Abstract
Bovine anaplasmosis is an infectious vector-borne disease caused by bacteria of the genus Anaplasma, which have a wide global distribution and represent a high economic burden for agriculture. The use of molecular genetic techniques has increased our knowledge of the species diversity of Anaplasma spp. and naturally susceptible animals. Monitoring studies allow us to assess the level of infection in herds, as well as the involvement of natural vectors in the processes of maintaining and spreading infection. Despite the high prevalence of Theileria and Babesia in cattle in Kazakhstan, there is no information on the distribution and species diversity of Anaplasma spp in this country. As part of this work, 7027 DNA samples isolated from the whole blood of cattle from 175 settlements in all 17 Kazakhstan regions were PCR-tested for the presence of Anaplasma spp. Anaplasma carriers were found in 1.3% (90 out of 7027) of the tested animals in 9 of the 17 regions of Kazakhstan. The highest percentage of infected animals was recorded in Turkistan (South Kazakhstan) and North Kazakhstan with 4.46% and 2.48% positive samples, respectively. The partial sequencing of 16S rRNA and the groEL gene allowed us to identify five species of Anaplasma: A. centrale, A. marginale, Candidatus Anaplasma Mongolica, A. ovis, and Unknown Anaplasma with infection rates of 0.63%, 0.44%, 0.13%, 0.01%, and 0.01%, respectively.
1. Introduction
The genus Anaplasma (family Anaplasmataceae, order Rickettsiales) includes obligate intracellular alphaproteobacteria that reproduce in membrane-bound vacuoles and are transmitted to vertebrate hosts by ixodic mites. Since the last reclassification of Anaplasmataceae 20 years ago, two new species of Anaplasma have been identified. To date, the Anaplasma genus includes eight species, A. phagocytophilum, A. marginale, A. centrale, A. ovis, A. bovis, A. platys, A. odocoilei, and A. capra, and a large number of unclassified genovariants that cannot be assigned to known species [1]. Anaplasma species have a global distribution and cause anaplasmosis, a disease with a high negative impact in veterinary and public health [2].
The species Anaplasma phagocytophilum is the most significant in terms of public health. It is most common in the northern hemisphere and causes granulocytic anaplasmosis in humans, horses, and dogs. It is the cause of “tick-borne fever” (TBF) in domestic ruminants [3]. Anaplasma marginale is the dominant cause of anaplasmosis in cattle and other ruminants, and is found in tropical and subtropical regions of the world, including South and Central America, as well as in the United States (USA), southern Europe, Africa, Asia, and Australia [4]. It is more dangerous in animals over two years of age, especially those imported from non-endemic regions. The disease is clinically manifested by anorexia, jaundice, abortion, weight loss, reduced meat and milk production, and possibly death [5]. The species A. centrale infects various species of domestic and wild ruminants [6,7], but does not cause severe infection. Other Anaplasma species including A. platys, A. bovis, and Anaplasma sp. ‘Omatjenne’ have also been reported to infect cattle [8,9,10]. Anaplasma platys is a pathogen that primarily affects dogs. In cattle, the disease often proceeds subclinically, presenting with thrombocytopenia [11]. Anaplasma bovis, first discovered in Brazil, is also widespread in Africa, Asia, the Americas, and southern Europe. It affects cattle, buffaloes, sheep, goats, dogs, cats, and small mammals, with reported cases of infection in monkeys and humans as well [12]. In cattle, the infection may cause fever, decreased productivity, seizures, anemia, weight loss, and enlarged lymph nodes [13]. Anaplasma sp. ‘Omatjenne’ was first detected in healthy Boer goats in southern Africa, and was subsequently identified in cattle and buffalo in Africa and the Mediterranean Basin, but the clinical impact of this pathogen is still unclear [14].
Economic losses to livestock associated with Anaplasma spp. infection, the increasing incidence of human infections, and the discovery of new types of pathogens underscore the necessity of understanding the epidemiological situation. Diagnostic studies are essential for comprehending the epidemiology of anaplasmosis. However, serological methods present several limitations in the diagnosis of anaplasmosis, including the absence of antibodies in the early stages of the disease and low specificity due to cross-serological reactions among Anaplasma species [15]. Light microscopy is a simple and low-cost laboratory test with, however, limited applicability for animals that are chronic carriers of pathogens with low bacterial counts, which can lead to false negative results. Additionally, these tests are not very effective for leukocytic species such as A. platys, which is associated with thrombocytopenia, and for granulocytic species like A. phagocytophilum [16]. Molecular studies provide more accurate and reliable results and are based on the detection of gene markers such as 16S rRNA, groEL, gltA, and major surface protein (msp) genes. For PCR screening, multi-copy msp genes are preferred, while for the identification of Anaplasma species based on nucleotide sequences, rrs, and groEL genes are currently considered the best choices [17].
