Intraperitoneal Administration of 17-DMAG as an Effective Treatment against Leishmania braziliensis Infection in BALB/c Mice: A Preclinical Study
Abstract
1. Introduction
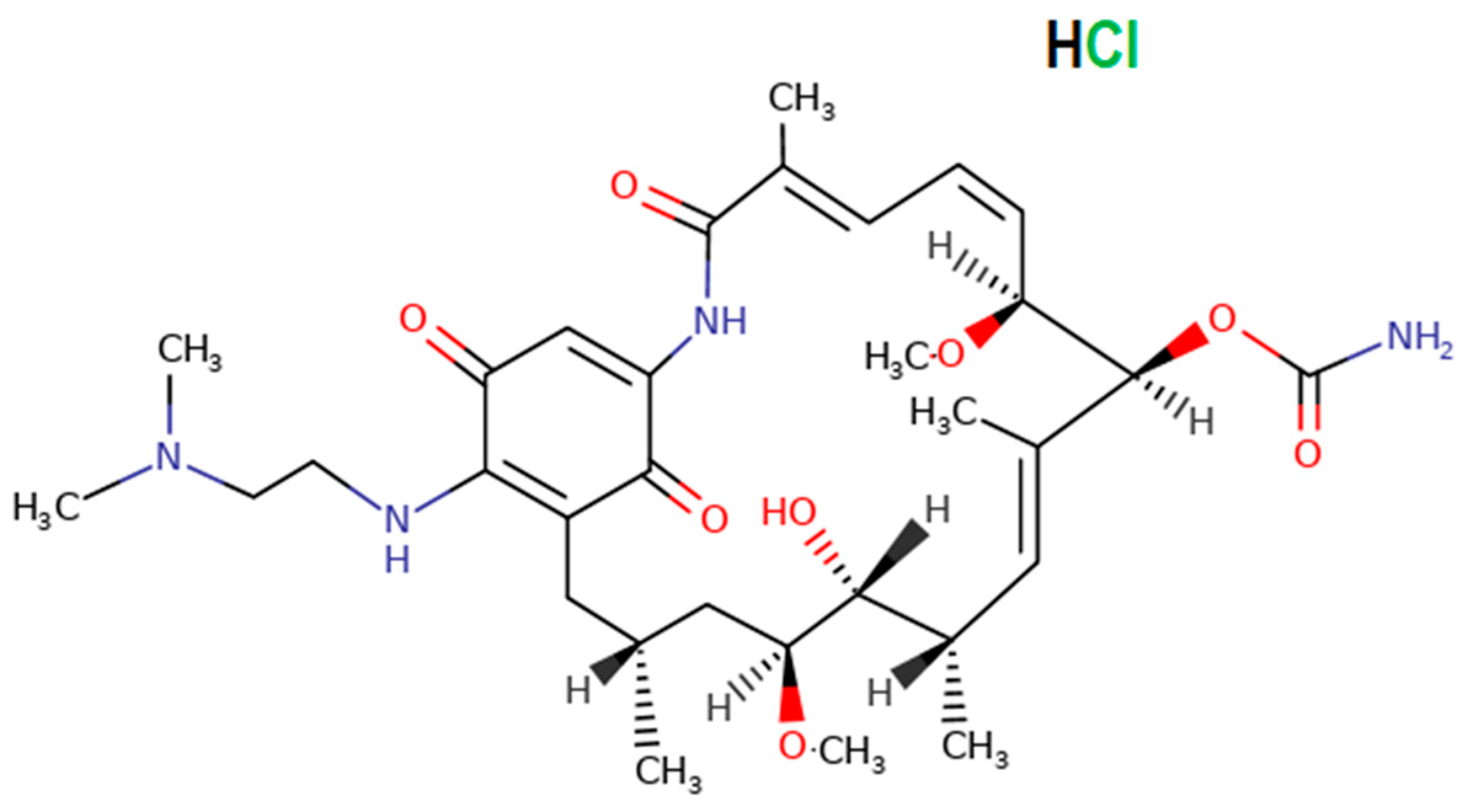
2. Materials and Methods
2.1. Animals
2.2. Parasites
2.3. Isolation and Differentiation of Bone Marrow Precursors into Macrophages (BMMΦ)
2.4. Assessment of the Cytotoxicity of 17-DMAG against BMMΦ In Vitro
2.5. Assessment of the Antileishmanial Efficacy of 17-DMAG against L. braziliensis Promastigotes In Vitro
2.6. Evaluation of the Antileishmanial Efficacy of 17-DMAG against Intracellular Amastigote of L. braziliensis In Vitro
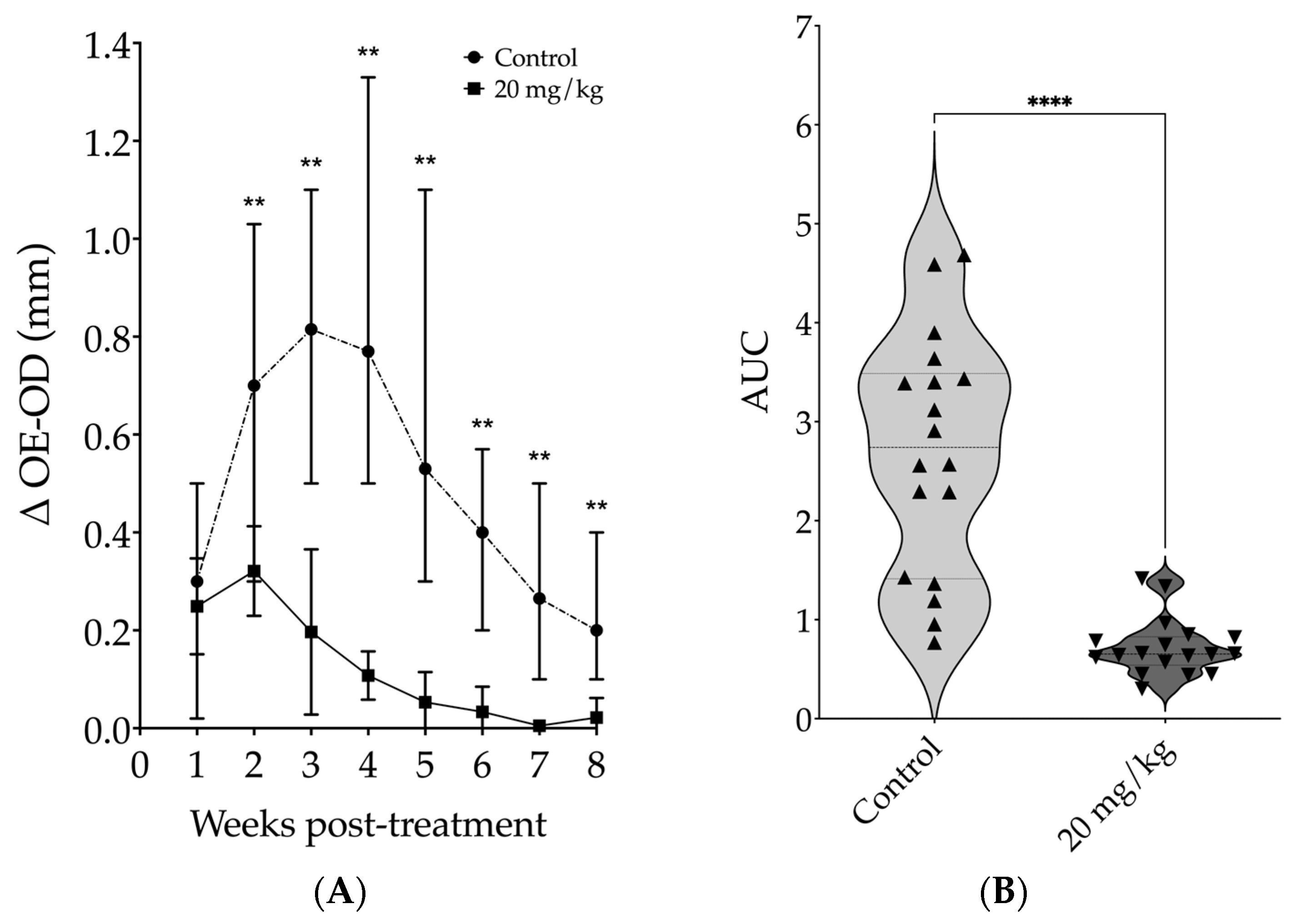
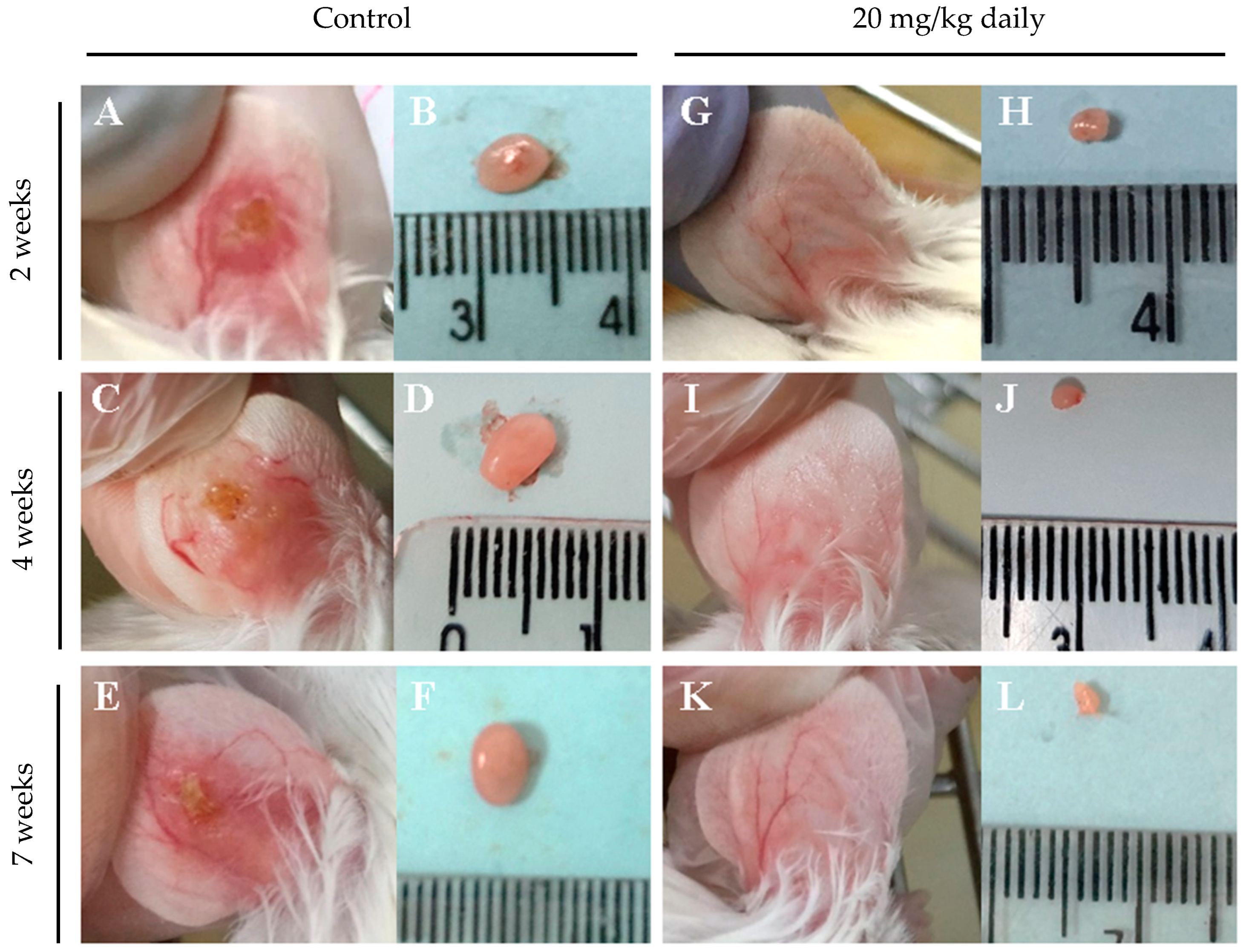
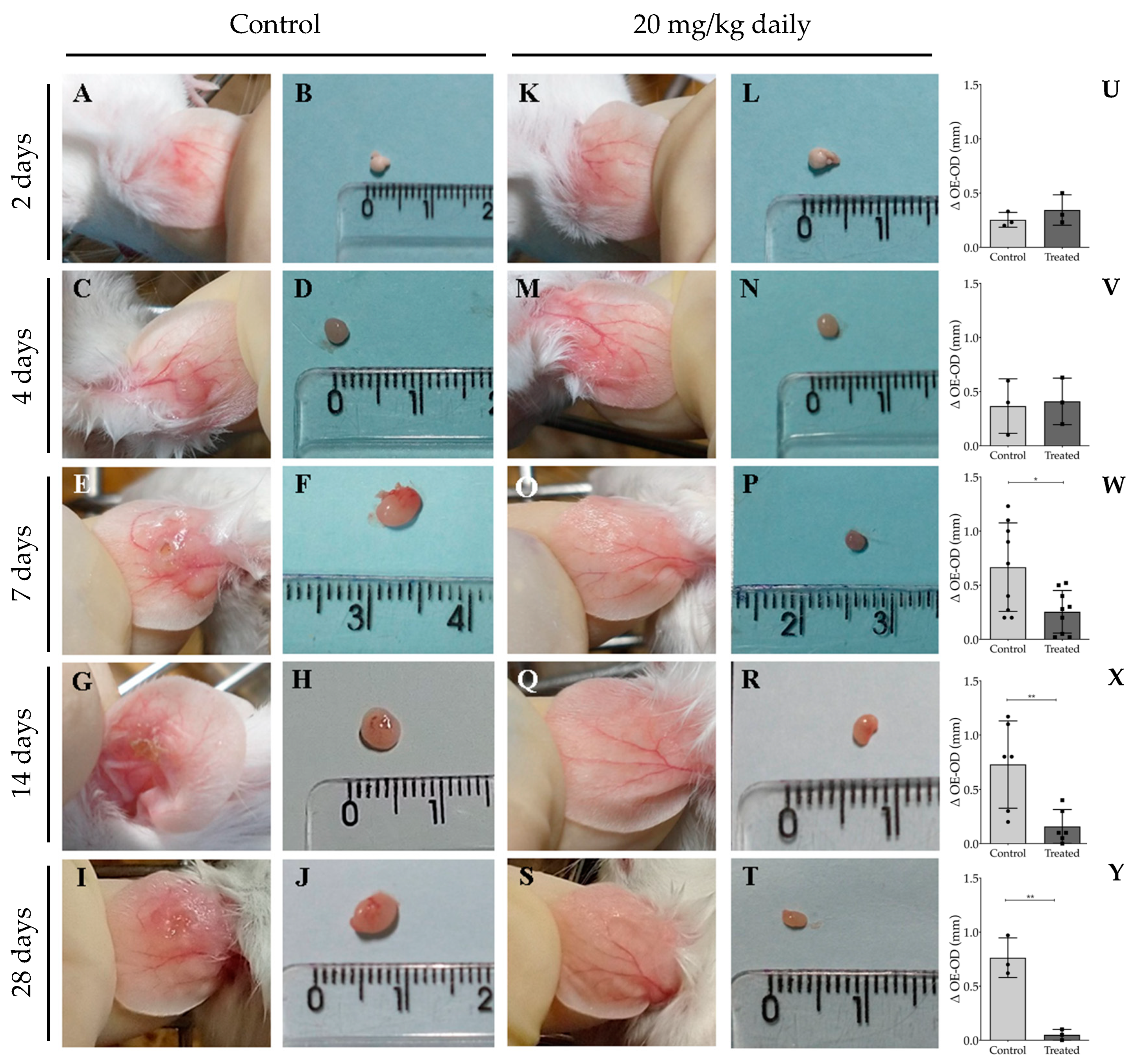
2.7. Long-Term Post-Treatment Observation of Intraperitoneally Administered 17-DMAG to L. braziliensis Infected BALB/c Mice
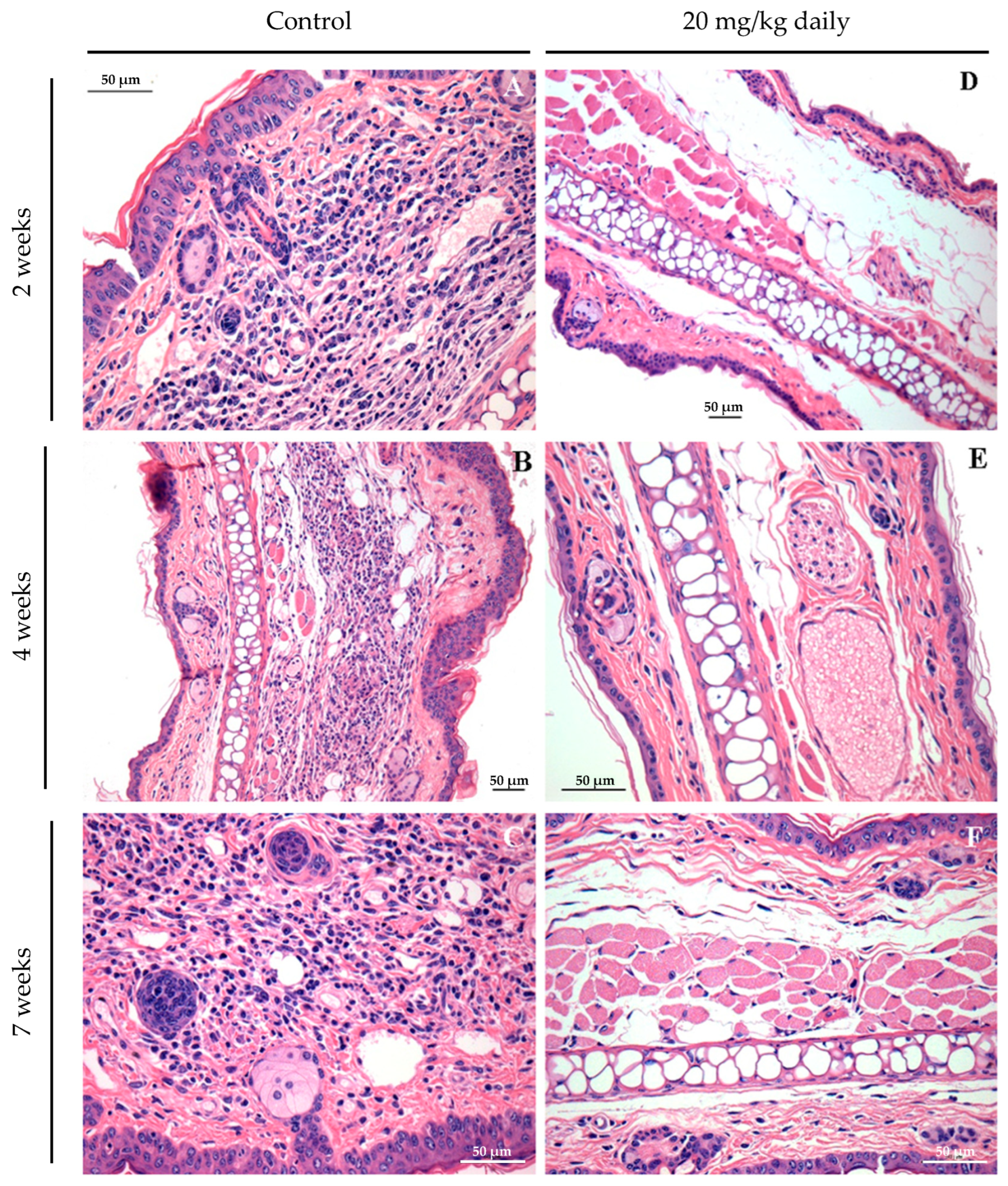
2.8. Evaluation of the Effect of 17-DMAG Treatment on the Inflammatory Infiltrate in Lesions of L. braziliensis-Infected BALB/c Mice
2.9. Evaluation of 17-DMAG Treatment on Pro-Inflammatory Cytokine Release by Lymph Node Cells from L. braziliensis-Infected BALB/c Mice
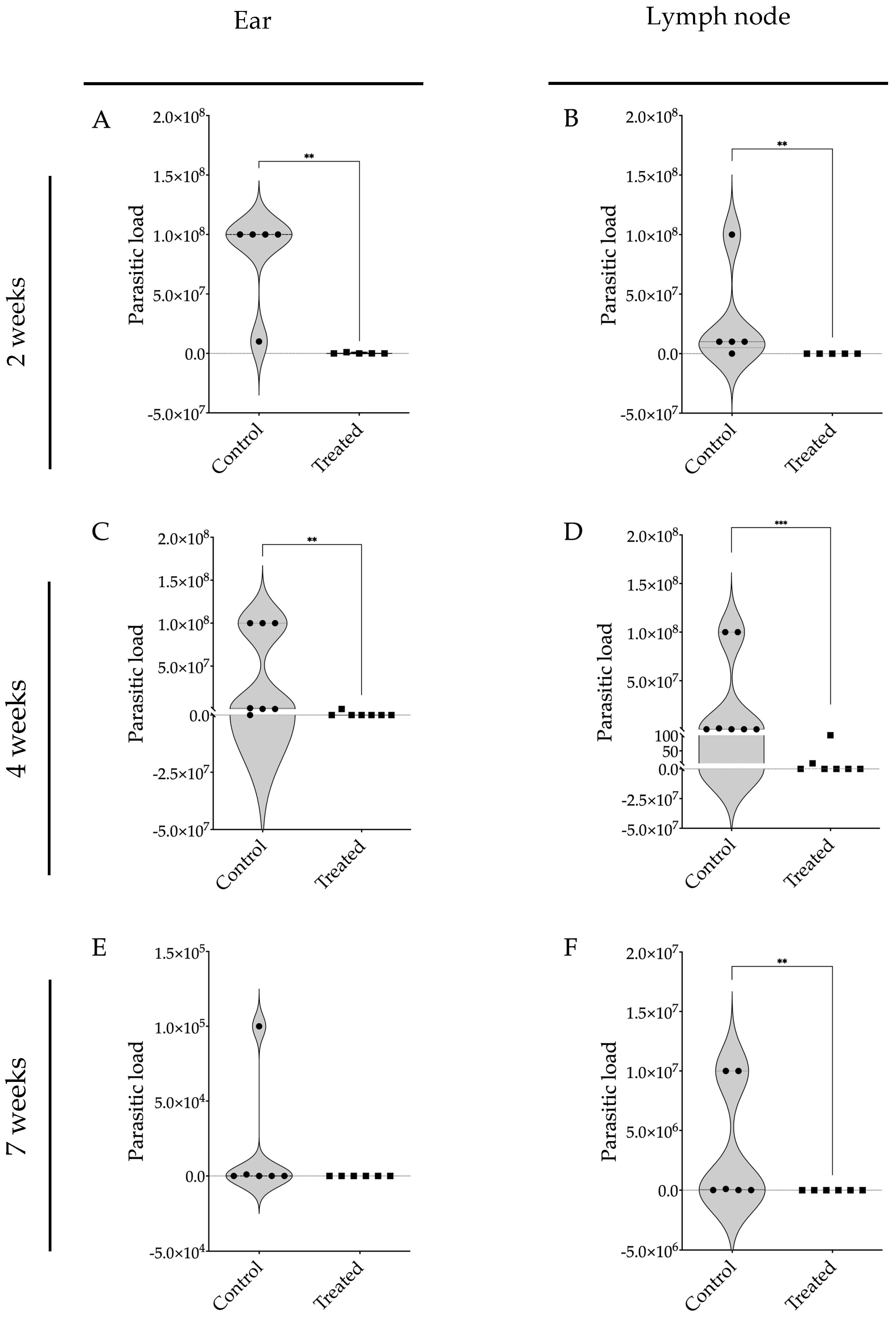
2.10. Quantification of Parasite Burden in BALB/c Mice Infected with L. braziliensis Using Limiting Dilution Assay
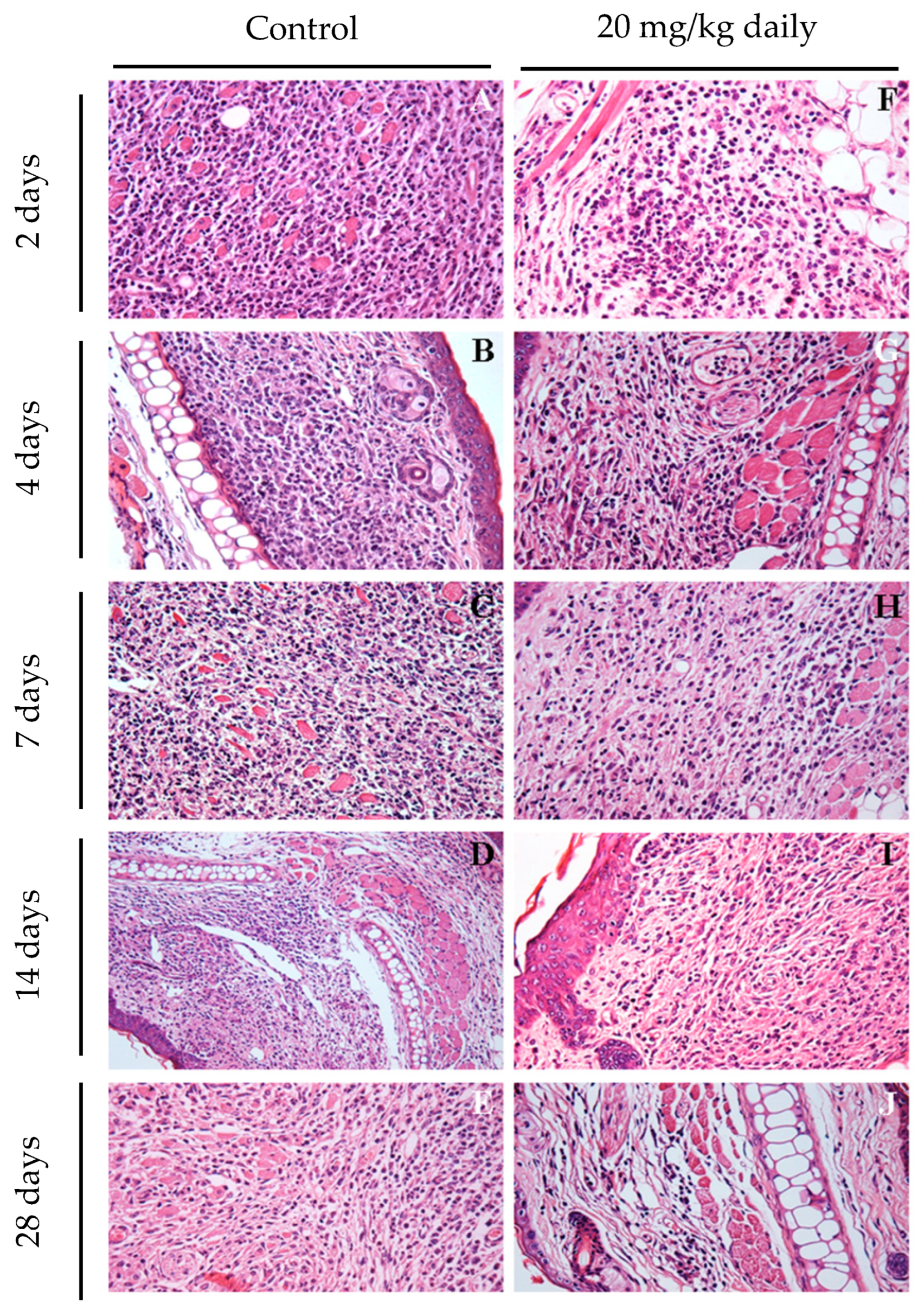
2.11. Histopathological Characterization of Lesions in BALB/c Mice Infected with L. braziliensis and Treated with 17-DMAG
