Differential TLR-ERK1/2 Activity Promotes Viral ssRNA and dsRNA Mimic-Induced Dysregulated Immunity in Macrophages
Abstract
1. Introduction
2. Materials and Methods
3. Results
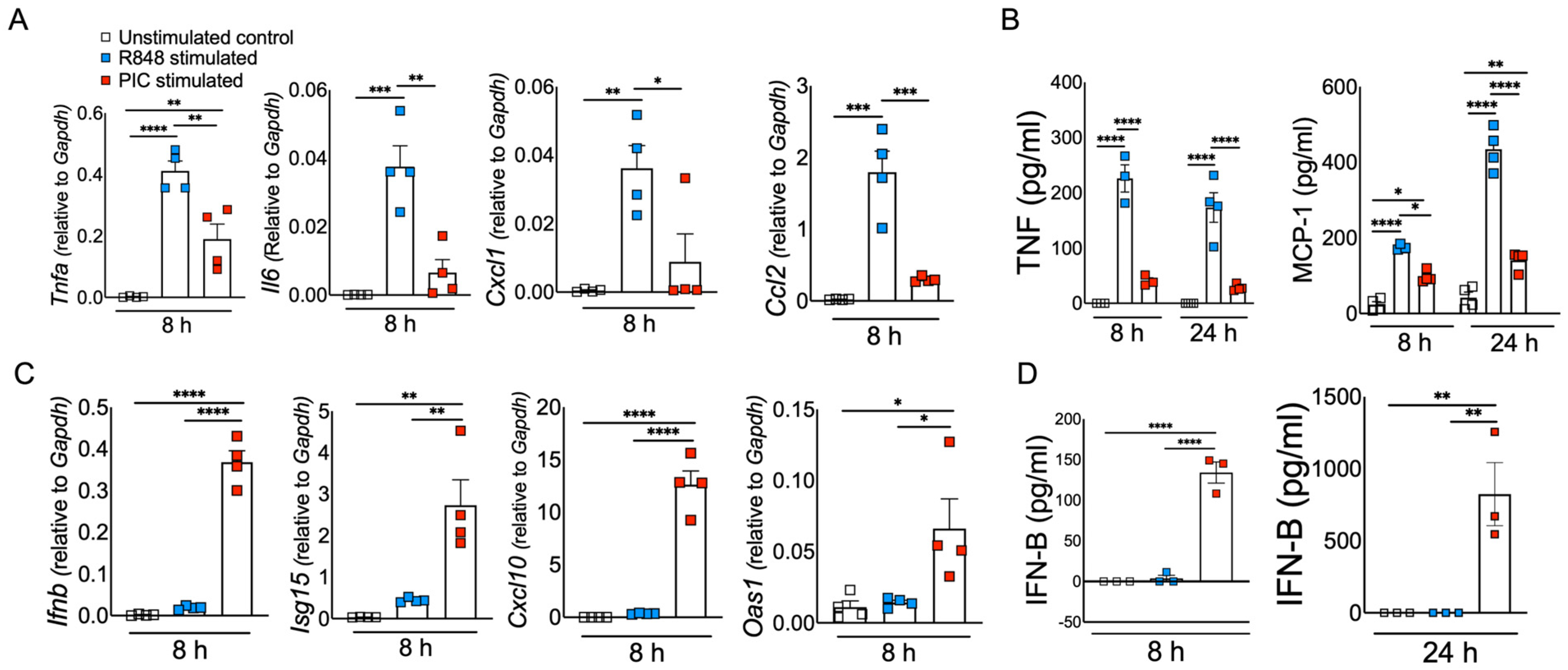
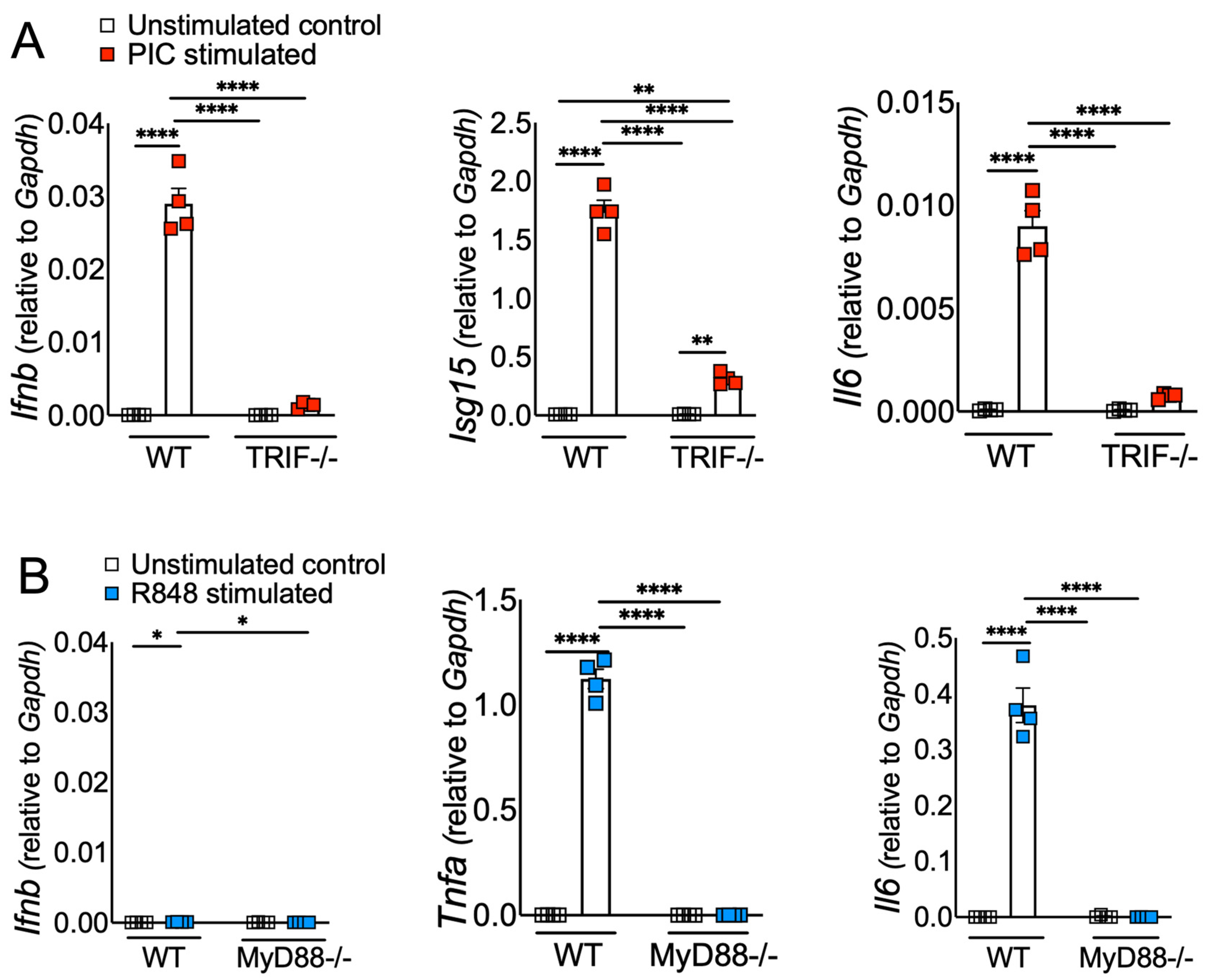
3.1. Viral ssRNA and dsRNA Mimics Induce Differential Inflammatory and Antiviral Responses
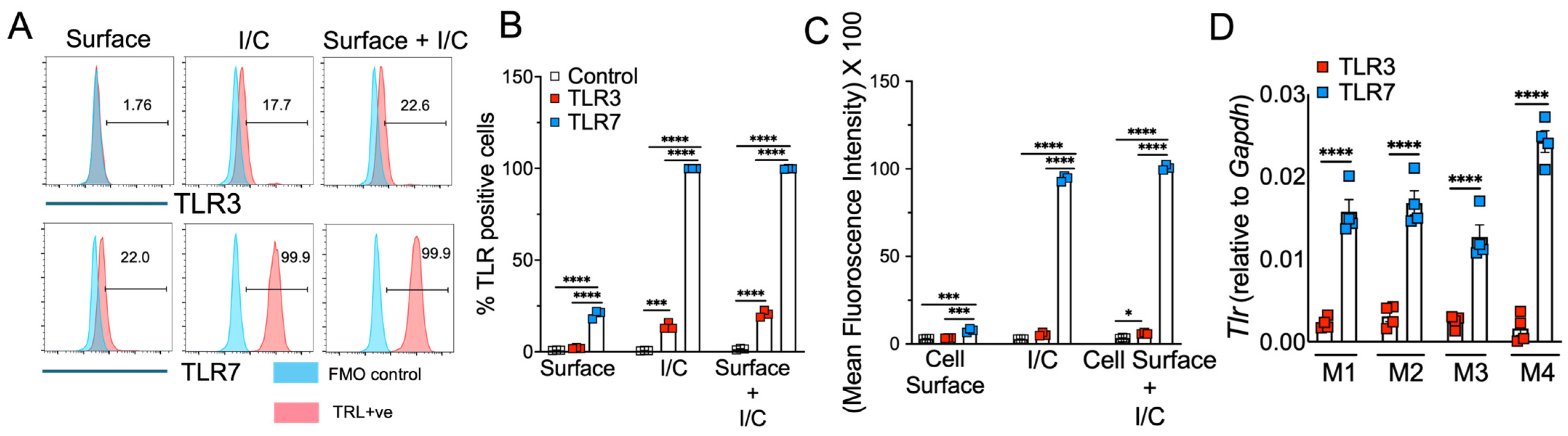
3.2. Macrophages Express Differential Levels of Cell-Surface and Endosomal TLR3 and TLR7
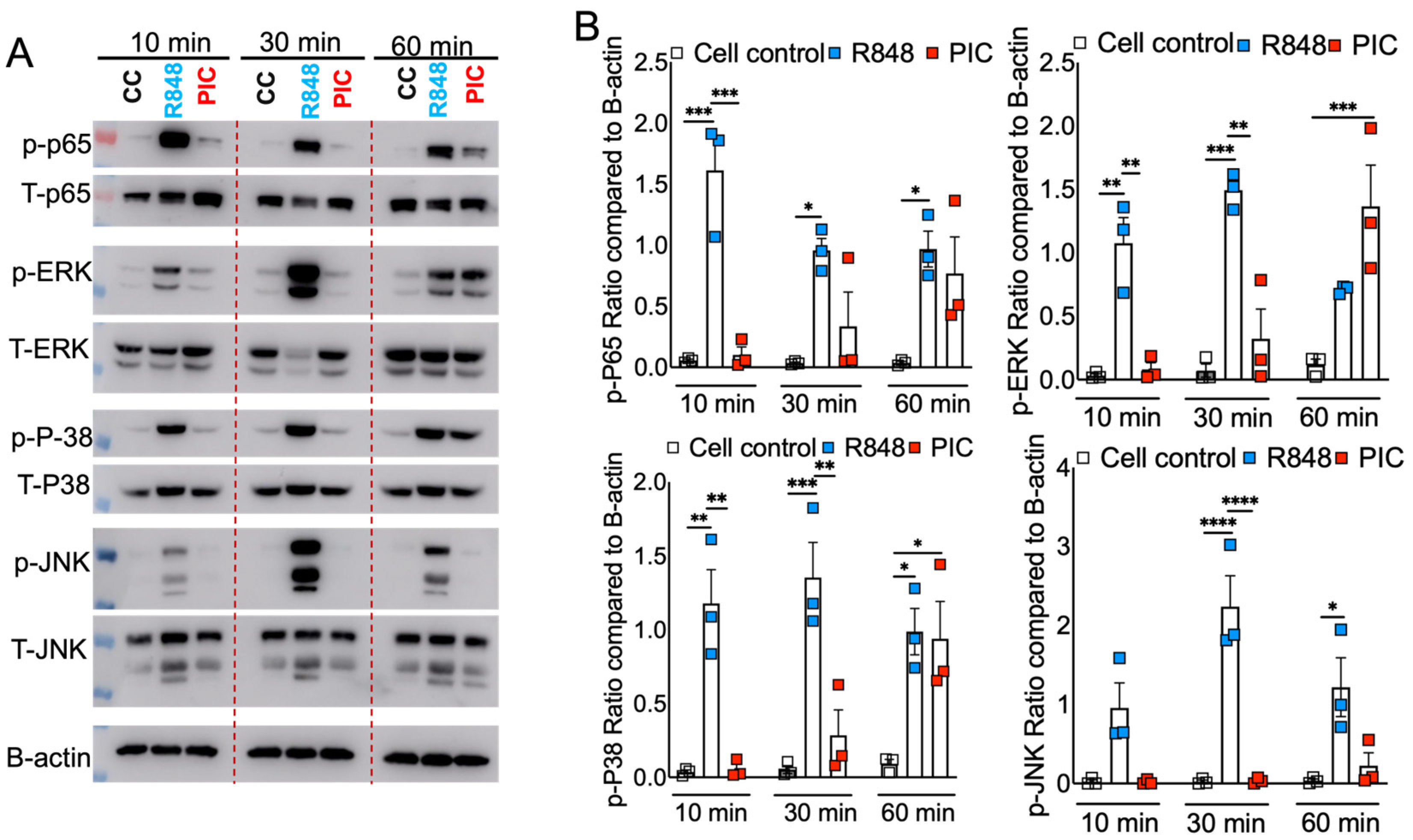
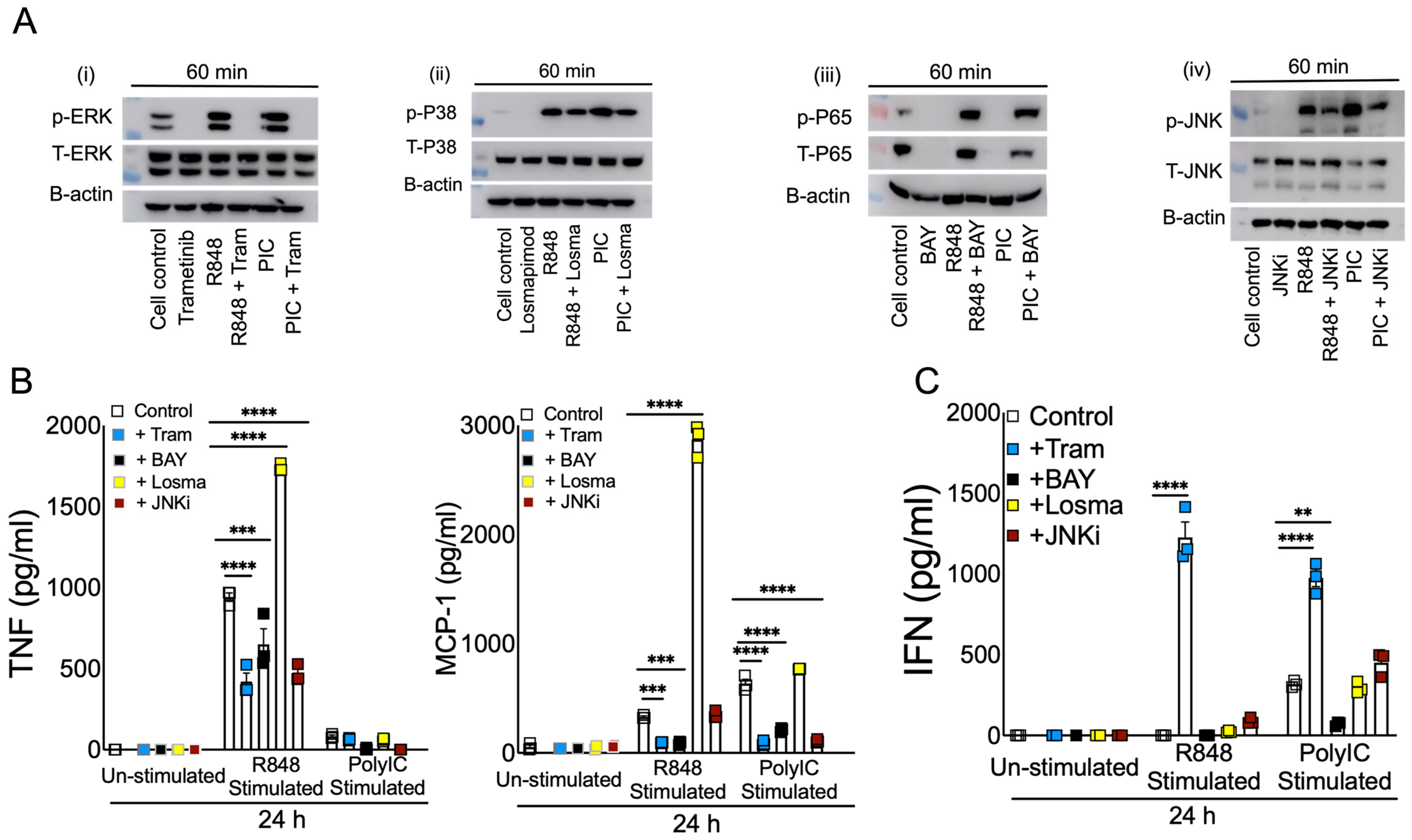
3.3. R848 and Poly I:C Induce Differential MAPK and NF-kB Activation in Macrophages
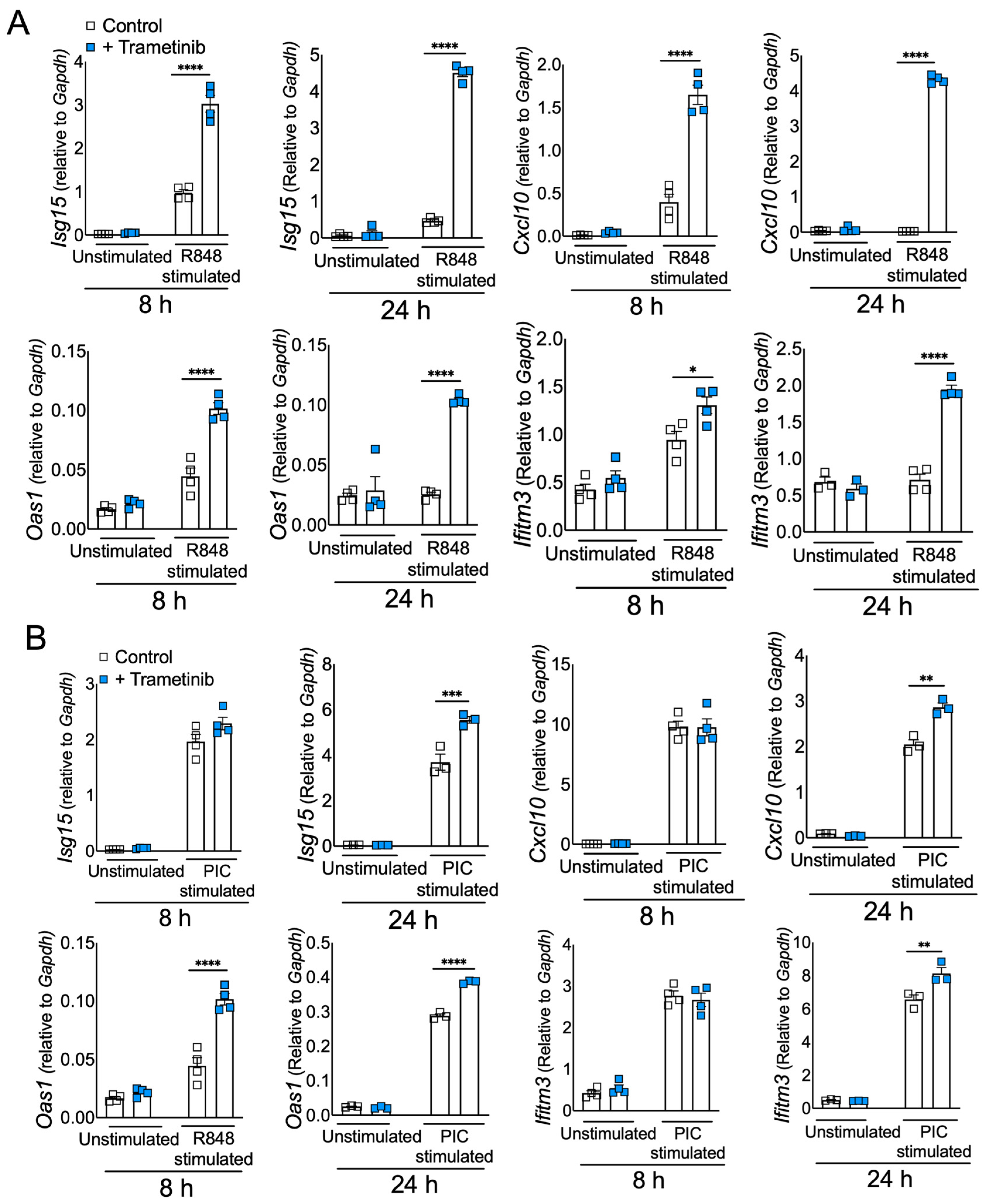
3.4. Blocking ERK1/2 Reduces Inflammation and Improves IFN in Viral RNA Mimic-Treated Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdelwhab, E.M.; Mettenleiter, T.C. Zoonotic Animal Influenza Virus and Potential Mixing Vessel Hosts. Viruses 2023, 15, 980. [Google Scholar] [CrossRef] [PubMed]
- Woolhouse, M.E.J.; Gowtage-Sequeria, S. Host Range and Emerging and Reemerging Pathogens. Emerg. Infect. Dis. 2005, 11, 1842–1847. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.H.; Latham, S.M.; Woolhouse, M.E. Risk factors for human disease emergence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 983–989. [Google Scholar] [CrossRef]
- Ge, X.-Y.; Li, J.-L.; Yang, X.-L.; Chmura, A.A.; Zhu, G.; Epstein, J.H.; Mazet, J.K.; Hu, B.; Zhang, W.; Peng, C.; et al. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature 2013, 503, 535–538. [Google Scholar] [CrossRef]
- Daep, C.A.; Muñoz-Jordán, J.L.; Eugenin, E.A. Flaviviruses, an expanding threat in public health: Focus on Dengue, West Nile, and Japanese encephalitis virus. J. Neurovirol. 2014, 20, 539–560. [Google Scholar] [CrossRef]
- Martina, B.E.; Osterhaus, A.D. “Filoviruses”: A real pandemic threat? EMBO Mol. Med. 2009, 1, 10–18. [Google Scholar] [CrossRef]
- Li, W.; Shi, Z.; Yu, M.; Ren, W.; Smith, C.; Epstein, J.H.; Wang, H.; Crameri, G.; Hu, Z.; Zhang, H.; et al. Bats Are Natural Reservoirs of SARS-Like Coronaviruses. Science 2005, 310, 676–679. [Google Scholar] [CrossRef]
- Lau, S.K.P.; Woo, P.C.Y.; Li, K.S.M.; Huang, Y.; Tsoi, H.-W.; Wong, B.H.L.; Wong, S.S.Y.; Leung, S.-Y.; Chan, K.-H.; Yuen, K.-Y. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc. Natl. Acad. Sci. USA 2005, 102, 14040–14045. [Google Scholar] [CrossRef]
