An Unusual Case of Serologically Confirmed Post-Partum Lyme Disease Following an Asymptomatic Borrelia burgdorferi Infection Acquired during Pregnancy and Lacking Vertical Transmission in Utero
Abstract
1. Introduction
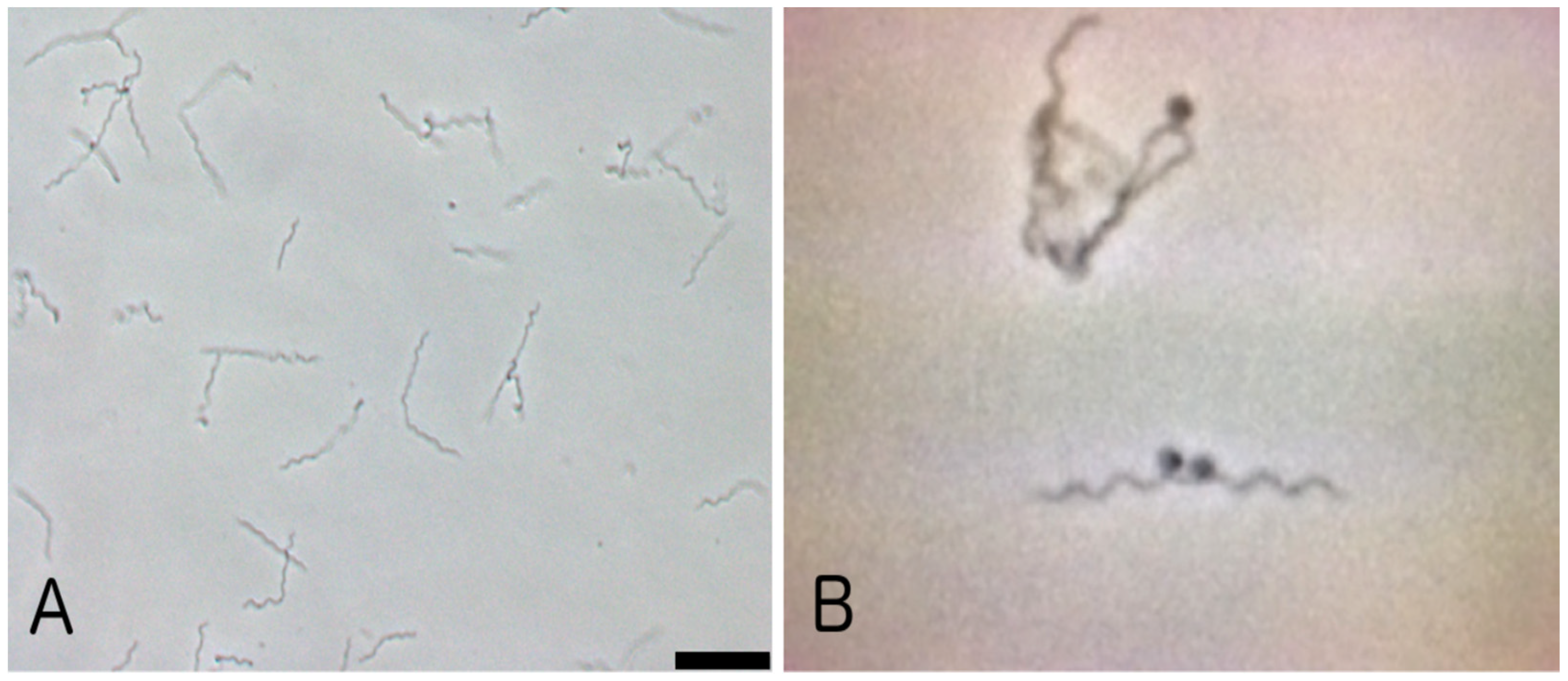
2. Case Report
3. Discussion
3.1. Factors That Could Explain the Favorable Outcome of the Mother and Her Child
- (1)
- Since many of the Lyme disease-associated symptoms [13,14] can be attributed to a Borrelia-elicited immune/inflammatory-mediated reaction rather than a direct attack on host cells by a toxin or other virulence factor, one possible mechanism could include maternal downregulation of certain immunologic responses during various stages of gestation [15,16,17,18,19]. Supporting this possibility are the well-documented findings, some of which are based on the pioneering work of Stites, Siiteri, and Pavia, outlined more than 40 years ago [16,17,18], showing that certain pregnancy-associated hormones, especially those produced by placental trophoblastic cells, may exert various immune-modulating properties [16,17]. These include human chorionic gonadotropin (hCG) [20,21], human placental lactogen (hPL) [21], estrogens, progestogens (especially progesterone) [22], relaxin [23,24], and estradiol [16,17,25]. As soon as the pregnancy ends, these hormones no longer operate at immune-inhibitory/regulatory concentrations, thus allowing for the re-emergence of a previously inactive infection or a dormant inflammatory state or other immunomodulatory activities [26,27]. Years later, these initial and breakthrough findings were subsequently extended in studies using animal infection models of Lyme disease, where it was shown that gestational attenuation of Lyme arthritis is mediated by progesterone and interleukin-4 [28], and that IL-10 can play a dual role in murine Lyme disease by regulating the severity of arthritis and host defense [29].
