Effects of Thifluzamide Treatment on the Production of Cell Wall Degrading Enzymes in Rhizoctonia solani and Phenylpropane Metabolism in Pear Fruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Pathogen
2.2. Fruit and Treatment
2.3. Sample Collection
2.4. Preparation of Crude Enzyme
2.5. Assay of the Activity of CWDE
2.6. Measurements of Total Phenolic and Flavonoid Contents
2.7. Assays of the Activities of Key Enzymes Involved in Phenylpropane Metabolism
2.8. Total RNA Extraction and Real-Time Quantitative PCR (RT-qPCR) Analysis
2.9. Data Analyses
3. Results
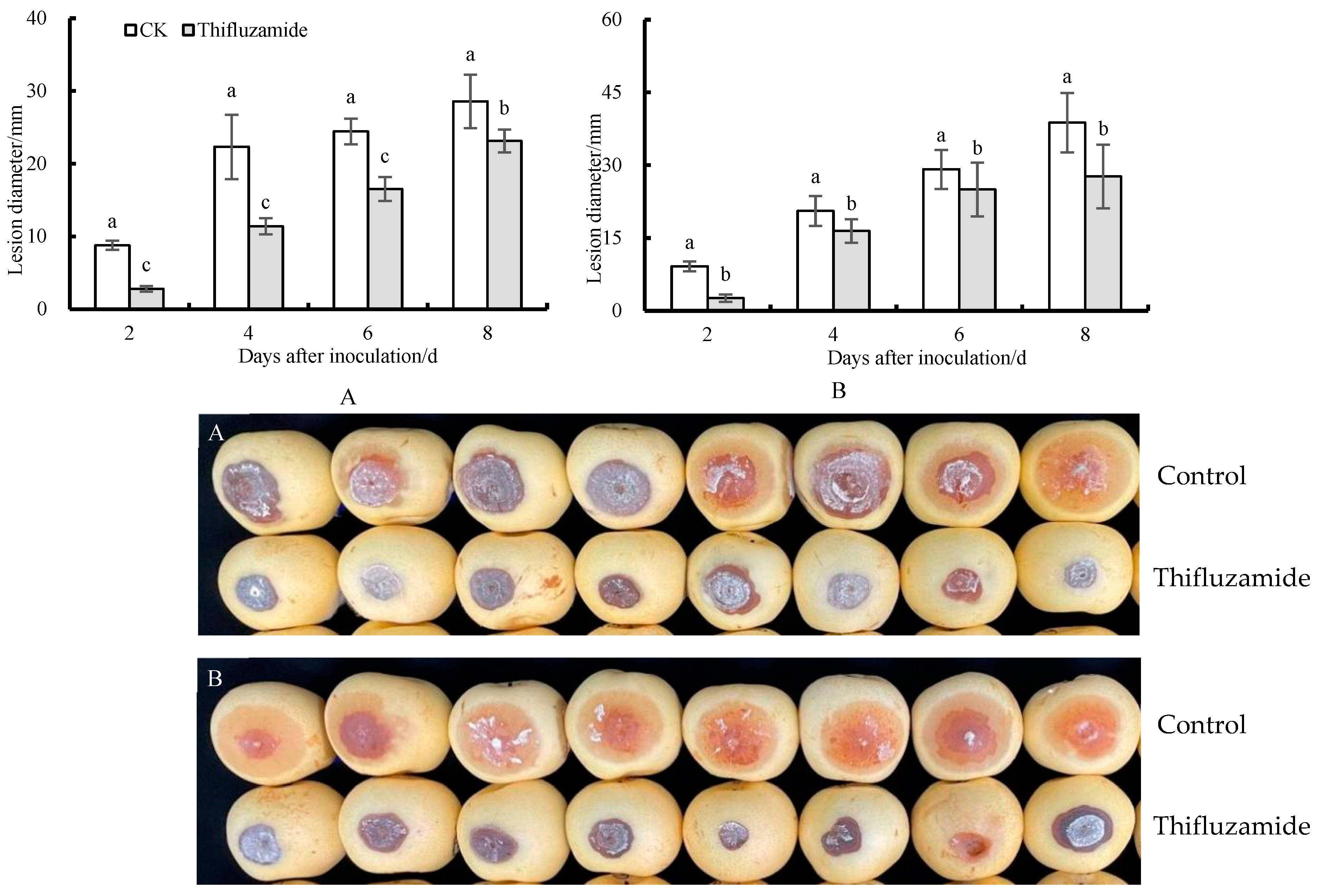
3.1. Symptom on ‘Huangguan’ Pear Fruit Caused by R. solani
3.2. Therapeutic and Protective Effects of Thifluzamide on Pear Fruit Rot
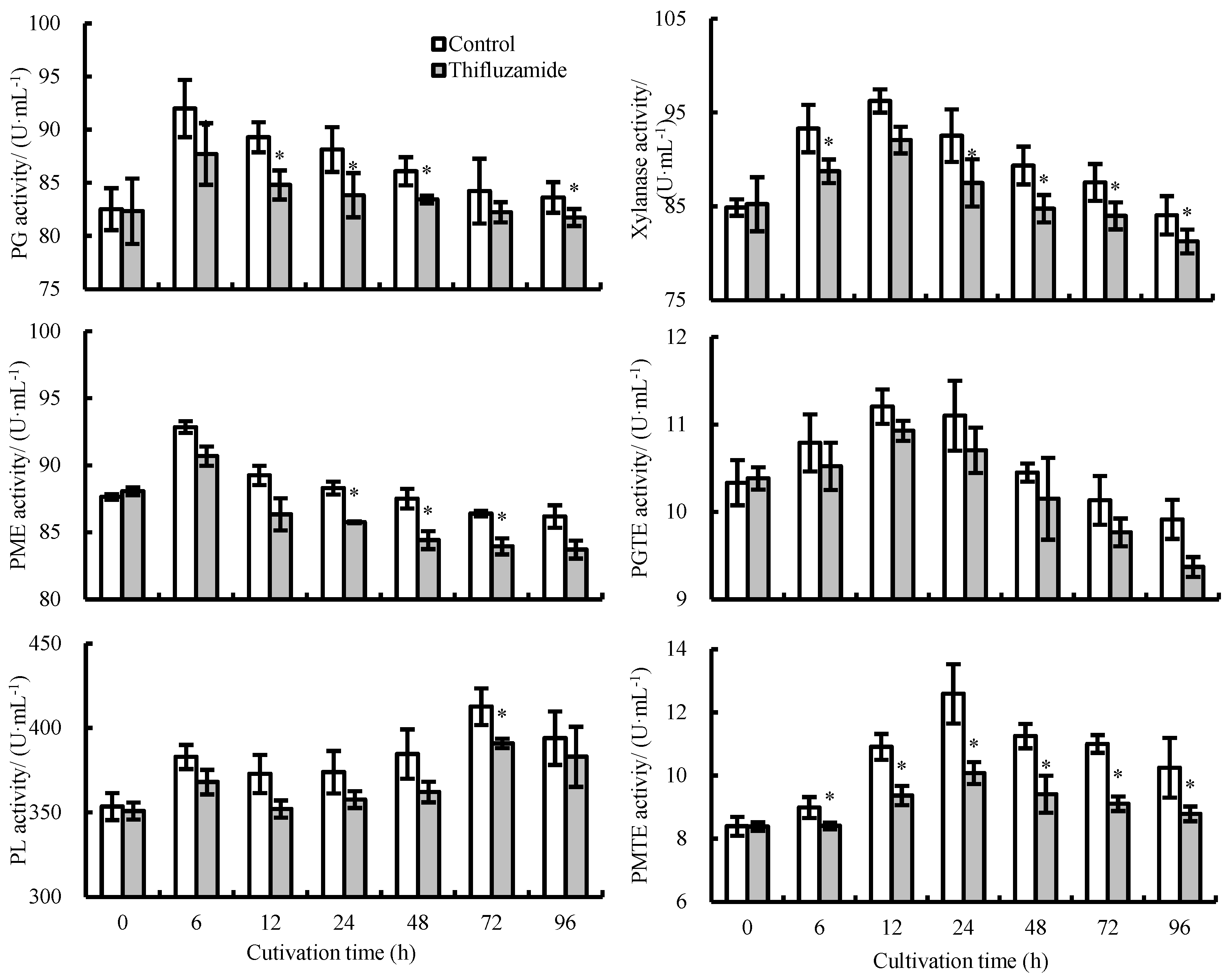
3.3. Activities of CWDEs Secreted by R. solani
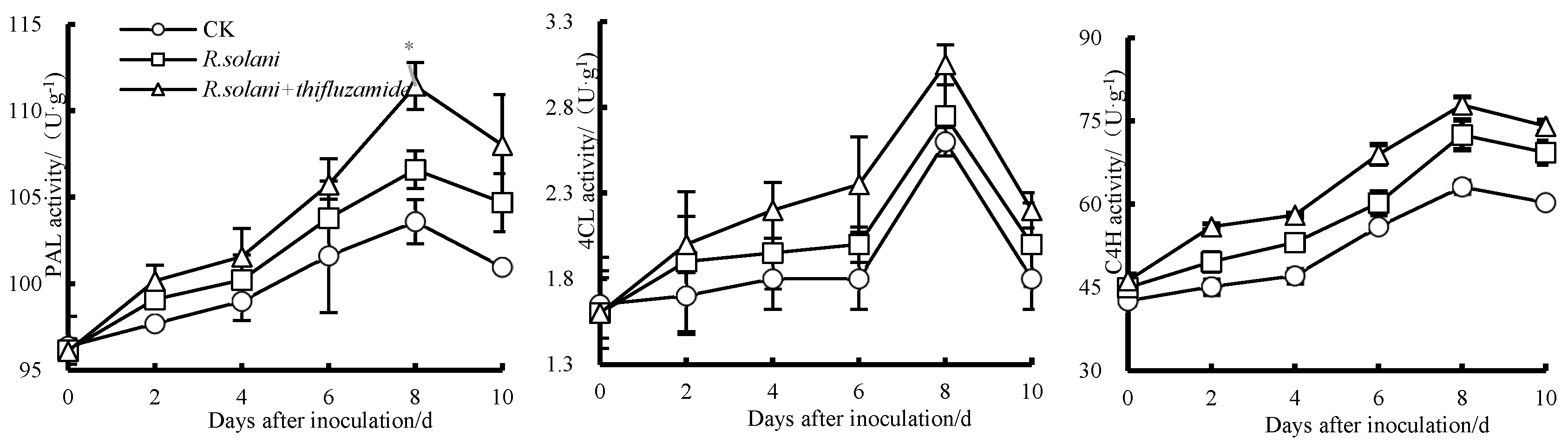
3.4. Effects of Thifluzamide Treatment on PAL, C4H, and 4CL Activities in Pears
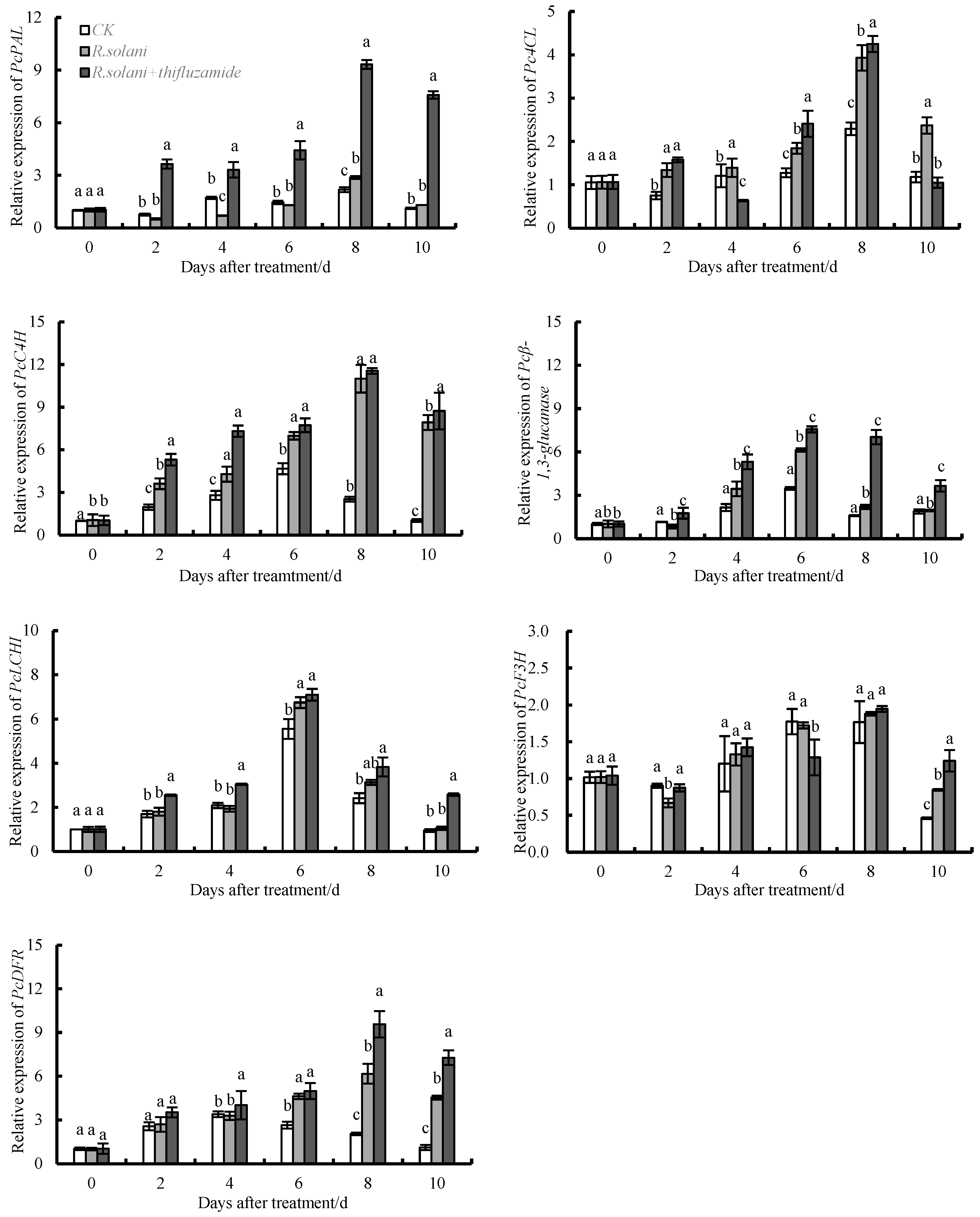
3.5. Effects of Thifluzamide Treatment on the Expression of PcPAL, PcC4H, Pc4CL, Pcβ-1,3-GA, PcLCH, PcF3H, and PcDFR in Pears
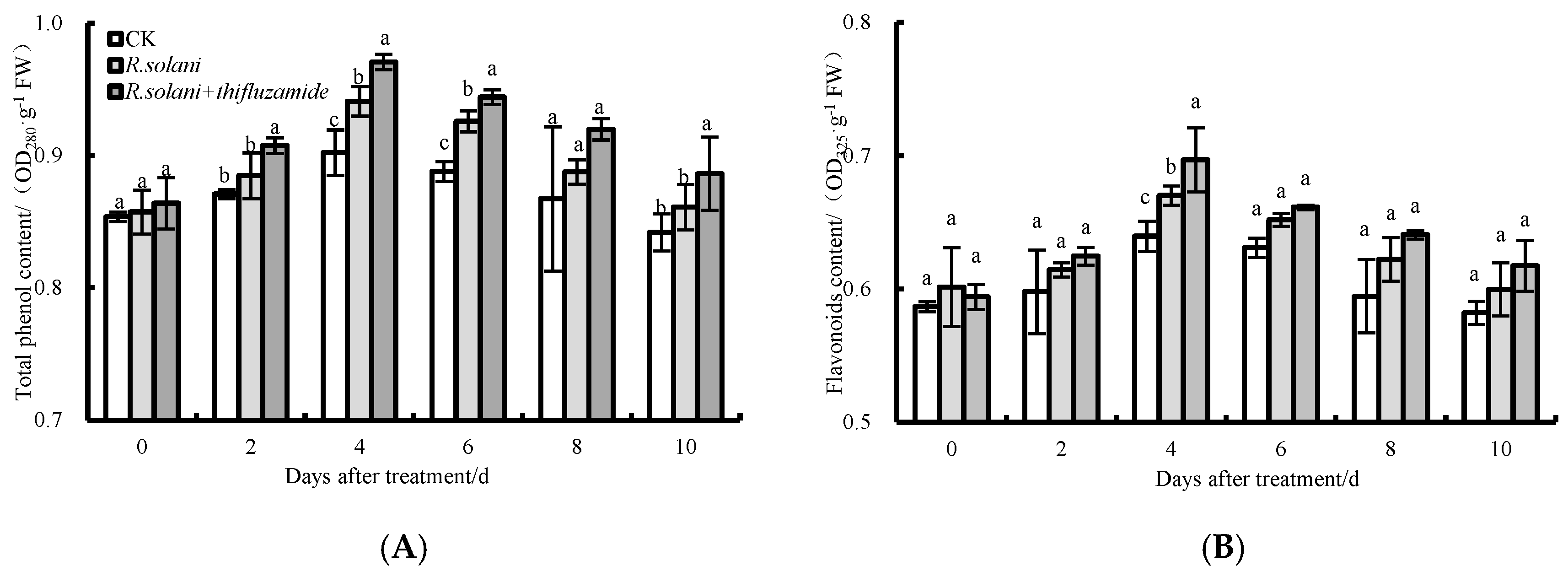
3.6. Total Phenolic and Flavonoid Contents
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joyce. Garden. 2022. Available online: https://www.garden.eco/where-do-pears-grow (accessed on 19 December 2022).
- Warmly, J. Northern Nester. 2022. Available online: https://northernnester.com/types-of-pears (accessed on 19 December 2022).
