Development of a Specific PCR Assay for Theileria sp. Yokoyama and Assessment of Its Potential to Cause Anemia in Cattle
Abstract
1. Introduction
2. Materials and Methods
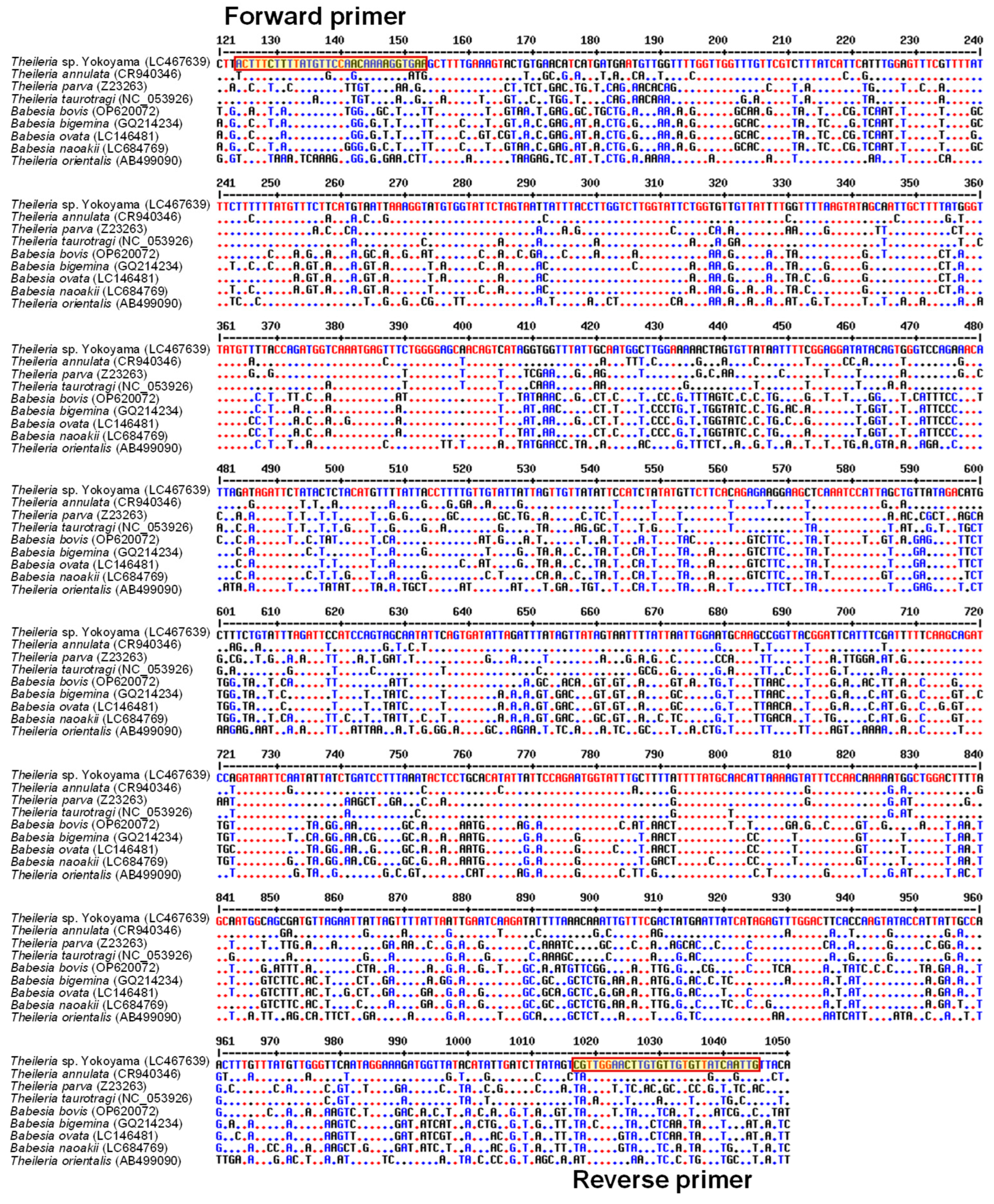
2.1. Development of a PCR Assay Specific for Theileria sp. Yokoyama
2.2. Blood Sampling
2.3. Measurement of RBC Indices
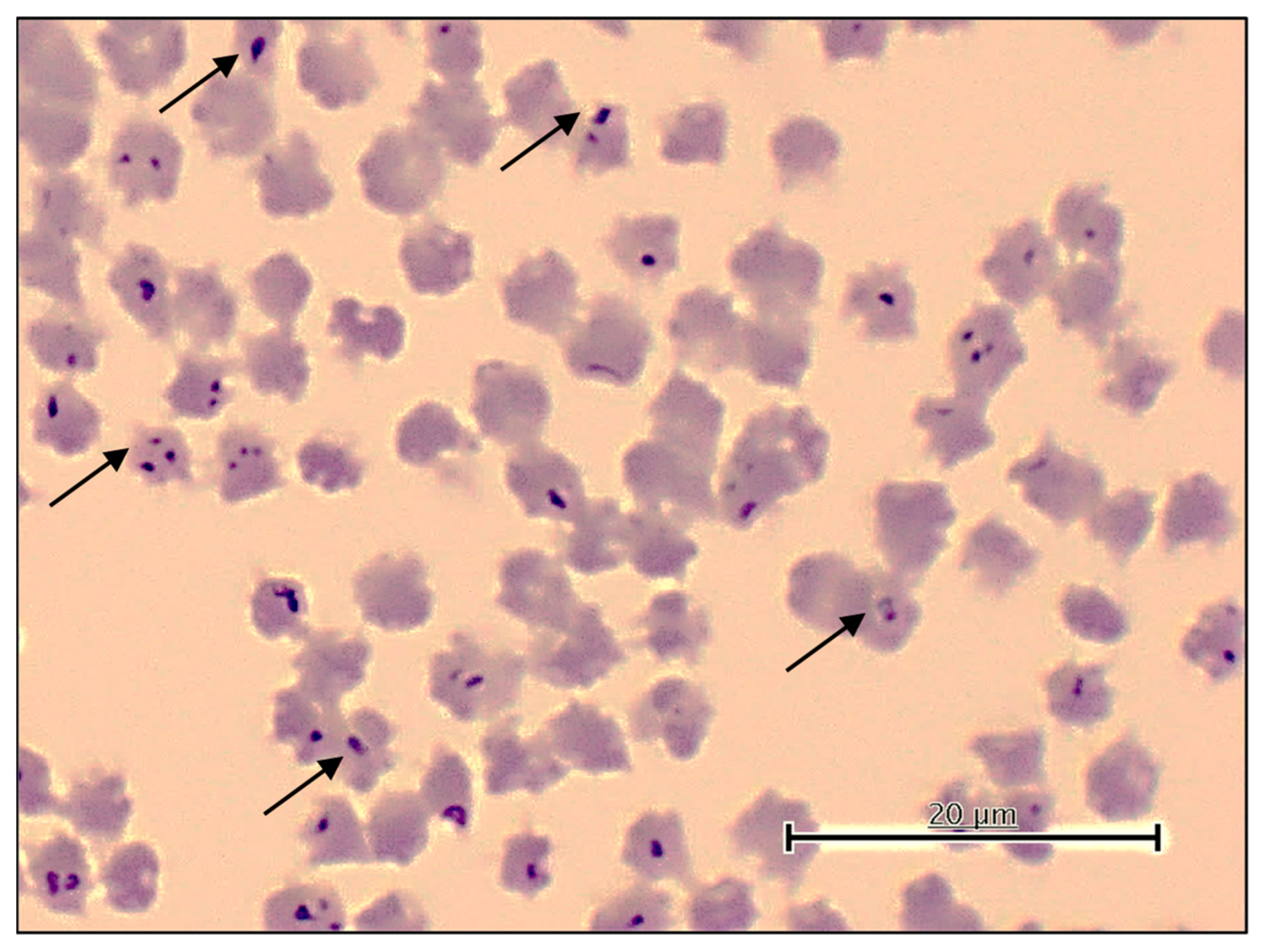
2.4. Microscopic Detection of Theileria, Babesia, and Anaplasma Species
2.5. PCR Detection of Theileria sp. Yokoyama and Other Bovine Hemopathogens
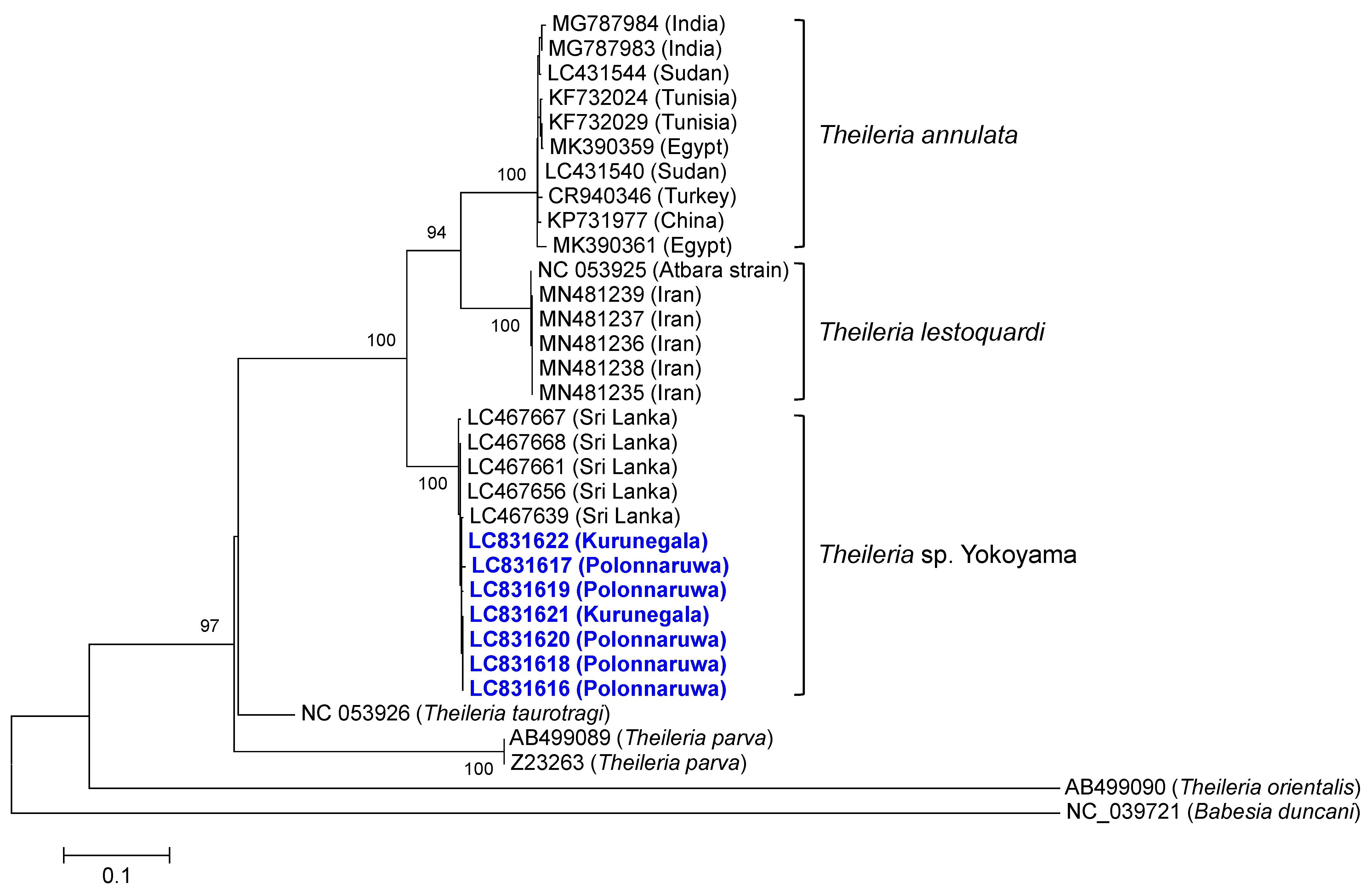
2.6. Sequencing and Phylogenetic Analyses
2.7. Statistical Analyses
3. Results
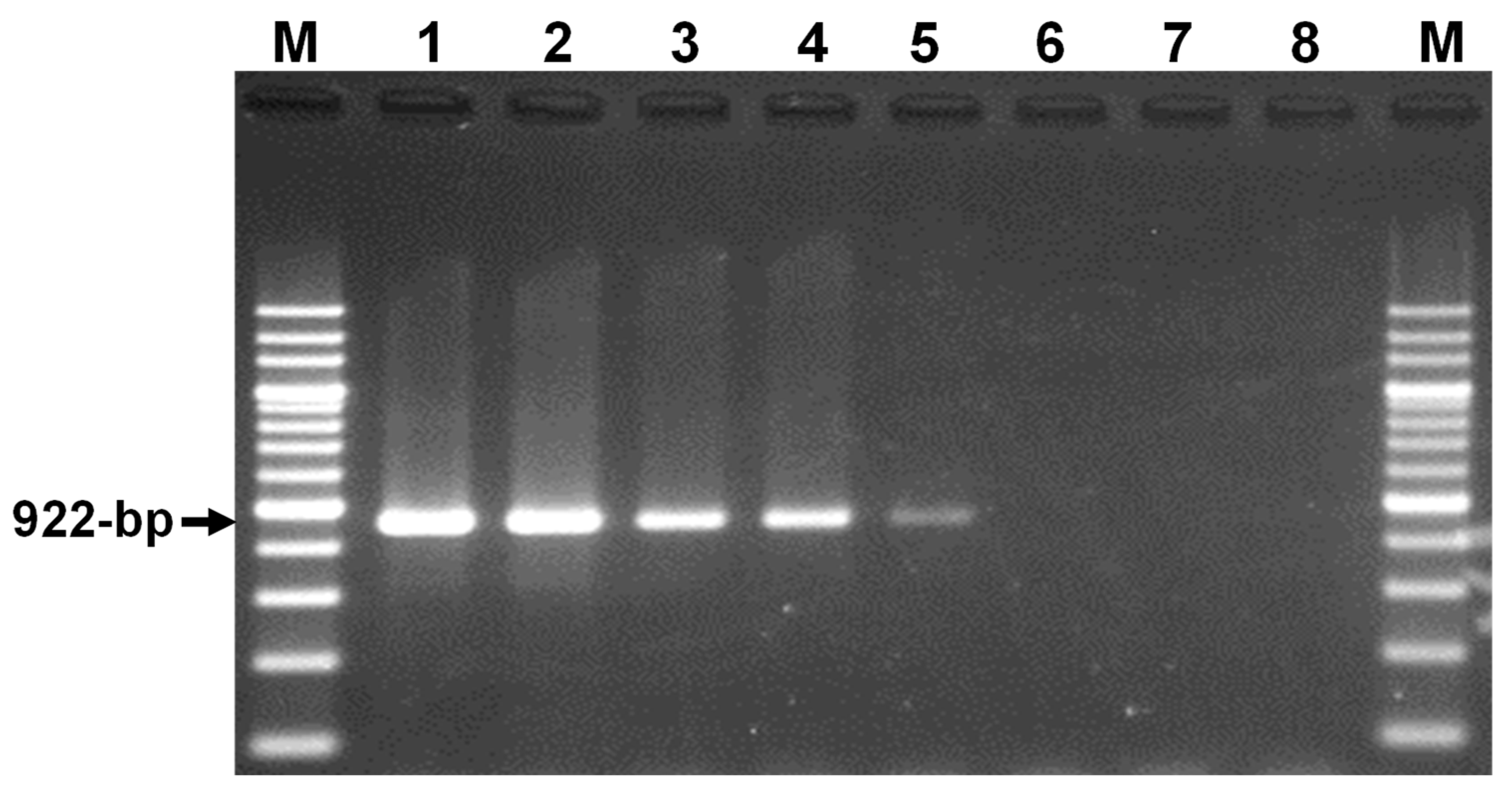
3.1. Development of a Theileria sp. Yokoyama-Specific PCR Assay
3.2. Microscopic and PCR Detection of Theileria sp. Yokoyama and Other Bovine Hemopathogens
3.3. Potential Risk Factors for Theileria sp. Yokoyama Infection
3.4. Anemia Status of Theileria sp. Yokoyama-Infected Cattle
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mans, B.J.; Pienaar, R.; Latif, A.A. A review of Theileria diagnostics and epidemiology. Int. J. Parasitol. Parasites Wildl. 2015, 4, 104–118. [Google Scholar] [CrossRef]
- Gebrekidan, H.; Perera, P.K.; Ghafar, A.; Abbas, T.; Gasser, R.B.; Jabbar, A. An appraisal of oriental theileriosis and the Theileria orientalis complex, with an emphasis on diagnosis and genetic characterisation. Parasitol. Res. 2020, 119, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Gill, B.S.; Bhattacharyulu, Y.; Kaur, D. Symptoms and pathology of experimental bovine tropical theileriosis (Theileria annulata infection). Ann. Parasitol. Hum. Comp. 1977, 52, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Vimonish, R.; Dinkel, K.D.; Fry, L.M.; Johnson, W.C.; Capelli-Peixoto, J.; Bastos, R.G.; Scoles, G.A.; Knowles, D.P.; Madder, M.; Chaka, G.; et al. Isolation of infectious Theileria parva sporozoites secreted by infected Rhipicephalus appendiculatus ticks into an in vitro tick feeding system. Parasit. Vectors 2021, 14, 616. