Survival of Escherichia coli in Edible Land Snails: Implications for Heliciculture and Public Health
Abstract
1. Introduction
2. Materials and Methods
2.1. E. coli Isolates and Inoculum Pre-Preparation
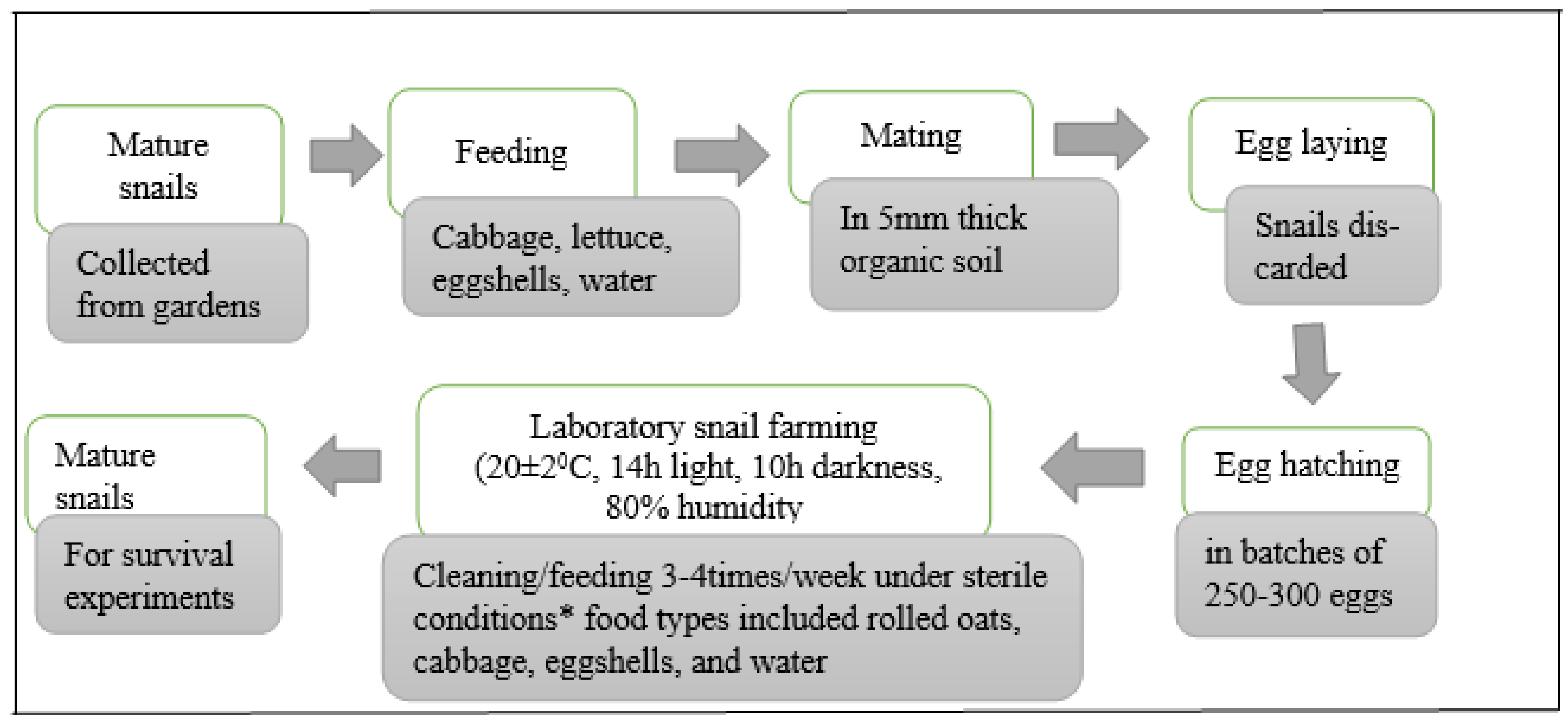
2.2. Preparation of Experimental Snails
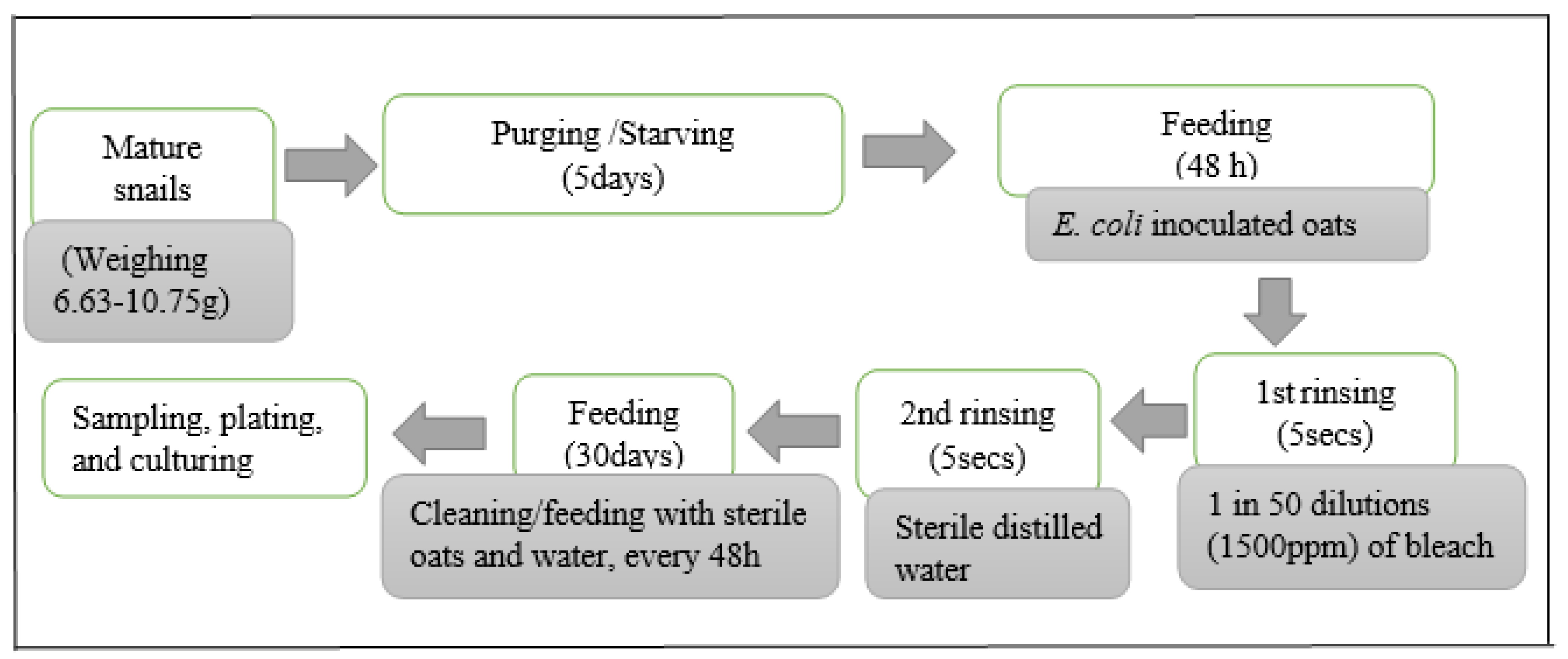
2.3. Inoculation of Experimental Snails
2.4. E. coli Survival Experiments
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paszkiewicz, W.; Kozyra, I.; Rzeżutka, A. A Refinement of an International Standard Method (ISO/TS 15216–2:2013) to Allow Extraction and Concentration of Human Enteric Viruses from Tissues of Edible Snail Species. Food Anal. Methods 2014, 8, 799–806. [Google Scholar] [CrossRef]
- Okafor, F.C. The Varied Roles of Snails (Gastropod Molluscs) in the Dynamics of Human Existence. Master’s Thesis, University of Lagos, Lagos, Nigeria, 2009; 153p. [Google Scholar]
- APHA. The Animal and Plant Health Agency. Import of Snail Meat for Human Consumption. Import Information Note (IIN) BAL/3. Available online: http://apha.defra.gov.uk/official-vets/Guidance/bip/iin/animal-prod-hum-cons.htm (accessed on 7 May 2022).
