Effect of Live and Fragmented Saccharomyces cerevisiae in the Feed of Pigs Challenged with Mycoplasma hyopneumoniae
Abstract
1. Introduction
2. Materials and Methods
3. Results
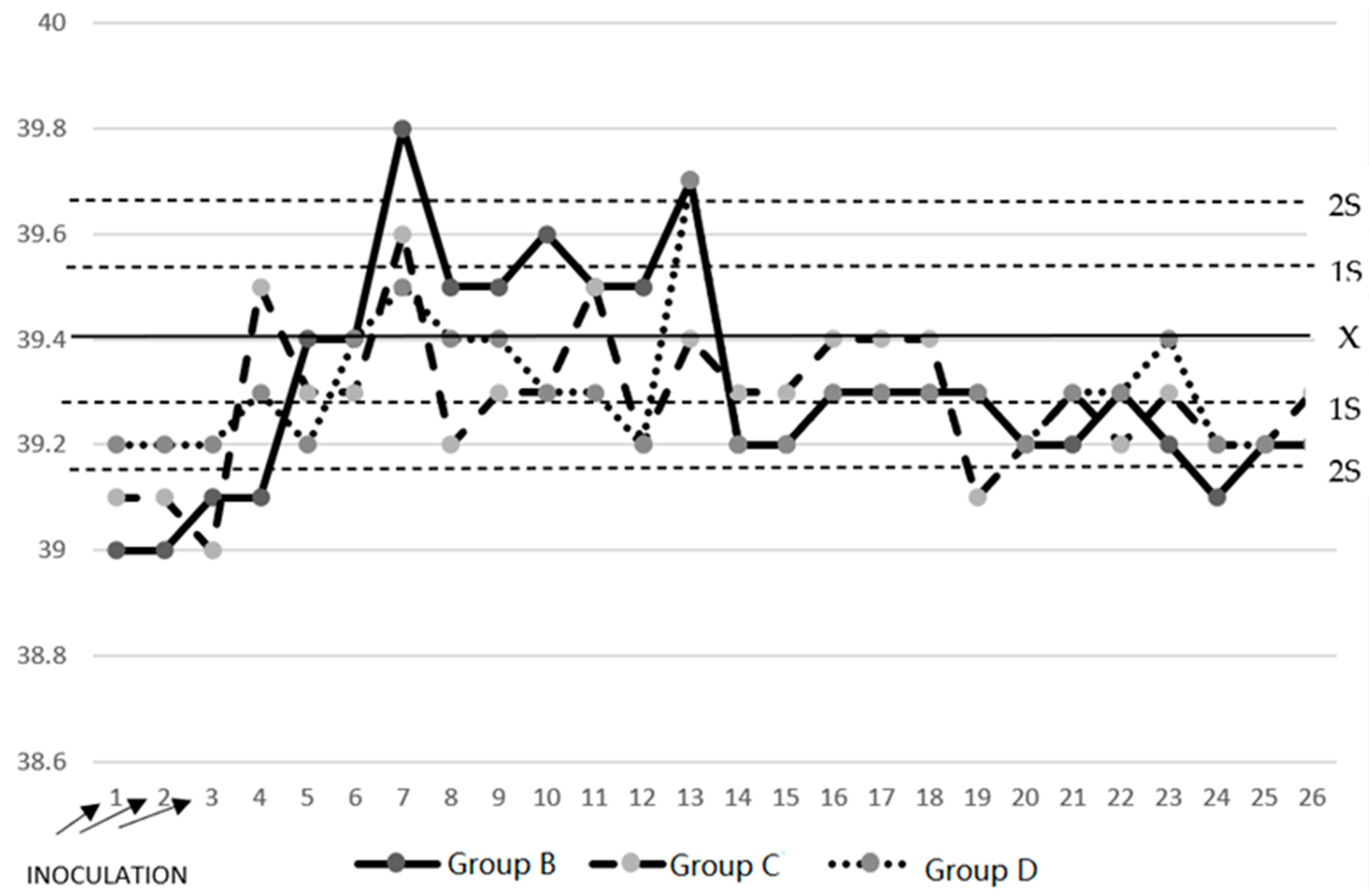
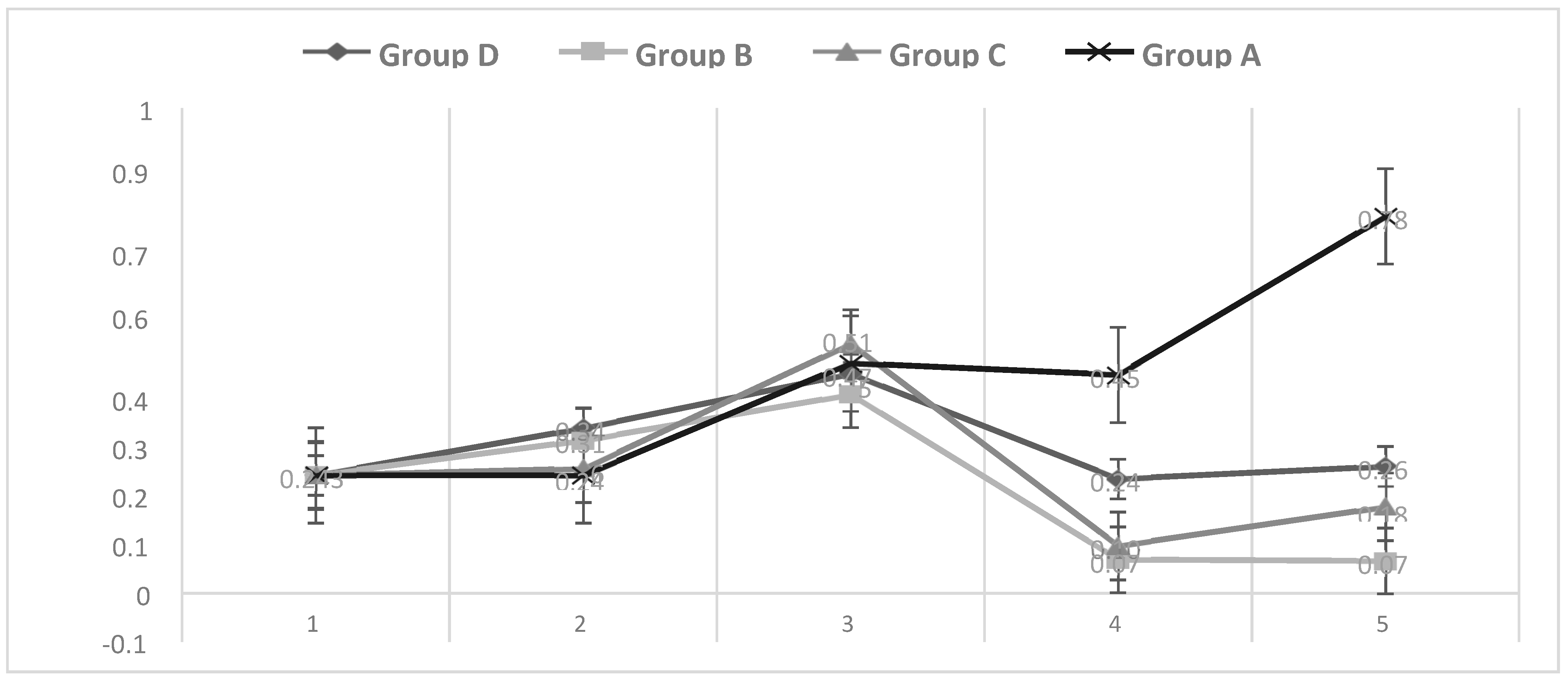
3.1. Temperature
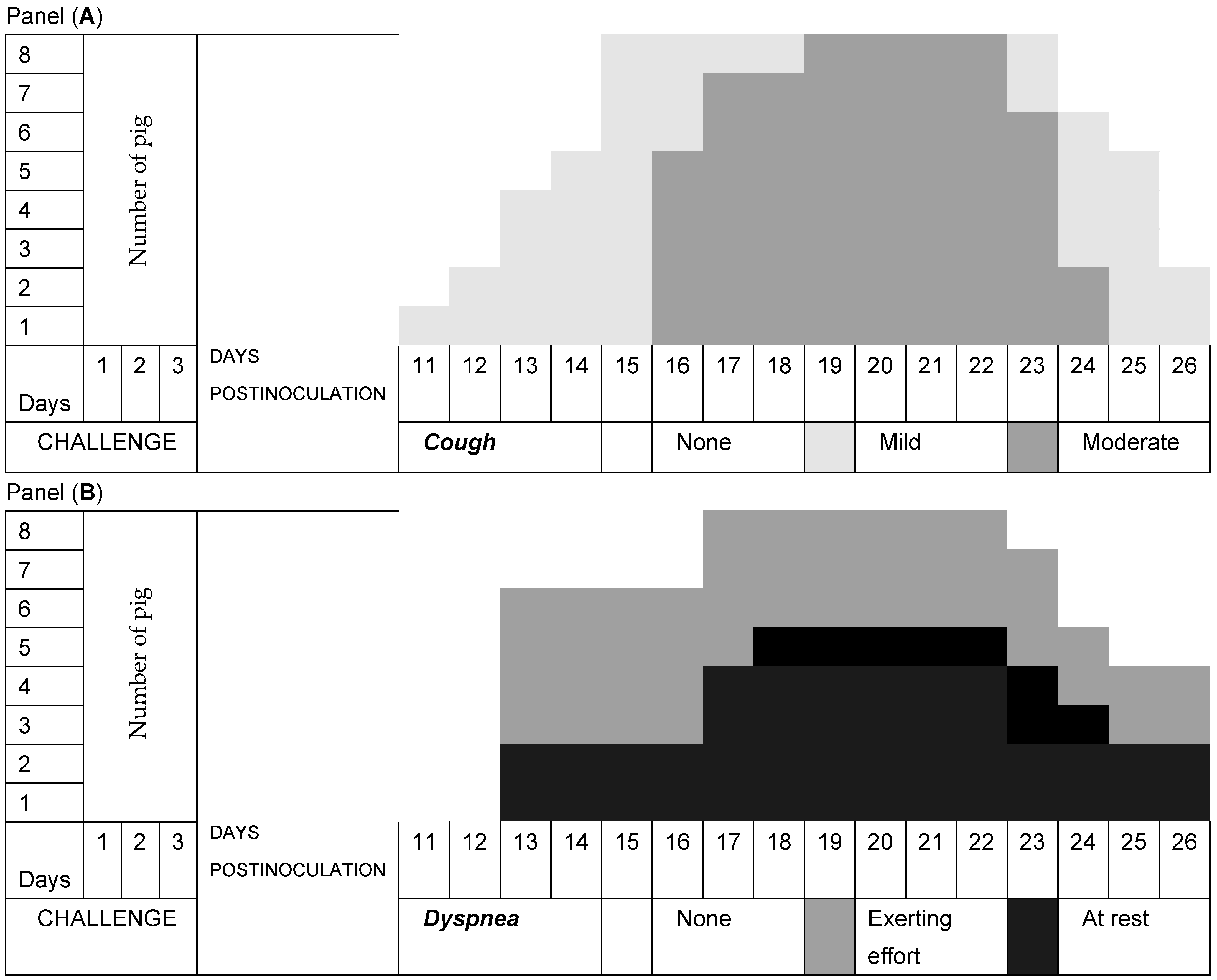
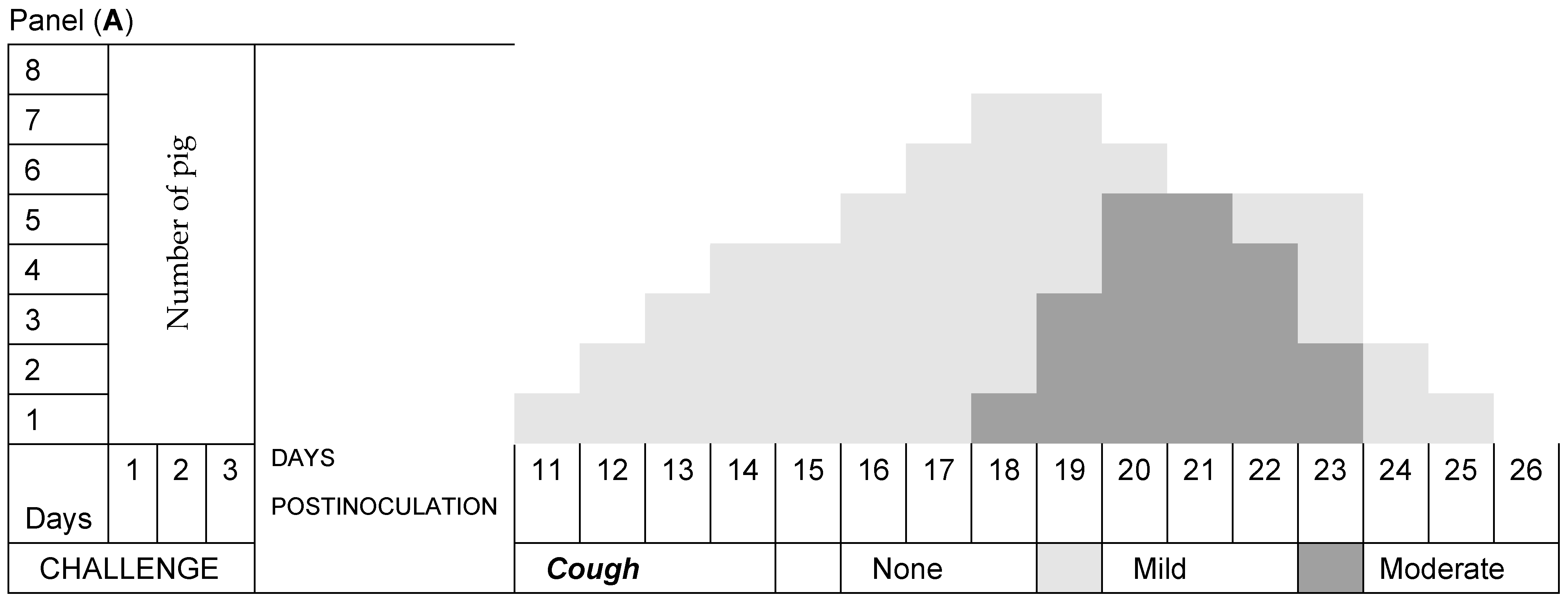
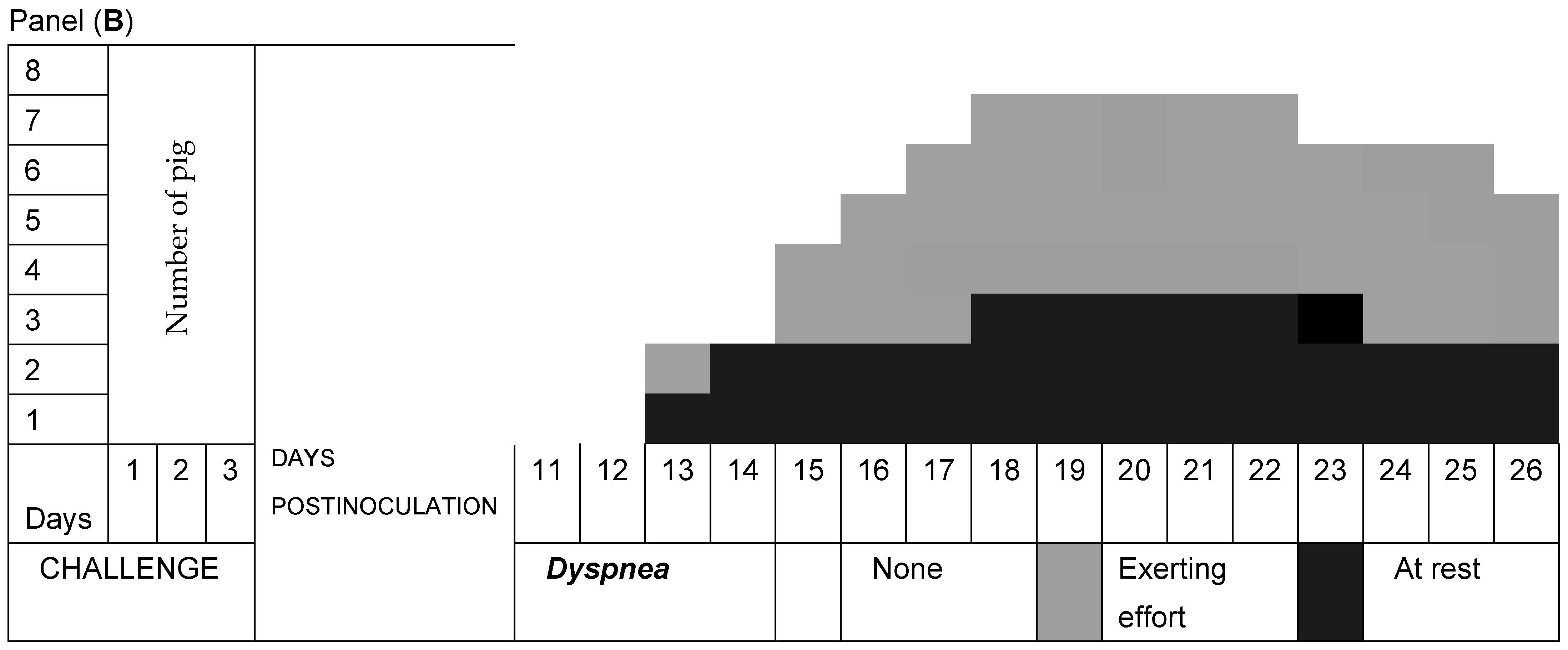
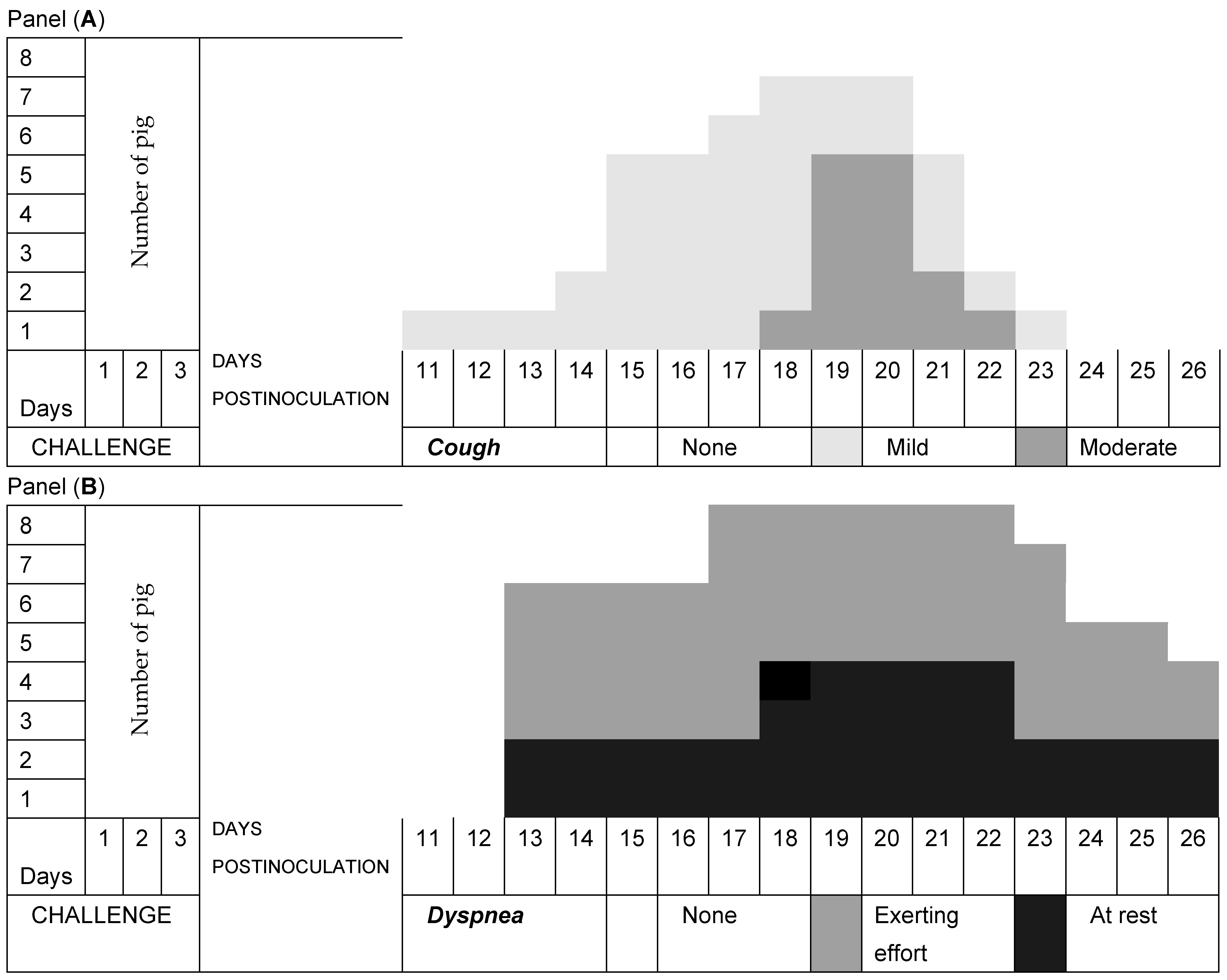
3.2. Respiratory Signs
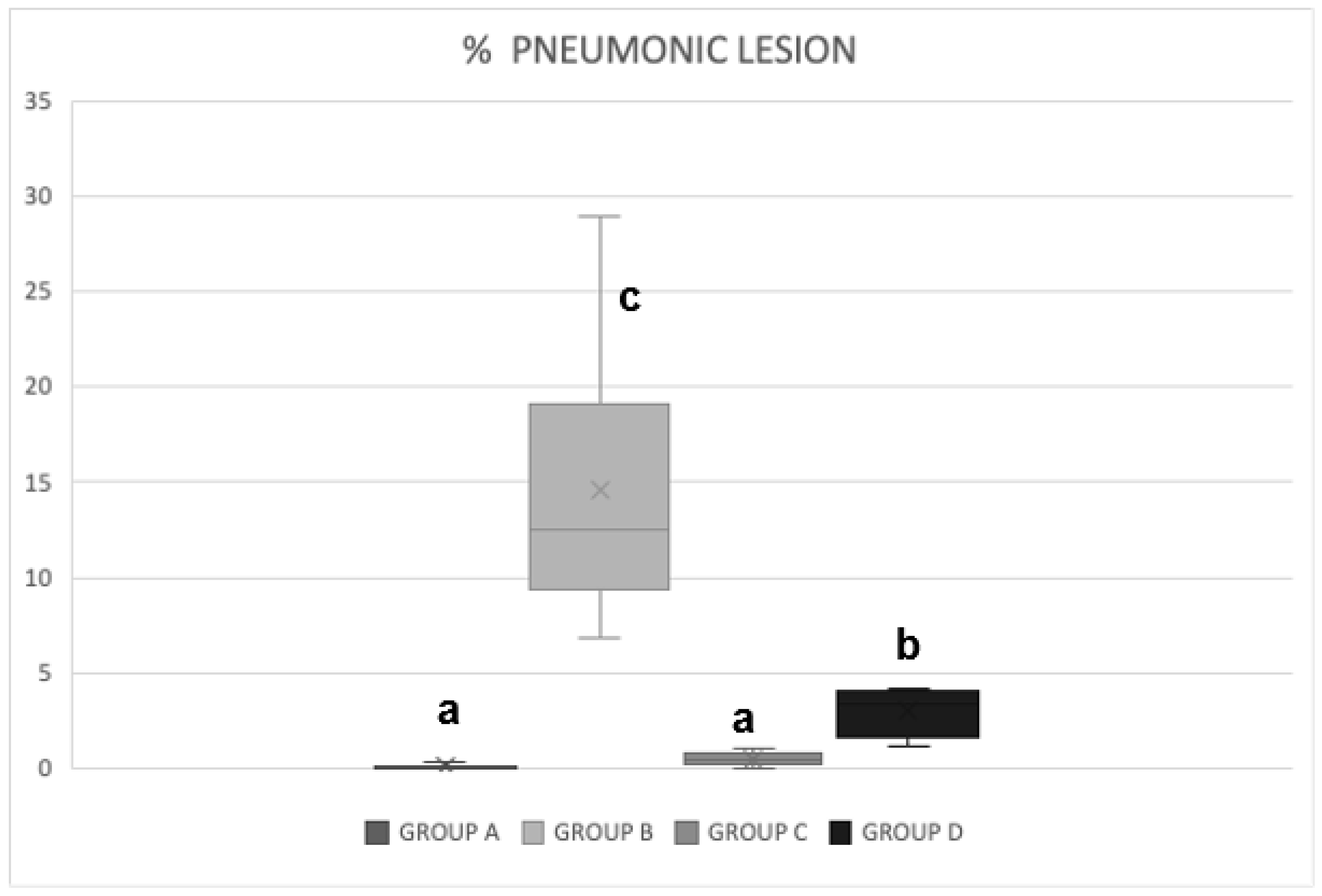
3.3. Pathological Pulmonary Lesions
3.4. Distribution of Pulmonary Injuries
3.5. Recovery of the Microorganisms
3.6. Growth Performance
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thacker, E.L. Mycoplasmal diseases. In Diseases of Swine, 9th ed.; Straw, B.E., Zimmerman, J.J.D., Alleire, S.D., Taylor, D.J., Eds.; Blackwell Publishing: Hoboken, NJ, USA, 2006; Chapter 42. [Google Scholar]
- Maes, D.; Segales, J.; Meyns, T.; Sibila, M.; Pieters, M.; Haesebrouck, F. Control of Mycoplasma hyopneumoniae infections in pigs. Vet. Microbiol. 2008, 126, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Thacker, E.L.; Thacker, B.J.; Wolff, T. Efficacy of aureomycin chlortetracycline granular premix against experimental Mycoplasma hyopneumoniae challenge. In Proceedings of the American Association of Swine Veterinary, Nashville, TN, USA, 24–27 February 2001. [Google Scholar]
- Thacker, E.L.; Young, T.F.; Ericson, B.Z.; DeBey, M.C. Evaluation of tilmicosin’s ability to prevent adherence of Mycoplasma hyopneumoniae to cilia using a differentiated swine epithelial culture system. Vet. Ther. 2001, 2, 293–300. [Google Scholar] [PubMed]