- Wang, L.F.; Eaton, B.T. Bats, Civets and the Emergence of SARS. In Wildlife and Emerging Zoonotic Diseases: The Biology, Circumstances and Consequences of Cross-Species Transmission; Childs, J.E., Mackenzie, J.S., Richt, J.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 325–344. [Google Scholar] [CrossRef]
- Plowright, R.K.; Eby, P.; Hudson, P.J.; Smith, I.L.; Westcott, D.; Bryden, W.L.; Middleton, D.; Reid, P.A.; McFarlane, R.A.; Martin, G.; et al. Ecological dynamics of emerging bat virus spillover. Proc. R. Soc. B: Biol. Sci. 2015, 282, 20142124. [Google Scholar] [CrossRef]
- Nelemans, T.; Kikkert, M. Viral Innate Immune Evasion and the Pathogenesis of Emerging RNA Virus Infections. Viruses 2019, 11, 961. [Google Scholar] [CrossRef]
- Channappanavar, R.; Fehr, A.R.; Vijay, R.; Mack, M.; Zhao, J.; Meyerholz, D.K.; Perlman, S. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe 2016, 19, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Peiris, J.; Hui, K.P.; Yen, H.L. Host response to influenza virus: Protection versus immunopathology. Curr. Opin. Immunol. 2010, 22, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.-C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020, 181, 1036–1045.e9. [Google Scholar] [CrossRef] [PubMed]
- Werling, D.; Jungi, T.W. TOLL-like receptors linking innate and adaptive immune response. Vet. Immunol. Immunopathol. 2003, 91, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Gale, M. Principles of intracellular viral recognition. Curr. Opin. Immunol. 2007, 19, 17–23. [Google Scholar] [CrossRef]
- Zhong, B.; Tien, P.; Shu, H.B. Innate immune responses: Crosstalk of signaling and regulation of gene transcription. Virology 2006, 352, 14–21. [Google Scholar] [CrossRef]
- Meylan, E.; Tschopp, J. Toll-Like Receptors and RNA Helicases: Two Parallel Ways to Trigger Antiviral Responses. Mol. Cell. 2006, 22, 561–569. [Google Scholar] [CrossRef]
- Majde, J.A.; Guha-Thakurta, N.; Chen, Z.; Bredow, S.; Krueger, J.M. Spontaneous release of stable viral double-stranded RNA into the extracellular medium by influenza virus-infected MDCK epithelial cells: Implications for the viral acute phase response. Arch. Virol. 1998, 143, 2371–2380. [Google Scholar] [CrossRef]
- Deng, X.; Hackbart, M.; Mettelman, R.C.; O’Brien, A.; Mielech, A.M.; Yi, G.; Kao, C.C.; Baker, S.C. Coronavirus nonstructural protein 15 mediates evasion of dsRNA sensors and limits apoptosis in macrophages. Proc. Natl. Acad. Sci. USA 2017, 114, E4251–E4260. [Google Scholar] [CrossRef]
