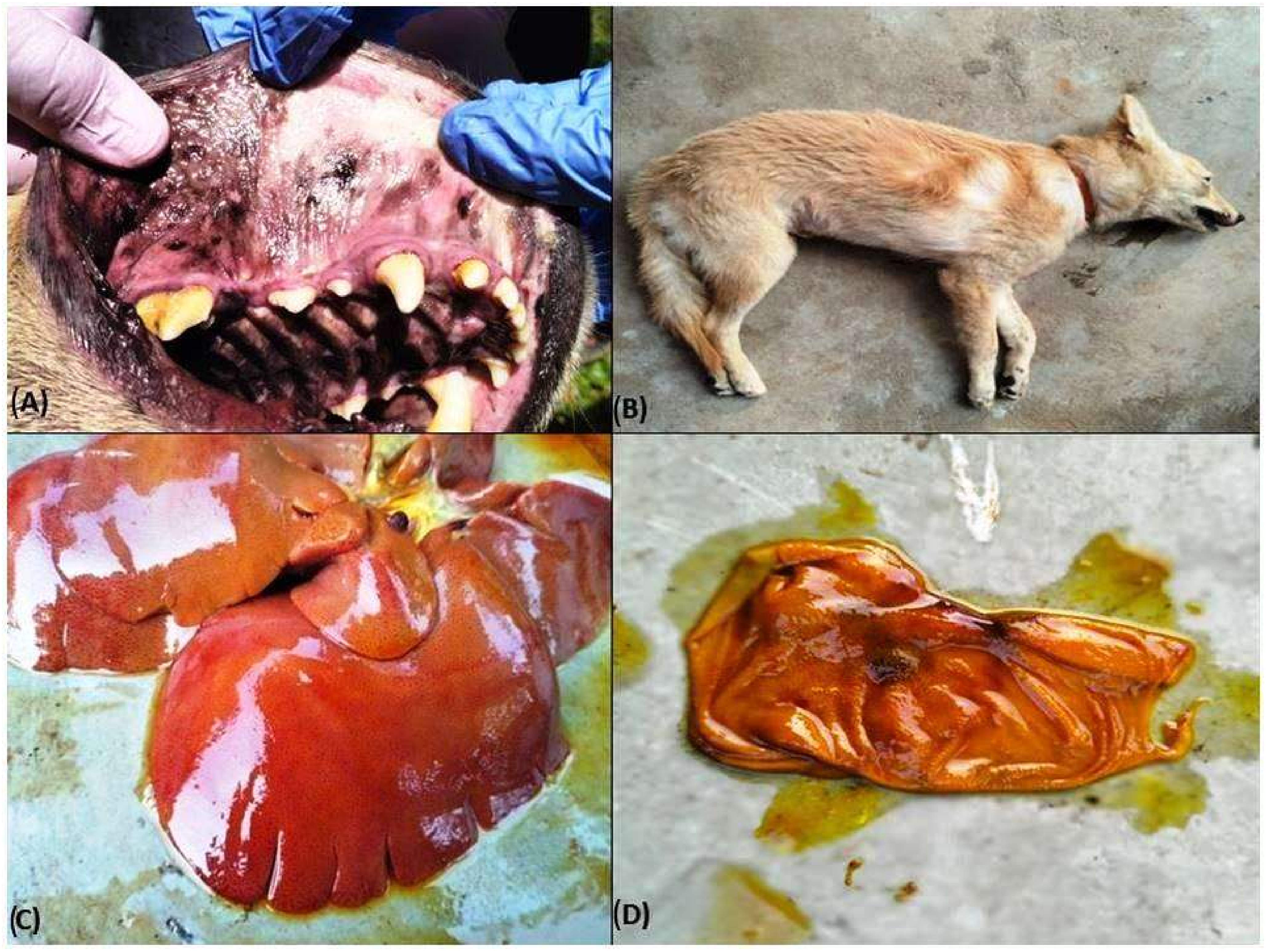
3.2. Clinical Signs
3.3. Haemato-Biochemical Parameters
3.4. Urine Examination
3.5. Ultrasonography
3.6. Pathology
3.6.1. Gross Pathology
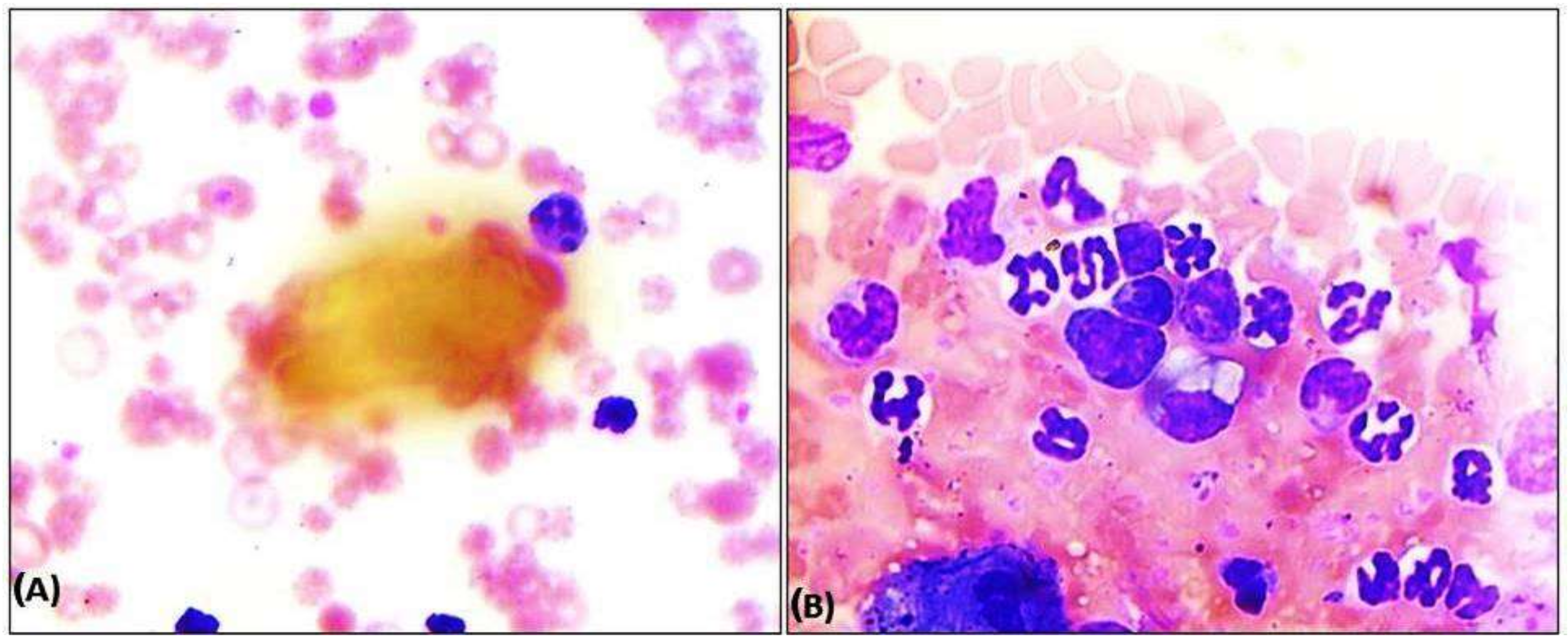
3.6.2. Histopathology
3.7. Identification of Ticks through Scanning Electron Microscopy (SEM)
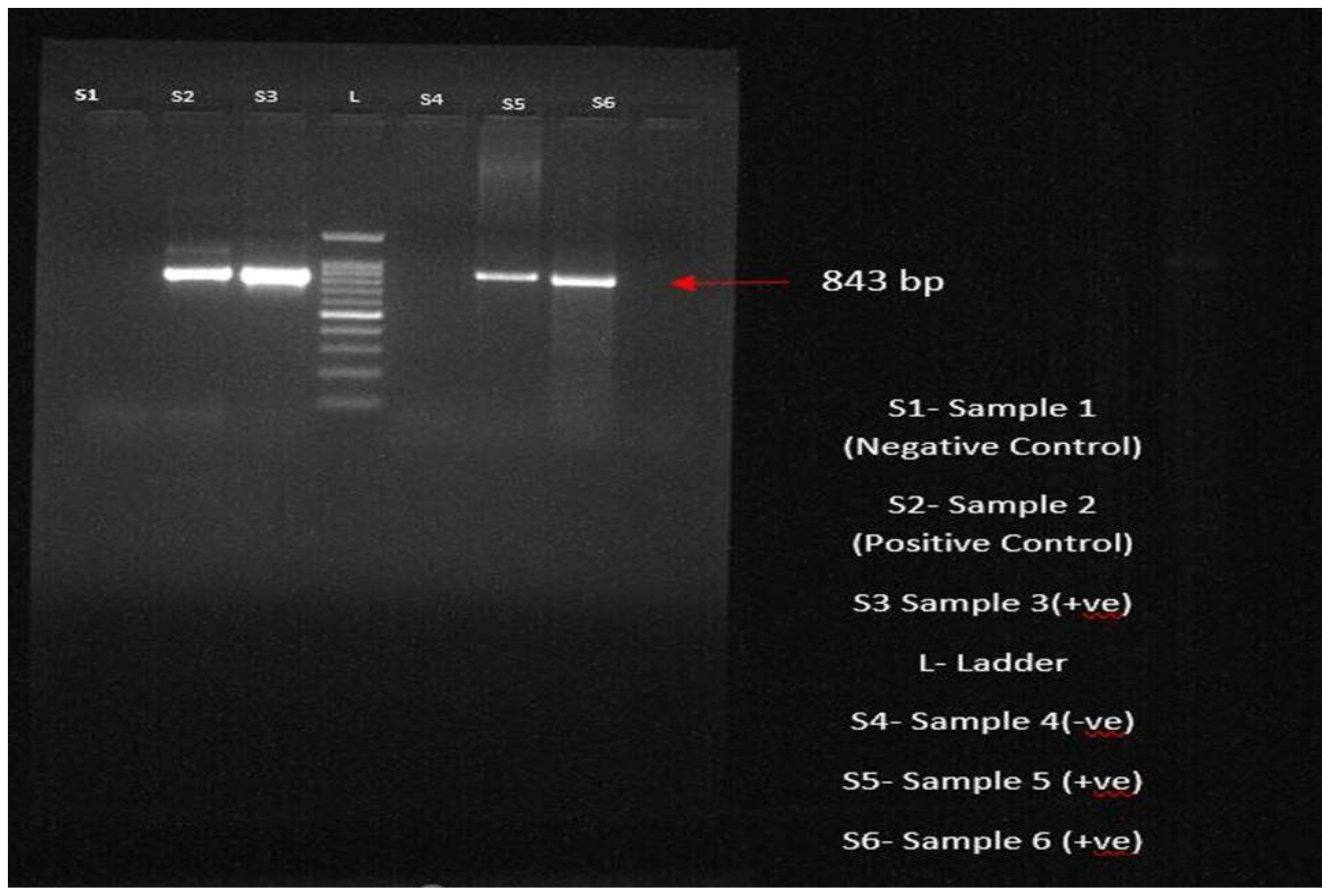
3.8. Molecular Confirmation through PCR
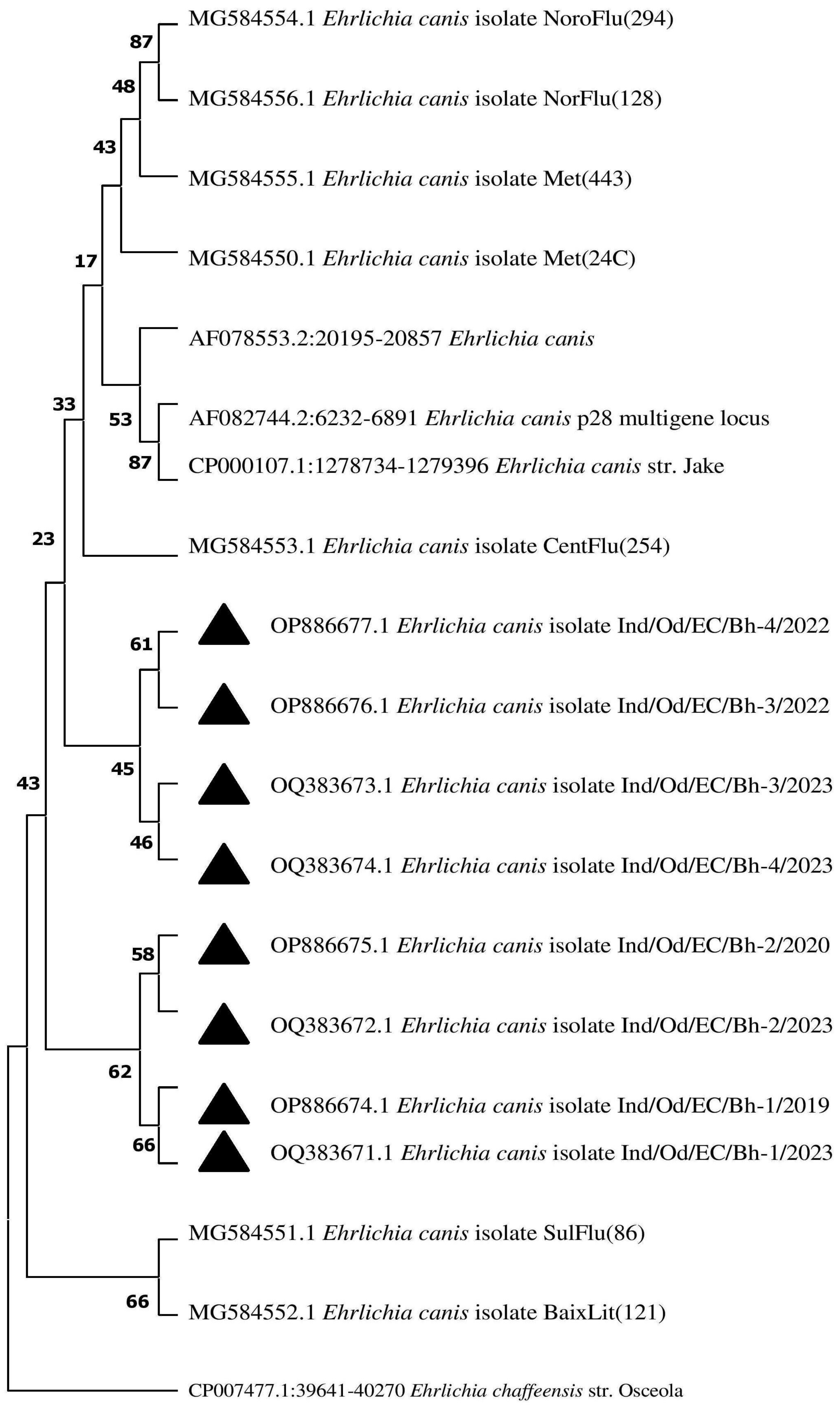
3.9. Phylogenetic Analysis
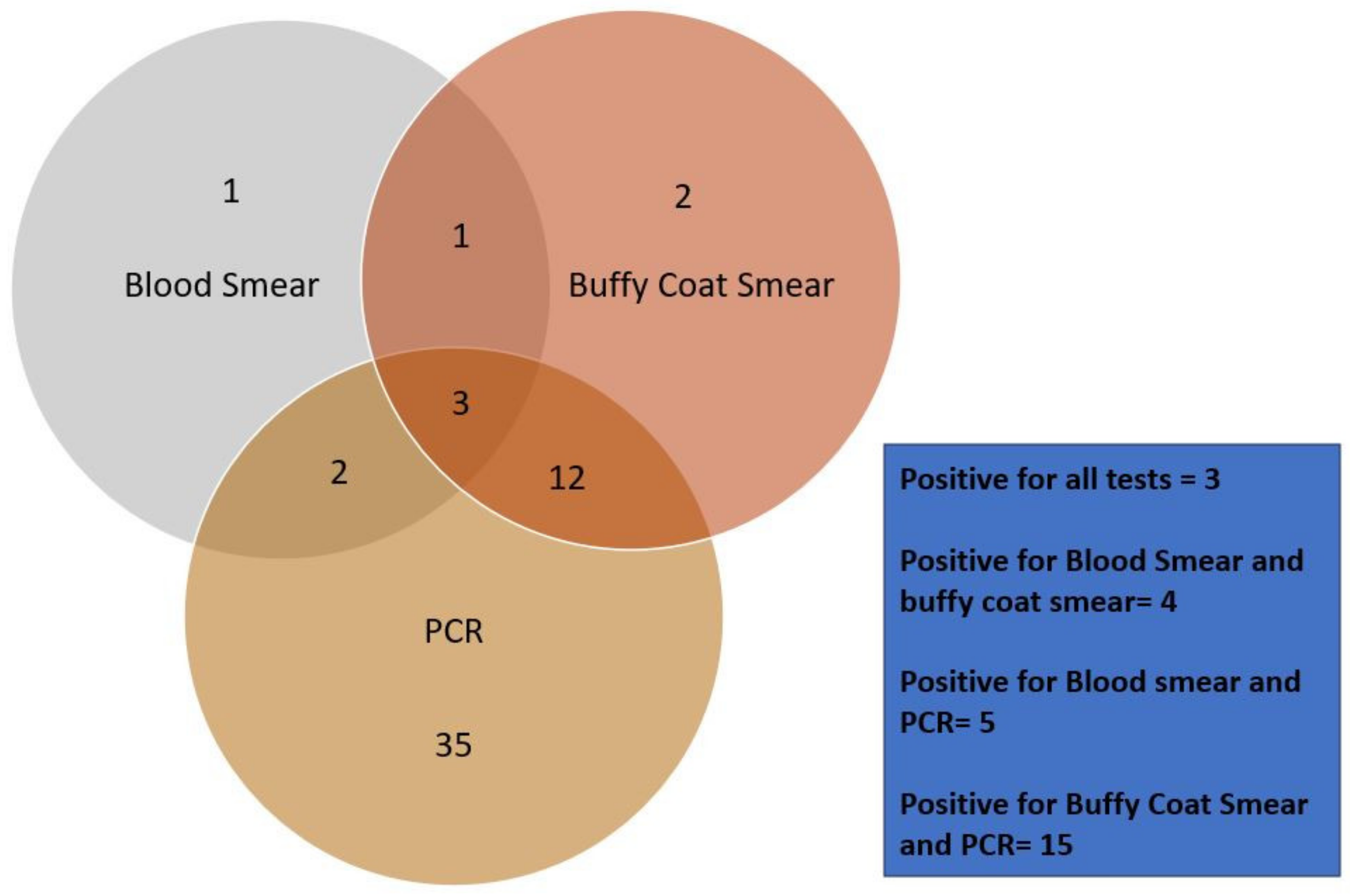
3.10. Comparative Analysis of the Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donatien, A.; Lestoquard, F. Existence en Algérie d’une Ricketttsia du chien. Bull. Soc. Pathol. Exot. 1935, 28, 418–419. [Google Scholar]
- Selim, A.; Abdelhady, A.; Alahadeb, J. Prevalence and first molecular characterization of Ehrlichia canis in Egyptian dogs. Pak. Vet. J. 2020, 41, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, S.S.; Aguiar, D.M.; Aquino, S.F.; Orlandelli, R.C.; Fernandes, A.R.F.; Uchôa, I.C.P. Soroprevalência e fatores de risco associados à soropositividade para Ehrlichia canis em cães do semiárido da Paraíba. Braz. J. Oveterinary Res. Anim. Sci. 2011, 48, 14–18. [Google Scholar] [CrossRef]
- Oliveira, D.; Nishimori, C.T.; Costa, M.T.; Machado, R.Z.; Castro, M.B. Anti-Ehrlichia canis antibodies detection by “Dot-ELISA” in naturally infected dogs. Rev. Bras. Parasitol. Vet. 2000, 9, 1–5. [Google Scholar]
- Lorsirigool, A.; Pumipuntu, N. A retrospective study of dogs infected with Ehrlichia canis from 2017–2019 in the Thonburi area of Bangkok province, Thailand. Int. J. Vet. Sci. 2020, 9, 578–580. [Google Scholar]