- Ciprián, C.A.; Palacios, J.M.; Quintanar, D.; Batista, L.; Colmenares, G.; Cruz, T.; Romero, A.; Schnitzlein, W.; Mendoza, E.S. Florfenicol feed supplemented decrease the clinical effects of Mycoplasma hyopneumoniae experimental infection in swine in México. Res. Vet. Sci. 2012, 92, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Rhouma, M.; Morris, F.J.; Beaudry, F.; Letellier, A. Post weaning diarrhea in pigs: Risk factors and non-colistin-based control strategies. Acta Vet. Scand. 2017, 59, 31. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.; Bello, F.D.; O’Doherty, J.; Arendt, E.K.; Sweeney, T.; Coffey, A. Analysis of bacterial community shifts in the gastrointestinal tract of pigs fed diets supplemented with b-glucan from Laminaria digitata, Laminaria hyperborea and Saccharomyces cerevisiae. Animal 2013, 7, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Dibner, J.J.; Richards, J.D. Antibiotic growth promoters in agriculture: History and mode of action. Poult. Sci. 2005, 84, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Wenk, C. Recent advance in animal feed additives such as metabolic modifiers, antimicrobial agents, probiotics, enzymes and highly available minerals review. Asian-Australas. J. Anim. Sci. 2000, 13, 86–95. [Google Scholar] [CrossRef]
- Zanello, G.; Meurens, F.; Serreau, D.; Chevaleyre, C.; Melo, S.; Berri, M.; D’Inca, R.; Auclair, E.; Salmon, H. Effects of dietary yeast strains on immunoglobulin in colostrum and milk of sows. Vet. Immunol. Immunopathol. 2013, 152, 20–27. [Google Scholar] [CrossRef]
- Trckova, M.; Faldyna, M.; Alexa, P.; Sramkova Zajacova, Z.; Gopfert, E.; Auclair, E. The effects of live yeast Saccharomyces cerevisiae on postweaning diarrhea, immune response, and growth performance in weaned piglets. J. Anim. Sci. 2014, 92, 767–774. [Google Scholar] [CrossRef]