- Tomasz, C.; Jan, O.; Ireneusz, K.; Sabina, L. Effect of abiotic stress factors on polyphenolic content in the skin and flesh of pear by UPLC-PDA-Q/TOF-MS. Eur. Food Res. Technol. 2019, 245, 2715–2725. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Zhang, M.; Jia, B.; Heng, W.; Ye, Z.; Zhu, L.; Xu, X. Transcriptome sequencing analysis of two different genotypes of Asian pear reveals potential drought stress genes. Tree Genet. Genomes 2018, 14, 40. [Google Scholar] [CrossRef]
- Kou, X.H.; Li, Y.F.; Zhang, Y.; Jiang, B.L.; Xue, Z.H. Gene expression and activity of enzymes involved in sugar metabolism and accumulation during “Huangguan” and “Yali” pear fruit development. Trans. Tianjin Univ. 2018, 24, 101–110. [Google Scholar] [CrossRef]
- Ma, Y.R.; Yang, M.G.; Wang, J.J.; Jiang, C.Z.; Wang, Q.G. Application of Exogenous Ethylene Inhibits Postharvest Peel Browning of ‘Huangguan’ Pear. Front. Plant Sci. 2016, 7, 2029. [Google Scholar] [CrossRef]
- Jia, X.H.; Xu, J.N.; Wu, Y.S.; Zhang, X.N.; Du, Y.M.; Wang, W.H. Isolation, identification and artificial inoculation of Rhizoctonia solani on pear during storage. Hortic. Plant J. 2022, 9, 73–76. [Google Scholar] [CrossRef]
- Ning, X.X.; Su, Y.; Ma, Y.; Ning, X.Y.; Zhang, J.H. Advances in research on Rhizoctonia solani. Heilongjiang Agric. Sci. 2019, 2, 140–143, (In Chinese with English abstract). [Google Scholar]
- Zhang, X.Y.; Huo, H.L.; Xi, X.M.; Liu, L.L.; Yu, Z.; Hao, J.J. Histological observation of potato in response to Rhizoctonia solani infection. Eur. J. Plant Pathol. 2016, 145, 289–303. [Google Scholar] [CrossRef]