2.12. Statistical Analysis
3. Results
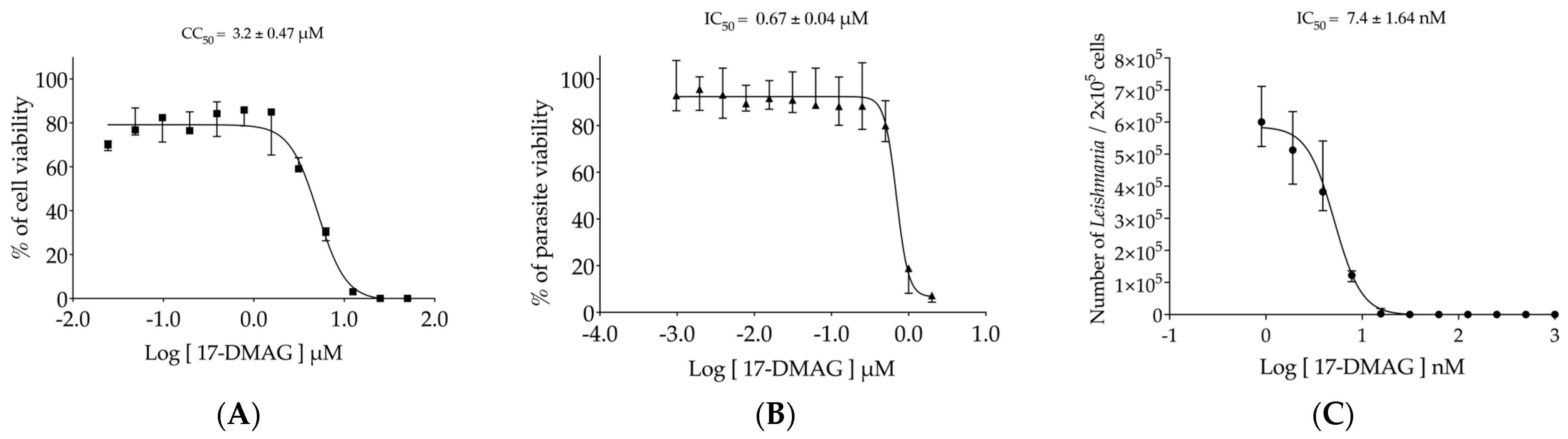
3.1. Assessment of the 17-DMAG Effectiveness against L. braziliensis In Vitro
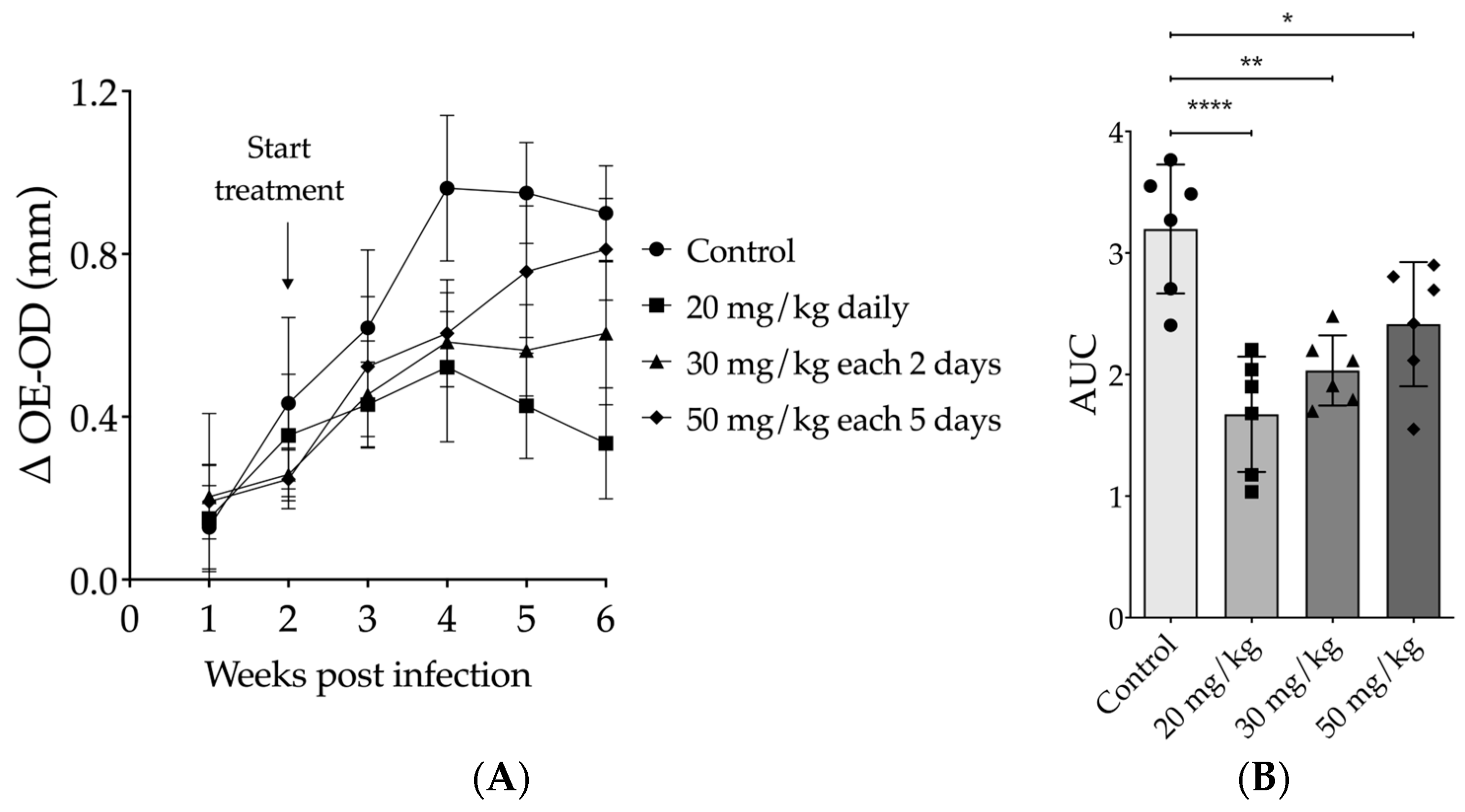
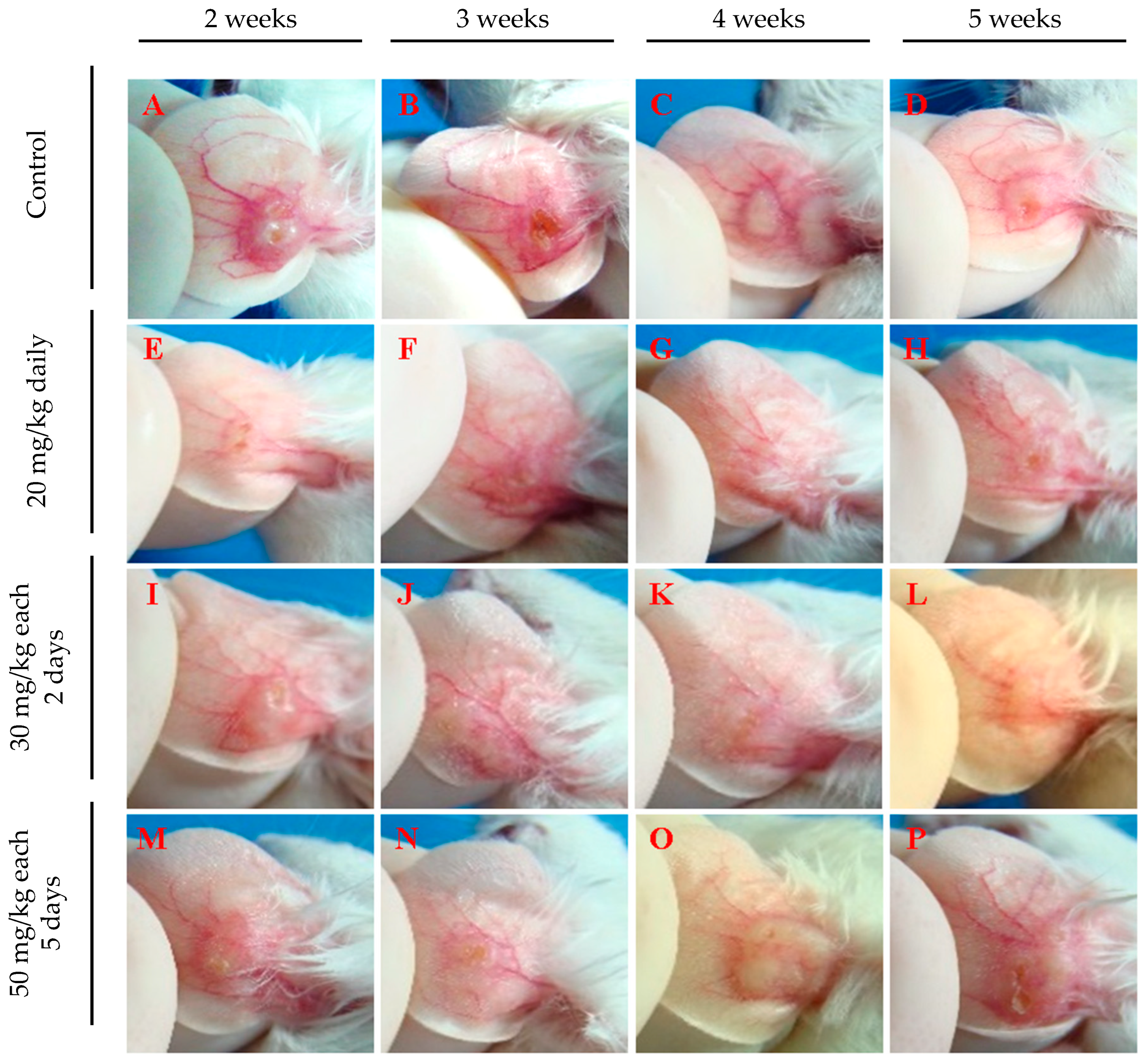
3.2. Evaluation of Intraperitoneally Administered 17-DMAG Treatment against L. braziliensis Infection in BALB/c Mice
3.3. Evaluation of 17-DMAG Treatment on Pro-Inflammatory Cytokines Released by Lymph Node Cells from L. braziliensis-Infected BALB/c Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Word Health Organization. Weekly Epidemiological Record. PLoS Negl. Trop. Dis. 2021, 4, 73–80. [Google Scholar]
- mondiale de la Santé, O.; World Health Organization. Weekly Epidemiological Record, 2016, Vol. 91, 39 [Full Issue]. Wkly. Epidemiol. Rec. Relev. Épidémiol. Hebd. 2016, 91, 441–460. [Google Scholar]
- de Menezes, J.P.B.; Guedes, C.E.S.; Petersen, A.L.d.O.A.; Fraga, D.B.M.; Veras, P.S.T. Advances in Development of New Treatment for Leishmaniasis. Biomed. Res. Int. 2015, 2015, 815023. [Google Scholar] [CrossRef] [PubMed]
- Solit, D.B.; Chiosis, G. Development and Application of Hsp90 Inhibitors. Drug Discov. Today 2008, 13, 38–43. [Google Scholar] [CrossRef]
- Schopf, F.H.; Biebl, M.M.; Buchner, J. The HSP90 Chaperone Machinery. Nat. Rev. Mol. Cell Biol. 2017, 18, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Pallavi, R.; Roy, N.; Nageshan, R.K.; Talukdar, P.; Pavithra, S.R.; Reddy, R.; Venketesh, S.; Kumar, R.; Gupta, A.K.; Singh, R.K. Heat