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.K. The same but different: The biology of Theileria sporozoite entry into bovine cells. Int. J. Parasitol. 1997, 27, 457–474. [Google Scholar] [CrossRef]
- Dobbelaere, D.; Heussler, V. Transformation of leukocytes by Theileria parva and T. annulata. Annu. Rev. Microbiol. 1999, 53, 1–42. [Google Scholar] [CrossRef]
- von Schubert, C.; Xue, G.; Schmuckli-Maurer, J.; Woods, K.L.; Nigg, E.A.; Dobbelaere, D.A. The transforming parasite Theileria co-opts host cell mitotic and central spindles to persist in continuously dividing cells. PLoS Biol. 2010, 8, e1000499. [Google Scholar] [CrossRef]
- Hayashida, K.; Hara, Y.; Abe, T.; Yamasaki, C.; Toyoda, A.; Kosuge, T.; Suzuki, Y.; Sato, Y.; Kawashima, S.; Katayama, T.; et al. Comparative genome analysis of three eukaryotic parasites with differing abilities to transform leukocytes reveals key mediators of Theileria-induced leukocyte transformation. mBio 2012, 3, e00204–e00212. [Google Scholar] [CrossRef]
- Watts, J.G.; Playford, M.C.; Hickey, K.L. Theileria orientalis: A review. N. Z. Vet. J. 2016, 64, 3–9. [Google Scholar] [CrossRef]
- Bishop, R.; Musoke, A.; Morzaria, S.; Gardner, M.; Nene, V. Theileria: Intracellular protozoan parasites of wild and domestic ruminants transmitted by ixodid ticks. Parasitology 2004, 129, S271–S283. [Google Scholar] [CrossRef]
