Molecular Characterization of Anaplasma spp. in Cattle from Kazakhstan
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
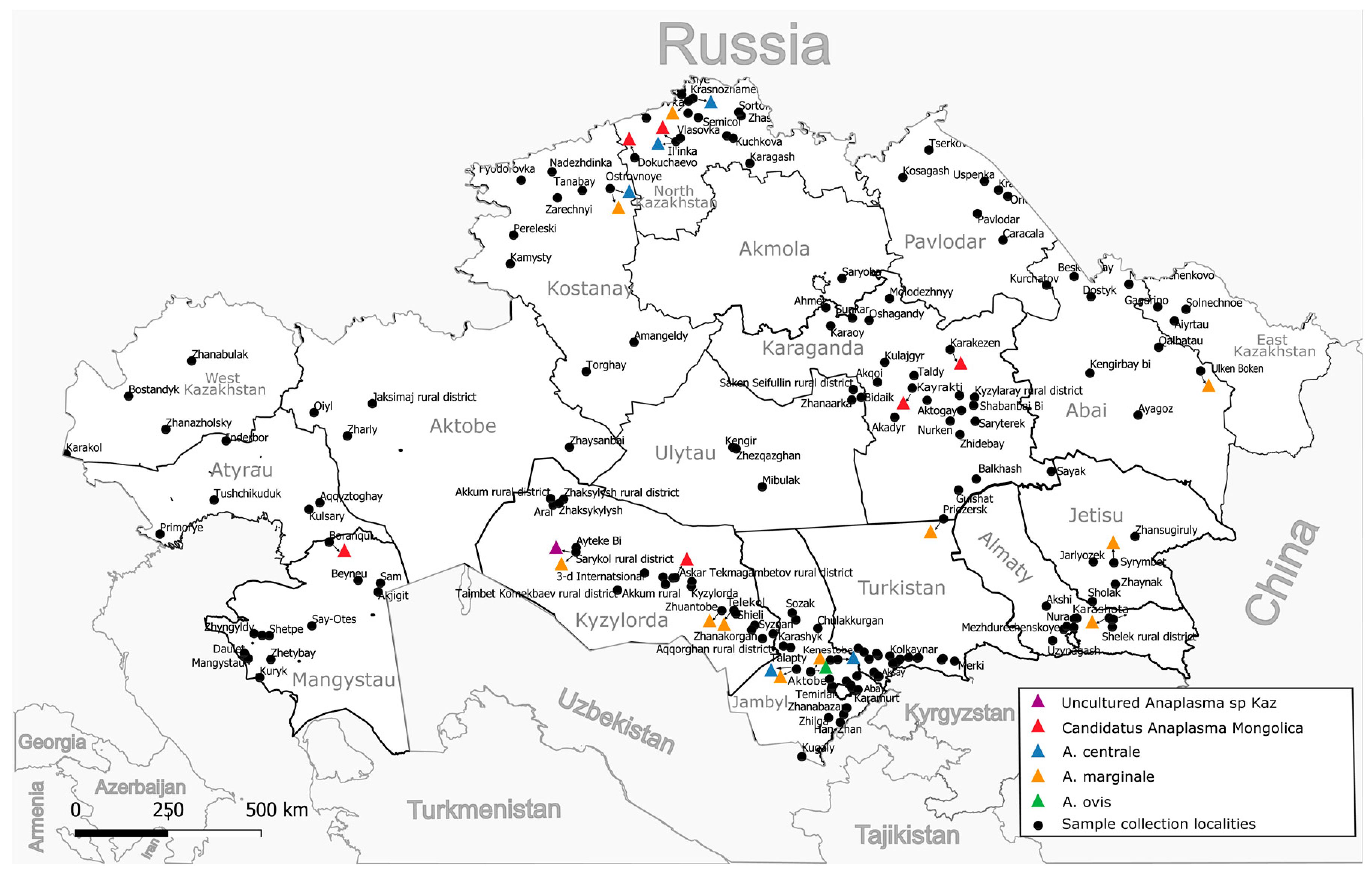
2.2. Collecting Samples
2.3. DNA Isolation
2.4. Amplification of the groEL Gene Fragment
2.5. Nested Amplification 16S rRNA
2.6. Sequencing and Analysis
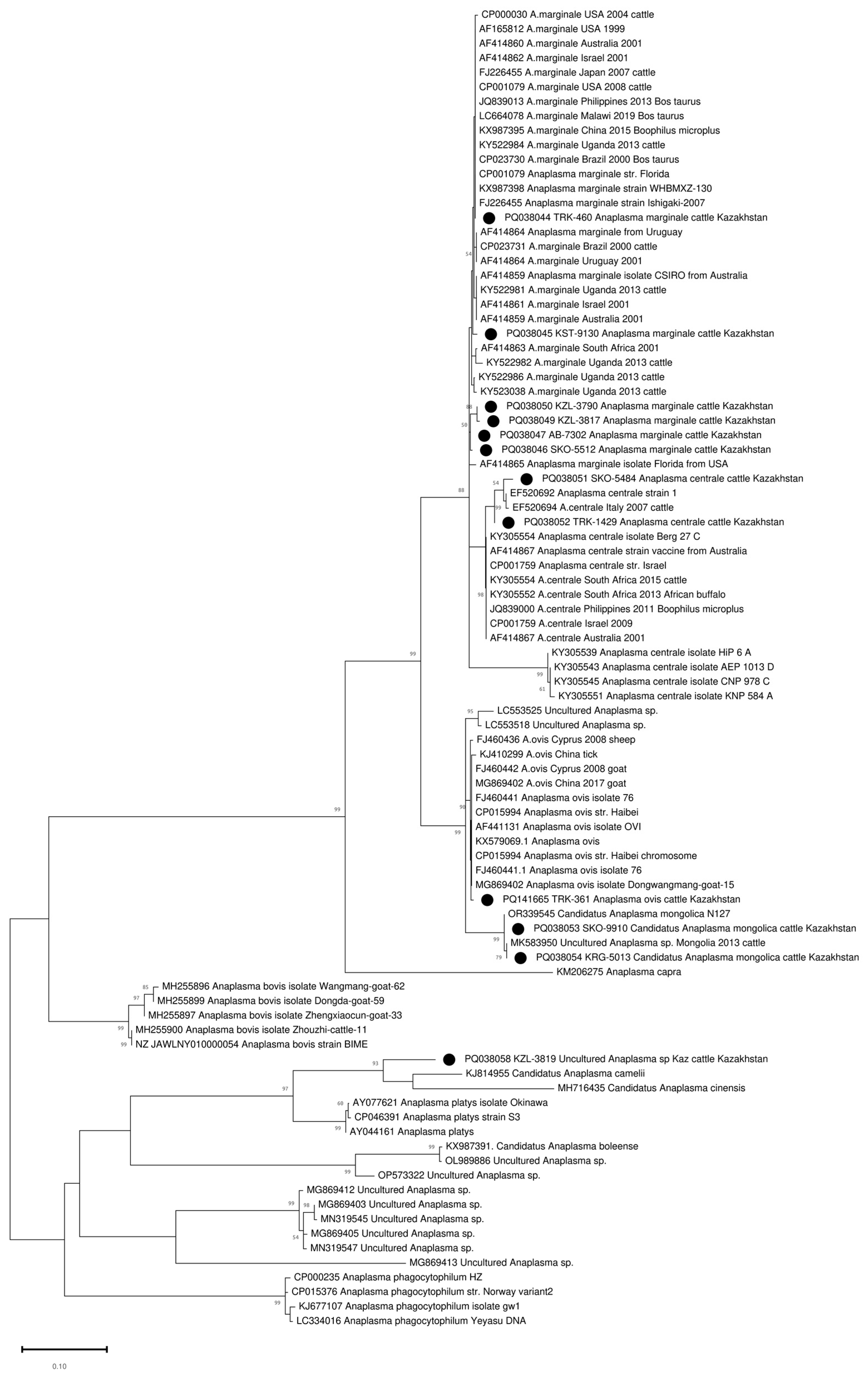
3. Results
3.1. Detection and Species Identification of Anaplasma spp. by the groEL Gene
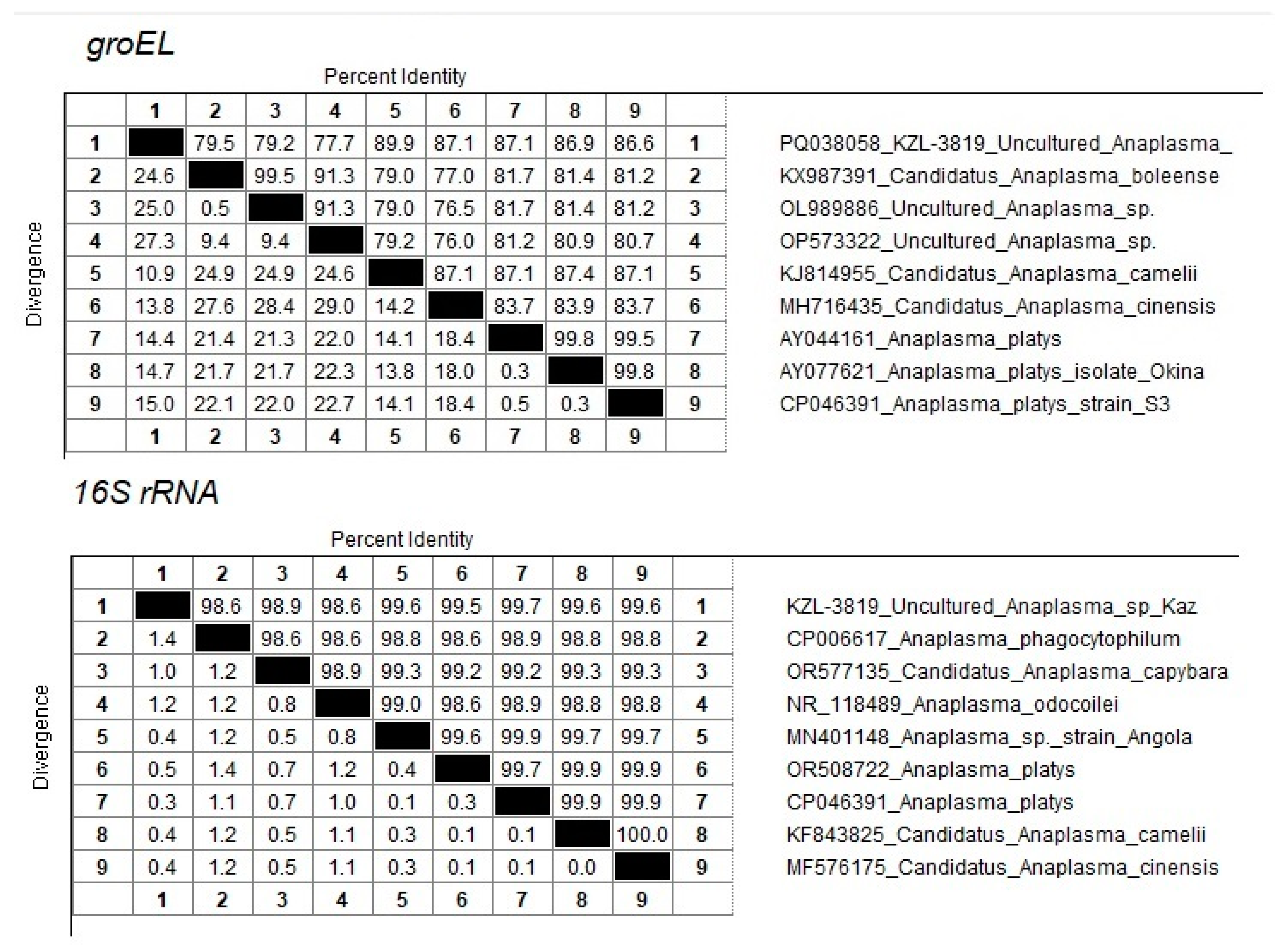
3.2. Identification of Anaplasma spp. by 16S rRNA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rar, V.; Tkachev, S.; Tikunova, N. Genetic diversity of Anaplasma bacteria: Twenty years later. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2021, 91, 104833. [Google Scholar] [CrossRef] [PubMed]
- Atif, F.A. Alpha proteobacteria of genus Anaplasma (Rickettsiales: Anaplasmataceae): Epidemiology and characteristics of Anaplasma species related to veterinary and public health importance. Parasitology 2016, 143, 659–685. [Google Scholar] [CrossRef]
- Matei, I.A.; Estrada-Peña, A.; Cutler, S.J.; Vayssier-Taussat, M.; Varela-Castro, L.; Potkonjak, A.; Zeller, H.; Mihalca, A.D. A review on the eco-epidemiology and clinical management of human granulocytic anaplasmosis and its agent in Europe. Parasites Vectors 2019, 12, 599. [Google Scholar] [CrossRef]
- Aubry, P.; Geale, D.W. A review of bovine anaplasmosis. Transbound. Emerg. Dis. 2011, 58, 1–30. [Google Scholar] [CrossRef]
- Salinas-Estrella, E.; Amaro-Estrada, I.; Cobaxin-Cárdenas, M.E.; Preciado de la Torre, J.F.; Rodríguez, S.D. Bovine Anaplasmosis: Will there ever be an almighty effective vaccine? Front. Vet. Sci. 2022, 9, 946545. [Google Scholar] [CrossRef] [PubMed]
- Khumalo, Z.T.; Catanese, H.N.; Liesching, N.; Hove, P.; Collins, N.E.; Chaisi, M.E.; Gebremedhin, A.H.; Oosthuizen, M.C.; Brayton, K.A. Characterization of Anaplasma marginale subsp. centrale Strains by Use of msp1aS Genotyping Reveals a Wildlife Reservoir. J. Clin. Microbiol. 2016, 54, 2503–2512. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yoshikawa, Y.; Kawamori, F.; Ikegaya, A.; Ohtake, M.; Ohashi, M.; Shimada, M.; Takada, A.; Iwai, K.; Ohashi, N. A Molecular and Serological Survey of Rickettsiales Bacteria in Wild Sika Deer (Cervus nippon nippon) in Shizuoka Prefecture, Japan: High Prevalence of Anaplasma Species. Jpn. J. Infect. Dis. 2015, 68, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Chien, N.T.H.; Nguyen, T.L.; Bui, K.L.; Nguyen, T.V.; Le, T.H. Anaplasma marginale and A. platys Characterized from Dairy and Indigenous Cattle and Dogs in Northern Vietnam. Korean J. Parasitol. 2019, 57, 43–47. [Google Scholar] [CrossRef]
