Enterobacteriaceae in Sewage Sludge and Digestate Intended for Soil Fertilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Determination of Bacteria Concentration Using Culture-Based Methods
2.1.1. Sample Collection
2.1.2. Microbiological Culture
2.2. Determination of Bacteria Species via Biochemical and Molecular Methods
2.2.1. Biochemical Tests
2.2.2. Molecular Tests
2.3. The Survival of E. coli Present in Organic Fertilizers on a Laboratory Scale
2.3.1. Samples
2.3.2. Inoculum Preparation
2.3.3. Main Experiment
2.3.4. Control Group
3. Results
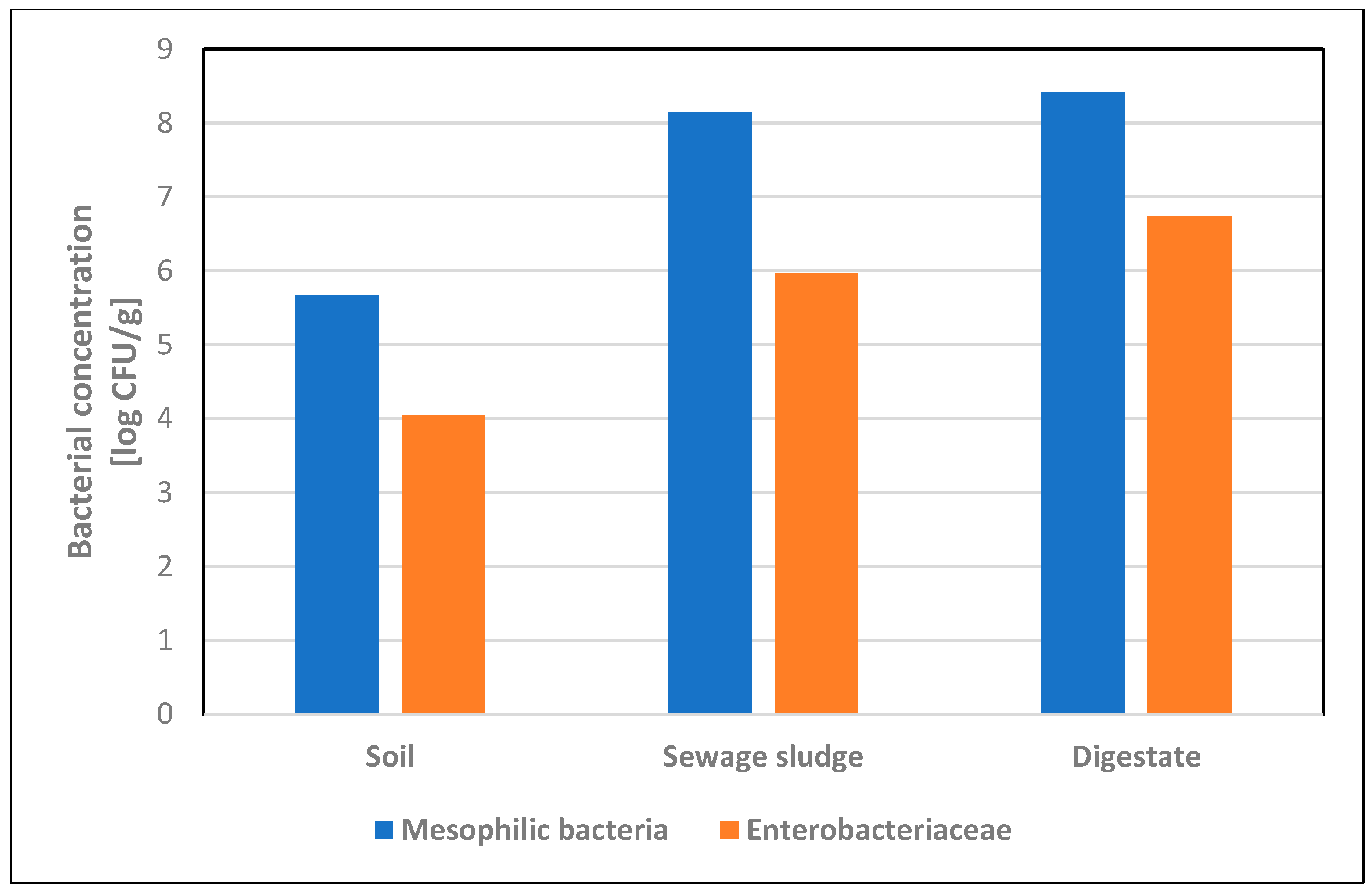
3.1. Bacterial Concentration in Soil, Sewage Sludge and Digestate Samples
3.2. Species Diversity of Enterobacteriaceae Isolated from Soil, Sewage Sludge and Digestate
| Enterobacteriaceae Isolated from Soil Samples (n = 82) | Sequence Result (Similarity with 16S rRNA) | Impact on Human Health | ||||
|---|---|---|---|---|---|---|
| Aeromonas spp. (n = 13) | A. hydrophila | 100% | MT572504.1 | opportunistic pathogen | emerging foodborne pathogen capable of causing human gastroenteritis | [19] |
| A. sobria | 99.79% | OL778934.1 | food-borne illness, severe extraintestinal diseases: sepsis, peritonitis, meningitis | [20] | ||
| A. media | 99.82% | CP038448.1 | food-borne illness, diarrhea | [21] | ||
| A. veronii | 99.41% | KF853564.1 | gastroenteritis, bacteremia, septicemia, wound infections | [22] | ||
| A. salmonicida | 99.54% | MT576565.1 | strain isolated from the blood of the patient with endocarditis | [23] | ||
| A. encheleia | 100% | MT436428.1 | - | - | ||
| Achromobacter spp. (n = 2) | A. xylosoxidans subsp. xylosoxidans | 99.77% | CP054571.1 | opportunistic pathogen | bacteremia, meningitis, urinary tract infection, endocarditis, pneumonia, especially in immunocompromised patients | [24] |
| Buttiauxella spp. (n = 4) | B. agrestis | 99.57% | AP023184.1 | rare opportunistic pathogen | B. agrestis infection occurring at a post-cesarean surgical site | [25] |
| B. gaviniae | 100% | MK905440.1 | - | clinical strain isolated from a urine sample from a spinal cord patient with urinary bladder pathology | [26] | |
| Citrobacter spp. (n = 27) | C. braakii | 99.57% | MT534007.1 | rare pathogen, nosocomial infections | bacteremia in immunocompromised patients | [27,28] |
| C. freundii | 100% | MH045703.1 | opportunistic pathogen of a wide spectrum of nosocomial infections | food poisoning, diarrhea, urinary tract infections | [29,30] | |
| Enterobacter spp. (n = 37) | E. cloacae subsp. cloacae | 99.83% | MN181145.1 | opportunistic pathogen, nosocomial infections | lower respiratory tract infections, bacteremia | [31] |
| E. ludwigii | 99.71% | MN826154.1 | unusual human pathogen | nosocomial bloodstream infection | [32] | |
| E. asburiae | 99.67% | MN709316.1 | opportunistic pathogen | nosocomial infections | [33] | |
| E. amnigenus biovar 1 | 100% | MN658356.1 | unusual human pathogen | nosocomial infections | [34] | |
| Escherichia spp. (n = 14) | E. coli | 100% | MT192520.1 | opportunistic pathogen | enteritis, urinary tract infection, septicemia, neonatal meningitis, diarrhea | [35] |
| E. vulneris | 99.57% | KX357823.1 | possible opportunistic pathogen | wound infections; complicated diarrhea and sepsis in an infant | [36,37] | |
| Ewingella spp. (n = 1) | E. americana | 100% | MT998223.1 | rare opportunistic pathogen | bacteremia, pneumonia, conjunctivitis, Waterhouse–Friderichsen syndrome, peritonitis | [38] |
| Gibbsiella spp. (n = 1) | G. quercinecans | 99.78% | MN822736.1 | - | - | - |
| Hafnia spp. (n = 2) | H. alvei | 99.78% | CP050150.1 | potentially opportunistic pathogen | rare pneumonia cases | [39,40] |
| Klebsiella spp. (12) | K. pneumoniae subsp. pneumoniae | 99.79% | OM017200.1 | opportunistic pathogen | urinary and respiratory tract infections, liver abscess, endophthalmitis, meningitis | [41] |
| K. oxytoca | 100% | MT568561.1 | opportunistic pathogen | colitis, infective endocarditis, urinary and respiratory tract infections associated with nosocomial infections | [42] | |