- (2)
- A possible protective mechanism for the newborn pertains to the potential phagocytic/borreliacidal activity and immunocompetence of the cells located on the surface and internally of the placental trophoblast within the placenta as it matures and grows, as previously reported [30,31]. It was shown that, when cultured in vitro, mouse trophoblast cells were able to phagocytize and kill malaria parasites; in addition, placental lymphocytes from Balb/c mice expressed a weak-to-moderate proliferative response to concanavalin A (Con A), phytohemagglutinin (PHA), and pokeweed mitogen when compared to the mitogenic responses of adult spleen cells. The stimulatory effects of Con A and PHA were abrogated after depleting the T cells from the placental lymphocyte preparation. Lipopolysaccharide-induced reactivity was similar for both cell populations.
3.2. Evidence for Either in Utero Transmission or Lack of B. burgdorferi during Pregnancy
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alexander, J.M.; Cox, S.M. Lyme disease and pregnancy. Infect. Dis. Obstet. Gynecol. 1995, 3, 256–261. [Google Scholar] [CrossRef]
- Waddell, L.A.; Greig, J.; Lindsay, L.R.; Hinckley, A.F.; Nicholas, H.; Ogden, N.H. A systematic review on the impact of gestational Lyme disease in humans on the fetus and newborn. PLoS ONE 2018, 13, e0207067. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.A.; Mayer, E.W.; Baxi, L.V. Lyme disease in pregnancy: Case report and review of the literature. Obstet. Gynecol. Surv. 2007, 62, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.S. An Overview of Tickborne Infections in Pregnancy and Outcomes in the Newborn: The Need for Prospective Studies. Front. Med. 2020, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Hinckley, A.F.; Connally, N.P.; Meek, J.I.; Johnson, B.J.; Kemperman, M.M.; Feldman, K.A.; White, J.L.; Mead, P.S. Lyme disease testing by large commercial laboratories in the United States. Clin. Infect. Dis. 2014, 59, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Strobino, B.A.; Williams, C.L.; Abid, S.; Ghalson, R.; Spierling, P. Lyme disease and pregnancy outcome: A prospective study of two thousand prenatal patients. Am. J. Obstet. Gynecol. 1993, 169 Pt 1, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Remington, J.S.; Klein, J.O.; Wilson, C.B.; Baker, C.J. Remington and Klein’s Infectious Diseases of the Fetus and Newborn Infant, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Larsson, C.; Andersson, M.; Guo, B.P.; Nordstrand, A.; Hägerstrand, I.; Carlsson, S.; Bergström, S. Complications of pregnancy and transplacental transmission of relapsing-fever borreliosis. J. Infect. Dis. 2006, 194, 1367–1374. [Google Scholar] [CrossRef]
- Shaked, Y.; Shpilberg, O.; Samra, D.; Samra, Y. Leptospirosis in pregnancy and its effect on the fetus: Case report and review. Clin. Infect. Dis. 1993, 17, 241–243. [Google Scholar] [CrossRef] [PubMed]
- White, J.L. Ticks and Tick-Borne Diseases in New York State. Available online: https://www.health.ny.gov/diseases/communicable/lyme/working_group/docs/ticks_and_tick-borne_disease_in_nys.pdf (accessed on 15 November 2023).
- McGraw, N. Tick Transmitted Diseases Rising. Sullivan County Health Department. 2021. Available online: https://www.sullivanny.us/news/tick-transmitted-diseases-rising (accessed on 15 November 2023).
