Concomitant Syndromic Diagnosis of Mpox and Other Vesicular Viruses in Patients with Skin and Genital Lesions
Abstract
1. Introduction
2. Materials and Methods
2.1. Procedure
2.2. Sequencing and Typing of Enterovirus
2.3. Microbiological Diagnosis
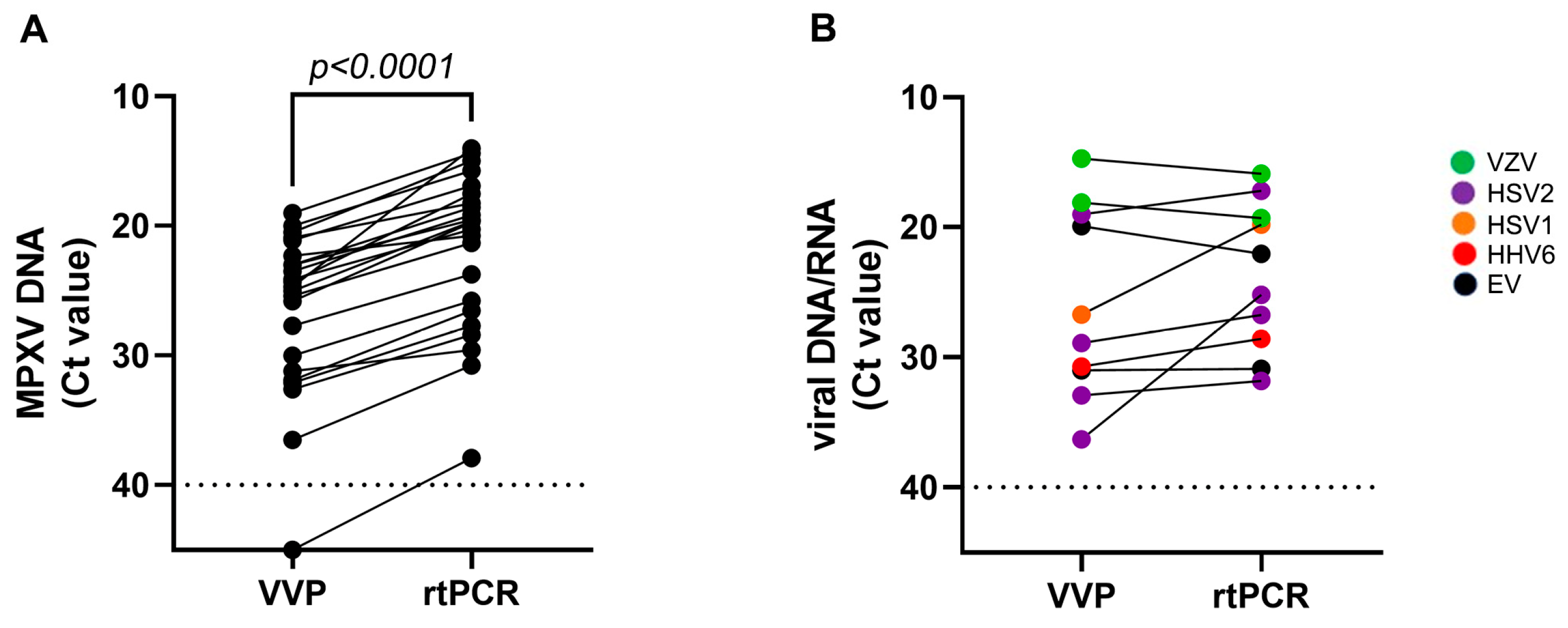
3. Results
3.1. Qualitative Performance of VVP
3.2. Quantitative Performance of VVP
3.3. Characteristics of the Patients Tested with the VVP
3.4. EV Genotyping
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The changing epidemiology of human monkeypox—A potential threat? A systematic review. PLoS Negl. Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef] [PubMed]
- Isidro, J.; Borges, V.; Pinto, M.; Sobral, D.; Santos, J.D.; Nunes, A.; Mixão, V.; Ferreira, R.; Santos, D.; Duarte, S.; et al. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat. Med. 2022, 28, 1569–1572. [Google Scholar] [CrossRef]
- Ulaeto, D.; Agafonov, A.; Burchfield, J.; Carter, L.; Happi, C.; Jakob, R.; Krpelanova, E.; Kuppalli, K.; Lefkowitz, E.J.; Mauldin, M.R.; et al. New nomenclature for mpox (monkeypox) and monkeypox virus clades. Lancet Infect. Dis. 2023, 23, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Von Magnus, P.; Andersen, E.K.; Petersen, K.B.; Birch-Andersen, A. A pox-like disease in cynomolgus monkeys. Acta Pathol. Microbiol. Scand. 1959, 46, 156–176. [Google Scholar] [CrossRef]
- Parker, S.; Buller, M.R. A review of experimental and natural infections of animals with monkeypox virus between 1958 and 2012. Future Virol. 2013, 8, 129–157. [Google Scholar] [CrossRef] [PubMed]
- Beer, E.M.; Rao, V.B. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl. Trop. Dis. 2019, 13, e0007791. [Google Scholar] [CrossRef]
- Reynolds, M.G.; Davidson, W.B.; Curns, A.T.; Conover, C.S.; Huhn, G.; Davis, J.P.; Wegner, M.; Croft, D.R.; Newman, A.; Obiesie, N.N.; et al. Spectrum of infection and risk factors for human monkeypox, United States, 2003. Emerg. Infect. Dis. 2007, 13, 1332e9. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Monkeypox Multi-Country Outbreak; ECDC: Stockholm, Sweden, 2022. Available online: https://www.ecdc.europe.eu/en/pubblications-data/risk-assessment-monkeypox-multi-country-outbreak (accessed on 23 May 2022).