- Makgabo, S.M.; Brayton, K.A.; Oosthuizen, M.C.; Collins, N.E. Unravelling the diversity of Anaplasma species circulating in selected African wildlife hosts by targeted 16S microbiome analysis. Curr. Res. Microb. Sci. 2023, 5, 100198. [Google Scholar] [CrossRef]
- Priyanka, M.; Dhanalakshmi, H.; Rakesh, R.L.; Thimmareddy, P.M.; Narayana Bhat, M. Monocytic anaplasmosis in a cow: A case report. J. Parasit. Dis. Off. Organ Indian Soc. Parasitol. 2017, 41, 687–688. [Google Scholar] [CrossRef]
- Al-Saadi, M.; Al-Sallami, D.; Alsultan, A. Molecular identification of Anaplasma platys in cattle by nested PCR. Iran. J. Microbiol. 2023, 15, 433. [Google Scholar] [CrossRef] [PubMed]
- Han, X.-Y.; Du, L.-F.; Lin, Z.-T.; Li, C.; Xiong, T.; Zhu, W.-J.; Ye, R.-Z.; Wang, N.; Wang, Y.-F.; Gao, W.-Y.J.E.M.; et al. Genomic characters of Anaplasma bovis and genetic diversity in China. Emerg. Microbes Infect. 2024, 13, 2323153. [Google Scholar] [CrossRef]
- Park, J.H.; Han, D.G.; Ryu, J.H.; Chae, J.B.; Chae, J.S.; Yu, D.H.; Park, B.K.; Kim, H.C.; Choi, K.S. Molecular detection of Anaplasma bovis in Holstein cattle in the Republic of Korea. Acta Vet. Scand. 2018, 60, 15. [Google Scholar] [CrossRef]
- Teshale, S.; Geysen, D.; Ameni, G.; Dorny, P.; Berkvens, D.J.P. Survey of Anaplasma phagocytophilum and Anaplasma sp.‘Omatjenne’infection in cattle in Africa with special reference to Ethiopia. Parasites Vectors 2018, 11, 162. [Google Scholar] [CrossRef]
- Sharma, A.; Singla, L.D.; Kaur, P.; Bal, M.S. PCR and ELISA vis-à-vis microscopy for detection of bovine anaplasmosis: A study on associated risk of an upcoming problem in North India. Sci. World J. 2015, 2015, 352519. [Google Scholar] [CrossRef]
- Silaghi, C.; Santos, A.S.; Gomes, J.; Christova, I.; Matei, I.A.; Walder, G.; Domingos, A.; Bell-Sakyi, L.; Sprong, H.; Von Loewenich, F.D.J.V.-B.; et al. Guidelines for the direct detection of Anaplasma spp. in diagnosis and epidemiological studies. Vector-Borne Zoonotic Dis. 2017, 17, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.-P.; Wang, X.; Li, Y.-N.; Xu, G.; Wang, Y.-H.; Zhou, E.-M. GroEL gene typing and genetic diversity of Anaplasma bovis in ticks in Shaanxi, China. Infect. Genet. Evol. 2019, 74, 103927. [Google Scholar] [CrossRef] [PubMed]