- Prashantha, S.T.; Bashyal, B.M.; Krishnan, S.G.; Dubey, H.; Solanke, A.U.; Prakash, G.; Aggarwalet, R. Identification and expression analysis of pathogenicity-related genes of Rhizoctonia solani anastomosis groups infecting rice. 3 Biotech 2021, 11, 394. [Google Scholar] [CrossRef]
- Joomdok, J.; Saepaisan, S.; Sunpapao, A.; Pongpisutta, R.; Monkham, T.; Sanitchon, J.; Chankaew, S. Identification of Rhizoctonia solani, as the cause of rice sheath blight and the source of its resistance, from Thai indigenous lowland rice germplasm. Euphytica 2021, 218, 6. [Google Scholar] [CrossRef]
- Brito, N.; Espino, J.J.; González, C. The endo-beta-1,4-xylanase xyn11A is required for virulence in Botrytis cinerea. Mol. Plant-Microbe Interact. 2006, 19, 25–32. [Google Scholar] [CrossRef]
- Siah, A.; Deweer, C.; Duymec, F.; Sanssené, J.; Durand, R.; Halama, P.; Reignault, P. Correlation of in planta endo-beta-1,4-xylanase activity with the necrotrophic phase of the hemibiotrophic fungus Mycosphaerella graminicola. Plant Pathol. 2010, 59, 661–670. [Google Scholar] [CrossRef]
- Ge, Y.H.; Wei, M.L.; Li, C.Y.; Chen, Y.R.; Lv, J.Y.; Meng, K.; Wang, W.H.; Li, J.R. Reactive oxygen species metabolism and phenylpropanoid pathway are involved in disease resistance against Penicillium expansum in apple fruit induced by ϵ-poly-l-lysine. J. Sci. Food Agric. 2018, 98, 5082–5088. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Wang, D.J.; Gao, X.X.; Yue, Z.Y.; Zhou, H.L. Exogenous caffeic acid and epicatechin enhance resistance against Botrytis cinerea through activation of the phenylpropanoid pathway in apples. Sci. Hortic. 2020, 268, 109348. [Google Scholar] [CrossRef]