Shock Protein 90 as a Drug Target against Protozoan Infections: Biochemical Characterization of HSP90 from Plasmodium Falciparum and Trypanosoma Evansi and Evaluation of Its Inhibitor as a Candidate Drug. J. Biol. Chem. 2010, 285, 37964–37975. [Google Scholar] [CrossRef]
- Zilberstein, D.; Shapira, M. The Role of pH and Temperature in the Development of Leishmania Parasites. Annu. Rev. Microbiol. 1994, 48, 449–470. [Google Scholar] [CrossRef]
- Graefe, S.E.B.; Wiesgigl, M.; Gaworski, I.; Macdonald, A.; Clos, J. Inhibition of HSP90 in Trypanosoma Cruzi Induces a Stress Response but No Stage Differentiation. Eukaryot. Cell 2002, 1, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Nageshan, R.K.; Ranade, S.; Tatu, U. Heat Shock Protein 90 from Neglected Protozoan Parasites. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2012, 1823, 707–711. [Google Scholar] [CrossRef]
- Hombach, A.; Ommen, G.; MacDonald, A.; Clos, J. A Small Heat Shock Protein Is Essential for Thermotolerance and Intracellular Survival of Leishmania Donovani. J. Cell Sci. 2014, 127, 4762–4773. [Google Scholar] [CrossRef]
- Guswanto, A.; Nugraha, A.B.; Tuvshintulga, B.; Tayebwa, D.S.; Rizk, M.A.; Batiha, G.E.-S.; Gantuya, S.; Sivakumar, T.; Yokoyama, N.; Igarashi, I. 17-DMAG Inhibits the Multiplication of Several Babesia Species and Theileria Equi on in Vitro Cultures, and Babesia Microti in Mice. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Solano, C.; Dong, C.; Sanchez, C.G.; Pizarro, J.C. Identification and Characterization of the Antiplasmodial Activity of Hsp90 Inhibitors. Malar. J. 2017, 16, 292. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.J.; Shapiro, T.A. Potent Antitrypanosomal Activities of Heat Shock Protein 90 Inhibitors in Vitro and in Vivo. J. Infect. Dis. 2013, 208, 489–499. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Palma, L.C.; Ferreira, L.F.G.R.; Petersen, A.L.d.O.A.; Dias, B.R.S.; Menezes, J.P.B.d.; Moreira, D.R.d.M.; Hernandes, M.Z.; Veras, P.S.T. A Docking-Based Structural Analysis of Geldanamycin-Derived Inhibitor Binding to Human or Leishmania Hsp90. Sci. Rep. 2019, 9, 14756. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.L.d.O.A.; Guedes, C.E.S.; Versoza, C.L.; Lima, J.G.B.; de Freitas, L.A.R.; Borges, V.M.; Veras, P.S.T. 17-AAG Kills Intracellular Leishmania Amazonensis While Reducing Inflammatory Responses in Infected Macrophages. PLoS ONE 2012, 7, e49496. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.M.; Petersen, A.L.O.A.; Celes, F.S.; Borges, V.M.; Veras, P.S.T.; de Oliveira, C.I. Chemotherapeutic Potential of 17-AAG against Cutaneous Leishmaniasis Caused by Leishmania (Viannia) Braziliensis. PLoS Negl. Trop. Dis. 2014, 8, e3275. [Google Scholar] [CrossRef] [PubMed]