- Akpomie, O.O. Assessment of health implication associated with snails and snail farm soils in Warri and Sapele, Delta state, Nigeria. Niger. J. Sci. Environ. 2013, 12, 50–56. [Google Scholar]
- Barimah, M.N.Y.S. Microbiological Quality of Edible Land Snails from Selected Markets in Ghana. Master’s Thesis, Department of Microbiology, University of Ghana Medical School, Accra, Ghana, 2013. [Google Scholar]
- Cobbinah, J.R.; Vink, A.; Onwuka, B. Snail Farming: Production, Processing and Marketing; Agromisa: Wageningen, The Netherlands, 2008. [Google Scholar]
- Mohammed, S.; Ahmed, A.; Adjei, D. Opportunities for Increasing Peasant Farmers Income through Snail Production in Ghana. Sch. J. Agric. Vet. Sci. 2014, 1, 195–200. [Google Scholar]
- Nyoagbe, L.A.; Appiah, V.; Josephine, N.; Daniel, L.; Isaac, A. Evaluation of African giant snails (Achatina and Archachatina) obtained from markets (wild) and breeding farms. Afr. J. Food Sci. 2016, 10, 94–104. [Google Scholar] [CrossRef]
- Kocatepe, D.; Çelik, Y. Snail Consumption Habits and Health. Poster in Ecology 2017 International Symposium. 2017. 2p. Available online: https://www.researchgate.net/publication/321330613_Snail_Consumption_Habits_And_Health#fullTextFileContent (accessed on 18 June 2022).
- Pissia, M.A.; Matsakidou, A.; Kiosseoglou, V. Raw materials from snails for food preparation. Future Foods 2021, 3, 100034. [Google Scholar] [CrossRef]
- Tardugno, R.; Virga, A.; Nava, V.; Mannino, F.; Salvo, A.; Monaco, F.; Giorgianni, M.; Cicero, N. Toxic and Potentially Toxic Mineral Elements of Edible Gastropods Land Snails (Mediterranean Escargot). Toxics 2023, 11, 317. [Google Scholar] [CrossRef]
- Ngenwi, A.A.; Mafeni, J.M.; Etchu, K.A.; Oben, F.T. Characteristics of Snail Farmers and Constraints to Increased Production in West and Central Africa. Afr. J. Environ. Sci. Technol. 2010, 4, 274–278. [Google Scholar]
- Parlapani, F.F.; Neofitou, C.; Boziaris, I.S. Microbiological quality of raw and processed wild and cultured edible snails: Microbiological quality of edible snails. J. Sci. Food Agric. 2014, 94, 768–772. [Google Scholar] [CrossRef]
- Waldhorn, D.R. Snails Used for Human Consumption: The Case of Meat and Slime. Available online: https://rethinkpriorities.org/publications/snails-used-for-human-consumption (accessed on 1 June 2022).