- Ge, Y.H.; Dong, X.; Wei, M.L.; Li, C.Y.; Jiang, C.N.; Chen, Y.R. Effect of chitosan combined with polylysine on blue mould of apple fruit and antioxidant enzymes and phenylpropanoid pathway. Sci. Technol. Food Ind. 2018, 39, 281–286, (In Chinese with English abstract). [Google Scholar]
- Gacnik, S.; Veberic, R.; Marinovic, S.; Halbwirth, H.; Mikulic-Petkovsek, M. Effect of pre-harvest treatments with salicylic and methyl salicylic acid on the chemical profile and activity of some phenylpropanoid pathway related enzymes in apple leaves. Sci. Hortic. 2021, 277, 109794. [Google Scholar] [CrossRef]
- Ge, Y.H.; Tang, Q.; Li, C.Y.; Duan, B.; Li, X.; Wei, M.L.; Li, J.R. Acibenzolar-S-methyl treatment enhances antioxidant ability and phenylpropanoid pathway of blueberries during low temperature storage. LWT-Food Sci. Technol. 2019, 110, 48–53. [Google Scholar] [CrossRef]
- Deng, Y.X.; Lu, S.F. Biosynthesis and regulation of phenylpropanoids in plants. Crit. Rev. Plant Sci. 2017, 36, 257–290. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, B.; Ma, L.; Zheng, X.Y.; Gong, D.; Xue, H.L.; Bi, Y.; Wang, Y.; Zhang, Z.; Prusky, D. Benzo-(1, 2, 3)-thiadiazole-7-carbothioic acid s-methyl ester (BTH) promotes tuber wound healing of potato by elevation of phenylpropanoid metabolism. Postharvest Biol. Technol. 2019, 153, 125–132. [Google Scholar] [CrossRef]
- Li, L.; Liu, Q.L.; Xue, H.L.; Bi, Y.; Raza, H.; Zhang, R.; Carelle, J.K.; Peng, H.; Long, H.T.; Prusky, D. Acetylsalicylic acid (ASA) suppressed Fusarium rot development and neosolaniol (NEO) accumulation by activating phenylpropane metabolism in muskmelon fruit. Eur. J. Plant Pathol. 2022, 163, 625–639. [Google Scholar] [CrossRef]