- Egorin, M.J.; Lagattuta, T.F.; Hamburger, D.R.; Covey, J.M.; White, K.D.; Musser, S.M.; Eiseman, J.L. Pharmacokinetics, Tissue Distribution, and Metabolism of 17-(Dimethylaminoethylamino)-17-Demethoxygeldanamycin (NSC 707545) in CD 2 F 1 Mice and Fischer 344 Rats. Cancer Chemother. Pharmacol. 2002, 49, 7–19. [Google Scholar] [CrossRef]
- Sausville, E.A. Geldanamycin Analogs. J. Chemother. 2004, 16, 68–69. [Google Scholar] [CrossRef] [PubMed]
- Whitesell, L.; Lin, N.U. HSP90 as a Platform for the Assembly of More Effective Cancer Chemotherapy. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2012, 1823, 756–766. [Google Scholar] [CrossRef]
- Pinto-da-Silva, L.H.; Camurate, M.; Costa, K.A.; Oliveira, S.M.P.; da Cunha-e-Silva, N.L.; Saraiva, E.M.B. Leishmania (Viannia) Braziliensis Metacyclic Promastigotes Purified Using Bauhinia Purpurea Lectin Are Complement Resistant and Highly Infective for Macrophages in vitro and Hamsters in vivo. Int. J. Parasitol. 2002, 32, 1371–1377. [Google Scholar] [CrossRef]
- Oliveira, C.I.d.; Barral Netto, M. O Modelo Experimental Nas Infecções Causadas Por L. Amazonensis e L. Braziliensis. Gaz. Méd. Bahia 2005, 75, 35–45. [Google Scholar]
- Debnath, A.; Shahinas, D.; Bryant, C.; Hirata, K.; Miyamoto, Y.; Hwang, G.; Gut, J.; Renslo, A.R.; Pillai, D.R.; Eckmann, L. Hsp90 Inhibitors as New Leads to Target Parasitic Diarrheal Diseases. Antimicrob. Agents Chemother. 2014, 58, 4138–4144. [Google Scholar] [CrossRef] [PubMed]
