Advances in Engineering Circular RNA Vaccines
Abstract
1. Introduction
2. The Biological Functions of Natural circRNA
3. Molecular Biology of circRNA Vaccines
3.1. Characterization of circRNA Vaccines
3.2. Mechanism of Immunotherapy Mediated by circRNA Vaccines
3.3. Enzymatic Degradation Mechanisms of circRNA
4. In Vitro Synthesis Process of circRNA
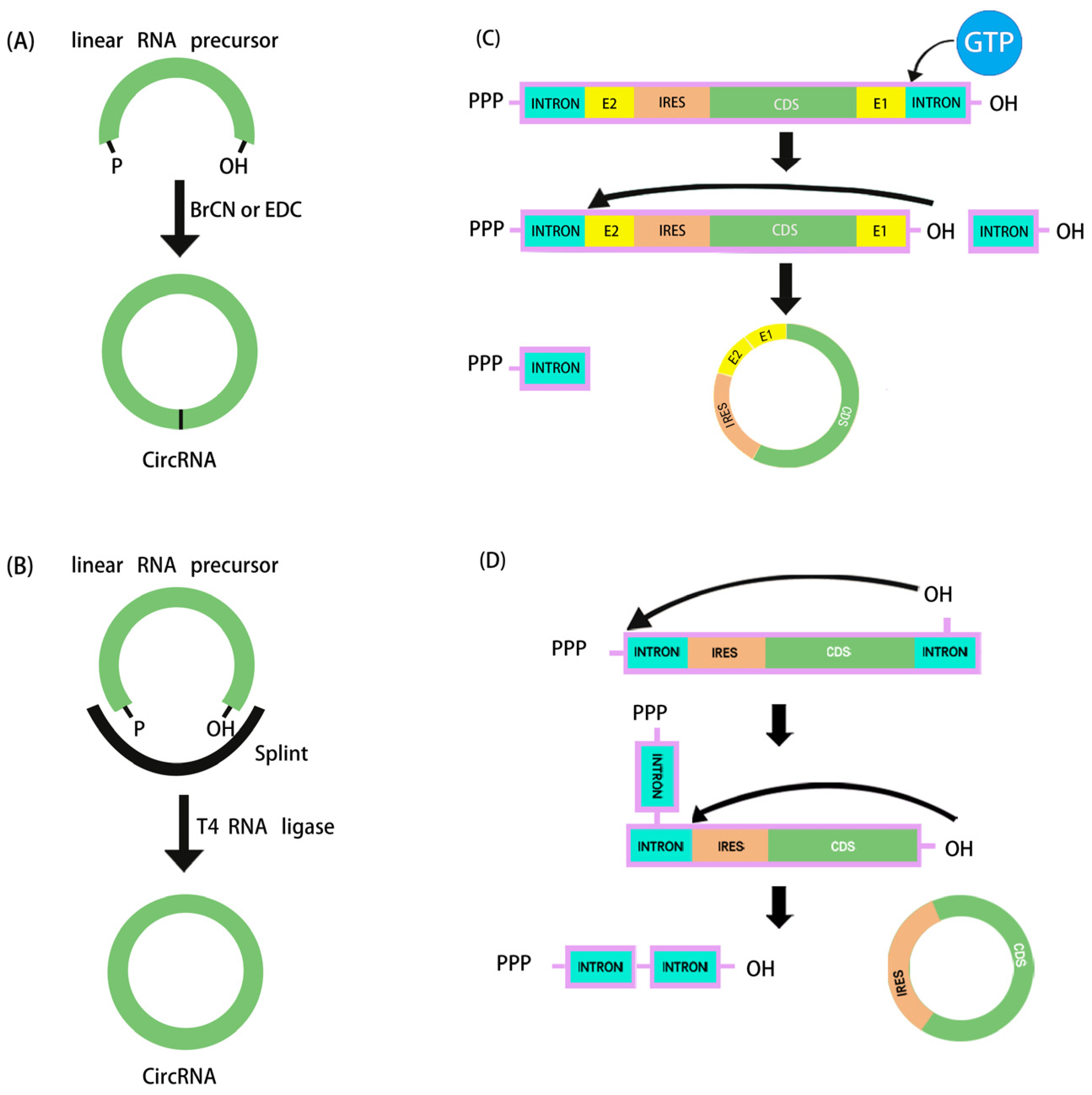
4.1. Design and Synthesis of Linear RNA Precursors
4.2. Circularization
4.2.1. Chemical Synthesis
4.2.2. Enzymatic Strategies
4.2.3. Ribozymatic Methods
4.3. Purification and Identification of circRNA Vaccines
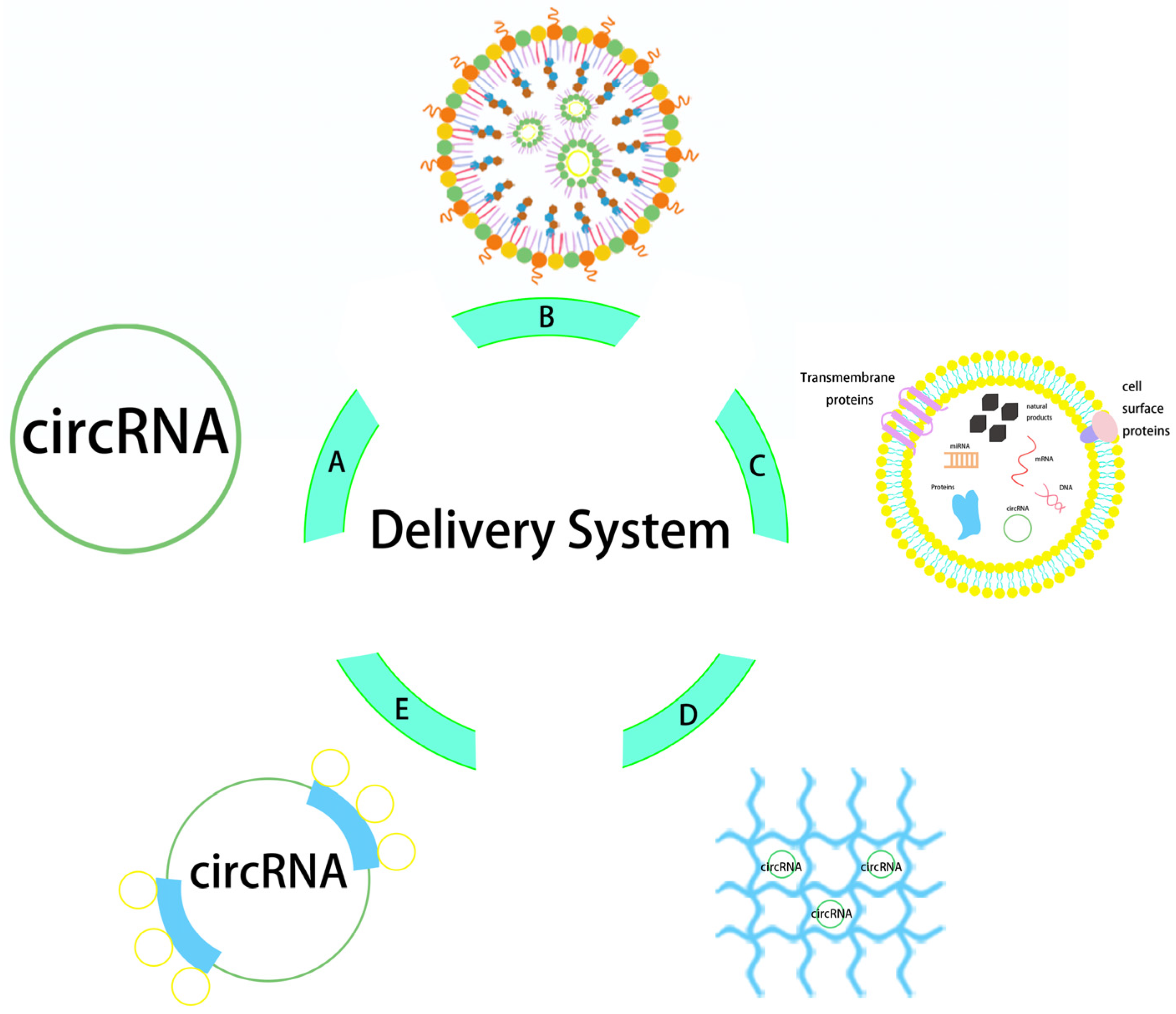
5. Delivery Strategies for circRNA Vaccines
5.1. Carrier-Free Delivery
5.2. Lipid Nanoparticle Delivery
| Vaccine | Antigen | IRES | Cyclization Method | Delivery | Research Team | Period | Reference |
|---|---|---|---|---|---|---|---|
| circRNA RBD | Encodes SARS-CoV-2 RBD receptor binding domain | CVB3 | Type I intron self-splicing | LNP | Therorna | March 2022 | [30] |
| circRNA RBD | Encodes SARS-CoV-2 RBD receptor binding domain | CVB3 | Type II intron self-splicing | LNP | Circode Bio | May 2022 | [55] |
| VFLIP-X | Encodes SARS-CoV-2 spiking protein | CVB3 | T4 RNA ligase | LNP | Patompon Wongtrakoongate et al. Mahidol University | March 2022 | [94] |
| circRNAOVA-Luc | Encodes OVA (257–264) luciferase | CVB3 | Type I intron self-splicing | LNP | Lin Xin et al. Tsinghua University School of Medicine | August 2022 | [9] |
| CircRNA encoding cytokines | Encodes IL-15, IL-12, GM-CSF, IFN-α2b | EV29 | Clean-PIE | LNP | CureMed | September 2022 | [45] |
| IL12-circRNA | IL-12 | CVB3 | Type I intron self-splicing | LNP | B. Li, University Health Network Toronto | April 2024 | [92] |
| cirA29L, cirA35R, cirB6R, and cirM1R | MPXV proteins A29L, A35R, B6R, and M1R | / | Type II intron self-splicing | LNP | Circode Bio | April 2024 | [95] |
5.3. Exosome Delivery
5.4. Other Potential Delivery Vectors
6. Applications of RNA Vaccines
6.1. Vaccines for Infectious Diseases
6.2. Anti-Tumor Vaccines
7. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, C.; Zhang, B.L. RNA therapeutics: Updates and future potential. Sci. China-Life Sci. 2023, 66, 12–30. [Google Scholar] [CrossRef]
- Sanger, H.L.; Klotz, G.; Riesner, D.; Gross, H.J.; Kleinschmidt, A.K. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc. Natl. Acad. Sci. USA 1976, 73, 3852–3856. [Google Scholar] [CrossRef] [PubMed]
- Patop, I.L.; Wüst, S.; Kadener, S. Past, present, and future of circRNAs. Embo J. 2019, 38, e100836. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Shi, S.; Zhang, W.; Li, C.; Sun, L.; Ge, Q.; Li, X. Circ_RPPH1 facilitates progression of breast cancer via miR-1296-5p/TRIM14 axis. Cancer Biol. Ther. 2024, 25, 2360768. [Google Scholar] [CrossRef]
- Li, F.; Zhang, L.; Li, W.; Deng, J.; Zheng, J.; An, M.; Lu, J.; Zhou, Y. Circular RNA ITCH has inhibitory effect on ESCC by suppressing the Wnt/β-catenin pathway. Oncotarget 2015, 6, 6001–6013. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Jin, P.; Chen, Y.; Zhang, C.; Geng, X.; Mun, K.S.; Phang, K.C. New insights into the potential of exosomal circular RNAs in mediating cancer chemotherapy resistance and their clinical applications. Biomed. Pharmacother. 2024, 177, 117027. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, X.J.; Mao, M.W.; Song, X.W.; Wu, P.; Zhang, Y.; Jin, Y.F.; Yang, Y.; Chen, L.L.; Wang, Y.; et al. Extensive translation of circular RNAs driven by N6-methyladenosine. Cell Res. 2017, 27, 626–641. [Google Scholar] [CrossRef] [PubMed]
- Wesselhoeft, R.A.; Kowalski, P.S.; Anderson, D.G. Engineering circular RNA for potent and stable translation in eukaryotic cells. Nat. Commun. 2018, 9, 2629. [Google Scholar] [CrossRef]
- Li, H.J.; Peng, K.; Yang, K.; Ma, W.B.; Qi, S.L.; Yu, X.Y.; He, J.; Lin, X.; Yu, G.C. Circular RNA cancer vaccines drive immunity in hard-to-treat malignancies. Theranostics 2022, 12, 6422–6436. [Google Scholar] [CrossRef]