- Silva, L.S.; Pinho, F.A.; Prianti, M.G.; Braga, J.F.; Pires, L.; França, S.A.; Silva, S.M. Renal histopathological changes in dogs naturally infected with Ehrlichia cani. Braz. J. Vet. Pathol. 2016, 9, 2–15. [Google Scholar]
- Niwetpathomwat, A.; Assarasakorn, S.; Techangarnsuwan, S.; Suvarnavibhaj, S.; Kaewthamasorn, M. Canine dirofilariasis and concurrent tick-borne transmitted diseases in Bangkok, Thailand. Comp. Clin. Pathol. 2006, 15, 249–253. [Google Scholar] [CrossRef]
- Angkanaporn, K.; Sanguanwai, J.; Baiyokvichit, T.O.; Vorrachotvarittorn, P.; Wongsompong, M.; Sukhumavasi, W. Retrospective analysis of canine monocytic ehrlichiosis in Thailand with emphasis on hematological and ultrasonographic changes. Vet. World 2022, 15, 1–9. [Google Scholar] [CrossRef]
- Lakkawar, A.W.; Nair, M.G.; Varshney, K.C.; Sreekrishnan, R.; Rao, V.N. Pathology of canine monocytic ehrlichiosis in a German Shepherd dog. Slov. Vet. Res. 2003, 40, 119–128. [Google Scholar]
- Waner, T.; Harrus, S. Canine monocytic ehrlichiosis—from pathology to clinical manifestations. Isr. J. Vet. Med. 2013, 68, 12–18. [Google Scholar]
- Parmar, C.; Pednekar, R.; Jayraw, A.; Gatne, M. Comparative diagnostic methods for canine ehrlichiosis. Turk. J. Vet. Anim. Sci. 2013, 37, 282–290. [Google Scholar] [CrossRef]