- Genoyer, E.; Wilson, J.; Ames, J.M.; Stokes, C.; Moreno, D.; Etzyon, N.; Oberst, A.; Gale, M. Exposure of negative-sense viral RNA in the cytoplasm initiates innate immunity to West Nile virus. bioRxiv 2024. [Google Scholar] [CrossRef]
- Kato, H.; Sato, S.; Yoneyama, M.; Yamamoto, M.; Uematsu, S.; Matsui, K.; Tsujimura, T.; Takeda, K.; Fujita, T.; Takeuchi, O.; et al. Cell Type-Specific Involvement of RIG-I in Antiviral Response. Immunity 2005, 23, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Andrejeva, J.; Childs, K.S.; Young, D.F.; Carlos, T.S.; Stock, N.; Goodbourn, S.; Randall, R.E. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-β promoter. Proc. Natl. Acad. Sci. USA 2004, 101, 17264–17269. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, M.; Kikuchi, M.; Natsukawa, T.; Shinobu, N.; Imaizumi, T.; Miyagishi, M.; Taira, K.; Akira, S.; Fujita, T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 2004, 5, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Kanno, A.; Tanimura, N.; Ishizaki, M.; Ohko, K.; Motoi, Y.; Onji, M.; Fukui, R.; Shimozato, T.; Yamamoto, K.; Shibata, T.; et al. Targeting cell surface TLR7 for therapeutic intervention in autoimmune diseases. Nat. Commun. 2015, 6, 6119. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen Recognition and Innate Immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Diebold, S.S.; Kaisho, T.; Hemmi, H.; Akira, S.; Reis e Sousa, C. Innate Antiviral Responses by Means of TLR7-Mediated Recognition of Single-Stranded RNA. Science 2004, 303, 1529–1531. [Google Scholar] [CrossRef]
- Lund, J.M.; Alexopoulou, L.; Sato, A.; Karow, M.; Adams, N.C.; Gale, N.W.; Iwasaki, A.; Flavell, R.A. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 2004, 101, 5598–5603. [Google Scholar] [CrossRef]
- Yamamoto, M.; Sato, S.; Mori, K.; Hoshino, K.; Takeuchi, O.; Takeda, K.; Akira, S. Cutting Edge: A Novel Toll/IL-1 Receptor Domain-Containing Adapter That Preferentially Activates the IFN-β Promoter in the Toll-Like Receptor Signaling1. J. Immunol. 2002, 169, 6668–6672. [Google Scholar] [CrossRef]
- Martin, M.U.; Kollewe, C. Interleukin-1 receptor-associated kinase-1 (IRAK-1): A self-regulatory adapter molecule in the signaling cascade of the Toll/IL-1 receptor family. Signal Transduct. 2001, 1, 37–50. [Google Scholar] [CrossRef]
- Morrison, D.K. MAP Kinase Pathways. Cold Spring Harb. Perspect. Biol. 2012, 4, a011254. [Google Scholar] [CrossRef]