| Kluyvera spp. (n = 2) | K. intermedia | 99.78% | LT899978.1 | potential opportunistic pathogen | soft tissue infections, urinary tract infections, intra-abdominal abscesses, catheter-associated bloodstream infections, septic shock in immunocompromised patients (nosocomial infections) | [43] |
| Pantoea spp. (n = 32) | P. agglomerans | 99.76% | MT635441.1 | opportunistic pathogen | septic arthritis, synovitis endophthalmitis, periostitis, endocarditis and osteomyelitis in the event of wound infection with plant material or as a hospital-acquired infection, mostly in immunocompromised individuals | [44] |
| Pseudomonas spp. (n = 25) | P. tolaasii | 99.56% | MT561438.1 | - | - | - |
| P. abietaniphila | 100% | MH379754.1 | - | - | - | |
| P. fluroescens | 99.73% | OM827287.1 | scarce clinical significance | nosocomial infections | [45] | |
| P. koreensis | 100% | MT501807.1 | - | possible nosocomial infections; a case of contact lens-related mixed infectious keratitis caused by A. fumigatus and P. koreensis | [46,47] | |
| P. brassicacearum subsp. neoaurantiaca | 99.80 | MT634587.1 | - | - | - | |
| P. chlororaphis | 100% | KJ530973.1 | - | - | - | |
| P. kilonensis | 99.57% | MT102732.1 | - | - | - | |
| Rahnella spp. (n = 18) | R. aquatilis | 99.77% | MN826573.1 | possible opportunistic pathogen | possible role as the pathogen responsible for ventilator-associated pneumonia associated with nosocomial infections in immunocompromised patients | [48] |
| R. victoriana | 99.78% | OK658118.1 | - | - | - | |
| Raoultella spp. (n = 5) | R. terrigena | 100% | MT545123.1 | opportunistic pathogen | cases of bloodstream, urinary tract, respiratory tract or bile tract infections mostly associated with nosocomial infections; subungual abscess caused by R. terrigena | [49,50] |
| R. ornithinolytica | 100% | MT568560.1 | ||||
| Serratia spp. (n = 44) | S. plymuthica | 99.58% | CP053398.1 | rare (unusual) human pathogen | associated with chronic osteomyelitis and cases of sepsis secondary to central venous catheter infection | [51] |
| S. liquefaciens | 100% | MT279350.1 | opportunistic pathogen | cause of transfusion-related sepsis, meningitis thrombophlebitis, corneal ulcers | [52] | |
| S. fonticola | 100% | MN227497.1 | rare (unusual) human pathogen | skin and soft tissue infections | [53,54] | |
| S. quinivorans | 99.71% | MT256279.1 | - | - | - | |
| S. proteamaculans | 100% | MK530287.1 | opportunistic pathogen | able to penetrate eukaryotic cells | [55] | |
| S. marcescens | 99.85% | MT598027.1 | opportunistic pathogen | urinary tract infections, pneumonia, intravenous catheter-associated infections, osteomyelitis, endocarditis | [51,56] | |
| S. entomophila | 99.83% | MK216954.1 | - | - | - | |
| Yersinia spp. (n = 2) | Y. enterocolitica | 99.14% | MN905014.1 | zoonotic pathogen | enteric infections, mesenteric lymphadenitis, reactive arthritis, erythema nodosum | [57] |
| Enterobacteriaceae Isolated from the Sewage Sludge and Digestate Samples (n = 18) | Sequence Result (Similarity with 16S rRNA) | Impact on Human Health | ||||
|---|---|---|---|---|---|---|
| Sewage Sludge Samples | Digestate Samples | |||||
| Aeromonas salmonicida | 100% | MT576565.1 | 100% | KF551980.1 | As in Table 2 | |
| Alcaligenes faecalis | 99.76% | MT277037.1 | - | - | sporadic cases of endocarditis, meningitis, chronic otitis, pyelonephritis, bacteremia, peritonitis, endophthalmitis, abscesses, often associated with nosocomial infections | [58] |
| Comamonas jiangduensis | 99.80% | NR_109655.1 | - | - | - | - |
| Citrobacter freundii | - | - | 100% | OM666544.1 | As in Table 2 | |
| Citrobacter gillenii | - | - | 100% | MT436425.1 | the strains were isolated from human stool, urine and blood | [59] |
| Enterobacter cloacae | 99.61% | MN006380.1 | - | - | As in Table 2 | |
| Escherichia coli | 100% | OM982954.1 | 100% | CP091756.1 | As in Table 2 | |
| Hafnia alvei | 99.39% | CP050150.1 | - | - | As in Table 2 | |
| Ignatzschineria indica | 99.75% | LC010924.1 | rare cases of bacteremia mostly associated with wound myiasis | [60] | ||
| Klebsiella oxytoca | 99.48% | MT509911.1 | - | - | As in Table 2 | |
| Klebsiella pneumoniae | - | - | 99.79% | OM978275.1 | As in Table 2 | |
| Morganella morganii subsp. morganii | 99.80% | CP043955.1 | - | - | opportunistic infections: urinary tract infection, wound infection, arthritis, prostatitis | [61] |