- Bai, L.; Goel, P.; Jhambh, R.; Kumar, P.; Joshi, V.G. Molecular prevalence and haemato-biochemical profile of canine monocytic ehrlichiosis in dogs in and around Hisar, Haryana, India. J. Parasit. Dis. 2017, 41, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Nakaghi, A.C.H.; Machado, R.Z.; Costa, M.T.; André, M.R.; Baldani, C.D. Canine ehrlichiosis: Clinical, haematological, serological and molecular aspects. Cienc. Rural 2008, 38, 766–770. [Google Scholar] [CrossRef]
- Nakaghi, A.C.H.; Machado, R.Z.; Ferro, J.A.; Labruna, M.B.; Chryssafidis, A.L.; André, M.R.; Baldani, C.D. Sensitivity evaluation of a single-step PCR assay using Ehrlichia canis p28 gene as a target and its application in diagnosis of canine ehrlichiosis. Rev. Bras. Parasitol. Vet. 2010, 19, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Costa, L.M., Jr.; Rembeck, K.; Ribeiro, M.F.B.; Beelitz, P.; Pfister, K.; Passos, L.M.F. Sero-prevalence and risk indicators for Canine Ehrlichiosis in three rural areas of Brazil. Vet. J. 2007, 174, 673–676. [Google Scholar] [CrossRef]
- Guedes, P.E.B.; Oliveira, T.N.D.A.; Carvalho, F.S.; Carlos, R.S.A.; Albuquerque, G.R.; Munhoz, A.D.; Silva, F.L. Canine ehrlichiosis: Prevalence and epidemiology in northeast Brazil. Rev. Bras. Parasitol. Vet. 2015, 24, 115–121. [Google Scholar] [CrossRef]
- Milanjeet, S.H.; Singh, N.K.; Singh, N.D.; Singh, C.; Rath, S.S. Molecular prevalence and risk factors for the occurrence of canine monocytic ehrlichiosis. Vet. Med. 2014, 59, 129–136. [Google Scholar] [CrossRef]
- Okubanjo, K.; Pyle, R.L.; Reddy, A.M. Rickettsia canis in Hyderabad. Indian Vet. J. 2014, 35, 63–68. [Google Scholar]
- Bhardwaj, R.K. Therapeutic management of acute canine monocytic ehrlichiosis. Indian Vet. J. 2013, 90, 138–139. [Google Scholar]