Despite the prevalence of tick-borne infectious diseases in cattle in Kazakhstan [18], our knowledge on the spread of anaplasmosis is currently limited to information obtained from the light microscopy observation of blood smears in southeastern Kazakhstan [19]. The aim of the present study was to assess the distribution and species diversity of Anaplasma spp. throughout Kazakhstan in order to provide a baseline of Anaplasma spp. presence in Kazakhstan for future epidemiological surveillance.
2. Materials and Methods
2.1. Ethical Approval
This study was approved by the Ethics Committee of the National Center for Biotechnology (Protocol No. 2 dated 4 April 2022). Cattle owners confirmed their consent to blood sampling.
2.2. Collecting Samples
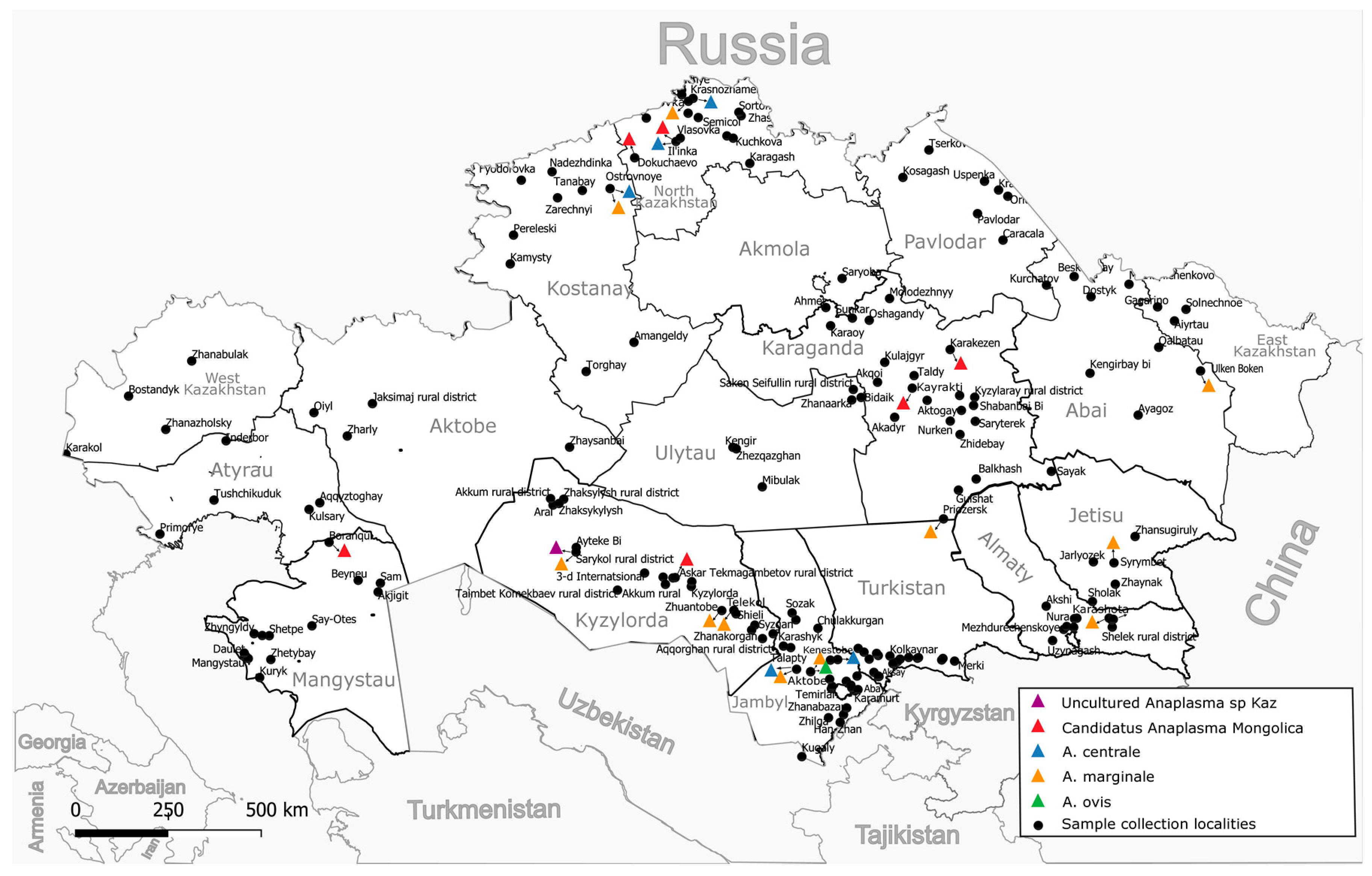
A total of 7027 whole blood samples were collected from cattle (cows) over three years of age from all 17 regions of Kazakhstan, 95 districts, and 175 settlements (Figure 1). The studied animals were kept in household farms and grazed in common herds of the respective settlement. The grazing of bulls is prohibited in common herds; therefore, the sample is limited to cows only. As a rule, the grazing of animals starts from 9 months of age and later; therefore, the inclusion of animals more than three years old guaranteed that the animals were on pasture for at least two grazing seasons from March to October (which may vary depending on the region). More detailed information about the blood collection season for the animals is provided in Supplementary Table S1. Blood was collected from the animals in July–August 2022 and 2023 using vacuum blood collection systems with di-potassium EDTA. Samples were transported to the laboratory within 48 h at 4 °C to 8 °C and were immediately used for DNA extraction.