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S.; et al. Monkeypox Virus Infection in Humans across 16 Countries—April-June 2022. N. Engl. J. Med. 2022, 387, 679–691. [Google Scholar] [CrossRef]
- Mileto, D.; Riva, A.; Cutrera, M.; Moschese, D.; Mancon, A.; Meroni, L.; Giacomelli, A.; Bestetti, G.; Rizzardini, G.; Gismondo, M.R.; et al. New challenges in human monkeypox outside Africa: A review and case report from Italy. Travel Med. Infect. Dis. 2022, 49, 02386. [Google Scholar] [CrossRef]
- Mitjà, O.; Ogoina, D.; Titanji, B.K.; Galvan, C.; Muyembe, J.J.; Marks, M.; Orkin, C.M. Monkeypox. Lancet 2023, 401, 60–74. [Google Scholar] [CrossRef]
- Patel, A.; Bilinska, J.; Tam, J.C.H.; Da Silva Fontoura, D.; Mason, C.Y.; Daunt, A.; Snell, L.B.; Murphy, J.; Potter, J.; Tuudah, C.; et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: Descriptive case series. BMJ 2022, 378, e072410. [Google Scholar] [CrossRef]
- Orviz, E.; Negredo, A.; Ayerdi, O.; Vázquez, A.; Muñoz-Gomez, A.; Monzón, S.; Clavo, P.; Zaballos, A.; Vera, M.; Sánchez, P.; et al. Grupo Viruela del Simio Madrid CNM/ISCIII/HCSC/Sandoval. Monkeypox outbreak in Madrid (Spain): Clinical and virological aspects. J. Infect. 2022, 85, 412–417. [Google Scholar] [CrossRef]
- Rizzo, A.; Pozza, G.; Salari, F.; Giacomelli, A.; Mileto, D.; Cossu, M.V.; Mancon, A.; Gagliardi, G.; Micol, B.; Micheli, V.; et al. Concomitant diagnosis of sexually transmitted infections and human monkeypox in patients attending a sexual health clinic in Milan, Italy. J. Med. Virol. 2023, 95, e28328. [Google Scholar] [CrossRef] [PubMed]
- AbdullGaffar, B.; Abdulrahman, S. Monkeypox virus, herpes simplex virus, and cytomegalovirus skin coinfections. J. Med. Virol. 2023, 95, e28500. [Google Scholar] [CrossRef] [PubMed]
- FindDx. 2022. Available online: https://www.finddx.org/mpx-test-directory/ (accessed on 3 January 2024).
- Chen, Q.; Gul, I.; Liu, C.; Lei, Z.; Li, X.; Raheem, M.A.; He, Q.; Haihui, Z.; Leeansyah, E.; Zhang, C.Y.; et al. CRISPR-Cas12-based field-deployable system for rapid detection of synthetic DNA sequence of the monkeypox virus genome. J. Med. Virol. 2023, 95, e28385. [Google Scholar] [CrossRef]
- Ramanan, P.; Bryson, A.L.; Binnicker, M.J.; Pritt, B.S.; Patel, R. Syndromic Panel-Based Testing in Clinical Microbiology. Clin. Microbiol. Rev. 2017, 31, e00024-17. [Google Scholar] [CrossRef]
- Available online: https://www.qiagen.com/us/products/diagnostics-and-clinical-research/infectious-disease/qiastat-dx-syndromic-testing/qiastat-dx-research (accessed on 3 January 2024).