- Ju, J.; Song, Y.N.; Chen, X.Z.; Wang, T.; Liu, C.Y.; Wang, K. circRNA is a potential target for cardiovascular diseases treatment. Mol. Cell. Biochem. 2022, 477, 417–430. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.W.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, Y.; Asemani, Y.; Aghamiri, S.; Ezzatifar, F.; Najafi, S. CiRS-7/CDR1as; An oncogenic circular RNA as a potential cancer biomarker. Pathol. Res. Pract. 2021, 227, 153639. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.Y.; Cao, Q.D.; Zhao, Z.; Song, C.L. Biogenesis, Features, Functions, and Disease Relationships of a Specific Circular RNA: CDR1as. Aging Dis. 2020, 11, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.Y.; Chen, J.R.; Wang, M.L.; Weng, W.Z.; Chen, Y.; Pan, Y.F. Rheumatoid arthritis fibroblast-like synoviocytes maintain tumor-like biological characteristics through ciRS-7-dependent regulation of miR-7. Mol. Biol. Rep. 2022, 49, 8473–8483. [Google Scholar] [CrossRef]
- Lei, M.; Zheng, G.T.; Ning, Q.Q.; Zheng, J.N.; Dong, D. Translation and functional roles of circular RNAs in human cancer. Mol. Cancer 2020, 19, 30. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, L.; Geng, S.; Yang, L.; Lv, X.; Xin, S.; Xu, T. CircMIB2 therapy can effectively treat pathogenic infection by encoding a novel protein. Cell Death Dis. 2023, 14, 578. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.F. Efficient backsplicing produces translatable circular mRNAs. Rna 2015, 21, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Sarnow, P. Initiation of protein synthesis by the eukaryotic translational apparatus on circular RNAs. Science 1995, 268, 415–417. [Google Scholar] [CrossRef]
- Zhang, L.L.; Hou, C.F.; Chen, C.; Guo, Y.X.; Yuan, W.T.; Yin, D.T.; Liu, J.B.; Sun, Z.Q. The role of N6-methyladenosine (m6A) modification in the regulation of circRNAs. Mol. Cancer 2020, 19, 105. [Google Scholar] [CrossRef]
- Duan, J.L.; Chen, W.; Xie, J.J.; Zhang, M.L.; Nie, R.C.; Liang, H.; Mei, J.; Han, K.; Xiang, Z.C.; Wang, F.W.; et al. A novel peptide encoded by N6-methyladenosine modified circMAP3K4 prevents apoptosis in hepatocellular carcinoma. Mol. Cancer 2022, 21, 93. [Google Scholar] [CrossRef]
- Chen, C.K.; Cheng, R.; Demeter, J.; Chen, J.; Weingarten-Gabbay, S.; Jiang, L.; Snyder, M.P.; Weissman, J.S.; Segal, E.; Jackson, P.K.; et al. Structured elements drive extensive circular RNA translation. Mol. Cell 2021, 81, 4300–4318.e4313. [Google Scholar] [CrossRef]
- Legnini, I.; Di Timoteo, G.; Rossi, F.; Morlando, M.; Briganti, F.; Sthandier, O.; Fatica, A.; Santini, T.; Andronache, A.; Wade, M.; et al. Circ-ZNF609 Is a Circular RNA that Can Be Translated and Functions in Myogenesis. Mol. Cell 2017, 66, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.F.; Du, H.Z.; Wu, X.W.; Guo, Q.; Zhang, F.H.; Gong, Z.Q.; Jiao, T.; Guo, J.; Kong, Y. FMRP ligand circZNF609 destabilizes RAC1 mRNA to reduce metastasis in acral melanoma and cutaneous melanoma. J. Exp. Clin. Cancer Res. 2022, 41, 170. [Google Scholar] [CrossRef]
- Wang, K.Q.; Ye, M.L.; Qiao, X.; Yu, Z.W.; Wu, C.X.; Zheng, J.F. Circular RNA Fibroblast Growth Factor Receptor 1 Promotes Pancreatic Cancer Progression by Targeting MicroRNA-532-3p/PIK3CB Axis. Pancreas 2022, 51, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Abe, N.; Matsumoto, K.; Nishihara, M.; Nakano, Y.; Shibata, A.; Maruyama, H.; Shuto, S.; Matsuda, A.; Yoshida, M.; Ito, Y.; et al. Rolling Circle Translation of Circular RNA in Living Human Cells. Sci. Rep. 2015, 5, 16435. [Google Scholar] [CrossRef]
- Hobernik, D.; Bros, M. DNA Vaccines-How Far From Clinical Use? Int. J. Mol. Sci. 2018, 19, 3605. [Google Scholar] [CrossRef]
- Ju, M.; Kim, D.; Son, G.; Han, J. Circular RNAs in and out of Cells: Therapeutic Usages of Circular RNAs. Mol. Cells 2023, 46, 33–40. [Google Scholar] [CrossRef]
- Chen, R.; Wang, S.K.; Belk, J.A.; Amaya, L.; Li, Z.J.; Cardenas, A.; Abe, B.T.; Chen, C.K.; Wender, P.A.; Chang, H.Y. Engineering circular RNA for enhanced protein production. Nat. Biotechnol. 2023, 41, 262–272. [Google Scholar] [CrossRef]