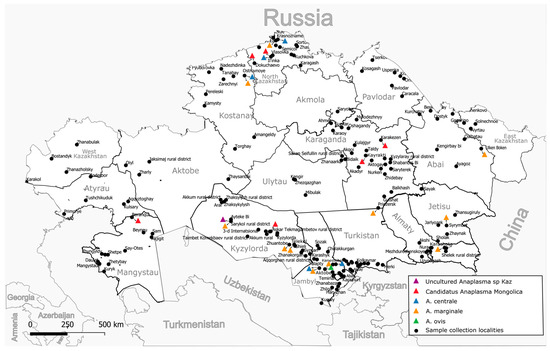
Figure 1.
Sampling locations and distribution by identified species.
2.3. DNA Isolation
Three hundred µL of blood were mixed with 900 µL of RBC buffer (Red Blood Cell Lysis Buffer, 150 mM NH4Cl (PanReac AppliChem, Darmstadt, Germany), 10 mM NaHCO3 (Thermo Fisher Scientific, Fair Lawn, NJ, USA), 1 mM EDTA (BioRad, Richmond, VA, USA), H2O). The mixtures were incubated for 5 min and centrifuged at 12,200× g for 5 min. The pellet was resuspended in 100 µL of buffer solution (400 mM NaCl (Titan Biotech Ltd., Rajasthan, India), 10 mM Tris-HCl pH 8.0 (BioRad, Richmond, VA, USA), 2 mM EDTA). Forty µL of 20% SDS (Sigma-Aldrich, Darmstadt, Germany) and 10 µL of 20 mg/mL Proteinase K (Magen, Guangzhou, China) were added and incubated at 50 °C for 2 h. A total of 500 µL of lysing solution was added (50 mm EDTA, 3.2 mM GuSCN (PanReac AppliChem, Darmstadt, Germany), 20 mM Tris-HCl (pH 7.4), 30% isopropanol (Sigma-Aldrich, St. Louis, MO, USA), 4% Triton X100 (Amresco, Solon, OH, USA), and the solution was stirred and incubated for 10 min at 60 °C. Seventy µL of sorbent (Silicon dioxide with a particle size of 0.5–10 µm (Sigma-Aldrich, St. Louis, MO, USA, S5631), prepared as described by R. Boom et al. [20], was added. The samples were incubated for 5 min at 60 °C, vortexing twice. They were kept at room temperature for 5 min and centrifuged at 600× g for 1 min. The precipitate was washed with 300 µL of buffer containing guanidine (3.2 M GuaSCN, 0.1 M Tris-HCl) and twice with 500 µL of 75% ethanol (75% ethanol (DOSFARM, Almaty, Kazakhstan), 10 mM Tris-HCl), each time carefully breaking up the sorbent. After each wash, the sorbent was pelleted by centrifugation at 1600× g for 1 min. Finally, the sorbent was dried at 60 °C for 3 min and DNA was eluted in 100 µL TE buffer (pH 8.0; 10 mM Tris, 1 mM EDTA, PanReac AppliChem, Darmstadt, Germany). DNA concentrations were measured using Nanodrop-1000 (Thermo Scientific, Wilmington, DE, USA).
2.4. Amplification of the groEL Gene Fragment
We designed new primers to amplify the groEL gene (Table 1). PCR amplification was performed in 25 µL reaction volume containing 12.5 µL BioMaster UDG HS-qPCR (2×) (Biolabmix, Novosibirsk, Russia), 1 µL (10 pmol/µL) of each primer, 5 µL DNA, and water up to 25 µL. Thermal cycling conditions using Mastercycler ProS (Eppendorf, Hamburg, Germany) were 2 min at 50 °C and 5 min at 95 °C initial denaturation, 35 cycles of 30 s at 95 °C, 40 s at 60 °C, and 50 s at 72 °C, followed by 5 min at 72 °C final elongation.