- Tanyitiku, M.N.; Nicholas, G.; Sullivan, J.J.; Njombissie Petcheu, I.C.; On, S.L.W. Snail Meat Consumption in Buea, Cameroon: The Methodological Challenges in Exploring Its Public Health Risks. Int. J. Qual. Methods 2022, 21, 16094069221078132. [Google Scholar] [CrossRef]
- Kiebre-Toe, M.; Borges, E.; Maurin, F.; Richard, Y.; Kodjo, A. Etude de la flore bacterienne a ‘erobie’ a Gram n ‘egatif de l’escargot d’ ‘elevage’ (Helix aspersa). Rev. De Médecine Vétérinaire 2003, 154, 606–610. [Google Scholar]
- Adegoke, A.A.; Bukola, A.T.C.; Comfort, I.U.; Olainka, A.A.; Amos, K.O. Snails as meat source: Epidemiological and nutritional perspectives. J. Microbiol. Antimicrob. 2010, 2, 001–005. [Google Scholar]
- Nwuzo, A.C.; Iroha, I.R.; Moses, I.B.; Ugbo, E.N.; Agumah, N.B.; Orji, J.; Ogene, L. Isolation and Characterization of Bacterial Species Associated with Edible Snails (Achatina achatina) sold in Major Markets within Abakaliki Metropolis. Biolife 2016, 4, 494–497. [Google Scholar]
- Tanyitiku, M.N.; Nicholas, G.; Njombissie Petcheu, I.C.; Sullivan, J.J.; On, S.L. Public health risk of foodborne pathogens in edible African land snails, Cameroon. Emerg. Infect. Dis. 2022, 28, 1715. [Google Scholar] [CrossRef]
- Temelli, S.; Dokuzlu, C.; Sen, M.K.C. Determination of microbiological contamination sources during frozen snail meat processing stages. Food Control 2006, 17, 22–29. [Google Scholar] [CrossRef]
- Capps, K.M.; Ludwig, J.B.; Shridhar, P.B.; Shi, X.; Roberts, E.; DebRoy, C.; Cernicchiaro, N.; Phebus, R.K.; Bai, J.; Nagaraja, T.G. Identification, Shiga toxin subtypes and prevalence of minor serogroups of Shiga toxin-producing Escherichia coli in feedlot cattle feces. Sci. Rep. 2021, 11, 8601. [Google Scholar] [CrossRef] [PubMed]
- Avery, S.M.; Moore, A.; Hutchison, M.L. Fate of Escherichia coli originating from livestock faeces deposited directly onto pasture. Lett. Appl. Microbiol. 2004, 38, 355–359. [Google Scholar] [CrossRef]
- Kudva, I.T.; Blanch, K.; Hovde, C.J. Analysis of Escherichia coli O157:H7 survival in ovine or bovine manure and manure slurry. Appl. Environ. Microbiol. 1998, 64, 3166–3174. [Google Scholar] [CrossRef]
- Moriarty, E.M.; Weaver, L.; Sinton, L.W.; Gilpin, B. Survival of Escherichia coli, Enterococci and Campylobacter jejuni in Canada Goose Faeces on Pasture. Zoonoses Public Health 2012, 59, 490–497. [Google Scholar] [CrossRef]
- Cízek, A.; Literák, I.; Scheer, P. Survival of Escherichia coli O157 in faeces of experimentally infected rats and domestic pigeons. Lett. Appl. Microbiol. 2000, 31, 349–352. [Google Scholar] [CrossRef]
- Brandt, S.M.; Paulin, S.M. Quantifying colonization potential of enterohemorrhagic Escherichia coli O157 using bovine in vitro organ culture and immunofluorescent staining. Foodborne Pathog. Dis. 2012, 9, 1064–1070. [Google Scholar] [CrossRef]
- Nakane, K.; Kawamura, K.; Goto, K.; Arakawa, Y. Long-Term Colonization by bla(CTX-M)-Harboring Escherichia coli in Healthy Japanese People Engaged in Food Handling. Appl. Environ. Microbiol. 2016, 82, 1818–1827. [Google Scholar] [CrossRef]
- Thorne, G.M.; Farrar, W.E. Genetic Properties of R Factors Associated with Epidemic Strains of Shigella dysenteriae Type I from Central America and Salmonella typhi from Mexico. J. Infect. Dis. 1973, 128, 132–136. [Google Scholar] [CrossRef]
- Garcia, A.; Perea, J.; Mayoral, A.; Acero, R.; Martos, J.; Gomez, G.; Peña, F. Laboratory rearing conditions for improved growth of juvenile Helix aspersa Muller snails. Lab. Anim. 2006, 40, 309–316. [Google Scholar] [CrossRef]
- Thompson, R.; Cheney, S. Raising Snails; U.S. Department of Agriculture, National Agricultural Library: Beltsville, MD, USA, 1996.
- Murphy, B. Breeding and Growing Snails Commercially in Australia: A Report for the Rural Industries Research and Development Corporation. Available online: http://www.rirdc.gov.au/reports/NAP/00-188.htm (accessed on 18 June 2022).
