Chronic Hepatitis C Virus Infection, Extrahepatic Disease and the Impact of New Direct-Acting Antivirals
Abstract
:1. Introduction
2. Hepatitis C Virus Biology and Antiviral Targets
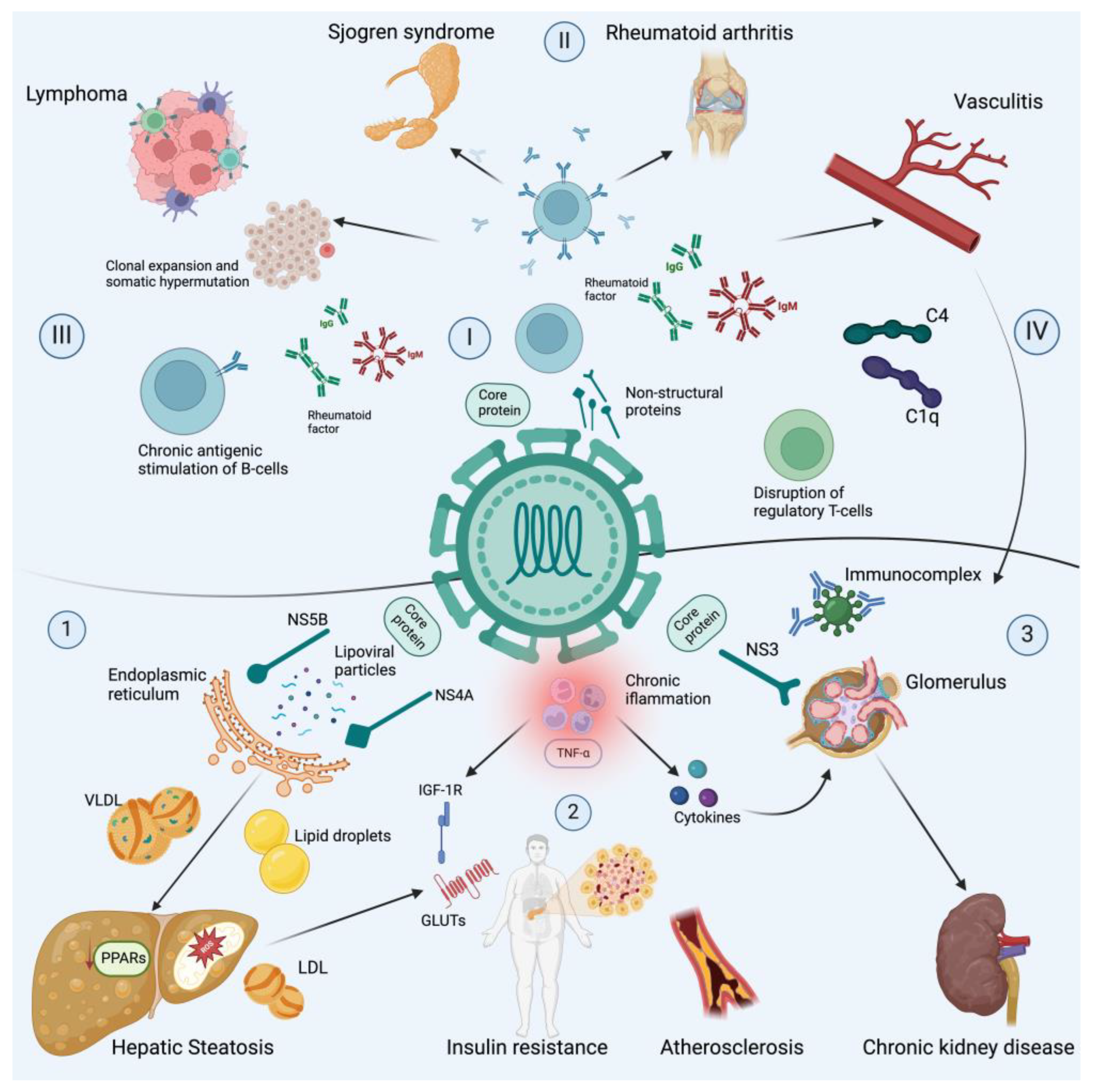
3. Mechanism of Chronic Hepatitis C Virus Infection and Extrahepatic Disease
3.1. Autoimmune Manifestations
3.2. Metabolic Manifestations
3.3. Neuropsychiatric Manifestations
3.4. Kidney Manifestations
3.5. Cancer Manifestations
3.6. Deficiency of Vitamin D
4. Impact of Direct-Acting Antiviral Agents on Extrahepatic Disease
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Global Health Estimates: Leading Causes of Death. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (accessed on 6 February 2024).