- Preston, P.M.; Brown, C.G.; Bell-Sakyi, L.; Richardson, W.; Sanderson, A. Tropical theileriosis in Bos taurus and Bos taurus cross Bos indicus calves: Response to infection with graded doses of sporozoites of Theileria annulata. Res. Vet. Sci. 1992, 53, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, T.; Hayashida, K.; Sugimoto, C.; Yokoyama, N. Evolution and genetic diversity of Theileria. Infect. Genet. Evol. 2014, 27, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Liyanaarachchi, D.R.; Rajakaruna, R.S.; Dikkumbura, A.W.; Rajapakse, R.P. Ticks infesting wild and domestic animals and humans of Sri Lanka with new host records. Acta. Trop. 2015, 142, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, T.; Kothalawala, H.; Abeyratne, S.A.; Vimalakumar, S.C.; Meewewa, A.S.; Hadirampela, D.T.; Puvirajan, T.; Sukumar, S.; Kuleswarakumar, K.; Chandrasiri, A.D.; et al. A PCR-based survey of selected Babesia and Theileria parasites in cattle in Sri Lanka. Vet. Parasitol. 2012, 190, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, T.; Kothalawala, H.; Weerasooriya, G.; Silva, S.S.P.; Puvanendiran, S.; Munkhjargal, T.; Igarashi, I.; Yokoyama, N. A longitudinal study of Babesia and Theileria infections in cattle in Sri Lanka. Vet. Parasitol. Reg. Stud. Rep. 2016, 6, 20–27. [Google Scholar]
- Gunasekara, E.; Sivakumar, T.; Kothalawala, H.; Abeysekera, T.S.; Weerasingha, A.S.; Vimalakumar, S.C.; Kanagaratnam, R.; Yapa, P.R.; Zhyldyz, A.; Igarashi, I.; et al. Epidemiological survey of hemoprotozoan parasites in cattle from low-country wet zone in Sri Lanka. Parasitol. Int. 2019, 71, 5–10. [Google Scholar] [CrossRef]
- Sivakumar, T.; Fujita, S.; Tuvshintulga, B.; Kothalawala, H.; Silva, S.S.P.; Yokoyama, N. Discovery of a new Theileria sp. closely related to Theileria annulata in cattle from Sri Lanka. Sci. Rep. 2019, 9, 16132. [Google Scholar]