- Costello, A.; Lao, N.T.; Barron, N.; Clynes, M. Continuous translation of circularized mRNA improves recombinant protein titer. Metab. Eng. 2019, 52, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Yi, Z.; Shen, Y.; Lin, L.; Chen, F.; Xu, Y.; Wu, Z.; Tang, H.; Zhang, X.; Tian, F.; et al. Circular RNA vaccines against SARS-CoV-2 and emerging variants. Cell 2022, 185, 1728–1744. [Google Scholar] [CrossRef]
- Chen, Y.G.; Kim, M.V.; Chen, X.; Batista, P.J.; Aoyama, S.; Wilusz, J.E.; Iwasaki, A.; Chang, H.Y. Sensing Self and Foreign Circular RNAs by Intron Identity. Mol. Cell 2017, 67, 228–238. [Google Scholar] [CrossRef]
- Ren, Y.; Manoharan, T.; Liu, B.J.; Cheng, C.Z.M.; Siew, B.E.; Cheong, W.K.; Lee, K.Y.; Tan, I.J.W.; Lieske, B.; Tan, K.K.; et al. Circular RNA as a source of neoantigens for cancer vaccines. J. Immunother. Cancer 2024, 12, e008402. [Google Scholar] [CrossRef]
- Hu, Z.T.; Leet, D.E.; Allesoe, R.L.; Oliveira, G.; Li, S.Q.; Luoma, A.M.; Liu, J.Y.; Forman, J.; Huang, T.; Iorgulescu, J.B.; et al. Personal neoantigen vaccines induce persistent memory T cell responses and epitope spreading in patients with melanoma. Nat. Med. 2021, 27, 515–525. [Google Scholar] [CrossRef]
- Mork, S.K.; Kadivar, M.; Bol, K.F.; Draghi, A.; Westergaard, M.C.W.; Skadborg, S.K.; Overgaard, N.; Sorensen, A.B.; Rasmussen, I.S.; Andreasen, L.V.; et al. Personalized therapy with peptide-based neoantigen vaccine (EVX-01) including a novel adjuvant, CAF®®09b, in patients with metastatic melanoma. Oncoimmunology 2022, 11, 2023255. [Google Scholar] [CrossRef]
- Rabu, C.; Rangan, L.; Florenceau, L.; Fortu, A.; Charpentier, M.; Dupré, E.; Paolini, L.; Beauvillain, C.; Dupel, E.; Latouche, J.B.; et al. Cancer vaccines: Designing artificial synthetic long peptides to improve presentation of class I and class II T cell epitopes by dendritic cells. Oncoimmunology 2019, 8, 1560919. [Google Scholar] [CrossRef]
- Lv, Z.; Zhang, X.; Zhao, K.; Du, L.; Wang, X.; Chu, Y.; Huang, T. Co-immunization with DNA vaccines encoding yidR and IL-17 augments host immune response against Klebsiella pneumoniae infection in mouse model. Virulence 2024, 15, 2345019. [Google Scholar] [CrossRef]
- Zhu, L.F.; Cui, X.J.; Yan, Z.L.; Tao, Y.F.; Shi, L.; Zhang, X.W.; Yao, Y.F.; Shi, L. Design and evaluation of a multi-epitope DNA vaccine against HPV16. Hum. Vaccines Immunother. 2024, 20, 2352908. [Google Scholar] [CrossRef] [PubMed]
- Jaber, H.M.; Ebdah, S.; Mahmoud, S.A.A.; Abu-Qatouseh, L.; Jaber, Y.H. Comparison of T cells mediated immunity and side effects of mRNA vaccine and conventional COVID-19 vaccines administrated in Jordan. Hum. Vaccines Immunother. 2024, 20, 2333104. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.M.; Liu, X.L.; Wang, Y.J.; Pan, M.L.; Wang, S.Q.; Shi, L.; Ni, B.B. Long-term stability and immunogenicity of lipid nanoparticle COVID-19 mRNA vaccine is affected by particle size. Hum. Vaccines Immunother. 2024, 20, 2342592. [Google Scholar] [CrossRef]
- Yao, R.H.; Xie, C.Y.; Xia, X.J. Recent progress in mRNA cancer vaccines. Hum. Vaccines Immunother. 2024, 20, 2307187. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Wang, C.; Lu, Y. Tactics targeting circular mRNA biosynthesis. Biotechnol. Bioeng. 2023, 120, 1975–1985. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.W.; Wang, Z.M.; Wang, L.L.; Wu, L.Q.; Zhang, C.G.; Zhou, M.; Fu, Z.F.; Zhao, L. Circular RNA vaccines with long-term lymph node-targeting delivery stability after lyophilization induce potent and persistent immune responses. Mbio 2024, 15, e01775-23. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cai, G.; Wang, Y.; Zhuang, Q.; Cai, Z.; Li, Y.; Gao, S.; Li, F.; Zhang, C.; Zhao, B.; et al. Circular RNA-based neoantigen vaccine for hepatocellular carcinoma immunotherapy. MedComm 2024, 5, e667. [Google Scholar] [CrossRef] [PubMed]