| Proteus mirabilis | - | - | 99.63% | OM882519.1 | catheter-associated urinary tract infections | [62] |
| Salmonella enterica subsp. enterica serovar Johannesburg | - | - | 100% | CP049308.1 | gastrointestinal infection—salmonellosis | [63] |
| Yersinia enterocolitica | - | - | 99.71% | MK910030.1 | As in Table 2 | |
| Yersinia frederiksenii | 100% | KC776774.1 | - | - | possible diarrheal diseases | [64] |
| Yersinia intermedia | - | - | 99.82% | MN416246.1 | strains were isolated from human stool and urine samples and from wound infections but rarely associated with human diseases | [65] |
3.3. The Survival of E. coli in Soil Samples Fertilized with Sewage Sludge and Digestate in Laboratory Conditions
3.3.1. Main Experiment
3.3.2. Control Experiment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samaddar, S.; Karp, D.S.; Schmidt, R.; Devarajan, N.; McGarvey, J.A.; Pires, A.F.A.; Scow, K. Role of soil in the regulation of human and plant pathogens: Soils’ contributions to people. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2021, 376, 20200179. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.; Skrzypczak, D.; Szopa, D.; Izydorczyk, G.; Moustakas, K.; Witek-Krowiak, A. Management of biological sewage sludge: Fertilizer nitrogen recovery as the solution to fertilizer crisis. J. Environ. Manag. 2023, 15, 116602. [Google Scholar] [CrossRef] [PubMed]
- Regulation of the Minister of the Environment of 6 February 2015 Regarding Municipal Sewage Sludge (Journal of Laws 2015, Item 257). Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=wdu20150000257 (accessed on 10 September 2024). (In Polish)
- Council Directive of 12 June 1986 on the protection of the environment, and in particular of the soil, when sewage sludge is used in agriculture (86/278/EEC). Off. J. Eur. Communities 1986, 181, 6–12.
- Hudcová, H.; Vymazal, J.; Rozkošný, M. Present restrictions of sewage sludge application in agriculture within the European Union. Soil Water Res. 2019, 14, 104–120. [Google Scholar] [CrossRef]
- Estrada, I.B.; Aller, A.; Aller, F.; Gómez, X.; Morán, A. The survival of Escherichia coli, faecal coliforms and enterobacteriaceae in general in soil treated with sludge from wastewater treatment plants. Bioresour. Technol. 2004, 93, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Stańczyk-Mazanek, E.; Stępniak, L. Analysis of migration of pathogenic drug-resistant bacteria to soils and groundwater after fertilization with sewage sludge. PLoS ONE 2021, 16, e0256936. [Google Scholar] [CrossRef]
- Michelon, W.; Peter, N.R.W.; Schneider, T.M.; Segalla, D.C.; Viancelli, A. Enterobacteria survival, percolation, and leaching on soil fertilized with swine manure. Int. J. Environ. Res. Public Health 2023, 20, 5283. [Google Scholar] [CrossRef]
- Regulation of the Minister of Agriculture and Rural Development of 18 June 2008 on the Implementation of Certain Provisions of the Act on Fertilizers and Fertilization. (Journal of Laws 2008 No. 119, Item 765). Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=wdu20081190765 (accessed on 10 September 2024). (In Polish)
- Act of 10 July 2007 on Fertilizers and Fertilization (Journal of Laws of 2024, item 105, as Amended). Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20240000105 (accessed on 10 September 2024). (In Polish)
- Regulation of the Minister of Agriculture and Rural Development of 9 August 2024 on the Implementation of Certain Provisions of the Act on Fertilizers and Fertilization (Journal of Laws 2024, Item 1261). Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20240001261 (accessed on 10 September 2024). (In Polish)
- PN-Z-19000–1/2001; Jakość gleby. Ocena Stanu Sanitarnego Gleby: Wykrywanie Bakterii z Rodzaju Salmonella [Soil Quality—Assessment of the Soil Sanitary Conditions—Detection of the Salmonella Genus Bacteria]. Polish Standardization Committee: Warsaw, Poland, 2001. (In Polish)
- PN-ISO 16649-2:2004; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of β-glucuronidase-Positive Escherichia coli—Part 2: Colony-Count Technique at 44 °C Using 5-bromo-4-chloro-3-indolyl β-D-glucuronide. ISO: Geneva, Switzerland, 2004.