- Himalini; Singh, R.; Bharadwaj, R.K.; Gupta, A.K. Prevalence and Molecular Characterization of Ehrlichia canis in dogs of Jammu region. J. Anim. Res. 2018, 8, 923–927. [Google Scholar]
- Bhadesiya, C.M.; Raval, S.K. Hematobiochemical changes in ehrlichiosis in dogs of Anand region, Gujarat. Vet. World 2015, 8, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Labruna, M.B.; Pereira, M.D.C. Carrapato em cães no Brasil. Clin. Vet. 2001, 6, 24–32. [Google Scholar]
- O’Dwyer, L.H. Diagno’stico de Hemoparasitos e Carrapatos de Cães Procedentes de A’reas Rurais Procedentes de Três Mesorregiões do Estado do Rio de Janeiro, Brasil. Ph.D. Thesis, Universidade Federal Rural do Rio de Janeiro-UFRRJ, Serope’dica, Brazil, 2000. [Google Scholar]
- Dantas-Torres, F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasites Vectors 2010, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Tuna, G.E.; Bakirci, S.; Dinler, C.; Karagenc, T.; Ulutas, B. Monocytic ehrlichiosis in Aegean region dogs: Clinical and haematological findings. Ataturk Univ. Vet. Bilim. Derg. 2019, 14, 8–14. [Google Scholar]
- Haritha, G.S.; Nirosha, M.; Shaik, S.; Venkatesh, K.; Kumari, K.N. Clinical and hemato-biochemical studies on ehrlichiosis and babesiosis in canines. Intas Polivet 2018, 19, 260–263. [Google Scholar]
- Rungsipipat, A.; Oda, M.; Kumpoosiri, N.; Wangnaitham, S.; Poosoonthontham, R.; Komkaew, W.; Ryoji, Y. Clinicopathological study of experimentally induced canine monocytic ehrlichiosis. Comp. Clin. Pathol. 2009, 18, 13–22. [Google Scholar] [CrossRef]