- Amaya, L.; Grigoryan, L.; Li, Z.J.; Lee, A.; Wender, P.A.; Pulendran, B.; Chang, H.Y. Circular RNA vaccine induces potent T cell responses. Proc. Natl. Acad. Sci. USA 2023, 120, e2302191120. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.; Zhu, J.F.; Sun, J.J.; Chen, Y.Y.; Du, Y.R.; Tan, Y.L.; Wu, L.P.; Zhai, M.T.; Wei, L.X.; Li, N.; et al. Intratumoral delivered novel circular mRNA encoding cytokines for immune modulation and cancer therapy. Mol. Ther. Nucleic Acids 2022, 30, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, D.; Tsanova, B.; Barbas, A.; Reis, F.P.; Dastidar, E.G.; Sanchez-Rotunno, M.; Arraiano, C.M.; van Hoof, A. The exosome contains domains with specific endoribonuclease, exoribonuclease and cytoplasmic mRNA decay activities. Nat. Struct. Mol. Biol. 2009, 16, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Xiao, M.S.; Li, Z.G.; Huang, C. New progresses of circular RNA biology: From nuclear export to degradation. Rna Biol. 2021, 18, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Vergnes, J.N. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2021, 384, 1577. [Google Scholar] [CrossRef]
- Vishweshwaraiah, Y.L.; Dokholyan, N.V. mRNA vaccines for cancer immunotherapy. Front. Immunol. 2022, 13, 1029069. [Google Scholar] [CrossRef]
- Liu, J.; Guo, C.; Fu, J.; Liu, D.; Liu, G.; Sun, B.; Deng, M.; Guo, Y.; Li, Y. Identification and Functional Analysis of circRNAs during Goat Follicular Development. Int. J. Mol. Sci. 2024, 25, 7548. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.K.; Dwivedi, P.; Bhushan, R.; Maurya, P.K.; Kumar, A.; Dakal, T.C. Engineering circular RNA for molecular and metabolic reprogramming. Funct. Integr. Genom. 2024, 24, 117. [Google Scholar] [CrossRef] [PubMed]
- Su, C.I.; Chuang, Z.S.; Shie, C.T.; Wang, H.I.; Kao, Y.T.; Yu, C.Y. A cis-acting ligase ribozyme generates circular RNA in vitro for ectopic protein functioning. Nat. Commun. 2024, 15, 6607. [Google Scholar] [CrossRef] [PubMed]
- Unti, M.J.; Jaffrey, S.R. Highly efficient cellular expression of circular mRNA enables prolonged protein expression. Cell Chem. Biol. 2024, 31, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wei, H.; Zhang, K.; Li, Z.; Wei, T.; Tang, C.; Yang, Y.; Wang, Z.J.B. A flexible, efficient, and scalable platform to produce circular RNAs as new therapeutics. BioRxiv 2022. [Google Scholar] [CrossRef]
- Jackson, R.J.; Hellen, C.U.T.; Pestova, T.V. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol. 2010, 11, 113–127. [Google Scholar] [CrossRef]
- Rong, M.; He, B.; McAllister, W.T.; Durbin, R.K. Promoter specificity determinants of T7 RNA polymerase. Proc. Natl. Acad. Sci. USA 1998, 95, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Usman, N.; Cedergren, R. Exploiting the chemical synthesis of RNA. Trends Biochem. Sci. 1992, 17, 334–339. [Google Scholar] [CrossRef]
- Obi, P.; Chen, Y.G. The design and synthesis of circular RNAs. Methods 2021, 196, 85–103. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhang, X.Y.; Zou, Y.X.Y.; Li, J.; Chang, L.; He, Y.C.; Jin, Q.H.; Ye, J.R. Effective synthesis of circRNA via a thermostable T7 RNA polymerase variant as the catalyst. Front. Bioeng. Biotechnol. 2024, 12, 1356354. [Google Scholar] [CrossRef]
- Chen, X.; Lu, Y. Circular RNA: Biosynthesis in vitro. Front. Bioeng. Biotechnol. 2021, 9, 787881. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Liu, D.; He, Q.; Liu, J.Y.; Mao, Q.Y.; Liang, Z.L. Research progress on circular RNA vaccines. Front. Immunol. 2023, 13, 1091797. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Kim, S.; Lee, S.W. Pros and Cons of In Vitro Methods for Circular RNA Preparation. Int. J. Mol. Sci. 2022, 23, 13247. [Google Scholar] [CrossRef]
- Costello, A.; Lao, N.T.; Barron, N.; Clynes, M. Reinventing the Wheel: Synthetic Circular RNAs for Mammalian Cell Engineering. Trends Biotechnol. 2020, 38, 217–230. [Google Scholar] [CrossRef]