- PN-EN ISO 4833-2:2013-12/AC; Microbiology of the Food Chain. Horizontal Method for the Enumeration of Microorganisms. Part 2: Colony Count at 30 °C by the Surface Plating Technique. ISO: Geneva, Switzerland, 2013.
- PN-EN ISO 21528-2:2017-08; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae. ISO: Geneva, Switzerland, 2017.
- Chun, J.; Goodfellow, M. A phylogenetic analysis of the genus Nocardia with 16S rRNA gene sequences. Int. J. Syst. Bacteriol. 1995, 45, 240–245. [Google Scholar] [CrossRef]
- Farian, E.; Wójcik-Fatla, A.; Kłapeć, T.; Zdybel, J.; Kowalczyk, K.; Sroka, J.; Cencek, T. Soil, sewage sludge and digestate samples contamination with Gram-negative bacteria from Enterobacteriaceae. In Proceedings of the 34th Annual Conference of the International Society for Environmental Epidemiology (ISEE 2022), Athens, Greece, 18–21 September 2022. [Google Scholar] [CrossRef]
- Wójcik-Fatla, A.; Kowalczyk, K.; Kłapeć, T.; Zdybel, J.; Farian, E.; Sroka, J.; Cencek, T. Species diversity of bacteria isolated from soil, sludge sewage and digestate. In Proceedings of the 34th Annual Conference of the International Society for Environmental Epidemiology (ISEE 2022), Athens, Greece, 18–21 September 2022. [Google Scholar] [CrossRef]
- Park, S.M.; Kim, H.W.; Choi, C.; Rhee, M.S. Pathogenicity and seasonal variation of Aeromonas hydrophila isolated from seafood and ready-to-eat sushi in South Korea. Food Res. Int. 2021, 147, 110484. [Google Scholar] [CrossRef]
- Kobayashi, H.; Seike, S.; Yamaguchi, M.; Ueda, M.; Takahashi, E.; Okamoto, K.; Yamanaka, H. Aeromonas sobria serine protease decreases epithelial barrier function in T84 cells and accelerates bacterial translocation across the T84 monolayer in vitro. PLoS ONE 2019, 14, e0221344. [Google Scholar] [CrossRef]
- Talagrand-Reboul, E.; Roger, F.; Kimper, J.L.; Colston, S.M.; Graf, J.; Latif-Eugenín, F.; Figueras, M.J.; Petit, F.; Marchandin, H.; Jumas-Bilak, E.; et al. Delineation of taxonomic species within complex of species: Aeromonas media and related species as a test case. Front. Microbiol. 2017, 8, 621. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bravo, A.; Figueras, M.J. Immune response of the monocytic cell line THP-1 against six Aeromonas spp. Front. Immunol. 2022, 13, 875689. [Google Scholar] [CrossRef]
- Salehi, M.R.; Shadvar, S.; Sadeghian, M.; Doomanlou, M.; Abdollahi, A.; Manshadi, S.A.D.; Sardari, A.; Rahdar, H.A.; Feizabadi, M.M. Endocarditis with Aeromonas salmonicida. IDCases 2019, 18, e00625. [Google Scholar] [CrossRef]
- Pickrum, A.M.; DeLeon, O.; Dirck, A.; Tessmer, M.H.; Riegert, M.O.; Biller, J.A.; Ledeboer, N.A.; Kirby, J.R.; Frank, D.W. Achromobacter xylosoxidans cellular pathology is correlated with activation of a type III secretion. Syst. Infect. Immun. 2020, 88, e00136-20. [Google Scholar] [CrossRef] [PubMed]
- Antonello, V.S.; Dallé, J.; Domingues, G.C.; Ferreira, J.A.; Fontoura Mdo, C.; Knapp, F.B. Post-cesarean surgical site infection due to Buttiauxella agrestis. Int. J. Infect. Dis. 2014, 22, 65–66. [Google Scholar] [CrossRef]
- De Baere, T.; Wauters, G.; Kämpfer, P.; Labit, C.; Claeys, G.; Verschraegen, G.; Vaneechoutte, M. Isolation of Buttiauxella gaviniae from a spinal cord patient with urinary bladder pathology. J. Clin. Microbiol. 2002, 40, 3867–3870. [Google Scholar] [CrossRef]
- Hirai, J.; Uechi, K.; Hagihara, M.; Sakanashi, D.; Kinjo, T.; Haranaga, S.; Fujita, J. Bacteremia due to Citrobacter braakii: A case report and literature review. J. Infect. Chemother. 2016, 22, 819–821. [Google Scholar] [CrossRef]
- Oyeka, M.; Antony, S. Citrobacter braakii bacteremia: Case report and review of the literature. Infect. Disord. Drug Targets 2017, 17, 59–63. [Google Scholar] [CrossRef]
- Liu, L.; Song, L.; Deng, R.; Lan, R.; Jin, W.; Tran Van Nhieu, G.; Cao, H.; Liu, Q.; Xiao, Y.; Li, X.; et al. Citrobacter freundii activation of NLRP3 inflammasome via the type VI secretion system. J. Infect. Dis. 2021, 223, 2174–2185. [Google Scholar] [CrossRef]
- Yang, L.; Li, P.; Liang, B.; Hu, X.; Li, J.; Xie, J.; Yang, C.; Hao, R.; Wang, L.; Jia, L.; et al. Multidrug-resistant Citrobacter freundii ST139 co-producing NDM-1 and CMY-152 from China. Sci. Rep. 2018, 8, 10653. [Google Scholar] [CrossRef]