- Reimann, T.; Büscher, D.; Hipskind, R.A.; Krautwald, S.; Lohmann-Matthes, M.L.; Baccarini, M. Lipopolysaccharide induces activation of the Raf-1/MAP kinase pathway. A putative role for Raf-1 in the induction of the IL-1 beta and the TNF-alpha genes. J. Immunol. 1994, 153, 5740–5749. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.J.; Vos, H.L.; Bertina, R.M. Lipopolysaccharide Induction of Tissue Factor in THP-1 Cells Involves Jun Protein Phosphorylation and Nuclear Factor κB Nuclear Translocation. J. Biol. Chem. 1999, 274, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Sato, S.; Hemmi, H.; Hoshino, K.; Kaisho, T.; Sanjo, H.; Takeuchi, O.; Sugiyama, M.; Okabe, M.; Takeda, K.; et al. Role of Adaptor TRIF in the MyD88-Independent Toll-Like Receptor Signaling Pathway. Science 2003, 301, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Marshall, J.A.; Bowden, D.S. Characterization of Rubella Virus Replication Complexes Using Antibodies to Double-Stranded RNA. Virology 1994, 200, 307–312. [Google Scholar] [CrossRef]
- Stollar, B.D.; Stollar, V. Immunofluorescent demonstration of double-stranded RNA in the cytoplasm of sindbis virus-infected cells. Virology 1970, 42, 276–280. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Innate immune recognition of viral infection. Nat. Immunol. 2006, 7, 131–137. [Google Scholar] [CrossRef]
- Gantier, M.P.; Williams, B.R.G. The response of mammalian cells to double-stranded RNA. Cytokine Growth Factor Rev. 2007, 18, 363–371. [Google Scholar] [CrossRef]
- Griffin, D.E. Why does viral RNA sometimes persist after recovery from acute infections? PLoS Biol. 2022, 20, e3001687. [Google Scholar] [CrossRef]
- Sow, M.S.; Etard, J.-F.; Baize, S.; Magassouba, N.; Faye, O.; Msellati, P.; Touré, A., II; Savane, I.; Barry, M.; Delaporte, E.; et al. New Evidence of Long-lasting Persistence of Ebola Virus Genetic Material in Semen of Survivors. J. Infect. Dis. 2016, 214, 1475–1476. [Google Scholar] [CrossRef]
- Lupi, L.; Vitiello, A.; Parolin, C.; Calistri, A.; Garzino-Demo, A. The Potential Role of Viral Persistence in the Post-Acute Sequelae of SARS-CoV-2 Infection (PASC). Pathogens 2024, 13, 388. [Google Scholar] [CrossRef]