- Czech, A.; Grela, E.R.; Mokrzycka, A.; Pejsak, Z. Efficacy of man-nanoligosaccharides additive to sows diets on colostrum, blood immunoglobulin content and production parameters of piglets. Pol. J. Vet. Sci. 2010, 13, 525–531. [Google Scholar]
- O’Quinn, P.R. Effects of dietary supplementation with mannan oligosaccharides on sow and litter performance in a commercial production system. J. Anim. Sci. 2001, 79, 212. [Google Scholar]
- Jurgens, M.H.; Rikabi, R.A.; Zimmerman, D.R. The effect of dietary active dry yeast supplement on performance of sows during gestation–lactation and their pigs. J. Anim. Sci. 1997, 75, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Kiros, T.G.; Derakhshani, H.; Pinloche, E.; D’Inca, R.; Marshall, J.; Auclair, E.; Khafipour, E.; Van Kessel, A. Effect of live yeast Saccharomyces cerevisiae (Actisaf Sc 47) supplementation on the performance and hindgut microbiota composition of weanling pigs. Sci. Rep. 2018, 8, 5315. [Google Scholar] [CrossRef] [PubMed]
- Scharek, L.; Filter, M.; Taras, D.; Wrede, P.; Schmidt, M.F.G. Influence of an Enterococcus faecium probiotic on the development of Peyer’s patches B cells in piglets. Arch. Anim. Nutr. 2009, 63, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Fooks, L.J.; Gibson, G.R. Probiotics as modulators of the gut flora. Br. J. Nutr. 2002, 88, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Paineau, D.; Carcano, D.; Leyer, D.; Darquy, S.; Alyanakian, M.A.; Simoneau, G.; Bergmann, J.F.; Brassart, D.; Bornet, F.; Ouwehand, A.C. Effects of seven potential probiotic strains on specific immune responses in healthy adults: A double-blind, randomized, controlled trial. FEMS Immunol. Med. Microbiol. 