- Silva, N.d.; Marta, T.H.; Valeria, J.C.A.; Neliane, S.F.A.; Margarete, O.M.; Renato, G.A.R. Microbiological Examination Methods of Food and Water: A Laboratory Manual, 2nd ed.; CRC Press/Balkema: Leiden, The Netherlands; Boca Raton, FL, USA, 2018. [Google Scholar]
- Vergine, P.; Salerno, C.; Barca, E.; Berardi, G.; Pollice, A. Identification of the faecal indicator Escherichia coli in wastewater through the -D-glucuronidase activity: Comparison between two enumeration methods, membrane filtration with TBX agar, and Colilert(R)-18. J. Water Health 2016, 15, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Edelson-Mammel, S.G.; Buchanan, R.L. Thermal inactivation of Enterobacter sakazakii in rehydrated infant formula. J. Food Prot. 2004, 67, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Meffowoet, C.P.; Kouam, K.M.; Kana, J.R.; Tchakounte, F.M. Infestation rate of African giant snails (Achatina fulica and Archachatina marginata) by parasites during the rainy season in three localities of Cameroon. J. Vet. Med. Res. 2020, 7, 1–8. [Google Scholar]
- Scott, L.; McGee, P.; Minihan, D.; Sheridan, J.J.; Earley, B.; Leonard, N. The characterisation of E. coli O157:H7 isolates from cattle faeces and feedlot environment using PFGE. Vet. Microbiol. 2006, 114, 331–336. [Google Scholar] [CrossRef]
- CDC. Center for Disease Control 2021 Outbreaks. Available online: https://www.cdc.gov/ecoli/outbreaks.html (accessed on 18 June 2022).
- Mukherjee, A.; Cho, S.; Scheftel, J.; Jawahir, S.; Smith, K.; Diez-Gonzalez, F. Soil survival of Escherichia coli O157:H7 acquired by a child from garden soil recently fertilized with cattle manure. J. Appl. Microbiol. 2006, 101, 429–436. [Google Scholar] [CrossRef]