Table 1.
Primers used for amplification of groEL and 16S rRNA.
2.5. Nested Amplification 16S rRNA
Amplification of 16S rRNA was performed by nested PCR using two pairs of primers (Table 1). Nested PCR was used to increase stage specificity, with primers specific to the genus Anaplasma spp. utilized at each stage. The PCR reaction was performed in a 25 µL reaction volume containing 12.5 µL BioMaster UDG HS-qPCR (2×), 1 µL (10 pmol/µL) of each primer for the appropriate round of amplification, and 5 µL DNA for round I PCR or 3 µL round I PCR product for round II PCR. Thermal cycling conditions using Mastercycler ProS (Eppendorf, Hamburg, Germany) were 2 min at 50 °C and 5 min at 95 °C initial denaturation, 30 cycles of 30 s at 95 °C, 40 s at 63 °C, 2 min for round I and 1.5 min for round II at 72 °C, followed by 5 min at 72 °C as final elongation.
2.6. Sequencing and Analysis
PCR products were run on a 1.5% agarose gel with ethidium bromide as the intercalating dye. PCR products were purified with magnetic particles, as described earlier [21]. Sanger sequencing was performed using the Big Dye Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific, Vilnius, Lithuania) according to the manufacturer’s instructions. Sequencing reaction products were resolved on a GeneticAnalyzer 3730 xl (Applied Biosystems, Carlsbad, CA, USA). Sequences from the two strands were assembled using SeqMan (Lasergene, DNASTAR) [22]. Sequences were aligned using ClustalW. The phylogenetic analysis was conducted using the maximum likelihood method [23] and Tamura 3-parameter model with the discrete Gamma distribution with invariant sites with 5 rate categories. Bootstrap support was computed by comparing 100 replications. Trees were visualized using Mega 11 software v.11.0.13 [24]. MegAlignTM (Lasergene, DNASTAR) was used to determine the percentage of identity between sequences.