- Jia, L.L.; Li, Y.; Liu, G.S.; He, J.G. Acidic electrolyzed water improves the postharvest quality of jujube fruit by regulating antioxidant activity and cell wall metabolism. Sci. Hortic. 2022, 304, 111253. [Google Scholar] [CrossRef]
- Yuan, H.B.; Yuan, M.J.; Shi, B.K.; Wang, Z.N.; Huang, T.X.; Qin, G.H.; Hou, H.; Wang, L.; Tu, H.T. Biocontrol activity and action mechanism of Paenibacillus polymyxa strain Nl4 against pear Valsa canker caused by Valsa pyri. Front. Microbiol. 2022, 13, 950742. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.E.; Wang, M.H.; Lee, A.Y.; Hwang, Y.S. Effects of short-term treatment of high-pressure CO2 on the changes in fruit quality during the storage of ‘Maehyang’ strawberries. Korean J. Agric. Sci. 2014, 41, 9–16. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Lan, B.; Jian, Y.L.; Chang, D.D.; Zhang, S.L.; Li, X.M. Infection process and pathogenic mechanism of Phomopsis asparagi, the Asparagus stem blight pathogen. Phytoparasitica 2015, 44, 11–18. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, C.; Li, J.; Huang, S.; Xu, B.; Jin, Z. Activation of salicylic acid metabolism and signal transduction can enhance resistance to Fusarium wilt in banana (Musa acuminata L. AAA group, cv. Cavendish). Funct. Integr. Genom. 2015, 15, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, C.Y.; Fan, Y.T.; Qu, L.H.; Huang, R.; Liu, J.X.; Zhang, C.Y.; Ge, Y.H. γ-Aminobutyric acid regulates mitochondrial energy metabolism and organic acids metabolism in apples during postharvest ripening. Postharvest Biol. Technol. 2022, 186, 111846. [Google Scholar] [CrossRef]