- Alberts, C.J.; Clifford, G.M.; Georges, D.; Negro, F.; Lesi, O.A.; Hutin, Y.J.; de Martel, C. Worldwide prevalence of hepatitis B virus and hepatitis C virus among patients with cirrhosis at country, region, and global levels: A systematic review. Lancet Gastroenterol. Hepatol. 2022, 7, 724–735. [Google Scholar] [CrossRef]
- Huang, D.Q.; Terrault, N.A.; Tacke, F.; Gluud, L.L.; Arrese, M.; Bugianesi, E.; Loomba, R. Global epidemiology of cirrhosis—Aetiology, trends and predictions. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 388–398. [Google Scholar] [CrossRef]
- Liu, Y.B.; Chen, M.K. Epidemiology of liver cirrhosis and associated complications: Current knowledge and future directions. World J. Gastroenterol. 2022, 28, 5910–5930. [Google Scholar] [CrossRef]
- Gonzalez-Chagolla, A.; Olivas-Martinez, A.; Ruiz-Manriquez, J.; Servín-Rojas, M.; Kauffman-Ortega, E.; Chávez-García, L.C.; Juárez-León, O.; Cordova-Gallardo, J.; Díaz-García, J.D.; Gonzalez-Huezo, M.S.; et al. Cirrhosis etiology trends in developing countries: Transition from infectious to metabolic conditions. Report from a multicentric cohort in central Mexico. Lancet Reg. Health Am. 2022, 7, 100151. [Google Scholar] [CrossRef]
- Manns, M.P.; Buti, M.; Gane, E.; Pawlotsky, J.M.; Razavi, H.; Terrault, N.; Younossi, Z. Hepatitis C virus infection. Nat. Rev. Dis. Primers 2017, 3, 17006. [Google Scholar] [CrossRef]
- Manns, M.P.; Maasoumy, B. Breakthroughs in hepatitis C research: From discovery to cure. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 533–550. [Google Scholar] [CrossRef]
- Kiser, J.J.; Flexner, C. Direct-acting antiviral agents for hepatitis C virus infection. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 427–449. [Google Scholar] [CrossRef]
- Holmes, J.A.; Rutledge, S.M.; Chung, R.T. Direct-acting antiviral treatment for hepatitis C. Lancet 2019, 393, 1392–1394. [Google Scholar] [CrossRef]
- Morozov, V.A.; Lagaye, S. Hepatitis C virus: Morphogenesis, infection and therapy. World J. Hepatol. 2018, 10, 186–212. [Google Scholar] [CrossRef]
- Dubuisson, J.; Cosset, F.L. Virology and cell biology of the hepatitis C virus life cycle: An update. J. Hepatol. 2014, 61, S3–S13. [Google Scholar] [CrossRef]
- Alazard-Dany, N.; Denolly, S.; Boson, B.; Cosset, F.L. Overview of HCV Life Cycle with a Special Focus on Current and Possible Future Antiviral Targets. Viruses 2019, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Dustin, L.B.; Bartolini, B.; Capobianchi, M.R.; Pistello, M. Hepatitis C virus: Life cycle in cells, infection and host response, and analysis of molecular markers influencing the outcome of infection and response to therapy. Clin. Microbiol. Infect. 2016, 22, 826–832. [Google Scholar] [CrossRef]
- Pecoraro, V.; Banzi, R.; Cariani, E.; Chester, J.; Villa, E.; D’Amico, R.; Bertele, V.; Trenti, T. New Direct-Acting Antivirals for the Treatment of Patients With Hepatitis C Virus Infection: A Systematic Review of Randomized Controlled Trials. J. Clin. Exp. Hepatol. 2019, 9, 522–538. [Google Scholar] [CrossRef]