- Giannini, G.; Battistuzzi, G. Exploring in Vitro and in Vivo Hsp90 Inhibitors Activity against Human Protozoan Parasites. Bioorg. Med. Chem. Lett. 2015, 25, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Zininga, T.; Shonhai, A. Small Molecule Inhibitors Targeting the Heat Shock Protein System of Human Obligate Protozoan Parasites. Int. J. Mol. Sci. 2019, 20, 5930. [Google Scholar] [CrossRef]
- Petersen, A.L.d.O.A.; Cull, B.; Dias, B.R.S.; Palma, L.C.; Luz, Y.d.S.; de Menezes, J.P.B.; Mottram, J.C.; Veras, P.S.T. 17-AAG-Induced Activation of the Autophagic Pathway in Leishmania Is Associated with Parasite Death. Microorganisms 2021, 9, 1089. [Google Scholar] [CrossRef]
- Mansfield, C.R.; Quan, B.; Chirgwin, M.E.; Eduful, B.; Hughes, P.F.; Neveu, G.; Sylvester, K.; Ryan, D.H.; Kafsack, B.F.C.; Haystead, T.A.J. Selective Targeting of Plasmodium Falciparum Hsp90 Disrupts the 26S Proteasome. Cell Chem. Biol. 2024, 31, 729–742. [Google Scholar] [CrossRef]
- Kiang, J.G.; Agravante, N.G.; Smith, J.T.; Bowman, P.D. 17-DMAG Diminishes Hemorrhage-Induced Small Intestine Injury by Elevating Bcl-2 Protein and Inhibiting INOS Pathway, TNF-α Increase, and Caspase-3 Activation. Cell Biosci. 2011, 1, 21. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Shen, H.-H.; Cheng, P.-Y.; Chu, Y.-J.; Hwang, H.-R.; Lam, K.-K.; Lee, Y.-M. 17-DMAG, an HSP90 Inhibitor, Ameliorates Multiple Organ Dysfunction Syndrome via Induction of HSP70 in Endotoxemic Rats. PLoS ONE 2016, 11, e0155583. [Google Scholar] [CrossRef] [PubMed]
- Tukaj, S.; Węgrzyn, G. Anti-Hsp90 Therapy in Autoimmune and Inflammatory Diseases: A Review of Preclinical Studies. Cell Stress Chaperones 2016, 21, 213–218. [Google Scholar] [CrossRef]