- Kuibagarov, M.; Makhamed, R.; Zhylkibayev, A.; Berdikulov, M.; Abdrakhmanov, S.; Kozhabayev, M.; Akhmetollayev, I.; Mukanov, K.; Ryskeldina, A.; Ramankulov, Y.; et al. Theileria and Babesia infection in cattle—First molecular survey in Kazakhstan. Ticks Tick-Borne Dis. 2023, 14, 102078. [Google Scholar] [CrossRef]
- Shabdarbayeva, G.S.; Abdybekova, A.M. Identification of foci of blood-parasitic diseases of ruminants in the south of Kazakhstan. Eurasian Union Sci. 2016, 33, 17–21. [Google Scholar]
- Boom, R.; Sol, C.J.; Salimans, M.M.; Jansen, C.L.; Wertheim-van Dillen, P.M.; van der Noordaa, J. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 1990, 28, 495–503. [Google Scholar] [CrossRef]
- Berdimuratova, K.; Amirgazin, A.; Kuibagarov, M.; Lutsay, V.; Mukanov, K.; Shevtsov, A. Optimization of PCR Purification Using Silica-Coated Magnetic Beads. Eurasian J. Appl. Biotechnol. 2020, 1, 11. [Google Scholar] [CrossRef]
- Burland, T.G. DNASTAR’s Lasergene sequence analysis software. Methods Mol. Biol. 2000, 132, 71–91. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Reller, M.E.; Dumler, J.S. Ehrlichia, Anaplasma, and Related Intracellular Bacteria. In Manual of Clinical Microbiology; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 1135–1149. [Google Scholar]
- Railey, A.F.; Marsh, T.L. Economic benefits of diagnostic testing in livestock: Anaplasmosis in cattle. Front. Vet. Sci. 2021, 8, 626420. [Google Scholar] [CrossRef]
- Perfilyeva, Y.V.; Shapiyeva, Z.Z.; Ostapchuk, Y.O.; Berdygulova, Z.A.; Bissenbay, A.O.; Kulemin, M.V.; Ismagulova, G.A.; Skiba, Y.A.; Sayakova, Z.Z.; Mamadaliyev, S.M.; et al. Tick-borne pathogens and their vectors in Kazakhstan—A review. Ticks Tick-Borne Dis. 2020, 11, 101498. [Google Scholar] [CrossRef] [PubMed]
- Miranda, E.A.; Han, S.W.; Cho, Y.K.; Choi, K.S.; Chae, J.S. Co-Infection with Anaplasma Species and Novel Genetic Variants Detected in Cattle and Goats in the Republic of Korea. Pathogens 2021, 10, 28. [Google Scholar] [CrossRef]