- Nicholson, F.; Meetoo, G.; Aiyar, S.; Banatvala, J.E.; Muir, P. Detection of enterovirus RNA in clinical samples by nested polymerase chain reaction for rapid diagnosis of enterovirus infection. J. Virol. Meth. 1994, 48, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Nix, A.W.; Oberste, M.S.; Pallansch, M.A. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J. Clin. Microbiol. 2006, 44, 2698–2704. [Google Scholar] [CrossRef]
- Kroneman, A.; Vennema, H.; Deforche, K.; Avoort, H.V.; Penaranda, S.; Oberste, M.S.; Vinje, J. Koopmans. An automated genotyping tool for enteroviruses and noroviruses. J. Clin. Virol. 2011, 51, 121–125. [Google Scholar] [CrossRef]
- Robinet, S.; Parisot, F. Performance assessment of the Allplex™ STI Essential real-time PCR assay for the diagnosis of Neisseria gonorrhoeae and Chlamydia trachomatis infections in genital and extra-genital sites. J. Labor. Med. 2019, 43, 191–200. [Google Scholar] [CrossRef]
- Tuddenham, S.; Hamill, M.M.; Ghanem, K.G. Diagnosis and Treatment of Sexually Transmitted Infections: A Review. JAMA 2022, 327, 161–172. [Google Scholar] [CrossRef]
- Dechamps, C.; Peigue-Lafeuille, H.H.; Laveran, H.; Beytout, J.; Roger, H.; Beytout, D. Four cases of vesicular lesions in adults caused by enterovirus infections. J. Clin. Microbiol. 1988, 26, 2182–2183. [Google Scholar] [CrossRef] [PubMed]
- Lizasoain, A.; Piegas, S.; Victoria, M.; Da Silva, E.E.; Colina, R. Hand-foot-and-mouth disease in Uruguay: Coxsackievirus A6 identified as causative of an outbreak in a rural childcare center. J. Med. Virol. 2020, 92, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Luchs, A.; Azevedo, L.S.; Souza, E.V.; Medeiros, R.S.; Souza, Y.F.V.P.; Teixeira, D.L.F.; Carneiro, T.F.d.O.; de Alencar, G.M.F.; Morais, F.L.d.S.L.; Pinto, D.d.F.A.; et al. Coxsackievirus A6 strains causing an outbreak of hand-foot-and-mouth disease in Northeastern Brazil in 2018. Rev. Inst. Med. Trop. São Paulo 2022, 64, e16. [Google Scholar] [CrossRef] [PubMed]
| Patient | VVP Assay Result | Ct VVP | Ct PCR | Specimen |
|---|---|---|---|---|
| #1 | MPXV2 | 23.5 | 19.1 | Skin lesion |
| #2 | MPXV2 | 20.0 | 15.7 | Anal swab |
| #3 | MPXV2 | 25.0 | 19.6 | Skin lesion |
| #4 | MPXV2 | 19.0 | 14.3 | Skin lesion |
| #5 | MPXV2 | 24.3 | 17.5 | Anal swab |
| #6 | MPXV2 | 24.1 | 20.2 | Skin lesion |
| #7 | MPXV2 | 30.0 | 25.7 | Anal swab |
| #8 | MPXV2 | 31.2 | 29.5 | Skin lesion |
| #9 | MPXV2 | 31.9 | 26.5 | Skin lesion |
| #10 | MPXV2 | 24.7 | 14.0 | Anal swab |
| #11 | MPXV2 | 20.5 | 14.9 | Anal swab |
| #12 | MPXV2 | 22.3 | 20.7 | Anal swab |
| #13 | MPXV2 | 36.5 | 30.7 | Skin lesion |
| MPXV2 | 32.6 | 28.3 | Genital swab | |
| #14 | MPXV2 | 32.1 | 27.7 | Genital swab |
| #15 | MPXV2 | 21.1 | 16.8 | Genital swab |
| #16 | MPXV2 | 23.0 | 19.5 | Genital swab |
| #17 | MPXV2 | 20.9 | 18.2 | Anal swab |
| #18 | MPXV2 | 27.7 | 23.7 | Genital swab |
| #19 | Negative | NA | 37.9 | Genital swab |
| #20 | MPXV2 | 23.0 | 18.5 | |
| EV | 31.0 | 30.8 | Skin lesion | |
| #21 | MPXV2 | 25.8 | 19.7 | |
| HSV2 | 36.3 | 25.1 | Genital swab | |
| #22 | MPXV2 | 25.4 | 21.2 | |
| HSV2 | 32.9 | 31.8 | Anal swab | |
| HHV6 | 30.7 | 28.5 | ||