- Thongsahuan, S.; Chethanond, U.; Wasiksiri, S.; Saechan, V.; Thongtako, W.; Musikacharoen, T. Hematological profile of blood parasitic infected dogs in Southern Thailand. Vet. World 2020, 13, 2388–2394. [Google Scholar] [CrossRef]
- Siarkou, V.I.; Mylonakis, M.E.; Bourtzi-Hatzopoulou, E.; Koutinas, A.F. Sequence and phylogenetic analysis of the 16S rRNA gene of Ehrlichia canis strains in dogs with clinical monocytic ehrlichiosis. Vet. Microbiol. 2007, 125, 304–312. [Google Scholar] [CrossRef]
- Buhles, W.C., Jr.; Huxsoll, D.L.; Ristic, M. Tropical canine pancytopenia: Clinical, hematologic, and serologic response of dogs to Ehrlichia canis infection, tetracycline therapy and challenge inoculation. J. Infect. Dis. 1974, 130, 357–367. [Google Scholar] [CrossRef]
- Kottadamane, M.R.; Dhaliwal, P.S.; Singla, L.D.; Bansal, B.K.; Uppal, S.K. Clinical and haematobiochemical response in canine monocytic ehrlichiosis seropositive dogs of Punjab. Vet. World 2017, 10, 255. [Google Scholar] [CrossRef] [PubMed]
- Neave, M.J.; Mileto, P.; Joseph, A.; Reid, T.J.; Scott, A.; Williams, D.T.; Keyburn, A.L. Comparative genomic analysis of the first Ehrlichia canis detections in Australia. Ticks Tick Borne Dis. 2022, 13, 101909. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.L.M.; Munhoz, T.D.; João, C.F.; Vargas-Hernández, G.; André, M.R.; Pereira, W.A.B.; Tinucci-Costa, M. Ehrlichia canis (Jaboticabal Strain) induces the expression of TNF-α in leukocytes and splenocytes of experimentally infected dogs. Rev. Bras. Parasitol. Vet. 2011, 20, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Mylonakis, M.E.; Kritsepi-Konstantinou, M.; Dumler, J.S.; Diniz, P.P.V.; Day, M.J.; Siarkou, V.I.; Breitschwerdt, E.B.; Psychas, V.; Petanides, T.; Koutinas, A.F. Severe hepatitis associated with acute Ehrlichia canis infection in a dog. J. Vet. Intern. Med. 2010, 24, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.K.; Hoque, M.; Sharma, K.; Saravanan, M.; Monsang, S.W.; Mohanta, R.K. Abdominal Ultrasonography of Dogs with Canine Monocytic Ehrlichiosis. Indian Vet. J. 2012, 89, 148. [Google Scholar]
- Kumar, V.; Kumar, A.; Varshney, A.C.; Tyagi, S.P.; Kanwar, M.S.; Sharma, S.K. Diagnostic imaging of canine hepatobiliary affections: A review. Vet. Med. Int. 2012, 2012, 672107. [Google Scholar] [CrossRef]
- Mylonakis, M.E.; Harrus, S.; Breitschwerdt, E.B. An update on the treatment of canine monocytic ehrlichiosis (Ehrlichia canis). Vet. J. 2019, 246, 45–53. [Google Scholar] [CrossRef]



| Events | Temperature | Time | Cycles |
|---|---|---|---|
| Initial Denaturation | 95 °C | 5 min | 1 cycle |
| Denaturation, Annealing, and Extension | 95 °C | 30 s | 35 cycles |
| 55 °C | 1 min | ||
| 72 °C | 2 min | ||
| Extension | 72 °C | 5 min | 1 cycle |
| Variable | N | Positive | Prevalence (%) | OR | 95% CI | Chi Square | p Value | |
|---|---|---|---|---|---|---|---|---|
| Gender | Male | 115 | 38 | 33.04 | * | - | >0.9 | |
| Female | 57 | 18 | 31.57 | 1.06 | 0.50–2.24 | 0.9 | ||
| Age (years) | <1 | 51 | 23 | 45.09 | * | - | 0.045 | |
| 1 to 2 | 36 | 12 | 33.33 | 0.37 | 0.14–0.89 | 0.030 | ||