- Breuer, J.; Rossbach, O. Production and Purification of Artificial Circular RNA Sponges for Application in Molecular Biology and Medicine. Methods Protoc. 2020, 3, 42. [Google Scholar] [CrossRef]
- Carmona, E.M. Circular RNA: Design Criteria for Optimal Therapeutical Utility; Harvard University: Cambridge, MA, USA, 2019. [Google Scholar]
- Puttaraju, M.; Been, M.D. Group I permuted intron-exon (PIE) sequences self-splice to produce circular exons. Nucleic Acids Res. 1992, 20, 5357–5364. [Google Scholar] [CrossRef]
- Ford, E.; Ares, M., Jr. Synthesis of circular RNA in bacteria and yeast using RNA cyclase ribozymes derived from a group I intron of phage T4. Proc. Natl. Acad. Sci. USA 1994, 91, 3117–3121. [Google Scholar] [CrossRef]
- Liu, C.X.; Guo, S.K.; Nan, F.; Xu, Y.F.; Yang, L.; Chen, L.L. RNA circles with minimized immunogenicity as potent PKR inhibitors. Mol. Cell 2022, 82, 420–434. [Google Scholar] [CrossRef]
- Qiu, Z.; Zhao, Y.; Hou, Q.; Zhu, J.; Zhai, M.; Li, D.; Li, Y.; Liu, C.; Li, N.; Cao, Y.J.b. Clean-PIE: A novel strategy for efficiently constructing precise circRNA with thoroughly minimized immunogenicity to direct potent and durable protein expression. BioRxiv 2022. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Chen, L.L. Characterization of circular RNAs. Long Non Coding RNAs Methods Protocols 2016, 1402, 215–227. [Google Scholar]
- Zhang, N.N.; Li, X.F.; Deng, Y.Q.; Zhao, H.; Huang, Y.J.; Yang, G.; Huang, W.J.; Gao, P.; Zhou, C.; Zhang, R.R.; et al. A Thermostable mRNA Vaccine against COVID-19. Cell 2020, 182, 1271–1283. [Google Scholar] [CrossRef]
- Niu, M.T.; Wang, C.Y.; Chen, Y.J.; Zou, Q.; Xu, L. Identification, characterization and expression analysis of circRNA encoded by SARS-CoV-1 and SARS-CoV-2. Brief. Bioinform. 2024, 25, bbad537. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Li, Y.; Li, S.Q.; Jia, L.; Wang, H.M.; Li, M.; Deng, J.; Zhu, A.L.; Ma, L.Q.; Li, W.H.; et al. The nano delivery systems and applications of mRNA. Eur. J. Med. Chem. 2022, 227, 113910. [Google Scholar] [CrossRef] [PubMed]
- Paunovska, K.; Loughrey, D.; Dahlman, J.E. Drug delivery systems for RNA therapeutics. Nat. Rev. Genet. 2022, 23, 265–280. [Google Scholar] [CrossRef]
- Li, Y.D.; Chi, W.Y.; Su, J.H.; Ferrall, L.; Hung, C.F.; Wu, T.C. Coronavirus vaccine development: From SARS and MERS to COVID-19. J. Biomed. Sci. 2020, 27, 104. [Google Scholar] [CrossRef]
- Sun, X.; Zeng, L.; Huang, Y. Transcutaneous delivery of DNA/mRNA for cancer therapeutic vaccination. J. Gene Med. 2019, 21, e3089. [Google Scholar] [CrossRef]
- Husseini, R.A.; Abe, N.; Hara, T.; Abe, H.; Kogure, K. Use of Iontophoresis Technology for Transdermal Delivery of a Minimal mRNA Vaccine as a Potential Melanoma Therapeutic. Biol. Pharm. Bull. 2023, 46, 301–308. [Google Scholar] [CrossRef]
- Gregoriadis, G. Liposomes in Drug Delivery: How It All Happened. Pharmaceutics 2016, 8, 19. [Google Scholar] [CrossRef]
- Harashima, H.; Sakata, K.; Funato, K.; Kiwada, H. Enhanced hepatic uptake of liposomes through complement activation depending on the size of liposomes. Pharm. Res. 1994, 11, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. mRNA vaccines for infectious diseases: Principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021, 20, 817–838. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.C.; Zaks, T.; Langer, R.; Dong, Y.Z. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Han, X.X.; Zhang, H.W.; Butowska, K.; Swingle, K.L.; Alameh, M.G.; Weissman, D.; Mitchell, M.J. An ionizable lipid toolbox for RNA delivery. Nat. Commun. 2021, 12, 7233. [Google Scholar] [CrossRef] [PubMed]
- Nitika; Wei, J.; Hui, A.M. The Delivery of mRNA Vaccines for Therapeutics. Life 2022, 12, 1254. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fang, H.T.; Zhang, T.; Wang, Y.; Qi, T.T.; Li, B.; Jiao, H.P. Lipid-mRNA nanoparticles landscape for cancer therapy. Front. Bioeng. Biotechnol. 2022, 10, 1053197. [Google Scholar] [CrossRef] [PubMed]