| #23 | EV | 19.9 | 22.0 | Skin lesion |
| #24 | HSV1 | 26.7 | 19.8 | Anal swab |
| #25 | HSV2 | 28.9 | 26.7 | Genital swab |
| #26 | HSV2 | 19.0 | 17.2 | Anal swab |
| #27 | VZV | 14.7 | 15.8 | Genital swab |
| #28 | VZV | 18.1 | 19.2 | Skin lesion |
| #29 | Negative | NA | NA | Skin lesion |
| #30 | Negative | NA | NA | Skin lesion |
| #31 | Negative | NA | NA | Genital swab |
| #32 | Negative | NA | NA | Skin lesion |
| #33 | Negative | NA | NA | Skin lesion |
| #34 | Negative | NA | NA | Anal swab |
| #35 | Negative | NA | NA | Genital swab |
| Characteristics | Patient (35) |
|---|---|
| Age, median (IQR) | 37 (17–61) |
| Gender (%) | |
| Male | 33 (94%) |
| Female | 2 (6%) |
| Sexual Orientation | |
| MSM | 18 (51%) |
| Heterosexual | 1 (3%) |
| Unknown | 16 (46%) |
| Transmission Route | |
| Sexual close contact | 17 |
| Household | 1 |
| Unknown | 17 |
| HIV | |
| Positive | 12 |
| Negative | 16 |
| Unknown | 7 |
| Recent Travel | |
| Yes | 7 |
| No | 12 |
| Unknown | 19 |
| Previous STI (Last Year) | |
| Treponema pallidum | 12 |
| Neisseria gonorrhoeae | 1 |
| T. pallidum and N. gonorrhoeae | 1 |
| Negative | 3 |
| Unknown | 18 |
| Confirmed STI or Vesicular Virus | 18 (54%) |
| Treponema pallidum | 4 |
| N. gonorrhoeae | 3 |
| Mycoplasma spp. | 4 |
| Ureaplasma urealyticum | 2 |
| Chlamydia trachomatis | 1 |
| Other bacteria | 1 |
| EV | 2 |
| HSV 1 | 1 |
| HSV2 | 4 |
| HHV6 | 1 |
| VZV | 2 |
| Negative or unknown | 17 (46%) |
| Concomitant STI or viral infection–Nr/MPXV positive | 8/22 (36%) |
| Patients Positive: | All Patients (n = 35) | % |
|---|---|---|
| MPXV | 13 | 37 |
| MPX and vesicular virus | 3 | 8.6 |
| Vesicular virus | 6 | 17 |
| MPXV and bacterial STI | 5/27 | 18.5 * |
| Bacteria STI | 4/27 | 14.8 * |
| Other bacteria | 1/27 | 3.7 * |
| Patients negative | 3 (2 not tested for STI) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valli, M.B.; Vulcano, A.; Rueca, M.; Matusali, G.; Mazzotta, V.; Nicastri, E.; Girardi, E.; Fontana, C.; Antinori, A.; Maggi, F. Concomitant Syndromic Diagnosis of Mpox and Other Vesicular Viruses in Patients with Skin and Genital Lesions. Pathogens 2024, 13, 207. https://doi.org/10.3390/pathogens13030207
Valli MB, Vulcano A, Rueca M, Matusali G, Mazzotta V, Nicastri E, Girardi E, Fontana C, Antinori A, Maggi F. Concomitant Syndromic Diagnosis of Mpox and Other Vesicular Viruses in Patients with Skin and Genital Lesions. Pathogens. 2024; 13(3):207. https://doi.org/10.3390/pathogens13030207
Chicago/Turabian StyleValli, Maria Beatrice, Antonella Vulcano, Martina Rueca, Giulia Matusali, Valentina Mazzotta, Emanuele Nicastri, Enrico Girardi, Carla Fontana, Andrea Antinori, and Fabrizio Maggi. 2024. "Concomitant Syndromic Diagnosis of Mpox and Other Vesicular Viruses in Patients with Skin and Genital Lesions" Pathogens 13, no. 3: 207. https://doi.org/10.3390/pathogens13030207
APA StyleValli, M. B., Vulcano, A., Rueca, M., Matusali, G., Mazzotta, V., Nicastri, E., Girardi, E., Fontana, C., Antinori, A., & Maggi, F. (2024). Concomitant Syndromic Diagnosis of Mpox and Other Vesicular Viruses in Patients with Skin and Genital Lesions. Pathogens, 13(3), 207. https://doi.org/10.3390/pathogens13030207