- Chen, B.; Julg, B.; Mohandas, S.; Bradfute, S.B.; Force, R.M.P.T. Viral persistence, reactivation, and mechanisms of long COVID. eLife 2023, 12, e86015. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.; He, D.; Liang, C.; Du, S.; Hua, Z.; Nie, Q.; Zhou, X.; Yang, M.; Tan, H.; Xu, J.; et al. The persistence of SARS-CoV-2 in tissues and its association with long COVID symptoms: A cross-sectional cohort study in China. Lancet Infect. Dis. 2024, 24, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Khandelwal, N.; Thachamvally, R.; Tripathi, B.N.; Barua, S.; Kashyap, S.K.; Maherchandani, S.; Kumar, N. Role of MAPK/MNK1 signaling in virus replication. Virus Res. 2018, 253, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Parisien, J.-P.; Lau, J.F.; Rodriguez, J.J.; Sullivan, B.M.; Moscona, A.; Parks, G.D.; Lamb, R.A.; Horvath, C.M. The V Protein of Human Parainfluenza Virus 2 Antagonizes Type I Interferon Responses by Destabilizing Signal Transducer and Activator of Transcription 2. Virology 2001, 283, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Basler, C.F.; Mikulasova, A.; Martinez-Sobrido, L.; Paragas, J.; Mühlberger, E.; Bray, M.; Klenk, H.-D.; Palese, P.; García-Sastre, A. The Ebola Virus VP35 Protein Inhibits Activation of Interferon Regulatory Factor 3. J. Virol. 2003, 77, 7945–7956. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.H.; Yang, Z.Q. The cytokine storm of severe influenza development of immunomodulatory, therapy. Cell. Mol. Immunol. 2016, 13, 3–10. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Zhan, Y.; Wu, L.; Yu, X.; Zhang, W.; Ye, L.; Xu, S.; Sun, R.; Wang, Y.; et al. Analysis of Serum Cytokines in Patients with Severe Acute Respiratory Syndrome. Infect. Immunity 2004, 72, 4410–4415. [Google Scholar] [CrossRef]
- Karpus, O.N.; Heutinck, K.M.; Wijnker, P.J.M.; Tak, P.P.; Hamann, J. Triggering of the dsRNA Sensors TLR3, MDA5, and RIG-I Induces CD55 Expression in Synovial Fibroblasts. PLoS ONE 2012, 7, e35606. [Google Scholar] [CrossRef]
- Billack, B. Macrophage Activation: Role of Toll-like Receptors, Nitric Oxide, and Nuclear Factor kappa B. Am. J. Pharm. Educ. 2006, 70, 102. [Google Scholar] [CrossRef]
- West, A.P.; Koblansky, A.A.; Ghosh, S. Recognition and Signaling by Toll-Like Receptors. Annu. Rev. Cell Dev. Biol. 2006, 22, 409–437. [Google Scholar] [CrossRef]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Sugiyama, M.; Yamamoto, M.; Watanabe, Y.; Kawai, T.; Takeda, K.; Akira, S. Toll/IL-1 Receptor Domain-Containing Adaptor Inducing IFN-β (TRIF) Associates with TNF Receptor-Associated Factor 6 and TANK-Binding Kinase 1, and Activates Two Distinct Transcription Factors, NF-κB and IFN-Regulatory Factor-3, in the Toll-Like Receptor Signaling 1. J. Immunol. 2003, 171, 4304–4310. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K. Emerging and re-emerging fatal viral diseases. Exp. Mol. Med. 2021, 53, 711–712. [Google Scholar] [CrossRef] [PubMed]