- Bahekar, V.S.; Gonuguntla, H.N.; Sarangi, L.N.; Manasa, G.; Chandaka, K.D.; Rana, S.K.; Prasad, A.; Surendra, K.S.N.L.; Ponnanna, N.M.; Sharma, G.K. Detection and genetic characterization indicates circulation of a possible new Theileria species (Theileria sp. Yokoyama) in India. Vet. Parasitol. Reg. Stud. Rep. 2022, 34, 100765. [Google Scholar] [CrossRef]
- Salman, D.; Sivakumar, T.; Otgonsuren, D.; Mahmoud, M.E.; Elmahallawy, E.K.; Khalphallah, A.; Kounour, A.M.E.Y.; Bayomi, S.A.; Igarashi, M.; Yokoyama, N. Molecular survey of Babesia, Theileria, Trypanosoma, and Anaplasma infections in camels (Camelus dromedaries) in Egypt. Parasitol. Int. 2022, 90, 102618. [Google Scholar] [CrossRef]
- McKeever, D.J. Bovine immunity—A driver for diversity in Theileria parasites? Trends. Parasitol. 2009, 25, 269–276. [Google Scholar]
- Afshari, A.; Tavassoli, M.; Esmaeilnejad, B.; Habibi, G.; Esmaeilnia, K. Molecular characterization and phylogenetic analysis of pathogenic Theileria spp. isolated from cattle and sheep based on cytochrome b gene in Iran. Arch. Razi. Inst. 2021, 76, 243–252. [Google Scholar]
- Sivakumar, T.; Tuvshintulga, B.; Zhyldyz, A.; Kothalawala, H.; Yapa, P.R.; Kanagaratnam, R.; Vimalakumar, S.C.; Abeysekera, T.S.; Weerasingha, A.S.; Yamagishi, J.; et al. Genetic analysis of Babesia isolates from cattle with clinical babesiosis in Sri Lanka. J. Clin. Microbiol. 2018, 56, e00895-18. [Google Scholar] [CrossRef] [PubMed]
- Hematology and Blood Bank Technique. Available online: https://www.selfstudys.com/books/nios/state-books/vocational/hematology-blood-bank-technique-english-medium/4357 (accessed on 23 July 2024).
- BBCCL-116 Human Physiology. Available online: https://egyankosh.ac.in/handle/123456789/84525 (accessed on 23 July 2024).
- Jain, K. Essentials of Veterinary Hematology; Blackwell Publishing: Hoboken, NJ, USA, 1993. [Google Scholar]