- Cacoub, P.; Comarmond, C.; Domont, F.; Savey, L.; Desbois, A.C.; Saadoun, D. Extrahepatic manifestations of chronic hepatitis C virus infection. Ther. Adv. Infect. Dis. 2016, 3, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Cacoub, P.; Saadoun, D. Extrahepatic Manifestations of Chronic HCV Infection. N. Engl. J. Med. 2021, 384, 1038–1052. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Park, H.; Henry, L.; Adeyemi, A.; Stepanova, M. Extrahepatic Manifestations of Hepatitis C: A Meta-analysis of Prevalence, Quality of Life, and Economic Burden. Gastroenterology 2016, 150, 1599–1608. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Cheng, J.S.; Lin, C.H.; Chen, T.H.; Lee, K.C.; Chang, M.L. Rheumatoid factor and immunoglobulin M mark hepatitis C-associated mixed cryoglobulinaemia: An 8-year prospective study. Clin. Microbiol. Infect. 2020, 26, 366–372. [Google Scholar] [CrossRef]
- Lauletta, G.; Russi, S.; Conteduca, V.; Sansonno, L. Hepatitis C virus infection and mixed cryoglobulinemia. Clin. Dev. Immunol. 2012, 2012, 502156. [Google Scholar] [CrossRef]
- Saadoun, D.; Landau, D.A.; Calabrese, L.H.; Cacoub, P.P. Hepatitis C-associated mixed cryoglobulinaemia: A crossroad between autoimmunity and lymphoproliferation. Rheumatology 2007, 46, 1234–1242. [Google Scholar] [CrossRef]
- Gorevic, P.D. Rheumatoid factor, complement, and mixed cryoglobulinemia. Clin. Dev. Immunol. 2012, 2012, 439018. [Google Scholar] [CrossRef]
- El-Shamy, A.; Branch, A.D.; Schiano, T.D.; Gorevic, P.D. The Complement System and C1q in Chronic Hepatitis C Virus Infection and Mixed Cryoglobulinemia. Front. Immunol. 2018, 9, 1001. [Google Scholar] [CrossRef] [PubMed]
- Tasleem, S.; Sood, G.K. Hepatitis C Associated B-cell Non-Hodgkin Lymphoma: Clinical Features and the Role of Antiviral Therapy. J. Clin. Transl. Hepatol. 2015, 3, 134–139. [Google Scholar] [CrossRef]
- Armand, M.; Degaud, M.; Tesson, B.; Laurent, C.; Vavasseur, M.; Parisot, M.; Hoareau-Coudert, B.; Canioni, D.; Michot, J.M.; Charlotte, F.; et al. Exploring the genetic landscape of HCV-related B-cell lymphomas using whole exome sequencing. Leukemia 2023, 37, 1388–1391. [Google Scholar] [CrossRef]
- Couronné, L.; Bachy, E.; Roulland, S.; Nadel, B.; Davi, F.; Armand, M.; Canioni, D.; Michot, J.M.; Visco, C.; Arcaini, L.; et al. From hepatitis C virus infection to B-cell lymphoma. Ann. Oncol. 2018, 29, 92–100. [Google Scholar] [CrossRef]
- Libra, M.; Capello, D.; Gloghini, A.; Laura, P.; Berra, E.; Cerri, M.; Gasparotto, D.; Franca, S.; De Re, V.; Gaidano, G.; et al. Analysis of aberrant somatic hypermutation (SHM) in non-Hodgkin’s lymphomas of patients with chronic HCV infection. J. Pathol. 2005, 206, 87–91. [Google Scholar] [CrossRef]
- Brito-Zerón, P.; Gheitasi, H.; Retamozo, S.; Bové, A.; Londoño, M.; Sánchez-Tapias, J.M.; Caballero, M.; Kostov, B.; Forns, X.; Kaveri, S.V.; et al. How hepatitis C virus modifies the immunological profile of Sjögren syndrome: Analysis of 783 patients. Arthritis Res. Ther. 2015, 17, 250. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dou, H.; Liu, G.; Yu, L.; Chen, S.; Min, Y.; Zhao, K.; Wang, X.; Hu, C. Hepatitis C virus infection and the risk of Sjögren or sicca syndrome: A meta-analysis. Microbiol. Immunol. 2014, 58, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.M.; Al-Hnhna, A.A.; Al-Huraibi, B.S.; Assayaghi, R.M.; Al-Qahtani, T.Y.; Jahzar, K.H.; Al-Huthaifi, M.M. Prevalence of hepatitis C virus among patients with arthralgia: Is it logic for screening? Virol. J. 2023, 20, 162. [Google Scholar] [CrossRef]