2008, 53, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Rutherfurd, K.J.; Cross, M.L.; Gopal, P.K. Dietary probiotic supplementation to enhance cellular immunity in the elderly. Br. J. Biomed. Sci. 2001, 58, 94–96. [Google Scholar] [PubMed]
- Donnet-Hughes, A.; Rochat, F.; Serrant, P.; Aeschlimann, J.M.; Schiffrin, E.J. Modulation of nonspecific mechanisms of defense by lactic acid bacteria: Effective dose. J. Dairy Sci. 1999, 82, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Vignali, D.A.A. How many mechanisms do regulatory T cells need? Eur. J. Immunol. 2008, 38, 908–911. [Google Scholar] [CrossRef]
- Vignali, D.A.A.; Collison, L.W.; Workman, C.J. How regulatory T cells Work. Nat. Rev. Immunol. 2008, 8, 523–532. [Google Scholar] [CrossRef]
- Kondelková, K.; Vokurková, D.; Krejsek, J.; Borská, L.; Fiala, Z.; Ctirad, A. Regulatory T cells (TREG) and their roles in immune system with respect to immunopathological disorders. Acta Medica 2010, 53, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Friis, N.F. A selective medium for Mycoplasma suipneumoniae. Acta Vet. Scand. 1971, 12, 454–456. [Google Scholar] [CrossRef] [PubMed]
- Lara, P.H.; Mendoza, E.S.; Quesada, M.F.; Cortes, R.; Lozano, D.B.; Sarfati, M.D.; Soto, P.E.; Ciprián, C.A. Experimental infection with Mycoplasma hyopneumoniae in SPF pigs using an aerosol chamber. In Proceedings of the 20th International Pig Veterinary Society Congress (IPVS 2008), Durban, South Africa, 22–26 June 2008; p. 103. [Google Scholar]