- Tukaj, S.; Zillikens, D.; Kasperkiewicz, M. Inhibitory Effects of Heat Shock Protein 90 Blockade on Proinflammatory Human Th1 and Th17 Cell Subpopulations. J. Inflamm. 2014, 11, 10. [Google Scholar] [CrossRef]
- Shimp, S.K.; Chafin, C.B.; Regna, N.L.; Hammond, S.E.; Read, M.A.; Caudell, D.L.; Rylander, M.N.; Reilly, C.M. Heat Shock Protein 90 Inhibition by 17-DMAG Lessens Disease in the MRL/Lpr Mouse Model of Systemic Lupus Erythematosus. Cell. Mol. Immunol. 2012, 9, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Shimp, S.K.; Parson, C.D.; Regna, N.L.; Thomas, A.N.; Chafin, C.B.; Reilly, C.M.; Nichole Rylander, M. HSP90 Inhibition by 17-DMAG Reduces Inflammation in J774 Macrophages through Suppression of Akt and Nuclear Factor-ΚB Pathways. Inflamm. Res. 2012, 61, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Nimmanapalli, R.; O’Bryan, E.; Kuhn, D.; Yamaguchi, H.; Wang, H.G.; Bhalla, K.N. Regulation of 17-AAG-Induced Apoptosis: Role of Bcl-2, Bcl-XL, and Bax Downstream of 17-AAG-Mediated down-Regulation of Akt, Raf-1, and Src Kinases. Blood 2003, 102, 269–275. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, K.P.; Petersen, A.L.O.A.; Amorim, M.F.; Pinho, A.G.S.F.; Palma, L.C.; Dantas, D.A.S.; Silveira, M.R.G.; Silva, C.S.A.; Cordeiro, A.L.J.; Oliveira, I.G.; et al. Intraperitoneal Administration of 17-DMAG as an Effective Treatment against Leishmania braziliensis Infection in BALB/c Mice: A Preclinical Study. Pathogens 2024, 13, 630. https://doi.org/10.3390/pathogens13080630