- Elgretli, W.; Chen, T.; Kronfli, N.; Sebastiani, G. Hepatitis C Virus-Lipid Interplay: Pathogenesis and Clinical Impact. Biomedicines 2023, 11, 271. [Google Scholar] [CrossRef]
- Chaudhari, R.; Fouda, S.; Sainu, A.; Pappachan, J.M. Metabolic complications of hepatitis C virus infection. World J. Gastroenterol. 2021, 27, 1267–1282. [Google Scholar] [CrossRef]
- Huang, H.; Sun, F.; Owen, D.M.; Li, W.; Chen, Y.; Gale, M., Jr.; Ye, J. Hepatitis C virus production by human hepatocytes dependent on assembly and secretion of very low-density lipoproteins. Proc. Natl. Acad. Sci. USA 2007, 104, 5848–5853. [Google Scholar] [CrossRef]
- Mehta, S.H.; Brancati, F.L.; Strathdee, S.A.; Pankow, J.S.; Netski, D.; Coresh, J.; Szklo, M.; Thomas, D.L. Hepatitis C virus infection and incident type 2 diabetes. Hepatology 2003, 38, 50–56. [Google Scholar] [CrossRef]
- Desbois, A.C.; Cacoub, P. Diabetes mellitus, insulin resistance and hepatitis C virus infection: A contemporary review. World J. Gastroenterol. 2017, 23, 1697–1711. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Muratalla, J.; Sierra-Cruz, M.; Cordoba-Chacon, J. Role of hepatic peroxisome proliferator-activated receptor γ in non-alcoholic fatty liver disease. J. Endocrinol. 2023, 257, e220155. [Google Scholar] [CrossRef]
- Lorenzo, M.; Fernández-Veledo, S.; Vila-Bedmar, R.; Garcia-Guerra, L.; De Alvaro, C.; Nieto-Vazquez, I. Insulin resistance induced by tumor necrosis factor-alpha in myocytes and brown adipocytes. J. Anim. Sci. 2008, 86, E94–E104. [Google Scholar] [CrossRef]
- Alzahrani, N. Hepatitis C virus, insulin resistance, and diabetes: A review. Microbiol. Immunol. 2022, 66, 453–459. [Google Scholar] [CrossRef]
- Bose, S.K.; Shrivastava, S.; Meyer, K.; Ray, R.B.; Ray, R. Hepatitis C virus activates the mTOR/S6K1 signaling pathway in inhibiting IRS-1 function for insulin resistance. J. Virol. 2012, 86, 6315–6322. [Google Scholar] [CrossRef]
- Ishizaka, N.; Ishizaka, Y.; Takahashi, E.; Tooda, E.; Hashimoto, H.; Nagai, R.; Yamakado, M. Association between hepatitis C virus seropositivity, carotid-artery plaque, and intima-media thickening. Lancet 2002, 359, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Petta, S.; Maida, M.; Macaluso, F.S.; Barbara, M.; Licata, A.; Craxì, A.; Cammà, C. Hepatitis C Virus Infection Is Associated With Increased Cardiovascular Mortality: A Meta-Analysis of Observational Studies. Gastroenterology 2016, 150, 145–155.e4, quiz e15–e16. [Google Scholar] [CrossRef]
- Romano, C.; Cuomo, G.; Ferrara, R.; Del Mastro, A.; Esposito, S.; Sellitto, A.; Adinolfi, L.E. Uncommon immune-mediated extrahepatic manifestations of HCV infection. Expert Rev. Clin. Immunol. 2018, 14, 1089–1099. [Google Scholar] [CrossRef]
- Więdłocha, M.; Marcinowicz, P.; Sokalla, D.; Stańczykiewicz, B. The neuropsychiatric aspect of the HCV infection. Adv. Clin. Exp. Med. 2017, 26, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Tillmann, H.L. Hepatitis C virus infection and the brain. Metab. Brain. Dis. 2004, 19, 351–356. [Google Scholar] [CrossRef]