- Nichol, S.T.; Arikawa, J.; Kawaoka, Y. Emerging viral diseases. Proc. Natl. Acad. Sci. USA 2000, 97, 12411–12412. [Google Scholar] [CrossRef]
- Franks, T.J.; Chong, P.Y.; Chui, P.; Galvin, J.R.; Lourens, R.M.; Reid, A.H.; Selbs, E.; Mcevoy, C.P.L.; Hayden, C.D.L.; Fukuoka, J.; et al. Lung pathology of severe acute respiratory syndrome (SARS): A study of 8 autopsy cases from Singapore. Hum. Pathol. 2003, 34, 743–748. [Google Scholar] [CrossRef]
- Nicholls, J.M.; Poon, L.L.; Lee, K.C.; Ng, W.F.; Lai, S.T.; Leung, C.Y.; Chu, C.M.; Hui, P.K.; Mak, K.L.; Lim, W.; et al. Lung pathology of fatal severe acute respiratory syndrome. Lancet 2003, 361, 1773–1778. [Google Scholar] [CrossRef]
- Wong, C.K.; Lam, C.W.K.; Wu, A.K.L.; Ip, W.K.; Lee, N.L.S.; Chan, I.H.S.; Lit, L.C.W.; Hui, D.S.C.; Chan, M.H.M.; Chung, S.S.C.; et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004, 136, 95–103. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, H.; Shen, H.; Li, Z.; Geng, J.; Han, H.; Cai, J.; Li, X.; Kang, W.; Weng, D.; et al. The clinical pathology of severe acute respiratory syndrome (SARS): A report from China. J. Pathol. 2003, 200, 282–289. [Google Scholar] [CrossRef]
- Beignon, A.-S.; McKenna, K.; Skoberne, M.; Manches, O.; DaSilva, I.; Kavanagh, D.G.; Larsson, M.; Gorelick, R.J.; Lifson, J.D.; Bhardwaj, N. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor–viral RNA interactions. J. Clin. Invest 2005, 115, 3265–3275. [Google Scholar] [CrossRef]
- Channappanavar, R.; Perlman, S. Evaluation of Activation and Inflammatory Activity of Myeloid Cells During Pathogenic Human Coronavirus Infection. MERS Coronavirus 2019, 2099, 195–204. [Google Scholar] [CrossRef]
- Doyle, S.E.; Vaidya, S.A.; O’Connell, R.; Dadgostar, H.; Dempsey, P.W.; Wu, T.-T.; Rao, G.; Sun, R.; Haberland, M.E.; Modlin, R.L.; et al. IRF3 Mediates a TLR3/TLR4-Specific Antiviral Gene Program. Immunity 2002, 17, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Oshiumi, H.; Matsumoto, M.; Funami, K.; Akazawa, T.; Seya, T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3–mediated interferon-β induction. Nat. Immunol. 2003, 4, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Ahlquist, P. Parallels among positive-strand RNA viruses, reverse-transcribing viruses and double-stranded RNA viruses. Nat. Rev. Microbiol. 2006, 4, 371–382. [Google Scholar] [CrossRef]