| >2 | 79 | 21 | 26.58 | 0.39 | 0.17–0.87 | 0.022 | ||
| Ticks | Absent | 38 | 12 | 31.57 | * | - | >0.9 | |
| Present | 140 | 44 | 31.42 | 0.88 | 0.38–2.06 | 0.8 | ||
| Housing | Kaccha House with Field | 51 | 16 | 31.37 | * | - | >0.9 | |
| Pukka house with close confinement | 22 | 7 | 31.81 | 0.89 | 0.27–2.83 | 0.9 | ||
| Pukka house with access to open field | 105 | 33 | 31.42 | 1 | 0.47–2.19 | >0.9 | ||
| Body weight (kg) | <10 | 16 | 5 | 31.25 | * | - | >0.9 | |
| 10 to 20 | 29 | 9 | 31.03 | 1.08 | 0.28–4.51 | >0.9 | ||
| >20 | 133 | 42 | 31.57 | 0.88 | 0.28–3.11 | 0.8 | ||
| Season | Rainy | 54 | 17 | 31.48 | * | - | >0.9 | |
| Summer | 98 | 31 | 31.63 | 1.16 | 0.54–2.54 | 0.7 | ||
| Winter | 26 | 8 | 30.76 | 1.03 | 0.34–2.98 | >0.9 | ||
| Breed | G.S | 44 | 14 | 31.81 | * | - | >0.9 | |
| Labrador | 39 | 12 | 30.76 | 1 | 0.38–2.67 | >0.9 | ||
| Golden Retriever | 28 | 9 | 32.14 | 0.97 | 0.33–2.78 | >0.9 | ||
| Alsatian | 25 | 8 | 32 | 0.93 | 0.30–2.79 | >0.9 | ||
| Doberman | 19 | 6 | 31.57 | 1.11 | 0.31–3.74 | 0.9 | ||
| Spitz | 13 | 4 | 30.76 | 0.8 | 0.18–3.13 | 0.8 | ||
| Beagle | 10 | 3 | 30 | 0.99 | 0.18–4.42 | >0.9 | ||
| Parameters | Ehrlichiosis Negative (N = 122) | Ehrlichiosis Positive (N = 56) |
|---|---|---|
| Hb (g/dL) | 14.07 ± 0.30 a | 6.33 ± 0.22 b |
| TEC (×106 µL) | 7.43 ± 1.04 a | 3.47 ± 0.19 b |
| PCV (%) | 44.10 ± 0.88 a | 19.33 ± 0.74 b |
| TLC (×103 µL) | 10.87 ± 0.63 a | 9.40 ± 0.13 b |
| PLT | 532.20 ± 38.68 a | 74.40 ± 4.31 b |
| MCV (fl) | 59.81 ± 1.24 a | 64.14 ± 0.38 b |
| MCH (Pg) | 22.17 ± 0.13 a | 23.07 ± 0.08 b |