- Lempereur, L.; Beck, R.; Fonseca, I.; Marques, C.; Duarte, A.; Santos, M.; Zúquete, S.; Gomes, J.; Walder, G.; Domingos, A.; et al. Guidelines for the detection of Babesia and Theileria parasites. Vector Borne Zoonotic Dis. 2017, 17, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Kocan, K.M.; de la Fuente, J.; Blouin, E.F.; Garcia-Garcia, J.C. Anaplasma marginale (Rickettsiales: Anaplasmataceae): Recent advances in defining host-pathogen adaptations of a tick-borne rickettsia. Parasitology 2004, 129, S285–S300. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Felsenstein, J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 1981, 17, 368–376. [Google Scholar] [CrossRef]
- Hasegawa, M.; Kishino, H.; Yano, T. Dating the human-ape split by a molecular clock of mitochondrial DNA. J. Mol. Evol. 1985, 22, 160–174. [Google Scholar] [CrossRef]
- Campbell, I. Chi-squared and Fisher-Irwin tests of two-by-two tables with small sample recommendations. Stat. Med. 2007, 26, 3661–3675. [Google Scholar] [CrossRef]
- Richardson, J.T. The analysis of 2 × 2 contingency tables—Yet again. Stat. Med. 2011, 30, 890. [Google Scholar] [CrossRef]
- Moré, D.D.; Cardoso, F.F.; Mudadu, M.A.; Malagó-Jr, W.; Gulias-Gomes, C.C.; Sollero, B.P.; Ibelli, A.M.G.; Coutinho, L.L.; Regitano, L.C.A. Network analysis uncovers putative genes affecting resistance to tick infestation in Braford cattle skin. BMC Genom. 2019, 20, 998. [Google Scholar] [CrossRef] [PubMed]
- Bock, R.E.; de Vos, A.J.; Kingston, T.G.; McLellan, D.J. Effect of breed of cattle on innate resistance to infection with Babesia bovis, B bigemina and Anaplasma marginale. Aust. Vet. J. 1997, 75, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Roland, L.; Drillich, M.; Iwersen, M. Hematology as a diagnostic tool in bovine medicine. J. Vet. Diagn. Investig. 