- Joseph, A.K. A Study on the Biocontrol Activity of Rhodotorula mucilaginosa Combined with Salicylic Acid Against Penicillium digitatum Infection in Oranges. Jiangsu University, Zhenjiang, China, 2020. (In Chinese with English abstract). [Google Scholar]
- Chen, M.Y.; Lin, H.T.; Hung, Y.C.; Zhang, S.; Lin, Y.F.; Chen, Y.H. Regulation of 2,4-dinitrophenol and adenosine triphosphate on disease development, energy status, and respiratory metabolism of Phomopsis longanae chi-infected longan fruit. Mod. Food Sci. Technol. 2015, 31, 49–58. [Google Scholar] [CrossRef]
- Lampugnani, E.R.; Khan, G.A.; Somssich, M.; Persson, S. Building a plant cell wall at a glance. J. Cell Sci. 2018, 131, 309. [Google Scholar] [CrossRef]
- Bellincampi, D.; Cervone, F.; Lionetti, V. Plant cell wall dynamics and wall-related susceptibility in plant-pathogen interactions. Front. Plant Sci. 2014, 5, 228–235. [Google Scholar] [CrossRef]
- Zhang, J.R.; Zhang, L.L.; Chang, L.L.; Zhang, S.Y. Effect of chitosan combined with sodium silicate on phenylpropanoid metabolism and pathogenesis-related proteins of postharvest winter jujube. Sci. Technol. Food Ind. 2020, 41, 268–273, (In Chinese with English abstract). [Google Scholar]
- Wang, Y.; Ji, D.C.; Chen, T.; Li, B.Q.; Zhang, Z.Q.; Qin, G.Z.; Tian, S.P. Production, signaling, and scavenging mechanisms of reactive oxygen species in fruit-pathogen interactions. Int. J. Mol. Sci. 2019, 20, 2994. [Google Scholar] [CrossRef]