- Ciprián, C.A.; Pijoan, A.C.; Cruz, S.T.; Camacho, M.J.; Tortora, P.J.; Colmenares, V.G.; Lopez-Revilla, R.; De la Garza, M. Mycoplasma hyopneumoniae increases the susceptibility of pigs to experimental Pasteurella multocida pneumoniae. Can. J. Vet. Res. 1988, 52, 434–438. [Google Scholar] [PubMed]
- Amezcua, H.; Serrano, L.; Hernández, E.; Mendoza, S.; Ciprián, C.A. Comparison of isolates of Mycoplasma hyopneumoniae lesions in lungs and bronchial explants. In Proceedings of the 22nd International Pig Veterinary Society Congress (IPVS 2012), Jeju, Republic of Korea, 10–13 June 2012; p. 711. [Google Scholar]
- Vega, M.A.G.; Camacho, E.J.; Sotres, F.; Serrano, L.; Hernández, E.; Ciprián, A.; Mendoza, S.E. Study of isolates of Mycoplasma hyopneumoniae lesions in lungs and bronquial explants. In Proceedings of the 32nd World Veterinary Congress. Lütfi Kirdar Convention & Exhibition Centre, Istabul, Turkey, 13–17 September 2015; p. 470. [Google Scholar]
- Calsamiglia, M.; Collin, J.E.; Pijoan, C. Correlation between the presence of enzootic pneumonia lesions and detection of Mycoplasma hyopneumoniae in bronchial swabs by PCR. Vet. Microbiol. 2000, 76, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Laskowska, E.; Jarosz, L.S.; Grądzki, Z. Effect of the EM Bokashi Multimicrobial Probiotic Preparation on the Non-specific Immune Response in Pigs. Probiotics Antimicrob. Proteins 2019, 11, 1264–1277. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, A.J.; Gatto, D.; Sainsbury, E.; Harman, G.R.; Hengartner, H.; Zinkernagel, R.M. A primitive T Cell-Independent Mechanism of Intestinal Mucosal IgA Responses to Commensal Bacteria. Science 2000, 288, 2222–2226. [Google Scholar] [CrossRef] [PubMed]