- Wormser, G.P.; Dattwyler, R.J.; Shapiro, E.D.; Halperin, J.J.; Steere, A.C.; Klempner, M.S.; Krause, P.J.; Bakken, J.S.; Strle, F.; Stanek, G.; et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: Clinical practice guidelines by the infectious diseases society of america. Clin. Infect. Dis. 2006, 43, 1089–1134. [Google Scholar] [CrossRef]
- Steere, A.C. Lyme borreliosis in 2005, 30 years after initial observations in Lyme Connecticut. Wien. Klin. Wochenschr. 2006, 118, 625–633. [Google Scholar] [CrossRef]
- Steere, A.C.; Strle, F.; Wormser, G.P.; Hu, L.T.; Branda, J.A.; Hovius, J.W.R.; Li, X.; Mead, P.S. Lyme borreliosis. Nat. Rev. Dis. Primers 2016, 2, 16090. [Google Scholar] [CrossRef]
- Torry, D.S.; Mcintyre, J.A.; Faulk, W.P. Immunobiology of the Trophoblast: Mechanisms by Which Placental Tissues Evade Maternal Recognition and Rejection. In Current Topics in Microbiology and Immunology Book Series; Springer: Berlin/Heidelberg, Germany, 1997; Volume 222, pp. 127–141. [Google Scholar]
- Stites, D.P.; Pavia, C.S.; Clemens, L.E.; Kuhn, R.W.; Siiteri, P.K. Immunologic regulation in pregnancy. Arthritis Rheum. 1979, 22, 1300–1307. [Google Scholar] [CrossRef]
- Siiteri, P.K.; Stites, D.P. Immunologic and endocrine interrelationships in pregnancy. Biol. Reprod. 1982, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pavia, C.S.; Stites, D.P. Humoral and cellular regulation of alloimmunity in pregnancy. J. Immunol. 1979, 123, 2194–2200. [Google Scholar] [CrossRef]
- Pavia, C.; Siiteri, P.; Perlman, J.; Stites, D. Suppression of murine allogeneic cell interactions by sex hormones. J. Reprod. Immunol. 1979, 1, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, A. Human Chorionic Gonadotropin as a Pivotal Endocrine Immune Regulator Initiating and Preserving Fetal Tolerance. Int. J. Mol. Sci. 2017, 18, 2166. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.; Pauli, G.; Friedmann, W.; Dudenhausen, J.W. Human choriogonadotropin (hCG) and placental lactogen (hPL) inhibit interleukin-2 (IL-2) and increase interleukin-1β (IL-1β), -6 (IL-6) and tumor necrosis factor (TNF-α) expression in monocyte cell cultures. J. Perinat. Med. 1992, 20, 233–240. [Google Scholar] [CrossRef]
- Arck, P.; Hansen, P.J.; Jericevic, B.M.; Piccinni, M.; Szekeres-Bartho, J. Progesterone during pregnancy: Endocrine–Immune cross talk in mammalian species and the role of stress. Am. J. Reprod. Immunol. 2007, 58, 268–279. [Google Scholar] [CrossRef]
- Dschietzig, T.; Bartsch, C.; Stangl, V.; Baumann, G.; Stangl, K. Identification of the pregnancy hormone relaxin as glucocorticoid receptor agonist. FASEB J. 2004, 18, 1536–1538. [Google Scholar] [CrossRef]
- Bryant-Greenwood, G.D.; Yamamoto, S.Y.; Sadowsky, D.W.; Gravett, M.G.; Novy, M.J. Relaxin stimulates interleukin-6 and interleukin-8 secretion from the extraplacental chorionic cytotrophoblast. Placenta 2009, 30, 599–606. [Google Scholar] [CrossRef]
- Kovats, S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell. Immunol. 2015, 294, 63–69. [Google Scholar] [CrossRef]
- Chen, S.-J.; Liu, Y.-L.; Sytwu, H.-K. Immunologic Regulation in Pregnancy: From Mechanism to Therapeutic Strategy for Immunomodulation. J. Immunol. Res. 2011, 2012, 258391. [Google Scholar] [CrossRef]
- Yang, X.; Tian, Y.; Zheng, L.; Luu, T.; Kwak-Kim, J. The Update Immune-Regulatory Role of Pro- and Anti-Inflammatory Cytokines in Recurrent Pregnancy Losses. Int. J. Mol. Sci. 2022, 24, 132. [Google Scholar] [CrossRef]