3. Results
3.1. Detection and Species Identification of Anaplasma spp. by the groEL Gene
The groEL gene fragment was amplified in 90 out of 7027 samples, which is 1.3% of the samples examined (Table 2). Animals with positive results were identified in 9 out of 17 regions of Kazakhstan, with the highest percentage of infected animals registered in the regions of Turkistan (South Kazakhstan) and North Kazakhstan. In total, 46 (4.36%) and 9 (2.48%) of the animals tested positive in these regions, respectively (Table 2). In the remaining seven regions, the percentage of infected animals ranged from 0.5 to 1.4%.

Table 2.
Results of PCR tests for Anaplasma spp. in cattle in the regions of Kazakhstan and their species identification.
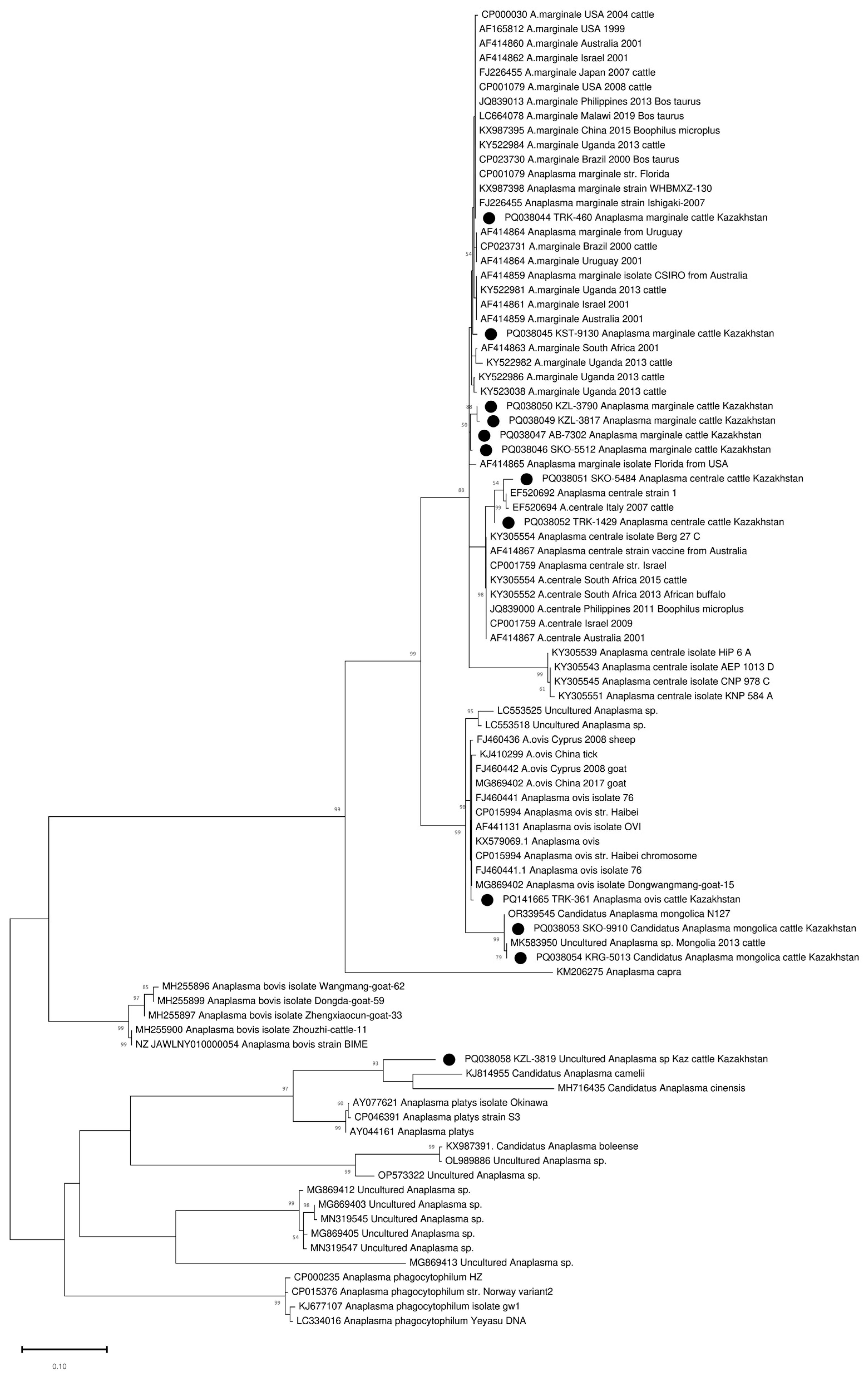
The amplified groEL gene fragment of 401–404 bp (excluding primers) was sequenced in all 90 positive samples. A heterozygous signal was found in 5–10 positions in four samples. The remaining 86 samples were clustered into five clades (Figure S1). Thirty-one samples were clustered with A. marginale sequences (Figure S1, Table 2). These A. marginale positive samples originate from eight regions of Kazakhstan (Figure 1, Table 2). The average A. marginale infection rate of livestock in Kazakhstan was 0.44%. The maximum percentage of A. marginale-infected animals was registered in the Almaty region (3 out of 248 or 1.21%). In the other positive regions, the percentage of A. marginale-infected animals ranged from 0.4 to 0.85%. Kazakhstan’s A. marginale sequences define six genotypes (Figure 2). The partial groEL sequence from nine samples is identical to PQ038044, which is present on five continents (Figure 2 and Figure S1). The other five are unique.
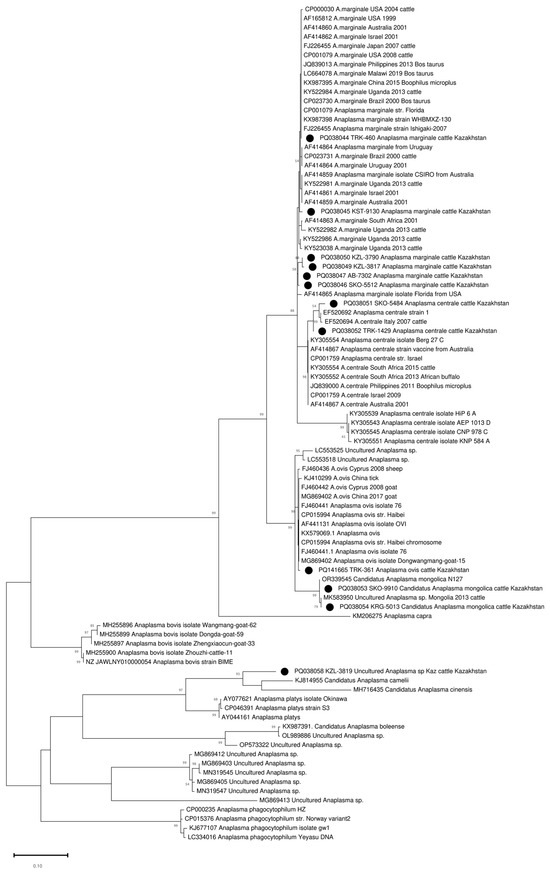
Figure 2.