- Rauch, M.; Lynch, S.V. Probiotic manipulation of the gastrointestinal microbiota. Gut Microbes 2010, 1, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Hou, C.; Zeng, X.; Qiao, S. The use of lactic acid bacteria as a probiotic in swine diets. Pathogens 2015, 4, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.J. Pig Diseases, 6th ed.; Glasgow University: Glasgow, UK, 1995. [Google Scholar]
- Vega, M.A.G.; Mendoza, S.E.; Ciprián, A.C.; Bárcenas, G.M.; Hernández, J.; Solorio, S. Evaluación de la respuesta de citocinas a una dosis baja de prebiótico en cerdos sanos. In Proceedings of the En Memoria del XXV Congreso de PANVET, Ciudad de Panamá, Panama, 3–7 October 2016. [Google Scholar]
- Thacker, E.L.; Tacker, B.J.; Kuhn, M.; Hawkins, P.A.; Waters, W.R. Evaluation of local and systemic immune responses induced by intramuscular injection of a Mycoplasma hyopneumoniae bacterin to pigs. Am. J. Vet. Res. 2000, 61, 1384–1389. [Google Scholar] [CrossRef]
- Thanawongnuwech, R.; Tacker, E.L. Interleukin 10, interleukin 12 and interferon gamma levels in the respiratory tract following Mycoplasma hyopneumoniae and PRRS infection in pigs. Viral Immunol. 2003, 16, 357–367. [Google Scholar] [CrossRef]
- Cruz, S.T.A.; Martínez, R.S.S.; Garrido, G.; Tórtora, J.; Mendoza, E.S.; Ciprián, C.A.; Hernández-Baumgartem, E.; Romero, R.A.; Vega-López, M.A. In situ analysis of CD4, CD8 and Mast Cells in Lung of Mycoplasma hyopneumoniae Experimentally Infected Pigs. J. Anim. Vet. Adv. 2008, 7, 752–758. [Google Scholar]
- Wise, D.J.; Carter, G.R. Immunology A Comprehensive Review; Iowa State University Press. A Blackwell Science Company: Ames, IA, USA, 2002. [Google Scholar]