2014, 26, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Liu, J.; Li, Z.; Xiang, Q.; Wang, J.; Liu, A.; Li, Y.; Yin, H.; Guan, G.; Luo, J. Clinical and pathological studies on cattle experimentally infected with Theileria annulata in China. Pathogens 2020, 9, 727. [Google Scholar] [CrossRef]
- Irvin, A.D.; Mwamachi, D.M. Clinical and diagnostic features of East Coast fever (Theileria parva) infection of cattle. Vet. Rec. 1983, 113, 192–198. [Google Scholar] [CrossRef]
| District | Veterinary Range | No. Animals | No. Microscopy Positive (%) | No. PCR Positive (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Theileria | Babesia | Anaplasma | Theileria sp. Yokoyama | T. orientalis | B. bovis | B. bigemina | A. marginale | |||
| Polonnaruwa | Medirigiriya | 21 | 9 (51.2) | 2 (23.8) | 8 (38.0) | 5 (23.8) | 8 (38.1) | 5 (23.8) | 3 (14.3) | 10 (47.6) |
| Polonnaruwa | 36 | 29 (80.5) | 5 (13.8) | 18 (50.0) | 23 (63.9) | 14 (38.9) | 1 (2.8) | 5 (13.9) | 24 (66.7) | |
| Hingurakgoda | 20 | 11(55.0) | 0 (0.0) | 3 (30.0) | 6 (30.0) | 11 (55.0) | 0 (0.0) | 0 (0.0) | 9 (45.0) | |
| Welikanda | 30 | 12 (40.0) | 1 (3.3) | 12 (40.0) | 3 (10.0) | 13 (43.3) | 0 (0.0) | 1 (3.3) | 16 (53.3) | |
| Bakamuna | 41 | 21(63.4) | 3 (7.3) | 15 (63.4) | 4 (9.8) | 23 (56.1) | 2 (4.9) | 2 (4.9) | 26 (63.4) | |
| Aralaganwila | 34 | 15 (44.1) | 3 (8.8) | 15 (44.2) | 4 (11.8) | 15 (44.1) | 0 (0.0) | 3 (8.8) | 19 (55.9) | |
| Kurunagela | Melsiripura | 24 | 13 (54.2) | 0 (0.0) | 14 (58.3) | 15 (62.5) | 14 (58.3) | 0 (0.0) | 0 (0.0) | 24 (100) |
| Total | 206 | 110 (53.3) | 17 (8.3) | 88 (42.7) | 60 (29.1) | 98 (47.6) | 8 (3.9) | 14 (6.8) | 128 (62.1) | |
| Breed | Jersey |
| Age | 3.5 years |
| Physiological status | Pregnant |
| Management | Extensive |
| Tick infestation | Yes |
| Rectal temperature | 38.8 °C |