Phylogenetic tree based on the analysis of the partial sequence of the groEL gene. Only one sample of each genotype is included in the analysis; a complete analysis of the 86 sequences is shown in Figure S1. The sequences obtained in this study are labelled with ●.
Forty-four sequences clustered with A. centrale and formed two separate genotypes. One genotype combined eight sequences from two regions of northern Kazakhstan (North Kazakhstan and Kostanay regions). The second genotype included 36 A. centrale sequences identified in the whole blood samples of the animals from the Turkistan region (southern region of Kazakhstan) (Figure 2 and Figure S1). The average infection rate of A. centrale was 0.63%, and the maximum prevalence of A. centrale was recorded in the Turkistan region (3.42%). In the North Kazakhstan and Kostanay regions, A. centrale was detected in 1.38% and 0.7% of animals, respectively.
Nine sequences defined two closely related alleles differing at one nucleotide position and identical to two published sequences (OR339545 and MK583950), classified as Candidatus Anaplasma Mongolica (Figure S1). Samples from this cluster were collected in four non-contiguous regions, and the distance between collection sites varied from 104.43 to 1574.92 km. The average infection rate was 0.13%, and the infection rate in some regions was as high as 1%.
One sequence from the Turkistan region (South Kazakhstan) clustered with A. ovis sequences (Figure 2).
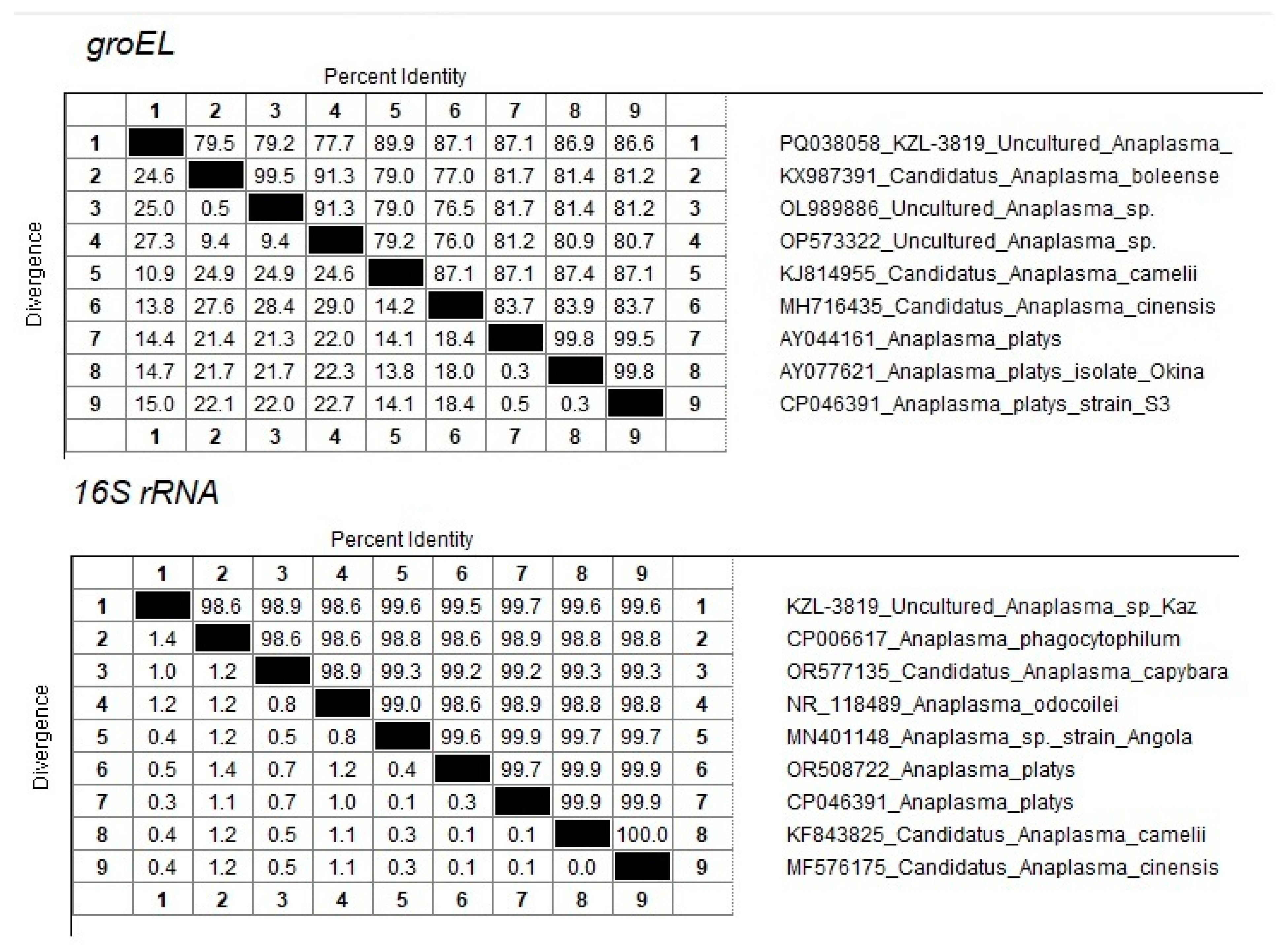
One sequence (PQ038058 KZL-3819 Uncultured Anaplasma sp. Kaz) from the Kyzylorda region (South Kazakhstan) represents a separate branch in the clade together with close neighbors KJ814955 Candidatus Anaplasma camelii, MH716435 Candidatus Anaplasma cinensis, AY044161 Anaplasma platys, AY077621 Anaplasma platys isolate Okinawa, and CP046391 Anaplasma platys strain S3 (Figure 2). At the same time, the maximum percentage of identity of 89.9% was established with Candidatus Anaplasma camelii (Figure 3).