- Rjeibi, M.R.; Ayadi, O.; Rekik, M.; Gharbi, M. Molecular survey and genetic characterization of Anaplasma centrale, A. marginale and A. bovis in cattle from Algeria. Transbound. Emerg. Dis. 2018, 65, 456–464. [Google Scholar] [CrossRef]
- Qi, Y.; Ai, L.; Zhu, C.; Lu, Y.; Lv, R.; Mao, Y.; Lu, N.; Tan, W. Co-existence of Multiple Anaplasma Species and Variants in Ticks Feeding on Hedgehogs or Cattle Poses Potential Threats of Anaplasmosis to Humans and Livestock in Eastern China. Front. Microbiol. 2022, 13, 913650. [Google Scholar] [CrossRef]
- Ashraf, S.; Parveen, A.; Asif, M.; Alanazi, A.D.; Alouffi, A.; Muhammad Awais, M.; Khan, A.; Aktas, M.; Ozubek, S.; Iqbal, F. First report regarding molecular epidemiology and novel variant identification of Anaplasma centrale in cattle from Pakistan. Saudi J. Biol. Sci. 2021, 28, 6488–6494. [Google Scholar] [CrossRef]
- Aktas, M.; Özübek, S. Bovine anaplasmosis in Turkey: First laboratory confirmed clinical cases caused by Anaplasma phagocytophilum. Vet. Microbiol. 2015, 178, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, K.; Sun, Y.; Shi, J.; Li, H.; Chen, Y.; Yang, H.; Li, X.; Wu, B.; Li, X.J.P.o. Molecular epidemiology and risk factors of Anaplasma spp., Babesia spp. and Theileria spp. infection in cattle in Chongqing, China. PloS ONE 2019, 14, e0215585. [Google Scholar] [CrossRef]
- Altay, K.; Erol, U.; Sahin, O.F.; Aytmirzakizi, A.J.T.; Diseases, T.-b. First molecular detection of Anaplasma species in cattle from Kyrgyzstan; molecular identification of human pathogenic novel genotype Anaplasma capra and Anaplasma phagocytophilum related strain. Ticks Tick-Borne Dis. 2022, 13, 101861. [Google Scholar] [CrossRef] [PubMed]
- De Wall, D.T. Anaplasmosis control and diagnosis in South Africa. Ann. N. Y. Acad. Sci. 2000, 916, 474–483. [Google Scholar] [CrossRef]
- Molad, T.; Mazuz, M.L.; Fleiderovitz, L.; Fish, L.; Savitsky, I.; Krigel, Y.; Leibovitz, B.; Molloy, J.; Jongejan, F.; Shkap, V. Molecular and serological detection of A. centrale- and A. marginale-infected cattle grazing within an endemic area. Vet. Microbiol. 2006, 113, 55–62. [Google Scholar] [CrossRef]