- Mathew, S.; Faheem, M.; Ibrahim, S.M.; Iqbal, W.; Rauff, B.; Fatima, K.; Qadri, I. Hepatitis C virus and neurological damage. World J. Hepatol. 2016, 8, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Rifai, M.A.; Gleason, O.C.; Sabouni, D. Psychiatric care of the patient with hepatitis C: A review of the literature. Prim. Care Companion J. Clin. Psychiatry 2010, 12, 27154. [Google Scholar] [CrossRef] [PubMed]
- Fishman, S.L.; Murray, J.M.; Eng, F.J.; Walewski, J.L.; Morgello, S.; Branch, A.D. Molecular and bioinformatic evidence of hepatitis C virus evolution in brain. J. Infect. Dis. 2008, 197, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, L.E.; Nevola, R.; Lus, G.; Restivo, L.; Guerrera, B.; Romano, C.; Zampino, R.; Rinaldi, L.; Sellitto, A.; Giordano, M.; et al. Chronic hepatitis C virus infection and neurological and psychiatric disorders: An overview. World J. Gastroenterol. 2015, 21, 2269–2280. [Google Scholar] [CrossRef]
- Zampino, R.; Marrone, A.; Restivo, L.; Guerrera, B.; Sellitto, A.; Rinaldi, L.; Romano, C.; Adinolfi, L.E. Chronic HCV infection and inflammation: Clinical impact on hepatic and extra-hepatic manifestations. World J. Hepatol. 2013, 5, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Rostoker, G.; Pawlotsky, J.M.; Bastie, A.; Weil, B.; Dhumeaux, D. Type I membranoproliferative glomerulonephritis and HCV infection. Nephrol. Dial. Transpl. 1996, 11 (Suppl. 4), 22–24. [Google Scholar] [CrossRef]
- Fabrizi, F. Hepatitis C virus, cryoglobulinemia, and kidney: Novel evidence. Scientifica 2012, 2012, 128382. [Google Scholar] [CrossRef]
- Barsoum, R.S.; William, E.A.; Khalil, S.S. Hepatitis C and kidney disease: A narrative review. J. Adv. Res. 2017, 8, 113–130. [Google Scholar] [CrossRef]
- Habas, E., Sr.; Farfar, K.L.; Errayes, N.; Habas, A.M.; Errayes, M.; Alfitori, G.; Rayani, A.; Elgara, M.; Al Adab, A.H.; Elzouki, A. Hepatitis Virus C-associated Nephropathy: A Review and Update. Cureus 2022, 14, e27322. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Lau, G.K.K.; Wei, L.; Moriyama, M.; Yu, M.L.; Chuang, W.L.; Ibrahim, A.; Lesmana, C.R.A.; Sollano, J.; Kumar, M.; et al. APASL clinical practice recommendation: How to treat HCV-infected patients with renal impairment? Hepatol. Int. 2019, 13, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Darvishian, M.; Tang, T.; Wong, S.; Binka, M.; Yu, A.; Alvarez, M.; Alexander Velásquez García, H.; Adu, P.A.; Jeong, D.; Bartlett, S.; et al. Chronic hepatitis C infection is associated with higher incidence of extrahepatic cancers in a Canadian population based cohort. Front. Oncol. 2022, 12, 983238. [Google Scholar] [CrossRef] [PubMed]
- Pol, S.; Vallet-Pichard, A.; Hermine, O. Extrahepatic cancers and chronic HCV infection. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.Y.; Sinn, D.H.; Kang, D.; Paik, S.W.; Guallar, E.; Cho, J.; Gwak, G.Y. Incidence of extrahepatic cancers among individuals with chronic hepatitis B or C virus infection: A nationwide cohort study. J. Viral Hepat. 2020, 27, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Lam, L.; Fontaine, H.; Lapidus, N.; Dorival, C.; Bellet, J.; Larrey, D.; Nahon, P.; Diallo, A.; Cagnot, C.; Lusivika-Nzinga, C.; et al. Impact of direct-acting antiviral treatment for hepatitis C on cardiovascular diseases and extrahepatic cancers. Pharmacoepidemiol. Drug Saf. 2023, 32, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Terrier, B.; Jehan, F.; Munteanu, M.; Geri, G.; Saadoun, D.; Sène, D.; Poynard, T.; Souberbielle, J.C.; Cacoub, P. Low 25-hydroxyvitamin D serum levels correlate with the presence of extra-hepatic manifestations in chronic hepatitis C virus infection. Rheumatolgy 2012, 51, 2083–2090. [Google Scholar] [CrossRef] [PubMed]