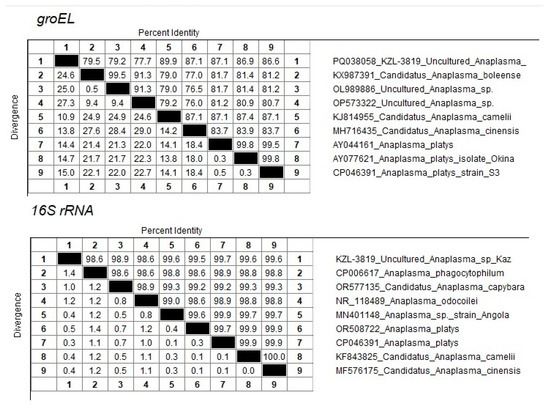
Figure 3.
Percentage of sequence identity/divergence of KZL-3819 Uncultured Anaplasma sp Kaz accession PQ133430 with close neighbors.
3.2. Identification of Anaplasma spp. by 16S rRNA
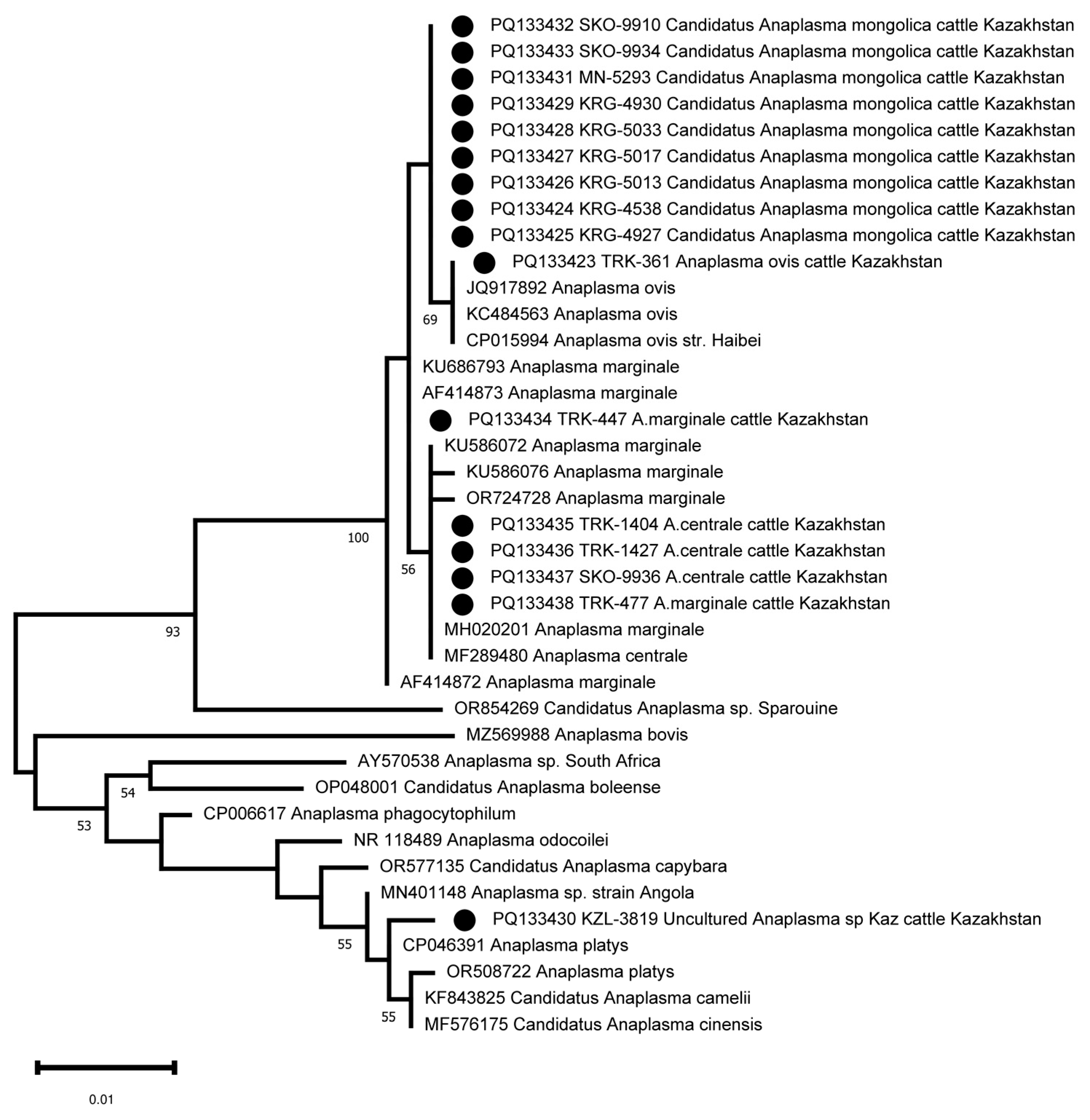
The amplification and sequencing of the 16S rRNA fragment was used to clarify the taxonomic position of Candidatus Anaplasma mongolica and KZL-3819 Uncultured Anaplasma sp. Kaz. Sixteen samples were selected for 16S sequencing, including the nine samples identified as Candidatus Anaplasma mongolica, the A. ovis sample, and the KZL-3819 Uncultured Anaplasma sp Kaz sample. Three A. centrale and two A. marginale samples were included as controls. The primers developed were able to amplify the 16S rRNA fragment in all 16 samples. The 16S sequences of the nine Candidatus Anaplasma mongolica samples are identical and differ at one position from the 16S sequence of the A. ovis sample and other public A. ovis 16S reference sequences (Figure 4). The five 16S sequences from the samples tentatively identified as A. centrale and A. marginale clustered as expected with public 16S sequences from A. centrale and A. marginale strains. The PQ133430 16S sequence from the KZL-3819 sample is located as a separate branch in a cluster with MN401148 Anaplasma sp. strain Angola, OR508722 