- Shkap, V.; Molad, T.; Fish, L.; Palmer, G.H. Detection of the Anaplasma centralevaccine strain and specific differentiation from Anaplasma marginale in vaccinated and infected cattle. Parasitol. Res. 2002, 88, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Khumalo, Z.T.H.; Brayton, K.A.; Collins, N.E.; Chaisi, M.E.; Quan, M.; Oosthuizen, M.C. Evidence confirming the phylogenetic position of Anaplasma centrale (ex Theiler 1911) Ristic and Kreier 1984. Int. J. Syst. Evol. Microbiol. 2018, 68, 2682–2691. [Google Scholar] [CrossRef]
- Hove, P.; Khumalo, Z.T.H.; Chaisi, M.E.; Oosthuizen, M.C.; Brayton, K.A.; Collins, N.E. Detection and Characterisation of Anaplasma marginale and A. centrale in South Africa. Vet. Sci. 2018, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Ben Said, M.; Belkahia, H.; Selmi, R.; Messadi, L. Computational selection of minimum length groESL operon required for Anaplasma species attribution and strain diversity analysis. Mol. Cell. Probes 2019, 48, 101467. [Google Scholar] [CrossRef]
- Caudill, M.T.; Brayton, K.A. The Use and Limitations of the 16S rRNA Sequence for Species Classification of Anaplasma Samples. Microorganisms 2022, 10, 605. [Google Scholar] [CrossRef]
- Zhyldyz, A.; Aitakin, K.; Atabek, B.; Elmurat, J.; Rysbek, N.; Jailobek, O.; Ahedor, B.; Otgonsuren, D.; Mumbi, N.N.M.; Guswanto, A.; et al. An epidemiological survey of vector-borne pathogens infecting cattle in Kyrgyzstan. Parasitol. Int. 2023, 97, 102791. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Jiang, Y.; Tao, D.; Zhao, A.; Qi, M.; Ning, C. Molecular detection of Anaplasma spp. in dairy cattle in southern Xinjiang, China. Vet. Parasitol. Reg. Stud. Rep. 2020, 20, 100406. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Liu, Z.; Liu, J.; Niu, Q.; Ren, Q.; Chen, Z.; Guan, G.; Luo, J.; Yin, H. Molecular detection and characterization of Anaplasma spp. in sheep and cattle from Xinjiang, northwest China. Parasites Vectors 2015, 8, 108. [Google Scholar] [CrossRef]
- Arkhipova, A.L.; Tagmazyan, P.P.; Kureev, N.; Brigida, A.V.; Kovalchuk, S. Distribution of bovine anaplasmosis in Russian Federation. Vet. Korml. 2019, 2, 23–25. [Google Scholar] [CrossRef]
- Rahr, V.A.; Marchenko, V.A.; Biryukov, I.V. On epizootology of anaplasmosis of ruminants in the south of west siberia. Bull. Altai State Agric. Univ. 2019, 7, 109–115. [Google Scholar]
- Tian, J.; Liu, J.; Zhao, H.; Chen, X.; Geng, X.; Lu, M.; Li, K. Molecular surveillance reveals a potential hotspot of tick-borne disease in Yakeshi City, Inner Mongolia. BMC Microbiol. 2023, 23, 359. [Google Scholar] [CrossRef]