- Ge, Y.H.; Chen, Y.R.; Li, C.Y.; Wei, M.L.; Li, X.; Tang, Q.; Duan, B. Effect of trisodium phosphate treatment on black spot of apple fruit and the roles of anti-oxidative enzymes. Physiol. Mol. Plant Pathol. 2019, 106, 226–231. [Google Scholar] [CrossRef]
- Ge, Y.H.; Duan, B.; Li, C.Y.; Tang, Q.; Li, X.; Wei, M.L.; Chen, Y.R.; Li, J.R. γ-Aminobutyric acid delays senescence of blueberry fruit by regulation of reactive oxygen species metabolism and phenylpropanoid pathway. Sci. Hortic. 2018, 240, 303–309. [Google Scholar] [CrossRef]
- Yu, C.; Zeng, L.Z.; Sheng, K.; Chen, F.X.; Zhou, T.; Zheng, X.D.; Yu, T. γ-Aminobutyric acid induces resistance against Penicillium expansum by priming of defence responses in pear fruit. Food Chem. 2014, 15, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.H.; Ma, J.H.; Xie, J.; Deng, L.L.; Yao, S.X.; Zeng, K.F. Transcriptomic and biochemical analysis of highlighted induction of phenylpropanoid pathway metabolism of citrus fruit in response to salicylic acid, Pichia membranaefaciens and oligochitosan. Postharvest Biol. Technol. 2018, 142, 81–92. [Google Scholar] [CrossRef]
- Liu, C.H.; Zheng, H.H.; Sheng, K.L.; Liu, W.; Zheng, L. Effects of postharvest UV-C irradiation on phenolic acids, flavonoids, and key phenylpropanoid pathway genes in tomato fruit. Sci. Hortic. 2018, 241, 107–114. [Google Scholar] [CrossRef]

| Gene | Primer Sequence | NCBI Number |
|---|---|---|
| PcPAL | F: CGTATGGTGGCGGAGTACAGAAAG | NM_001319807.1 |
| R: GCTATTGCTGCAACTTGGGAAATGG | ||
| PcC4H | F: GGCAGTTCACTCTCCCACACAAC | KF663548.1 |
| R: TTTCCAGGAGGAGGAGGTCCATTG | ||
| Pc4CL | F: CCTCTTCCCTCAAGCACCAATTCAG | XM_009372686.2 |
| R: ATGGGGATGTCGGGGAGTTTGG | ||