- Alsam, S.; Jeong, S.; Sissons, J.; Dudley, R.; Kim, K.; Khan, N. Escherichia coli interactions with Acanthamoeba: A symbiosis with environmental and clinical implications. J. Med. Microbiol. 2006, 55, 689–694. [Google Scholar] [CrossRef]
- Cerezal Mezquita, P.; Madariaga, P.; Segovia, Y. A practical approach to preparation and meat preservation by refrigeration or freezing of the land snail (helix aspersa müller). J. Muscle Foods 2009, 20, 401–419. [Google Scholar] [CrossRef]
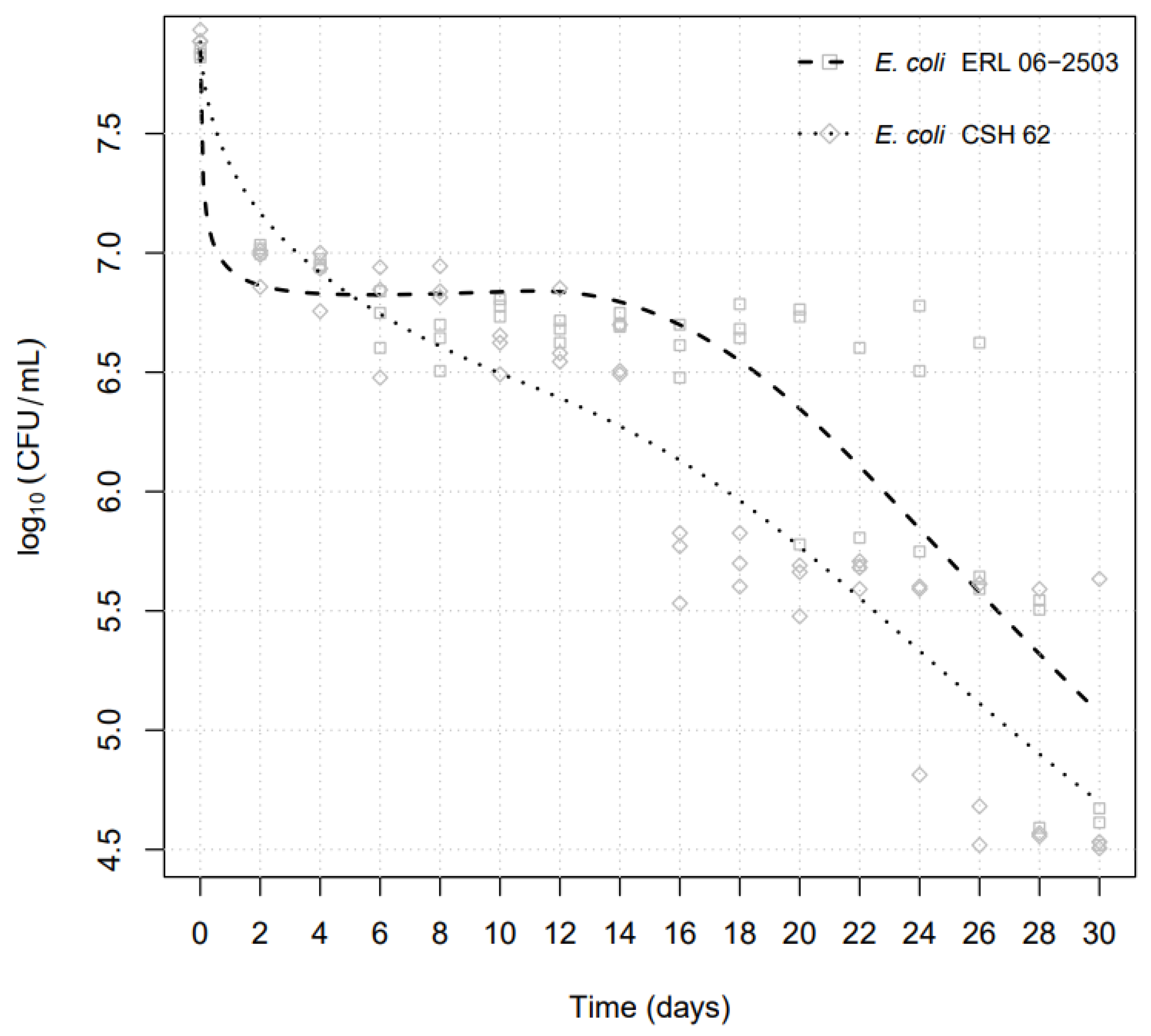
| E. coli Isolates | Day of ~1log Cycle | log10 CFUmL−1 | D-Value (Days) |
|---|---|---|---|
| E. coli CSH-62 | 2 | 6.952 | 1.59 |
| 18 | 5.675 | 14.62 | |
| 26 | 4.604 | 25.36 | |
| E. coli ERL 06-2503 | 2 | 6.795 | 1.38 |
| 22 | 5.765 | 18.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanyitiku, M.N.; Nicholas, G.; Sullivan, J.J.; Petcheu, I.C.N.; On, S.L.W. Survival of Escherichia coli in Edible Land Snails: Implications for Heliciculture and Public Health. Pathogens 2024, 13, 204. https://doi.org/10.3390/pathogens13030204
Tanyitiku MN, Nicholas G, Sullivan JJ, Petcheu ICN, On SLW. Survival of Escherichia coli in Edible Land Snails: Implications for Heliciculture and Public Health. Pathogens. 2024; 13(3):204. https://doi.org/10.3390/pathogens13030204
Chicago/Turabian StyleTanyitiku, Mary Nkongho, Graeme Nicholas, Jon J. Sullivan, Igor C. Njombissie Petcheu, and Stephen L. W. On. 2024. "Survival of Escherichia coli in Edible Land Snails: Implications for Heliciculture and Public Health" Pathogens 13, no. 3: 204. https://doi.org/10.3390/pathogens13030204
APA StyleTanyitiku, M. N., Nicholas, G., Sullivan, J. J., Petcheu, I. C. N., & On, S. L. W. (2024). Survival of Escherichia coli in Edible Land Snails: Implications for Heliciculture and Public Health. Pathogens, 13(3), 204. https://doi.org/10.3390/pathogens13030204