- Gitlin, L.; Barchet, W.; Gilfillan, S.; Cella, M.; Beutler, B.; Flavell, R.A.; Diamond, M.S.; Colonna, M. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc. Natl. Acad. Sci. USA 2006, 103, 8459–8464. [Google Scholar] [CrossRef]
- Kato, H.; Takeuchi, O.; Sato, S.; Yoneyama, M.; Yamamoto, M.; Matsui, K.; Uematsu, S.; Jung, A.; Kawai, T.; Ishii, K.J.; et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 2006, 441, 101–105. [Google Scholar] [CrossRef]
- Flory, E.; Kunz, M.; Scheller, C.; Jassoy, C.; Stauber, R.; Rapp, U.R.; Ludwig, S. Influenza Virus-induced NF-κB-dependent Gene Expression Is Mediated by Overexpression of Viral Proteins and Involves Oxidative Radicals and Activation of IκB Kinase. J. Biol. Chem. 2000, 275, 8307–8314. [Google Scholar] [CrossRef]
- de Magalhães, J.C.; Andrade, A.A.; Silva, P.N.G.; Sousa, L.P.; Ropert, C.; Ferreira, P.C.P.; Kroon, E.G.; Gazzinelli, R.T.; Bonjardim, C.A. A mitogenic signal triggered at an early stage of vaccinia virus infection: Implication of MEK/ERK and protein kinase A in virus multiplication. J. Biol. Chem. 2001, 276, 38353–38360. [Google Scholar] [CrossRef]
- Lomas, D.A.; Lipson, D.A.; Miller, B.E.; Willits, L.; Keene, O.; Barnacle, H.; Barnes, N.C.; Tal-Singer, R. An Oral Inhibitor of p38 MAP Kinase Reduces Plasma Fibrinogen in Patients with Chronic Obstructive Pulmonary Disease. J. Clin. Pharmacol. 2012, 52, 416–424. [Google Scholar] [CrossRef]
- Fisk, M.; Cheriyan, J.; Mohan, D.; Forman, J.; Mäki-Petäjä, K.M.; McEniery, C.M.; Fuld, J.; Rudd, J.H.F.; Hopkinson, N.S.; Lomas, D.A.; et al. The p38 mitogen activated protein kinase inhibitor losmapimod in chronic obstructive pulmonary disease patients with systemic inflammation, stratified by fibrinogen: A randomised double-blind placebo-controlled trial. PLoS ONE 2018, 13, e0194197. [Google Scholar] [CrossRef]
- Davies, J.M.; Carroll, M.L.; Li, H.; Poh, A.M.; Kirkegard, D.; Towers, M.; Upham, J.W. Budesonide and Formoterol Reduce Early Innate Anti-Viral Immune Responses In Vitro. PLoS ONE 2011, 6, e27898. [Google Scholar] [CrossRef]
- Thomas, B.J.; Porritt, R.A.; Hertzog, P.J.; Bardin, P.G.; Tate, M.D. Glucocorticosteroids enhance replication of respiratory viruses: Effect of adjuvant interferon. Sci. Rep. 2014, 4, 7176. [Google Scholar] [CrossRef]