| Hemoglobin concentration | 7.1 g/dL |
| Hematocrit | 21% |
| RBC counts | 3.32 × 106/μL |
| Parasitemia | 7.8% |
| Risk Factors | No. Animals | No. Positive (%) | p-Value |
|---|---|---|---|
| Sex | |||
| Female | 188 | 54 (28.7) | 0.6822 |
| Male | 18 | 6 (33.3) | |
| Age | |||
| 1–3 years | 165 | 47(28.48) | 0.6854 |
| >3 years | 41 | 13 (31.7) | |
| Breed a | |||
| Bos taurus | 6 | 3 (50) | 0.0473 (Bt vs. Bi) |
| Bos indicus | 72 | 12 (16.6) | 0.0054 (Bi vs. Cr) |
| Cross-bred | 128 | 45 (35.2) | 0.4601 (Bt vs. Cr) |
| Management b | |||
| Extensive | 93 | 59 (63.4) | <0.0001 (Ex vs. In) |
| Intensive | 24 | 0 (0) | 0.6041 (In vs. Si) |
| Semi-intensive | 89 | 1 (1.1) | <0.0001 (Ex vs. Si) |
| Ticks | |||
| Presence | 132 | 53 (40.2) | <0.0001 |
| Absence | 74 | 7 (9.5) |
| Pathogen | No. Animals (% a) | No. Anemic Animals (% b) |
|---|---|---|
| Non-infected | 29 (14.1) | 0 (0.0) |
| Single pathogen | ||
| Theileria sp. Yokoyama | 13 (6.3) | 3 (23.1) |
| T. orientalis | 23 (11.2) | 3 (13.0) |
| B. bovis | 1 (0.5) | 0 (0.0) |
| B. bigemina | 4 (1.9) | 1 (25.0) |
| A. marginale | 34 (16.5) | 0 (0.0) |
| Two pathogens | ||
| Theileria sp. Yokoyama + T. orientalis | 5 (2.4) | 0 (0.0) |
| Theileria sp. Yokoyama + A. marginale | 18 (8.7) | 6 (33.3) |
| T. orientalis + B. bovis | 1 (0.5) | 0 (0.0) |
| T. orientalis + B. bigemina | 2 (1.0) | 0 (0.0) |
| T. orientalis + A. marginale | 47 (22.8) | 10 (21.3) |
| B. bovis + A. marginale | 1 (0.5) | 0 (0.0) |
| B. bigemina + A. marginale | 2 (1.0) | 2 (100) |
| Three pathogens | ||
| Theileria sp. Yokoyama + T. orientalis + A. marginale | 19 (9.2) | 5 (26.3) |
| Theileria sp. Yokoyama + B. bovis + A. marginale | 1 (0.5) | 0 (0.0) |
| Theileria sp. Yokoyama + B. bigemina + A. marginale | 2 (1.0) | 0 (0.0) |