- Mustafa, A.; Ibrahim, M.; Rasheed, M.A.; Kanwal, S.; Hussain, A.; Sami, A.; Ahmed, R.; Bo, Z. Genome-wide analysis of four Enterobacter cloacae complex type strains: Insights into virulence and niche adaptation. Sci. Rep. 2020, 10, 8150. [Google Scholar] [CrossRef]
- Flores-Carrero, A.; Labrador, I.; Paniz-Mondolfi, A.; Peaper, D.R.; Towle, D.; Araque, M. Nosocomial outbreak of extended-spectrum β-lactamase-producing Enterobacter ludwigii co-harbouring CTX-M-8, SHV-12 and TEM-15 in a neonatal intensive care unit in Venezuela. J. Glob. Antimicrob. Resist. 2016, 7, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wang, S.; Li, O.; Hussain, A.; Hussain, A.; Shen, J.; Ibrahim, M. High-quality genome sequence of human pathogen Enterobacter asburiae type strain 1497-78T. J. Glob. Antimicrob. Resist. 2017, 8, 104–105. [Google Scholar] [CrossRef]
- Capdevila, J.A.; Bisbe, V.; Gasser, I.; Zuazu, J.; Olivé, T.; Fernández, F.; Pahissa Berga, A. Enterobacter amnigenus. An unusual human pathogen. Enferm. Infecc. Microbiol. Clin. 1998, 16, 364–366. [Google Scholar]
- Allocati, N.; Masulli, M.; Alexeyev, M.F.; Di Ilio, C. Escherichia coli in Europe: An overview. Int. J. Environ. Res. Public Health 2013, 10, 6235–6254. [Google Scholar] [CrossRef]
- Pérez, C.; Melo, P.; Besomi, J.; Porte, L.; Wilhelm, J.P. Escherichia vulneris etiologic agent of septic arthritis in a child. Rev. Chilena Infectol. 2018, 35, 80–82. [Google Scholar] [CrossRef]
- Jain, S.; Nagarjuna, D.; Gaind, R.; Chopra, S.; Debata, P.K.; Dawar, R.; Sardana, R.; Yadav, M. Escherichia vulneris: An unusual cause of complicated diarrhoea and sepsis in an infant. A case report and review of literature. New Microbes New Infect. 2016, 13, 83–86. [Google Scholar] [CrossRef]
- Abrantes, C.; Freitas, J.; Silva, T.; Marques da Silva, L.; Carvalho, M.J.; Rodrigues, A.; Cabrita, A. A case of peritoneal dialysis-related peritonitis caused by Ewingella americana. Case Rep. Infect. Dis. 2022, 2022, 5607080. [Google Scholar] [CrossRef]
- Ramos-Vivas, J. Microbiology of Hafnia alvei. Enferm. Infecc. Microbiol. Clin. 2020, 38, 1–6. [Google Scholar] [CrossRef]
- Severiche-Bueno, D.F.; Vargas-Cuervo, M.T.; Medina-Lee, L.; Oliver-Hernandez, G.; Buitrago-Toro, K.; Insignares, D.A.; Conde-Camacho, R. Hafnia alvei pneumonia: From bees to human beings. Germs 2021, 11, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Chew, K.L.; Octavia, S.; Lai, D.; Lin, R.T.P.; Teo, J.W.P. Genomic Characterization of Klebsiella quasipneumoniae from clinical specimens in Singapore. Antimicrob. Agents Chemother. 2021, 65, e0041221. [Google Scholar] [CrossRef]
- Neog, N.; Phukan, U.; Puzari, M.; Sharma, M.; Chetia, P. Klebsiella oxytoca and emerging nosocomial infections. Curr. Microbiol. 2021, 78, 1115–1123. [Google Scholar] [CrossRef]
- Thele, R.; Gumpert, H.; Christensen, L.B.; Worning, P.; Schønning, K.; Westh, H.; Hansen, T.A. Draft genome sequence of a Kluyvera intermedia isolate from a patient with a pancreatic abscess. J. Glob. Antimicrob. Resist. 2017, 10, 1–2. [Google Scholar] [CrossRef]
- Dutkiewicz, J.; Mackiewicz, B.; Lemieszek, M.; Golec, M.; Milanowski, J. Pantoea agglomerans: A mysterious bacterium of evil and good. Part III. Deleterious effects: Infections of humans, animals and plants. Ann. Agric. Environ. Med. 2016, 23, 197–205. [Google Scholar] [CrossRef]
- Donnarumma, G.; Buommino, E.; Fusco, A.; Paoletti, I.; Auricchio, L.; Tufano, M.A. Effect of temperature on the shift of Pseudomonas fluorescens from an environmental microorganism to a potential human pathogen. Int. J. Immunopathol. Pharmacol. 2010, 23, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Fluit, A.C.; Rogers, M.R.C.; Díez-Aguilar, M.; Cantón, R.; Benaissa-Trouw, B.J.; Bayjanov, J.R.; Ekkelenkamp, M.B. Draft genome sequence of the strain 16-537536, isolated from a patient with bronchiectasis and its relationship to the Pseudomonas koreensis group of the Pseudomonas fluorescens complex. BMC Res. Notes 2020, 13, 10. [Google Scholar] [CrossRef]
- Khoo, L.W.; Srinivasan, S.S.; Henriquez, F.L.; Bal, A.M. A rare case of mixed infectious keratitis caused by Pseudomonas koreensis and Aspergillus fumigatus. Case Rep. Ophthalmol. 2020, 11, 600–605. [Google Scholar] [CrossRef]