- Abbas, A.; Lichtman, A.; Pillai, S. Inmunologia Celular y Molecular, 9th ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Kursar, M.; Koch, M.; Mittrücker, H.-W.; Nouailles, G.; Bonhagen, K.; Kamradt, T.; Stefan, H.E.; Kaufmann, S.H.E. Cutting Edge: Regulatory T cells prevent efficient clearance of Mycobacterium tuberculosis. J. Immunol. 2007, 178, 2661–2665. [Google Scholar] [CrossRef] [PubMed]
- Caruso, J.P.; Ross, R.F. Effects of Mycoplasma hyopneumoniae and Actinobacillus (Haemophilus) pleuropneumoniae infections on alveolar macrophage functions in swine. Am. J. Vet. Res. 1990, 51, 227–231. [Google Scholar] [CrossRef]
- Procajlo, Z.; Szweda, W.; Mikulska-Skupien Platt-Samoraj, A. Effectiviness of coupled administration of bioimmuno inmmodulator and respisure one vaccinein the profiylaxis os mycoplasmal pneumonia of swine. Pol. J. Vet. Sci. 2010, 13, 325–331. [Google Scholar] [PubMed]
| Group a Treatment | Recovery of M. hyopneumoniae | ||||
|---|---|---|---|---|---|
| Lung Homogenates | Bronchial Explants | ||||
| Number of Positive Pigs | Mean Titer of Positives | Number of Positive Pigs | Mean Titer of Positives | ||
| A | None | 0 | 0 | 0 | 0 |
| B | M. hyopneumoniae alone | 8 | 103 | 8 | 105 |
| C | Sc disintegrated and M. hyopneumoniae | 4 | 101 | 4 | 103 |
| D | Sc live and M. hyopneumoniae | 6 | 102 | 6 | 103 |
| Group | Consolidated Lobes | Appearance of Lesions | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Apical | Cardiac | Diaphragmatic b | Accessory | Network | Reddish Gray | Pleural Adhesions | ||||
| R c | L c | R c | L c | R c | L c | |||||