Cruz KP, Petersen ALOA, Amorim MF, Pinho AGSF, Palma LC, Dantas DAS, Silveira MRG, Silva CSA, Cordeiro ALJ, Oliveira IG, et al. Intraperitoneal Administration of 17-DMAG as an Effective Treatment against Leishmania braziliensis Infection in BALB/c Mice: A Preclinical Study. Pathogens. 2024; 13(8):630. https://doi.org/10.3390/pathogens13080630
Chicago/Turabian StyleCruz, Kercia P., Antonio L. O. A. Petersen, Marina F. Amorim, Alan G. S. F. Pinho, Luana C. Palma, Diana A. S. Dantas, Mariana R. G. Silveira, Carine S. A. Silva, Ana Luiza J. Cordeiro, Izabella G. Oliveira, and et al. 2024. "Intraperitoneal Administration of 17-DMAG as an Effective Treatment against Leishmania braziliensis Infection in BALB/c Mice: A Preclinical Study" Pathogens 13, no. 8: 630. https://doi.org/10.3390/pathogens13080630
APA StyleCruz, K. P., Petersen, A. L. O. A., Amorim, M. F., Pinho, A. G. S. F., Palma, L. C., Dantas, D. A. S., Silveira, M. R. G., Silva, C. S. A., Cordeiro, A. L. J., Oliveira, I. G., Pita, G. B., Souza, B. C. A., Bomfim, G. C., Brodskyn, C. I., Fraga, D. B. M., Lima, I. S., de_Santana, M. B. R., Teixeira, H. M. P., de_Menezes, J. P. B., ... Veras, P. S. T. (2024). Intraperitoneal Administration of 17-DMAG as an Effective Treatment against Leishmania braziliensis Infection in BALB/c Mice: A Preclinical Study. Pathogens, 13(8), 630. https://doi.org/10.3390/pathogens13080630