- Fischer, T.; Myalkhaa, M.; Krücken, J.; Battsetseg, G.; Batsukh, Z.; Baumann, M.P.O.; Clausen, P.H.; Nijhof, A.M. Molecular detection of tick-borne pathogens in bovine blood and ticks from Khentii, Mongolia. Transbound. Emerg. Dis. 2020, 67 (Suppl. 2), 111–118. [Google Scholar] [CrossRef]
- Bauer, B.U.; Răileanu, C.; Tauchmann, O.; Fischer, S.; Ambros, C.; Silaghi, C.; Ganter, M. Anaplasma phagocytophilum and Anaplasma ovis-Emerging Pathogens in the German Sheep Population. Pathogens 2021, 10, 1298. [Google Scholar] [CrossRef] [PubMed]
- Renneker, S.; Abdo, J.; Salih, D.E.; Karagenç, T.; Bilgiç, H.; Torina, A.; Oliva, A.G.; Campos, J.; Kullmann, B.; Ahmed, J.; et al. Can Anaplasma ovis in small ruminants be neglected any longer? Transbound. Emerg. Dis. 2013, 60 (Suppl. 2), 105–112. [Google Scholar] [CrossRef]
- de la Fuente, J.; Ruiz-Fons, F.; Naranjo, V.; Torina, A.; Rodríguez, O.; Gortázar, C. Evidence of Anaplasma infections in European roe deer (Capreolus capreolus) from southern Spain. Res. Vet. Sci. 2008, 84, 382–386. [Google Scholar] [CrossRef]
- de la Fuente, J.; Atkinson, M.W.; Naranjo, V.; Fernández de Mera, I.G.; Mangold, A.J.; Keating, K.A.; Kocan, K.M. Sequence analysis of the msp4 gene of Anaplasma ovis strains. Vet. Microbiol. 2007, 119, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Z.; Liu, Z.; Liu, J.; Yang, J.; Li, Q.; Li, Y.; Luo, J.; Yin, H. Molecular Survey of Anaplasma and Ehrlichia of Red Deer and Sika Deer in Gansu, China in 2013. Transbound. Emerg. Dis. 2016, 63, e228–e236. [Google Scholar] [CrossRef] [PubMed]
- Haigh, J.C.; Gerwing, V.; Erdenebaatar, J.; Hill, J.E. A novel clinical syndrome and detection of Anaplasma ovis in Mongolian reindeer (Rangifer tarandus). J. Wildl. Dis. 2008, 44, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Noaman, V. Molecular Detection of Novel Genetic Variants Associated to Anaplasma ovis among Dromedary Camels in Iran. Arch. Razi Inst. 2018, 73, 11–18. [Google Scholar] [CrossRef]
- Selmi, R.; Ben Said, M.; Dhibi, M.; Ben Yahia, H.; Abdelaali, H.; Messadi, L. Genetic diversity of groEL and msp4 sequences of Anaplasma ovis infecting camels from Tunisia. Parasitol. Int. 2020, 74, 101980. [Google Scholar] [CrossRef]