| A | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| B | 8 | 8 | 8 | 8 | 7 | 6 | 3 | 2 | 6 | 2 |
| C | 3 | 3 | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 0 |
| D | 6 | 6 | 6 | 6 | 3 | 3 | 1 | 4 | 1 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vega-Munguía, G.; Vargas Sánchez, A.; Camacho-Medina, J.E.; Suárez-Vélez, L.; Bárcenas-Morales, G.; Quintar Guerrero, D.; Ciprian-Carrasco, A.; Mendoza Elvira, S. Effect of Live and Fragmented Saccharomyces cerevisiae in the Feed of Pigs Challenged with Mycoplasma hyopneumoniae. Pathogens 2024, 13, 322. https://doi.org/10.3390/pathogens13040322
Vega-Munguía G, Vargas Sánchez A, Camacho-Medina JE, Suárez-Vélez L, Bárcenas-Morales G, Quintar Guerrero D, Ciprian-Carrasco A, Mendoza Elvira S. Effect of Live and Fragmented Saccharomyces cerevisiae in the Feed of Pigs Challenged with Mycoplasma hyopneumoniae. Pathogens. 2024; 13(4):322. https://doi.org/10.3390/pathogens13040322
Chicago/Turabian StyleVega-Munguía, Gabriela, Alejandro Vargas Sánchez, Juan E. Camacho-Medina, Luis Suárez-Vélez, Gabriela Bárcenas-Morales, David Quintar Guerrero, Abel Ciprian-Carrasco, and Susana Mendoza Elvira. 2024. "Effect of Live and Fragmented Saccharomyces cerevisiae in the Feed of Pigs Challenged with Mycoplasma hyopneumoniae" Pathogens 13, no. 4: 322. https://doi.org/10.3390/pathogens13040322
APA StyleVega-Munguía, G., Vargas Sánchez, A., Camacho-Medina, J. E., Suárez-Vélez, L., Bárcenas-Morales, G., Quintar Guerrero, D., Ciprian-Carrasco, A., & Mendoza Elvira, S. (2024). Effect of Live and Fragmented Saccharomyces cerevisiae in the Feed of Pigs Challenged with Mycoplasma hyopneumoniae. Pathogens, 13(4), 322. https://doi.org/10.3390/pathogens13040322