- Eygeris, Y.; Gupta, M.; Kim, J.; Sahay, G. Chemistry of Lipid Nanoparticles for RNA Delivery. Acc. Chem. Res. 2022, 55, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Ball, R.L.; Hajj, K.A.; Vizelman, J.; Bajaj, P.; Whitehead, K.A. Lipid Nanoparticle Formulations for Enhanced Co-delivery of siRNA and mRNA. Nano Lett. 2018, 18, 3814–3822. [Google Scholar] [CrossRef] [PubMed]
- Cullis, P.R.; Hope, M.J. Lipid Nanoparticle Systems for Enabling Gene Therapies. Mol. Ther. 2017, 25, 1467–1475. [Google Scholar] [CrossRef]
- Wesselhoeft, R.A.; Kowalski, P.S.; Parker-Hale, F.C.; Huang, Y.X.; Bisaria, N.; Anderson, D.G. RNA Circularization Diminishes Immunogenicity and Can Extend Translation Duration In Vivo. Mol. Cell 2019, 74, 508–520. [Google Scholar] [CrossRef]
- Xu, S.F.; Xu, Y.; Solek, N.C.; Chen, J.A.; Gong, F.L.; Varley, A.J.; Golubovic, A.; Pan, A.N.; Dong, S.T.; Zheng, G.; et al. Tumor-Tailored Ionizable Lipid Nanoparticles Facilitate IL-12 Circular RNA Delivery for Enhanced Lung Cancer Immunotherapy. Adv. Mater. 2024, 36, 2400307. [Google Scholar] [CrossRef]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyártó, B.Z. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. Iscience 2021, 24, 103479. [Google Scholar] [CrossRef]
- Seephetdee, C.; Bhukhai, K.; Buasri, N.; Leelukkanaveera, P.; Lerdwattanasombat, P.; Manopwisedjaroen, S.; Phueakphud, N.; Kuhaudomlarp, S.; Olmedillas, E.; Saphire, E.O.; et al. A circular mRNA vaccine prototype producing VFLIP-X spike confers a broad neutralization of SARS-CoV-2 variants by mouse sera. Antivir. Res. 2022, 204, 105370. [Google Scholar] [CrossRef]
- Zhou, J.; Ye, T.; Yang, Y.; Li, E.; Zhang, K.; Wang, Y.; Chen, S.; Hu, J.; Zhang, K.; Liu, F.; et al. Circular RNA vaccines against monkeypox virus provide potent protection against vaccinia virus infection in mice. Mol. Ther. J. Am. Soc. Gene Ther. 2024, 32, 1779–1789. [Google Scholar] [CrossRef]
- Zhou, Q.; Fang, L.; Tang, Y.C.; Wang, Q.; Tang, X.; Zhu, L.X.; Peng, N.; Wang, B.Y.; Song, W.K.; Fu, H. Exosome-mediated delivery of artificial circular RNAs for gene therapy of bladder cancer. J. Cancer 2024, 15, 1770–1778. [Google Scholar] [CrossRef]
- Moon, B.; Chang, S. Exosome as a Delivery Vehicle for Cancer Therapy. Cells 2022, 11, 316. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Bagherifar, R.; Dezfouli, E.A.; Kiaie, S.H.; Jafari, R.; Ramezani, R. Exosomes as bio-inspired nanocarriers for RNA delivery: Preparation and applications. J. Transl. Med. 2022, 20, 125. [Google Scholar] [CrossRef]
- Sun, Y.F.; Pi, J.; Xu, J.F. Emerging Role of Exosomes in Tuberculosis: From Immunity Regulations to Vaccine and Immunotherapy. Front. Immunol. 2021, 12, 628973. [Google Scholar] [CrossRef]
- Li, Y.J.; Wu, J.Y.; Liu, J.H.; Xu, W.J.; Qiu, X.H.; Huang, S.; Hu, X.B.; Xiang, D.X. Artificial exosomes for translational nanomedicine. J. Nanobiotechnol. 2021, 19, 242. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Zhao, X.; Zhong, Y.X.; Shen, J.H.; An, W.L. Biomimetic Exosomes: A New Generation of Drug Delivery System. Front. Bioeng. Biotechnol. 2022, 10, 865682. [Google Scholar] [CrossRef]
- Lu, Y.C.; Huang, W.; Li, M.; Zheng, A.P. Exosome-Based Carrier for RNA Delivery: Progress and Challenges. Pharmaceutics 2023, 15, 598. [Google Scholar] [CrossRef] [PubMed]
- Du, W.W.; Fang, L.; Yang, W.N.; Wu, N.; Awan, F.M.; Yang, Z.G.; Yang, B.B. Induction of tumor apoptosis through a circular RNA enhancing Foxo3 activity. Cell Death Differ. 2017, 24, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.J.; Guo, C.; Sedgwick, A.; Kanagavelu, S.; Nice, J.; Shetty, S.; Landaverde, C.; Atai, N.A.; Gould, S.J. Exosome-mediated mRNA delivery for SARS-CoV-2 vaccination. BioRxiv 2020. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhang, H.Y.; Yang, H.O.; Bai, M.; Ning, T.; Deng, T.; Liu, R.; Fan, Q.; Zhu, K.G.; Li, J.L.; et al. Exosome-delivered circRNA promotes glycolysis to induce chemoresistance through the miR-122-PKM2 axis in colorectal cancer. Mol. Oncol. 2020, 14, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Han, B.; Zhang, Z.T.; Wang, S.G.; Bai, Y.; Zhang, Y.; Tang, Y.; Du, L.L.; Xu, L.; Wu, F.F.; et al. Extracellular Vesicle-Mediated Delivery of Circular RNA SCMH1 Promotes Functional Recovery in Rodent and Nonhuman Primate Ischemic Stroke Models. Circulation 2020, 142, 556–574. [Google Scholar] [CrossRef]