- Murayama, A.; Kato, T. Inhibition of hepatitis C virus by vitamin D. Vitam. Horm. 2021, 117, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Okubo, T.; Atsukawa, M.; Tsubota, A.; Shimada, N.; Abe, H.; Yoshizawa, K.; Arai, T.; Nakagawa, A.; Itokawa, N.; Kondo, C.; et al. Association between vitamin D deficiency and pre-existing resistance-associated hepatitis C virus NS5A variants. Hepatol. Res. 2017, 47, 641–649. [Google Scholar] [CrossRef]
- Hashimoto, S.; Yatsuhashi, H.; Abiru, S.; Yamasaki, K.; Komori, A.; Nagaoka, S.; Saeki, A.; Uchida, S.; Bekki, S.; Kugiyama, Y.; et al. Rapid Increase in Serum Low-Density Lipoprotein Cholesterol Concentration during Hepatitis C Interferon-Free Treatment. PLoS ONE 2016, 11, e0163644. [Google Scholar] [CrossRef]
- Carvalho, J.R.; Velosa, J.; Serejo, F. Lipids, glucose and iron metabolic alterations in chronic hepatitis C after viral eradication—Comparison of the new direct-acting antiviral agents with the old regimens. Scand. J. Gastroenterol. 2018, 53, 857–863. [Google Scholar] [CrossRef]
- Özdoğan, O.; Yaraş, S.; Ateş, F.; Üçbilek, E.; Sezgin, O.; Altıntaş, E. The impact of direct-acting antiviral treatment on lipid metabolism and insulin resistance in chronic hepatitis C patients: Temporary? permanent? Turk. J. Gastroenterol. 2020, 31, 384–392. [Google Scholar] [CrossRef]
- Di Minno, M.N.D.; Ambrosino, P.; Buonomo, A.R.; Pinchera, B.; Calcaterra, I.; Crispo, M.; Scotto, R.; Borgia, F.; Mattia, C.; Gentile, I. Direct-acting antivirals improve endothelial function in patients with chronic hepatitis: A prospective cohort study. Intern. Emerg. Med. 2020, 15, 263–271. [Google Scholar] [CrossRef]
- Petta, S.; Adinolfi, L.E.; Fracanzani, A.L.; Rini, F.; Caldarella, R.; Calvaruso, V.; Cammà, C.; Ciaccio, M.; Di Marco, V.; Grimaudo, S.; et al. Hepatitis C virus eradication by direct-acting antiviral agents improves carotid atherosclerosis in patients with severe liver fibrosis. J. Hepatol. 2018, 69, 18–24. [Google Scholar] [CrossRef]
- Adinolfi, L.E.; Petta, S.; Fracanzani, A.L.; Nevola, R.; Coppola, C.; Narciso, V.; Rinaldi, L.; Calvaruso, V.; Pafundi, P.C.; Lombardi, R.; et al. Reduced incidence of type 2 diabetes in patients with chronic hepatitis C virus infection cleared by direct-acting antiviral therapy: A prospective study. Diabetes Obes. Metab. 2020, 22, 2408–2416. [Google Scholar] [CrossRef]
- Russo, F.P.; Zanetto, A.; Gambato, M.; Bortoluzzi, I.; Al Zoairy, R.; Franceschet, E.; De Marchi, F.; Marzi, L.; Lynch, E.N.; Floreani, A.; et al. Hepatitis C virus eradication with direct-acting antiviral improves insulin resistance. J. Viral Hepat. 2020, 27, 188–194. [Google Scholar] [CrossRef]
- Villani, R.; Romano, A.D.; Sangineto, M.; Serviddio, G. Direct-acting antivirals improve kidney function in diabetic patients with HCV infection and chronic kidney disease. Intern. Emerg. Med. 2021, 16, 1239–1245. [Google Scholar] [CrossRef]