| MCHC (%) | 33.46 ± 0.38 a | 38.23 ± 0.21 b |
| N (%) | 57.20 ± 0.38 a | 78.37 ± 0.83 b |
| L (%) | 36.70 ± 0.35 a | 16.50 ± 0.79 b |
| E (%) | 4.47 ± 0.26 a | 1.53 ± 0.93 b |
| M (%) | 1.63 ± 0.89 a | 3.60 ± 0.13 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakraborty, A.; Rath, P.K.; Panda, S.K.; Mishra, B.P.; Dehuri, M.; Biswal, S.; Jena, M.K.; Sahu, B.P.; Paital, B.; Sahoo, D.K. Molecular Confirmation, Epidemiology, and Pathophysiology of Ehrlichia canis Prevalence in Eastern India. Pathogens 2024, 13, 803. https://doi.org/10.3390/pathogens13090803
Chakraborty A, Rath PK, Panda SK, Mishra BP, Dehuri M, Biswal S, Jena MK, Sahu BP, Paital B, Sahoo DK. Molecular Confirmation, Epidemiology, and Pathophysiology of Ehrlichia canis Prevalence in Eastern India. Pathogens. 2024; 13(9):803. https://doi.org/10.3390/pathogens13090803
Chicago/Turabian StyleChakraborty, Ankita, Prasana Kumar Rath, Susen Kumar Panda, Bidyut Prava Mishra, Manaswini Dehuri, Sangram Biswal, Manoj Kumar Jena, Basanta Pravas Sahu, Biswaranjan Paital, and Dipak Kumar Sahoo. 2024. "Molecular Confirmation, Epidemiology, and Pathophysiology of Ehrlichia canis Prevalence in Eastern India" Pathogens 13, no. 9: 803. https://doi.org/10.3390/pathogens13090803
APA StyleChakraborty, A., Rath, P. K., Panda, S. K., Mishra, B. P., Dehuri, M., Biswal, S., Jena, M. K., Sahu, B. P., Paital, B., & Sahoo, D. K. (2024). Molecular Confirmation, Epidemiology, and Pathophysiology of Ehrlichia canis Prevalence in Eastern India. Pathogens, 13(9), 803. https://doi.org/10.3390/pathogens13090803