- Martins, W.; Carvalhaes, C.G.; Cayô, R.; Gales, A.C.; Pignatari, A.C. Co-transmission of Rahnella aquatilis between hospitalized patients. Braz. J. Infect. Dis. 2015, 19, 648–650. [Google Scholar] [CrossRef]
- Appel, T.M.; Quijano-Martínez, N.; De La Cadena, E.; Mojica, M.F.; Villegas, M.V. Microbiological and clinical aspects of Raoultella spp. Front. Public Health 2021, 9, 686789. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, X.; Xu, Z.; Ying, C.; Yu, W.; Xiao, Y. Identification of Raoultella terrigena as a rare causative agent of subungual abscess based on 16S rRNA and housekeeping gene sequencing. Can. J. Infect. Dis. Med. Microbiol. 2016, 2016, 3879635. [Google Scholar] [CrossRef][Green Version]
- Carrero, P.; Garrote, J.A.; Pacheco, S.; García, A.I.; Gil, R.; Carbajosa, S.G. Report of six cases of human infection by Serratia plymuthica. J. Clin. Microbiol. 1995, 33, 275–276. [Google Scholar] [CrossRef] [PubMed]
- Remuzgo-Martínez, S.; Aranzamendi-Zaldunbide, M.; Pilares-Ortega, L.; Icardo, J.M.; Acosta, F.; Martínez-Martínez, L.; Ramos-Vivas, J. Interaction of macrophages with a cytotoxic Serratia liquefaciens human isolate. Microbes Infect. 2013, 15, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Aljorayid, A.; Viau, R.; Castellino, L.; Jump, R.L. Serratia fonticola, pathogen or bystander? A case series and review of the literature. IDCases 2016, 5, 6–8. [Google Scholar] [CrossRef]
- Hai, P.D.; Hoa, L.T.V.; Tot, N.H.; Phuong, L.L.; Quang, V.V.; Thuyet, B.T.; Son, P.N. First report of biliary tract infection caused by multidrug-resistant Serratia fonticola. New Microbes New Infect. 2020, 36, 100692. [Google Scholar] [CrossRef] [PubMed]
- Tsaplina, O.; Bozhokina, E. Bacterial outer membrane protein OmpX regulates β1 integrin and epidermal growth factor receptor (EGFR) involved in invasion of M-HeLa cells by Serratia proteamaculans. Int. J. Mol. Sci. 2021, 22, 13246. [Google Scholar] [CrossRef] [PubMed]
- Weakland, D.R.; Smith, S.N.; Bell, B.; Tripathi, A.; Mobley, H.L.T. The Serratia marcescens siderophore serratiochelin is necessary for full virulence during bloodstream infection. Infect. Immun. 2020, 88, e00117–e00120. [Google Scholar] [CrossRef]
- Leon-Velarde, C.G.; Jun, J.W.; Skurnik, M. Yersinia phages and food safety. Viruses 2019, 11, 1105. [Google Scholar] [CrossRef]
- Tena, D.; Fernández, C.; Lago, M.R. Alcaligenes faecalis: An unusual cause of skin and soft tissue infection. Jpn. J. Infect. Dis. 2015, 68, 128–130. [Google Scholar] [CrossRef]
- Brenner, D.J.; O’Hara, C.M.; Grimont, P.A.; Janda, J.M.; Falsen, E.; Aldova, E.; Ageron, E.; Schindler, J.; Abbott, S.L.; Steigerwalt, A.G. Biochemical identification of Citrobacter species defined by DNA hybridization and description of Citrobacter gillenii sp. nov. (formerly Citrobacter genomospecies 10) and Citrobacter murliniae sp. nov. (formerly Citrobacter genomospecies 11). J. Clin. Microbiol. 1999, 37, 2619–2624. [Google Scholar] [CrossRef]
- Deslandes, V.; Haney, C.; Bernard, K.; Desjardins, M. Ignatzschineria indica bacteremia in a patient with a maggot-infested heel ulcer: A case report and literature review. Access Microbiol. 2019, 2, acmi000078. [Google Scholar] [CrossRef]
- Li, X.; Chen, J. Septic shock induced by bacterial prostatitis with Morganella morganii subsp. morganii in a posttransplantation patient. Case Rep. Transplant. 2015, 2015, 850532. [Google Scholar] [CrossRef]
- Armbruster, C.E.; Mobley, H.L.T.; Pearson, M.M. Pathogenesis of Proteus mirabilis infection. EcoSal Plus 2018, 8, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Lamas, A.; Miranda, J.M.; Regal, P.; Vázquez, B.; Franco, C.M.; Cepeda, A. A comprehensive review of non-enterica subspecies of Salmonella enterica. Microbiol. Res. 2018, 206, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Yeung, E.Y.H. A case series of diarrheal diseases associated with Yersinia frederiksenii. Infect. Dis. Rep. 2021, 13, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Babujee, L.; Balakrishnan, V.; Kiley, P.J.; Glasner, J.D.; Perna, N.T. Transcriptome changes associated with anaerobic growth in Yersinia intermedia (ATCC29909). PLoS ONE 2013, 8, e76567. [Google Scholar] [CrossRef] [PubMed]