| Pcβ-1,3-GA | F: CTTGATGCCAGCCCTGCAAATAAC | JX127223.1 |
| R: GCCCGATGCCATTGCTTTTGTAC | ||
| PcLCH | F: AATGGCTTCTGCTTTTCTCAATGGC | KP202179.1 |
| R: TCACTATCTTCACCAACGGCTTCAC | ||
| PcF3H | F: TGGCTCCTGCTACTACGCTCAC | KC460396.1 |
| R: AACCTTTGGACGCTCGTCTTCG | ||
| PcDFR | F: CGAAACACCCAACCGTTTAGTTCAG | MF489221.1 |
| R: TTGTCCTTGCTCTTCAGTGCTCAC | ||
| Pcactin | F: AGAGCATCCAGTCCTCCTGACAG | AF386514.1 |
| R: GCCTGAATTGCAACGTACATAGCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Yan, W.; Sun, X.; Zhang, X.; Ge, Y.; Jia, X. Effects of Thifluzamide Treatment on the Production of Cell Wall Degrading Enzymes in Rhizoctonia solani and Phenylpropane Metabolism in Pear Fruit. Pathogens 2024, 13, 963. https://doi.org/10.3390/pathogens13110963
Wu Y, Yan W, Sun X, Zhang X, Ge Y, Jia X. Effects of Thifluzamide Treatment on the Production of Cell Wall Degrading Enzymes in Rhizoctonia solani and Phenylpropane Metabolism in Pear Fruit. Pathogens. 2024; 13(11):963. https://doi.org/10.3390/pathogens13110963
Chicago/Turabian StyleWu, Yushuo, Weiwei Yan, Xiaonan Sun, Xinnan Zhang, Yonghong Ge, and Xiaohui Jia. 2024. "Effects of Thifluzamide Treatment on the Production of Cell Wall Degrading Enzymes in Rhizoctonia solani and Phenylpropane Metabolism in Pear Fruit" Pathogens 13, no. 11: 963. https://doi.org/10.3390/pathogens13110963
APA StyleWu, Y., Yan, W., Sun, X., Zhang, X., Ge, Y., & Jia, X. (2024). Effects of Thifluzamide Treatment on the Production of Cell Wall Degrading Enzymes in Rhizoctonia solani and Phenylpropane Metabolism in Pear Fruit. Pathogens, 13(11), 963. https://doi.org/10.3390/pathogens13110963