- Cheng, Y.R.; Zeng, Q.Y.; Han, Q.; Xia, W.L. Effect of pH, temperature and freezing-thawing on quantity changes and cellular uptake of exosomes. Protein Cell 2019, 10, 295–299. [Google Scholar] [CrossRef]
- Loan Young, T.; Chang Wang, K.; James Varley, A.; Li, B. Clinical delivery of circular RNA: Lessons learned from RNA drug development. Adv. Drug Deliv. Rev. 2023, 197, 114826. [Google Scholar] [CrossRef]
- Jarzebska, N.T.; Mellett, M.; Frei, J.; Kundig, T.M.; Pascolo, S. Protamine-Based Strategies for RNA Transfection. Pharmaceutics 2021, 13, 877. [Google Scholar] [CrossRef]
- Fotin-Mleczek, M.; Duchardt, K.M.; Lorenz, C.; Pfeiffer, R.; Ojkic-Zrna, S.; Probst, J.; Kallen, K.J. Messenger RNA-based Vaccines With Dual Activity Induce Balanced TLR-7 Dependent Adaptive Immune Responses and Provide Antitumor Activity. J. Immunother. 2011, 34, 1–15. [Google Scholar] [CrossRef]
- Zhong, R.B.; Talebian, S.; Mendes, B.B.; Wallace, G.; Langer, R.; Conde, J.; Shi, J.J. Hydrogels for RNA delivery. Nat. Mater. 2023, 22, 818–831. [Google Scholar] [CrossRef]
- Flemming, A. mRNA vaccine shows promise in autoimmunity. Nat. Rev. Immunol. 2021, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.R.; Wang, X.L.; Li, M.C.; Yuan, Y.H.; Chen, Y.; Zou, D.D.; Bian, L.J.; Li, D.S. PDX-1 mRNA-induced reprogramming of mouse pancreas-derived mesenchymal stem cells into insulin-producing cells in vitro. Clin. Exp. Med. 2015, 15, 501–509. [Google Scholar] [CrossRef]
- Krienke, C.; Kolb, L.; Diken, E.; Streuber, M.; Kirchhoff, S.; Bukur, T.; Akilli-Öztürk, Ö.; Kranz, L.M.; Berger, H.; Petschenka, J.; et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science 2021, 371, 145–153. [Google Scholar] [CrossRef]
- Niu, D.; Wu, Y.R.; Lian, J.Q. Circular RNA vaccine in disease prevention and treatment. Signal Transduct. Target. Ther. 2023, 8, 341. [Google Scholar] [CrossRef]
- Essink, B.; Chu, L.R.C.; Seger, W.; Barranco, E.; Le Cam, N.; Bennett, H.; Faughnan, V.; Pajon, R.; Paila, Y.; Bollman, B.; et al. The safety and immunogenicity of two Zika virus mRNA vaccine candidates in healthy flavivirus baseline seropositive and seronegative adults: The results of two randomised, placebo-controlled, dose-ranging, phase 1 clinical trials. Lancet Infect. Dis. 2023, 23, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Chalkias, S.; Harper, C.; Vrbicky, K.; Walsh, S.R.; Essink, B.; Brosz, A.; McGhee, N.; Tomassini, J.E.; Chen, X.; Chang, Y.; et al. A Bivalent Omicron-Containing Booster Vaccine against COVID-19. N Engl. J. Med. 2022, 387, 1279–1291. [Google Scholar] [CrossRef]
- Huang, K.; Li, N.; Li, Y.; Zhu, J.; Fan, Q.; Yang, J.; Gao, Y.; Liu, Y.; Hou, Q.; Gao, S.; et al. Delivery of Circular mRNA via Degradable Lipid Nanoparticles against SARS-CoV-2 Delta Variant. BioRxiv 2022. [Google Scholar] [CrossRef]
- Arevalo, C.P.; Bolton, M.J.; Le Sage, V.; Ye, N.Q.; Furey, C.; Muramatsu, H.; Alameh, M.G.; Pardi, N.; Drapeau, E.M.; Parkhouse, K.; et al. A multivalent nucleoside-modified mRNA vaccine against all known influenza virus subtypes. Science 2022, 378, 899–904. [Google Scholar] [CrossRef]
- Lee, I.T.; Nachbagauer, R.; Ensz, D.; Schwartz, H.; Carmona, L.; Schaefers, K.; Avanesov, A.; Stadlbauer, D.; Henry, C.; Chen, R.; et al. Safety and immunogenicity of a phase 1/2 randomized clinical trial of a quadrivalent, mRNA-based seasonal influenza vaccine (mRNA-1010) in healthy adults: Interim analysis. Nat. Commun. 2023, 14, 3631. [Google Scholar] [CrossRef]
- Mallory, K.L.; Taylor, J.A.; Zou, X.Y.; Waghela, I.N.; Schneider, C.G.; Sibilo, M.Q.; Punde, N.M.; Perazzo, L.C.; Savransky, T.; Sedegah, M.; et al. Messenger RNA expressing PfCSP induces functional, protective immune responses against malaria in mice. Npj Vaccines 2021, 6, 84. [Google Scholar] [CrossRef] [PubMed]