- Ochirkhuu, N.; Konnai, S.; Odbileg, R.; Murata, S.; Ohashi, K. Molecular Epidemiological Survey and Genetic Characterization of Anaplasma Species in Mongolian Livestock. Vector Borne Zoonotic Dis. 2017, 17, 539–549. [Google Scholar] [CrossRef]
- Noaman, V.; Sazmand, A. Anaplasma ovis infection in sheep from Iran: Molecular prevalence, associated risk factors, and spatial clustering. Trop. Anim. Health Prod. 2021, 54, 6. [Google Scholar] [CrossRef]
- M’Ghirbi, Y.; Oporto, B.; Hurtado, A.; Bouattour, A. First Molecular Evidence for the Presence of Anaplasma phagocytophilum in Naturally Infected Small Ruminants in Tunisia, and Confirmation of Anaplasma ovis Endemicity. Pathogens 2022, 11, 315. [Google Scholar] [CrossRef]
- Enkhtaivan, B.; Narantsatsral, S.; Davaasuren, B.; Otgonsuren, D.; Amgalanbaatar, T.; Uuganbayar, E.; Zoljargal, M.; Myagmarsuren, P.; Suganuma, K.; Molefe, N.I.; et al. Molecular detection of Anaplasma ovis in small ruminants and ixodid ticks from Mongolia. Parasitol. Int. 2019, 69, 47–53. [Google Scholar] [CrossRef]
- Kuketova, A.A. Development of cattle breeding of the Kazakh steppe in the XVIII-XIX centuries in the works of Russian travelers: Historiographic analysis (on the example of Semirechye). Bull. Karaganda Univ. Hist. Philos. Ser. 2020, 100, 50–56. [Google Scholar]
- Mutaliyeva, A.; Yesbolova, A.; Dyrka, S.; Saparbayev, M.; Kazanbayeva, Z.; Balabekova, D.; Orazova, B. Formation and History of the Agrarian Economy of Kazakhstan: The State of Development Today. Acad. J. Interdiscip. Stud. 2023, 12, 401. [Google Scholar] [CrossRef]

| Target Gene | Primer Name | Primer Sequences (5′–3′) | PCR Size (bp) |
|---|---|---|---|
| groEL | groEL_Anapl_all_F | aaggatggatayaaggtmatgaa | about 445 |
| groEL_Anapl_all_R | cgcggwcaaactgcatac | ||
| 16S rRNA, round I | 16S_Anap_F150 | atctacctagtagtatgggatagccact | about 1293 |
| 16S_Anap_R1460 | ctgcctccttacggttggcg | ||
| 16S rRNA, round II | 16S_Anap_F397 | agctatgccgcgtgagtgag | about 914 |
| 16S_Anap_R1315 | atgccctcgagttgcagagga |
| Region | Number of Herds Studied | Total Samples Examined | Total Positive for PCR groEL | A. centrale | A. marginale | A. ovis | Candidatus Anaplasma mongolica | Unknown Anaplasma spp. | Anaplasma spp. Co-Infection |
|---|---|---|---|---|---|---|---|---|---|
| Turkistan | 23 | 1054 | 46 (4.36%) | 36 (3.42%) | 9 (0.85%) | 1 (0.09%) | 0 | 0 | 0 |
| North Kazakhstan | 15 | 362 | 9 (2.48%) | 5 (1.38%) | 2 (0.55%) | 0 | 2 (0.55%) | 0 | 0 |