- Moro, M.H.; Bjornsson, J.; Marietta, E.V.; Hofmeister, E.K.; Germer, J.J.; Bruinsma, E.; David, C.S.; Persing, D.H. Gestational attenuation of Lyme arthritis is mediated by progesterone and IL-4. J. Immunol. 2001, 166, 7404–7409. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.P.; Zachary, J.F.; Teuscher, C.; Weis, J.J.; Wooten, R.M. Dual Role of Interleukin-10 in Murine Lyme Disease: Regulation of Arthritis Severity and Host Defense. Infect. Immun. 1999, 67, 5142–5150. [Google Scholar] [CrossRef] [PubMed]
- Pavia, C.S.; Stites, D.P. Immunocompetence of murine placental lymphocytes. In vitro response to mitogens and allogeneic cells. Immunobiology 1981, 160, 228–240. [Google Scholar] [CrossRef]
- Pavia, C.S. Expression of cell-mediated antimicrobial immunity by mouse trophoblast monolayers. J. Infect. Dis. 1983, 147, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, P.A.; Duray, P.H.; Burke, B.A.; Steere, A.C.; Stillman, M.T. Maternal-fetal transmission of the Lyme disease spirochete, Borrelia burgdorferi. Ann. Intern. Med. 1985, 103, 67–68. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.B. Human fetal borreliosis, toxemia of pregnancy, and fetal death. Zentralblatt Bakteriol. Mikrobiol. Hygiene. Ser. A Med. Microbiol. Infect. Dis. Virol. Parasitol. 1986, 263, 189–200. [Google Scholar] [CrossRef]
- MacDonald, A.B.; Benach, J.L.; Burgdorfer, W. Stillbirth following maternal Lyme disease. NY State J. Med. 1987, 87, 615–616. [Google Scholar]
- MacDonald, A.B. Gestational Lyme borreliosis. Implications for the fetus. Rheum. Dis. Clin. N. Am. 1989, 15, 657–677. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Lyme Carditis. Available online: https://www.cdc.gov/lyme/treatment/lymecarditis.html (accessed on 15 November 2023).
- Weber, K.; Bratzke, H.J.; Neubert, U.; Wilske, B.; Duray, P.H. Borrelia burgdorferi in a newborn despite oral penicillin for Lyme borreliosis during pregnancy. Pediatr. Infect. Dis. J. 1988, 7, 286–289. [Google Scholar] [CrossRef]
- Liveris, D.; Wormser, G.P.; Nowakowski, J.; Nadelman, R.; Bittker, S.; Cooper, D.; Varde, S.; Moy, F.H.; Forseter, G.; Pavia, C.S.; et al. Molecular typing of Borrelia burgdorferi from Lyme disease patients by PCT-restriction fragment length polymorphogenic analyses. J. Clin. Microbiol. 1996, 34, 1306–1309. [Google Scholar] [CrossRef]
- Wormser, G.P.; Nowakowski, J.; Nadelman, R.N.; Bittker, S.; Cooper, D.; Pavia, C. Improving the yield of blood cultures in early Lyme disease. J. Clin. Microbiol. 1998, 36, 296–298. [Google Scholar] [CrossRef]
- Wormser, G.P.; Bittker, S.; Cooper, D.; Nowakowski, J.; Nadelman, R.B.; Pavia, C. Comparison of the yields of blood cultures using serum or plasma from patients with early Lyme disease. J. Clin. Microbiol. 2000, 38, 1648–1650. [Google Scholar] [CrossRef]
- Benach, J.L.; Bosler, E.M.; Hanrahan, J.P.; Coleman, J.L.; Habicht, G.S.; Bast, T.F.; Cameron, D.J.; Ziegler, J.L.; Barbour, A.G.; Burgdorfer, W.; et al. Spirochetes isolated from the blood of two patients with Lyme disease. N. Engl. J. Med. 1984, 308, 740–742. [Google Scholar] [CrossRef]
- Leavey, K.; MacKenzie, R.K.; Faber, S.; Lloyd, V.K.; Mao, C.; Wills, M.K.B.; Boucoiran, I.; Cates, E.C.; Omar, A.; Marquez, O.; et al. Lyme borreliosis in pregnancy and associations with parent and offspring health outcomes: An international cross-sectional survey. Front. Med. 2022, 9, 1022766. [Google Scholar] [CrossRef]
- Government of Canada. Lyme Disease: Surveillance. Available online: https://www.canada.ca/en/public-health/services/diseases/lyme-disease/surveillance-lyme-disease.html (accessed on 16 November 2023).