- Fabrizi, F.; Cerutti, R.; Dixit, V.; Messa, P. The impact of antiviral therapy for HCV on kidney disease: A systematic review and meta-analysis. Nefrología 2020, 40, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Gragnani, L.; Fognani, E.; Piluso, A.; Boldrini, B.; Urraro, T.; Fabbrizzi, A.; Stasi, C.; Ranieri, J.; Monti, M.; Arena, U.; et al. Long-term effect of HCV eradication in patients with mixed cryoglobulinemia: A prospective, controlled, open-label, cohort study. Hepatology 2015, 61, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Landau, D.A.; Saadoun, D.; Halfon, P.; Martinot-Peignoux, M.; Marcellin, P.; Fois, E.; Cacoub, P. Relapse of hepatitis C virus-associated mixed cryoglobulinemia vasculitis in patients with sustained viral response. Arthritis Rheum. 2008, 58, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Cacoub, P.; Si Ahmed, S.N.; Ferfar, Y.; Pol, S.; Thabut, D.; Hezode, C.; Alric, L.; Comarmond, C.; Ragab, G.; Quartuccio, L.; et al. Long-term Efficacy of Interferon-Free Antiviral Treatment Regimens in Patients With Hepatitis C Virus-Associated Cryoglobulinemia Vasculitis. Clin. Gastroenterol. Hepatol. 2019, 17, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, D.; Pol, S.; Ferfar, Y.; Alric, L.; Hezode, C.; Si Ahmed, S.N.; de Saint Martin, L.; Comarmond, C.; Bouyer, A.S.; Musset, L.; et al. Efficacy and Safety of Sofosbuvir Plus Daclatasvir for Treatment of HCV-Associated Cryoglobulinemia Vasculitis. Gastroenterology 2017, 153, 49–52.e45. [Google Scholar] [CrossRef] [PubMed]
- Gragnani, L.; Cerretelli, G.; Lorini, S.; Steidl, C.; Giovannelli, A.; Monti, M.; Petraccia, L.; Sadalla, S.; Urraro, T.; Caini, P.; et al. Interferon-free therapy in hepatitis C virus mixed cryoglobulinaemia: A prospective, controlled, clinical and quality of life analysis. Aliment. Pharmacol. Ther. 2018, 48, 440–450. [Google Scholar] [CrossRef]
- Bonacci, M.; Lens, S.; Londoño, M.C.; Mariño, Z.; Cid, M.C.; Ramos-Casals, M.; Sánchez-Tapias, J.M.; Forns, X.; Hernández-Rodríguez, J. Virologic, Clinical, and Immune Response Outcomes of Patients With Hepatitis C Virus-Associated Cryoglobulinemia Treated With Direct-Acting Antivirals. Clin. Gastroenterol. Hepatol. 2017, 15, 575–583.e571. [Google Scholar] [CrossRef] [PubMed]
- Kondili, L.A.; Monti, M.; Quaranta, M.G.; Gragnani, L.; Panetta, V.; Brancaccio, G.; Mazzaro, C.; Persico, M.; Masarone, M.; Gentile, I.; et al. A prospective study of direct-acting antiviral effectiveness and relapse risk in HCV cryoglobulinemic vasculitis by the Italian PITER cohort. Hepatology 2022, 76, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Danishwar, M.; Jamil, Z.; Khan, S.; Nakhla, M.; Ahmad, I.; Ali, M.A.; Lau, D.T.Y. Persistence of Cryoglobulinemic Vasculitis after DAA Induced HCV Cure. J. Clin. Med. 2022, 11, 984. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Chen, C.Y.; Yu, H.C.; Lin, P.C. Neuropsychiatric disorders in chronic hepatitis C patients after receiving interferon or direct-acting antivirals: A nationwide cohort study. Front. Pharmacol. 2023, 14, 1191843. [Google Scholar] [CrossRef]