| Kyzylorda | 21 | 980 | 12 (1.22%) | 0 | 10 (1.02%) | 0 | 1 (0.10%) | 1 (0.10%) | 0 |
| Karaganda | 23 | 500 | 7(1.40%) | 0 | 2 (0.40%) | 0 | 5 (1.00%) | 0 | 0 |
| Kostanay | 9 | 428 | 6 (1.40%) | 3 (0.70%) | 2 (0.47%) | 0 | 0 | 0 | 1 (0.23) |
| Abai | 8 | 400 | 4 (1.00%) | 0 | 2 (0.50%) | 0 | 0 | 0 | 2 (0.50%) |
| Mangystau | 15 | 200 | 2 (1.00%) | 0 | 0 | 1 (0.50%) | 1 (0.50%) | ||
| Almaty | 11 | 248 | 3 (1.21%) | 0 | 3 (1.21%) | 0 | 0 | 0 | 0 |
| Jetisu | 4 | 200 | 1 (0.50%) | 0 | 1 (0.50%) | 0 | 0 | 0 | 0 |
| Jambyl | 16 | 955 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ulytau | 6 | 400 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pavlodar | 7 | 300 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| West Kazakhstan | 4 | 200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aktobe | 4 | 200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Atyrau | 5 | 200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| East Kazakhstan | 3 | 200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Akmola | 1 | 200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 175 | 7027 | 90 (1.3%) | 44 (0.63%) | 31 (0.44%) | 1 (0.01%) | 9 (0.13%) | 1 (0.01%) | 4 (0.06%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadyrova, M.; Ostrovskii, A.; Mukanov, K.; Kassen, A.; Shevtsova, E.; Berdikulov, M.; Vergnaud, G.; Shevtsov, A. Molecular Characterization of Anaplasma spp. in Cattle from Kazakhstan. Pathogens 2024, 13, 894. https://doi.org/10.3390/pathogens13100894
Kadyrova M, Ostrovskii A, Mukanov K, Kassen A, Shevtsova E, Berdikulov M, Vergnaud G, Shevtsov A. Molecular Characterization of Anaplasma spp. in Cattle from Kazakhstan. Pathogens. 2024; 13(10):894. https://doi.org/10.3390/pathogens13100894
Chicago/Turabian StyleKadyrova, Madina, Alexandr Ostrovskii, Kassym Mukanov, Amirkhan Kassen, Elena Shevtsova, Maxat Berdikulov, Gilles Vergnaud, and Alexandr Shevtsov. 2024. "Molecular Characterization of Anaplasma spp. in Cattle from Kazakhstan" Pathogens 13, no. 10: 894. https://doi.org/10.3390/pathogens13100894
APA StyleKadyrova, M., Ostrovskii, A., Mukanov, K., Kassen, A., Shevtsova, E., Berdikulov, M., Vergnaud, G., & Shevtsov, A. (2024). Molecular Characterization of Anaplasma spp. in Cattle from Kazakhstan. Pathogens, 13(10), 894. https://doi.org/10.3390/pathogens13100894