| B. bovis + B. bigemina + A. marginale | 1 (0.5) | 0 (0.0) |
| Four pathogens | ||
| Theileria sp. Yokoyama + B. bovis + B. bigemina + A. marginale | 2 (1.0) | 1 (50.0) |
| T. orientalis + B. bovis + B. bigemina + A. marginale | 1 (0.5) | 0 (0.0) |
| Total | 206 | 31 (15.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amarasiri, I.D.; Nizanantha, K.; Mumbi, N.N.M.; Kothalawala, I.S.; Madusanka, S.; Perera, W.P.P.S.I.; Kothalawala, H.; Sivakumar, T.; Yokoyama, N. Development of a Specific PCR Assay for Theileria sp. Yokoyama and Assessment of Its Potential to Cause Anemia in Cattle. Pathogens 2024, 13, 735. https://doi.org/10.3390/pathogens13090735
Amarasiri ID, Nizanantha K, Mumbi NNM, Kothalawala IS, Madusanka S, Perera WPPSI, Kothalawala H, Sivakumar T, Yokoyama N. Development of a Specific PCR Assay for Theileria sp. Yokoyama and Assessment of Its Potential to Cause Anemia in Cattle. Pathogens. 2024; 13(9):735. https://doi.org/10.3390/pathogens13090735
Chicago/Turabian StyleAmarasiri, Iromy Dhananjani, Kalaichelvan Nizanantha, Ngigi Noel Muthoni Mumbi, Isuru Sachintha Kothalawala, Sampath Madusanka, Wettam Perumage Pavithra Sandamali Indrasiri Perera, Hemal Kothalawala, Thillaiampalam Sivakumar, and Naoaki Yokoyama. 2024. "Development of a Specific PCR Assay for Theileria sp. Yokoyama and Assessment of Its Potential to Cause Anemia in Cattle" Pathogens 13, no. 9: 735. https://doi.org/10.3390/pathogens13090735
APA StyleAmarasiri, I. D., Nizanantha, K., Mumbi, N. N. M., Kothalawala, I. S., Madusanka, S., Perera, W. P. P. S. I., Kothalawala, H., Sivakumar, T., & Yokoyama, N. (2024). Development of a Specific PCR Assay for Theileria sp. Yokoyama and Assessment of Its Potential to Cause Anemia in Cattle. Pathogens, 13(9), 735. https://doi.org/10.3390/pathogens13090735