- Peveling-Oberhag, J.; Arcaini, L.; Bankov, K.; Zeuzem, S.; Herrmann, E. The anti-lymphoma activity of antiviral therapy in HCV-associated B-cell non-Hodgkin lymphomas: A meta-analysis. J. Viral Hepat. 2016, 23, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Carrier, P.; Jaccard, A.; Jacques, J.; Tabouret, T.; Debette-Gratien, M.; Abraham, J.; Mesturoux, L.; Marquet, P.; Alain, S.; Sautereau, D.; et al. HCV-associated B-cell non-Hodgkin lymphomas and new direct antiviral agents. Liver Int. 2015, 35, 2222–2227. [Google Scholar] [CrossRef] [PubMed]
- Frigeni, M.; Besson, C.; Visco, C.; Fontaine, H.; Goldaniga, M.; Visentini, M.; Pulsoni, A.; Torres, H.A.; Peveling-Oberhag, J.; Rossotti, R.; et al. Interferon-free compared to interferon-based antiviral regimens as first-line therapy for B-cell lymphoproliferative disorders associated with hepatitis C virus infection. Leukemia 2020, 34, 1462–1466. [Google Scholar] [CrossRef]
- Merli, M.; Frigeni, M.; Alric, L.; Visco, C.; Besson, C.; Mannelli, L.; Di Rocco, A.; Ferrari, A.; Farina, L.; Pirisi, M.; et al. Direct-Acting Antivirals in Hepatitis C Virus-Associated Diffuse Large B-cell Lymphomas. Oncologist 2019, 24, e720–e729. [Google Scholar] [CrossRef] [PubMed]
- Persico, M.; Aglitti, A.; Caruso, R.; De Renzo, A.; Selleri, C.; Califano, C.; Abenavoli, L.; Federico, A.; Masarone, M. Efficacy and safety of new direct antiviral agents in hepatitis C virus-infected patients with diffuse large B-cell non-Hodgkin’s lymphoma. Hepatology 2018, 67, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Merli, M.; Rattotti, S.; Spina, M.; Re, F.; Motta, M.; Piazza, F.; Orsucci, L.; Ferreri, A.J.M.; Perbellini, O.; Dodero, A.; et al. Direct-Acting Antivirals as Primary Treatment for Hepatitis C Virus-Associated Indolent Non-Hodgkin Lymphomas: The BArT Study of the Fondazione Italiana Linfomi. J. Clin. Oncol. 2022, 40, 4060–4070. [Google Scholar] [CrossRef]
| NS3/4A Protease Inhibitor | NS5B Nucleotide Analogue Inhibitor | NS5B Non-nucleotide Inhibitor | NS5A Replication Complex Inhibitors |
|---|---|---|---|
| Simeprevir (SIM) | Sofosbuvir (SOF) | Dasabuvir (DSV) | Daclatasvir (DAC) |
| Asunaprevir (ASV) | Ombitasvir (OBV) | ||
| Paritaprevir (PTV) | Ledipasvir (LDV) | ||
| Grazoprevir (GZR) | Elbasvir (EBR) | ||
| Voxilaprevir (VOX) | Velpatasvir (VEL) | ||
| Glecaprevir (GLE) | Pibrentasvir (PIB) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méndez-Sánchez, N.; Coronel-Castillo, C.E.; Ramírez-Mejía, M.M. Chronic Hepatitis C Virus Infection, Extrahepatic Disease and the Impact of New Direct-Acting Antivirals. Pathogens 2024, 13, 339. https://doi.org/10.3390/pathogens13040339
Méndez-Sánchez N, Coronel-Castillo CE, Ramírez-Mejía MM. Chronic Hepatitis C Virus Infection, Extrahepatic Disease and the Impact of New Direct-Acting Antivirals. Pathogens. 2024; 13(4):339. https://doi.org/10.3390/pathogens13040339
Chicago/Turabian StyleMéndez-Sánchez, Nahum, Carlos E. Coronel-Castillo, and Mariana Michelle Ramírez-Mejía. 2024. "Chronic Hepatitis C Virus Infection, Extrahepatic Disease and the Impact of New Direct-Acting Antivirals" Pathogens 13, no. 4: 339. https://doi.org/10.3390/pathogens13040339
APA StyleMéndez-Sánchez, N., Coronel-Castillo, C. E., & Ramírez-Mejía, M. M. (2024). Chronic Hepatitis C Virus Infection, Extrahepatic Disease and the Impact of New Direct-Acting Antivirals. Pathogens, 13(4), 339. https://doi.org/10.3390/pathogens13040339