- Barbier, A.J.; Jiang, A.Y.J.; Zhang, P.; Wooster, R.; Anderson, D.G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 2022, 40, 840–854. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Oehm, P.; Derhovanessian, E.; Jabulowsky, R.A.; Vormehr, M.; Gold, M.; Maurus, D.; Schwarck-Kokarakis, D.; Kuhn, A.N.; Omokoko, T.; et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 2020, 585, 107–112. [Google Scholar] [CrossRef] [PubMed]
- He, K.; McKean, M.; Balaraman, R.; Shah, S.; Arrowsmith, E.; Peguero, J.; Hamm, J.; He, A.; Spira, A.I.; Joshi, R. 599 Single-agent safety and activities of target-preserving anti-CTLA-4 antibody gotistobart (ONC-392/BNT316) in PD-(L) 1 resistant metastatic NSCLC and population PK analysis in patients with solid tumors. BMJ Spec. J. 2023, 11, A1–A1731. [Google Scholar]
- Moore, K.N.; Sabanathan, D.; Du, Y.Q.; Duan, H.X.; Li, X.M.; Wang, F.; Marathe, O.; Yang, H.; Makker, V.; Growdon, W.; et al. Safety and efficacy of DB-1303 in patients with advanced/metastatic solid tumors: A multicenter, open-label, first-in-human, phase 1/2a study. J. Clin. Oncol. 2023, 41, 3023. [Google Scholar] [CrossRef]
- Palmer, C.D.; Rappaport, A.R.; Davis, M.J.; Hart, M.G.; Scallan, C.D.; Hong, S.J.; Gitlin, L.; Kraemer, L.D.; Kounlavouth, S.; Yang, A.R.; et al. Individualized, heterologous chimpanzee adenovirus and self-amplifying mRNA neoantigen vaccine for advanced metastatic solid tumors: Phase 1 trial interim results. Nat. Med. 2022, 28, 1619–1629. [Google Scholar] [CrossRef]
- Huang, D.; Zhu, X.; Ye, S.; Zhang, J.; Liao, J.; Zhang, N.; Zeng, X.; Wang, J.; Yang, B.; Zhang, Y.; et al. Tumour circular RNAs elicit anti-tumour immunity by encoding cryptic peptides. Nature 2024, 625, 593–602. [Google Scholar] [CrossRef]
| Type of Vaccine | Synthetic Long-Peptide Vaccine | DNA Vaccine | mRNA Vaccine | circRNA Vaccine |
|---|---|---|---|---|
| Synthesis | Synthetic long peptides | Plasmid DNA encoding the antigen | mRNA transcribed in vitro | CircRNA constructed in vitro |
| Advantages | Stable Safe Easy to purify Long immunization time | Stable (can be stored at room temperature) Simple production process Low cost Mass production | Fast production Low cost Cytoplasmic translation No risk of genomic integration Self-adjuvant effect | More stable and easy to store Cytoplasmic translation No risk of genomic integration Self-adjuvant effect High antigen expression efficiency |
| Disadvantages | Complex in vitro synthesis High cost Low immune response Potential adverse effects | Risk of genomic integration Must cross the nuclear membrane to be transcribed Carcinogenic risk | Poor stability Easily degradable Harsh storage conditions High risk of side effects | Currently not available for mass production Safety unknown |
| Improvement program | Use immune adjuvants | Vector and promoter optimization Improve inoculation route | Improvement of target gene expression Improve delivery system | Nucleotide modifications Improve cyclization efficiency Improve purification methods |
| Reference | [33,34,35] | [36,37] | [38,39,40] | [28,41,42,43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Fu, Y.; Ju, X.; Zhang, F.; Zhang, P.; He, M. Advances in Engineering Circular RNA Vaccines. Pathogens 2024, 13, 692. https://doi.org/10.3390/pathogens13080692
Zhang Z, Fu Y, Ju X, Zhang F, Zhang P, He M. Advances in Engineering Circular RNA Vaccines. Pathogens. 2024; 13(8):692. https://doi.org/10.3390/pathogens13080692
Chicago/Turabian StyleZhang, Zhongyan, Yuanlei Fu, Xiaoli Ju, Furong Zhang, Peng Zhang, and Meilin He. 2024. "Advances in Engineering Circular RNA Vaccines" Pathogens 13, no. 8: 692. https://doi.org/10.3390/pathogens13080692
APA StyleZhang, Z., Fu, Y., Ju, X., Zhang, F., Zhang, P., & He, M. (2024). Advances in Engineering Circular RNA Vaccines. Pathogens, 13(8), 692. https://doi.org/10.3390/pathogens13080692