- Miguel, N.; Sarasa, J.; López, A.; Gómez, J.; Mosteo, R.; Ormad, M.P. Study of evolution of microbiological properties in sewage sludge-amended soils: A pilot experience. Int. J. Environ. Res. Public Health 2020, 17, 6696. [Google Scholar] [CrossRef] [PubMed]
- Pleissner, D.; Händel, N. Reduction of the microbial load of digestate by the cultivation of Galdieria sulphuraria under acidic conditions. Waste Biomass Valor. 2023, 14, 2621–2627. [Google Scholar] [CrossRef]
- Bonetta, S.; Ferretti, E.; Bonetta, S.; Fezia, G.; Carraro, E. Microbiological contamination of digested products from anaerobic co-digestion of bovine manure and agricultural by-products. Lett. Appl. Microbiol. 2011, 53, 552–557. [Google Scholar] [CrossRef]
- Le Maréchal, C.; Druilhe, C.; Repérant, E.; Boscher, E.; Rouxel, S.; Le Roux, S.; Poëzévara, T.; Ziebal, C.; Houdayer, C.; Nagard, B.; et al. Evaluation of the occurrence of sporulating and nonsporulating pathogenic bacteria in manure and in digestate of five agricultural biogas plants. Microbiologyopen 2019, 8, e872. [Google Scholar] [CrossRef]
- Santos, F.A.; Santos, P.C.; Matos, M.A.; Cardoso, O.; Quina, J.M. Effect of thermal drying and chemical treatments with wastes on microbiological contamination indicators in sewage sludge. Microorganisms 2020, 8, 376. [Google Scholar] [CrossRef]
- Krzyzanowski, F., Jr.; Zappelini, L.; Martone-Rocha, S.; Dropa, M.; Matté, M.H.; Nacache, F.; Razzolini, M.T. Quantification and characterization of Salmonella spp. isolates in sewage sludge with potential usage in agriculture. BMC Microbiol. 2014, 14, 263. [Google Scholar] [CrossRef] [PubMed]
- Korzeniewska, E.; Korzeniewska, A.; Harnisz, M. Antibiotic resistant Escherichia coli in hospital and municipal sewage and their emission to the environment. Ecotoxicol. Environ. Saf. 2013, 91, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Flach, C.F.; Genheden, M.; Fick, J.; Joakim Larsson, D.G. A comprehensive screening of Escherichia coli isolates from Scandinavia’s largest sewage treatment plant indicates no selection for antibiotic resistance. Environ. Sci. Technol. 2018, 52, 11419–11428. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Azeem, M.; Zhang, H.; Yang, L.; Yang, S. Soil environment modulation by varying physicochemical attributes change the population dynamics of fecal Escherichia coli. Pol. J. Environ. Stud. 2013, 32, 225–232. [Google Scholar] [CrossRef]
- Alegbeleye, O.; Sant’Ana, A.S. Survival behavior of six enterotoxigenic Escherichia coli strains in soil and biochar-amended soils. Environ. Res. 2023, 223, 115443. [Google Scholar] [CrossRef]
- Bina, B.; Movahedian, H.I.; Kord, I. The effect of lime stabilization on the microbiological quality of sewage sludge. Iranian J. Environ. Health. Sci. Eng. 2004, 1, 34–38. [Google Scholar]
- Garrec, N.; Picard-Bonnaud, F.; Pourcher, A.M. Occurrence of Listeria sp and L monocytogenes in sewage sludge used for land application: Effect of dewatering, liming and storage in tank on survival of Listeria species. FEMS Immunol. Med. Microbiol. 2003, 35, 275–283. [Google Scholar] [CrossRef] [PubMed]
| Component | Total No. of Enterobacteriaceae [CFU/g] | Total No. of Escherichia coli [CFU/g] |
|---|---|---|
| Universal soil | 3.1 × 102 | <1 |
| Clay soil | 26 | <1 |
| Sewage sludge | 2.5 × 106 | 1.4 × 104 |
| Digestate | 1.3 × 106 | 1.0 × 104 |
| Type of Samples | Bacterial Concentration [CFU/g] | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total Mesophilic Bacteria | Enterobacteriaceae | Escherichia coli | |||||||
| Positive Samples | Median (Range) | Mean ± S.D. | Positive Samples | Median (Range) | Mean ± S.D. | Positive Samples | Median (Range) | Mean ± S.D. | |
| Soil n = 82 | 82 (100%) | 3.8 × 105 (1.1 × 105−4.6 × 106) | 4.6 × 105 ± 5.1 × 105 | 82 (100%) | 6.3 × 102 (1.2 × 101−3.2 × 105) | 1.1 × 104 ± 4.3 × 104 | 2 (2.4%) | − | − |
| Sewage sludge n = 9 | 8 (88.9%) | 3.7 × 107 (1.6 × 103−5.7 × 108) | 1.4 × 108 ± 2.1 × 108 | 6 (66.7%) | 7.2 × 105 (1.5 × 103−2.2 × 106) | 9.4 × 105 ± 9.9 × 105 | 4 (44.4%) | 1.2 × 104 (3.8 × 102−4.2 × 104) | 1.7 × 104 ± 2.0 × 104 |