| Primer | Forward Sequence | Reverse Sequence |
|---|---|---|
| GAPDH | 5′ATGACTCCACTCACGGCAAAT3′ | 5′GGGTCTCGCTCCTGGAAGAT3′ |
| TNF α | 5′GAACTGGCAGAAGAGGCACT3′ | 5′AGGGTCTGGGCCATAGAACT3′ |
| IL6 | 5′GAGGATACCACTCCCAACAGACC3′ | 5′AAGTGCATCATCGTTGTTCATACA3′ |
| CXCL1 | 5′GCTGGGATTCACCTCAAGAA3′ | 5′TCTCCGTTACTTGGGGACAC3′ |
| CCL2 | 5′CTTCTGGGCCTGCTGTTCA3′ | 5′CCAGCCTACTCATTGGGATCA3′ |
| IFN-B | 5′TCAGAATGAGTGGTGGTTGC3′ | 5′GACCTTTCAAATGCAGTAGATTCA3′ |
| ISG-15 | 5′GGCCACAGCAACATCTATGA3′ | 5′CGCAAATGCTTGATCACTGT3′ |
| CXCL10 | 5′GCCGTCATTTTCTGCCTCAT3′ | 5′GCTTCCCTATGGCCCTCATT3′ |
| OAS1 | 5′ATTACCTCCTTCCCGACACC3′ | 5′CAAACTCCACCTCCTGATGC3′ |
| IFITM3 | 5′GCCCCCAACTACGAAAGA3′ | 5′ATTGAACAGGGACCAGACCAC3′ |
| TLR3 | 5′GTCTTCTGCACGAACCTGACAG3′ | 5′TGGAGGTTCTCCAGTTGGACCC3′ |
| TLR7 | 5′GTGATGCTGTGTGGTTTGTCTGG3′ | 5′CCTTTGTGTGCTCCTGGACCTA3’ |
| Antibody | Detection | Source and Catalog Number |
|---|---|---|
| PECy7 anti-mouse CD45 | CD45 | Biolegend cat#103114/clone 30-F11 |
| Pacific Blue™ anti-mouse/human CD11b | CD11b | Biolegend cat#101224/clone M1/70 |
| APC anti-mouse F4/80 | F4/80 | Biolegend cat#123116/clone BM8 |
| PE anti-mouse CD283 (TLR3) | TLR3 | Biolegend cat#141904/clone 11F8 |
| BD Pharminogen™ PE Mouse Anti-Mouse TLR7 (CD287) | TLR7 | BD Biosciences cat#565557/clone A94B10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrestha, R.; Johnson, P.M.; Ghimire, R.; Whitley, C.J.; Channappanavar, R. Differential TLR-ERK1/2 Activity Promotes Viral ssRNA and dsRNA Mimic-Induced Dysregulated Immunity in Macrophages. Pathogens 2024, 13, 1033. https://doi.org/10.3390/pathogens13121033
Shrestha R, Johnson PM, Ghimire R, Whitley CJ, Channappanavar R. Differential TLR-ERK1/2 Activity Promotes Viral ssRNA and dsRNA Mimic-Induced Dysregulated Immunity in Macrophages. Pathogens. 2024; 13(12):1033. https://doi.org/10.3390/pathogens13121033
Chicago/Turabian StyleShrestha, Rakshya, Paige Marie Johnson, Roshan Ghimire, Cody John Whitley, and Rudragouda Channappanavar. 2024. "Differential TLR-ERK1/2 Activity Promotes Viral ssRNA and dsRNA Mimic-Induced Dysregulated Immunity in Macrophages" Pathogens 13, no. 12: 1033. https://doi.org/10.3390/pathogens13121033
APA StyleShrestha, R., Johnson, P. M., Ghimire, R., Whitley, C. J., & Channappanavar, R. (2024). Differential TLR-ERK1/2 Activity Promotes Viral ssRNA and dsRNA Mimic-Induced Dysregulated Immunity in Macrophages. Pathogens, 13(12), 1033. https://doi.org/10.3390/pathogens13121033