- De Luigi, G.; Meoli, M.; Zgraggen, L.; Kottanattu, L.; Simonetti, G.D.; Terrani, I.; Bianchetti, M.G.; Lava, S.A.; Milani, G.P. Mucosal Respiratory Syndrome: A Systematic Literature Review. Dermatology 2022, 238, 53–59. [Google Scholar] [CrossRef]
- Picone, O.; Fuchs, F.; Benoist, G.; Binquet, C.; Kieffer, F.; Wallon, M.; Wehbe, K.; Mandelbrot, L.; Villena, I. Toxoplasmosis screening during pregnancy in France: Opinion of an expert panel for the CNGOF. J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101814. [Google Scholar] [CrossRef] [PubMed]
- Maraspin, V.; Lusa, L.; Blejec, T.; Ružić-Sabljić, E.; Perme, M.P.; Strle, F. Course and Outcome of Erythema Migrans in Pregnant Women. J. Clin. Med. 2020, 9, 2364. [Google Scholar] [CrossRef] [PubMed]
- Seriburi, V.; Ndukwe, N.; Chang, Z.; Cox, M.; Wormser, G. High frequency of false positive IgM immunoblots for Borrelia burgdorferi in clinical practice. Clin. Microbiol. Infect. 2012, 18, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Lantos, P.M.; Lipsett, S.C.; Nigrovic, L.E. False positive Lyme disease IgM immunoblots in children. J. Pediatr. 2016, 174, 267–269.e9. [Google Scholar] [CrossRef] [PubMed]
- Webber, B.; Burganowski, R.; Colton, L.; Escobar, J.; Pathak, S.; Gambino-Shirley, K. Lyme disease overdiagnosis in a large healthcare system: A population-based, retrospective study. Clin. Microbiol. Infect. 2019, 25, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.J.; Gazumyan, A.; Schwartz, I. rRNA gene organization in the Lyme disease spirochete, Borrelia burgdorferi. J. Bacteriol. 1992, 174, 3757–3765. [Google Scholar] [CrossRef]
- Cleveland, D.W.; Anderson, C.C.; Brissette, C.A. Borrelia miyamotoi: A Comprehensive Review. Pathogens 2023, 12, 267. [Google Scholar] [CrossRef]
| Factor | Relevant References |
|---|---|
| Immunologic regulation caused by pregnancy-associated hormones a | [16,17,18,19,20,21,22,23,24,25,29] |
| Phagocytic/microbicidal activity by placental trophoblastic cells b | [31] |
| Immunocompetence of placental lymphocytes b | [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavia, C.S.; Plummer, M.M.; Varantsova, A. An Unusual Case of Serologically Confirmed Post-Partum Lyme Disease Following an Asymptomatic Borrelia burgdorferi Infection Acquired during Pregnancy and Lacking Vertical Transmission in Utero. Pathogens 2024, 13, 186. https://doi.org/10.3390/pathogens13030186
Pavia CS, Plummer MM, Varantsova A. An Unusual Case of Serologically Confirmed Post-Partum Lyme Disease Following an Asymptomatic Borrelia burgdorferi Infection Acquired during Pregnancy and Lacking Vertical Transmission in Utero. Pathogens. 2024; 13(3):186. https://doi.org/10.3390/pathogens13030186
Chicago/Turabian StylePavia, Charles S., Maria M. Plummer, and Alena Varantsova. 2024. "An Unusual Case of Serologically Confirmed Post-Partum Lyme Disease Following an Asymptomatic Borrelia burgdorferi Infection Acquired during Pregnancy and Lacking Vertical Transmission in Utero" Pathogens 13, no. 3: 186. https://doi.org/10.3390/pathogens13030186
APA StylePavia, C. S., Plummer, M. M., & Varantsova, A. (2024). An Unusual Case of Serologically Confirmed Post-Partum Lyme Disease Following an Asymptomatic Borrelia burgdorferi Infection Acquired during Pregnancy and Lacking Vertical Transmission in Utero. Pathogens, 13(3), 186. https://doi.org/10.3390/pathogens13030186