| Digestate n = 9 | 9 (100%) | 1.2 × 108 (1.0 × 107−9.8 × 108) | 2.6 × 108 ± 3.4 × 108 | 9 (100%) | 2.8 × 106 (3.1× 105−2.1 × 107) | 5.6 × 106 ± 7.3 × 106 | 6 (66.7%) | 3.1 × 104 (8.0 × 101−9.9 × 106) | 1.7 × 106 ± 4.0 × 106 |
| Type of Sample: Non-Sterile Soil + Sterile Sewage Sludge/Digestate + E. coli Suspension | Week | Enterobacteriaceae [CFU/g] | E. coli [CFU/g] |
|---|---|---|---|
| universal soil + sewage sludge + E. coli (8.3 dm3 + 14.2 g + 1.8 mL) | 0 | 3.3 × 103 | 1.1 × 103 |
| 1 | 1.3 × 103 | 5.7 × 103 | |
| 2 | 1.8 × 102 | <1 | |
| 3 | 41 | <1 | |
| universal soil + sewage sludge + E. coli (8.3 dm3 + 88.5 g + 11.1 mL) | 0 | 1.2 × 104 | 1.6 × 103 |
| 1 | 9.7 × 103 | 3.5 × 103 | |
| 2 | 8.0 × 102 | 2.4 × 103 | |
| 3 | 1.3 × 102 | <1 | |
| universal soil + digestate + E. coli (8.3 dm3 + 14.2 g + 1.8 mL) | 0 | 1.4 × 103 | 0.9 × 102 |
| 1 | 3.2 × 103 | 1.0 × 102 | |
| 2 | 2.3 × 102 | <1 | |
| 3 | 67 | <1 | |
| universal soil + digestate + E. coli (8.3 dm3 + 88.5 g + 11.1 mL) | 0 | 3.0 × 103 | 3.9 × 103 |
| 1 | 6.4 × 103 | 4.0 × 103 | |
| 2 | 1.8 × 102 | 1.0 × 102 | |
| 3 | 1.8 × 102 | <1 | |
| clay soil + sewage sludge + E. coli (8.3 dm3 + 14.2 g + 1.8 mL) | 0 | 2.2 × 103 | 1.8 × 103 |
| 1 | 2.4 × 102 | 1.3 × 102 | |
| 2 | 1.2 × 102 | 21 | |
| 3 | 83 | <1 | |
| clay soil + sewage sludge + E. coli (8.3 dm3 + 88.5 g + 11.1 mL) | 0 | 1.0 × 104 | 1.3 × 104 |
| 1 | 2.9 × 105 | 1.8 × 105 | |
| 2 | 1.6 × 104 | 5.3 × 103 | |
| 3 | 6.0 × 102 | 2.8 × 102 | |
| clay soil + digestate + E. coli (8.3 dm3 + 14.2 g + 1.8 mL) | 0 | 1.8 × 103 | 3.1 × 103 |
| 1 | 1.9 × 102 | 1.6 × 102 | |
| 2 | 41 | 13 | |
| 3 | 15 | <1 | |
| clay soil + digestate + E. coli (8.3 dm3 + 88.5 g + 11.1 mL) | 0 | 1.6 × 104 | 1.5 × 104 |
| 1 | 1.8 × 103 | 1.3 × 103 | |
| 2 | 2.1 × 102 | 25 | |
| 3 | 2.2 × 102 | 16 |
| Type of Sample: Non-Sterile Soil + Non-Sterile Sewage Sludge/Digestate | Week | Enterobacteriaceae [CFU/g] | E. coli [CFU/g] |
|---|---|---|---|
| universal soil + sewage sludge (8.3 dm3 + 14.2 g) | 0 | 1.3 × 104 | <1 |
| 1 | 3.5 × 102 | <1 | |
| 2 | 1.2 × 102 | <1 | |
| 3 | 1.1 × 102 | <1 | |
| universal soil + sewage sludge (8.3 dm3 + 88.5 g) | 0 | 3.5 × 103 | 2.5 × 102 |
| 1 | 9.5 × 102 | 65 | |
| 2 | 1.2 × 102 | <1 | |
| 3 | 1.7 × 102 | <1 | |
| universal soil + digestate (8.3 dm3 + 14.2 g) | 0 | 2.3 × 103 | <1 |
| 1 | 3.3 × 102 | <1 | |
| 2 | 37 | <1 | |
| 3 | 84 | <1 | |
| universal soil + digestate (8.3 dm3 + 88.5 g) | 0 | 1.3 × 103 | <1 |
| 1 | 4.6 × 102 | <1 | |
| 2 | 57 | <1 | |
| 3 | 97 | <1 | |
| clay soil + sewage sludge (8.3 dm3 + 14.2 g) | 0 | 95 | <1 |
| 1 | 1.3 × 103 | <1 | |
| 2 | 2.8 × 102 | <1 | |
| 3 | 32 | <1 | |
| clay soil + sewage sludge (8.3 dm3 + 88.5 g) | 0 | 117 | <1 |
| 1 | 9.3 × 103 | <1 | |
| 2 | 3.0 × 102 | <1 | |
| 3 | 55 | <1 | |
| clay soil + digestate (8.3 dm3 + 14.2 g) | 0 | 49 | <1 |
| 1 | 1.1 × 102 | <1 | |
| 2 | 11 | <1 | |
| 3 | 15 | <1 | |
| clay soil + digestate (8.3 dm3 + 88.5 g) | 0 | 1.3 × 102 | <1 |
| 1 | 1.5 × 103 | <1 | |
| 2 | 1.4 × 102 | <1 | |
| 3 | 1.8 × 102 | <1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójcik-Fatla, A.; Farian, E.; Kowalczyk, K.; Sroka, J.; Skowron, P.; Siebielec, G.; Zdybel, J.M.; Jadczyszyn, T.; Cencek, T. Enterobacteriaceae in Sewage Sludge and Digestate Intended for Soil Fertilization. Pathogens 2024, 13, 1056. https://doi.org/10.3390/pathogens13121056
Wójcik-Fatla A, Farian E, Kowalczyk K, Sroka J, Skowron P, Siebielec G, Zdybel JM, Jadczyszyn T, Cencek T. Enterobacteriaceae in Sewage Sludge and Digestate Intended for Soil Fertilization. Pathogens. 2024; 13(12):1056. https://doi.org/10.3390/pathogens13121056
Chicago/Turabian StyleWójcik-Fatla, Angelina, Ewelina Farian, Katarzyna Kowalczyk, Jacek Sroka, Piotr Skowron, Grzegorz Siebielec, Jolanta Małgorzata Zdybel, Tamara Jadczyszyn, and Tomasz Cencek. 2024. "Enterobacteriaceae in Sewage Sludge and Digestate Intended for Soil Fertilization" Pathogens 13, no. 12: 1056. https://doi.org/10.3390/pathogens13121056
APA StyleWójcik-Fatla, A., Farian, E., Kowalczyk, K., Sroka, J., Skowron, P., Siebielec, G., Zdybel, J. M., Jadczyszyn, T., & Cencek, T. (2024). Enterobacteriaceae in Sewage Sludge and Digestate Intended for Soil Fertilization. Pathogens, 13(12), 1056. https://doi.org/10.3390/pathogens13121056