Anaplasma platys, CP046391 Anaplasma platys, KF843825 Candidatus Anaplasma camelii, and MF576175 Candidatus Anaplasma cinensis (Figure 4). The closest neighbor of PQ133430 is CP046391 Anaplasma platys, which differs at two positions among 734 bps (99.7% sequence identity, Figure 3).
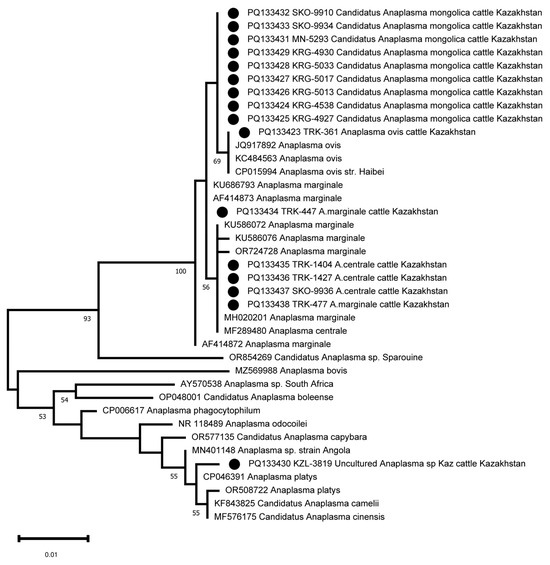
Figure 4.
Phylogenetic tree based on the analysis of the 16S rRNA fragment of 16 samples compared to published Anaplasma species 16S sequence data. The sequences obtained in this study are labelled with ●.
4. Discussion
Anaplasmosis is a vector-borne or mechanically transmitted disease caused by members of the genus Anaplasma of the order Rickettsiales [25]. Anaplasmosis causes significant damage to the livestock industry, with an estimated economic burden of USD 660 [26] per clinical case. Currently, there are no vaccines available to prevent the infection of livestock with anaplasmosis, but their use facilitates the clinical course of the disease [5]. In the absence of specific prevention, the focus is on the diagnosis, the culling of infected animals, and the exclusion of the import of infected animals into safe areas. Therefore, data on the distribution and species diversity of circulating Anaplasma spp. are needed. We conducted the first study in Kazakhstan to assess the spread of anaplasmosis in cattle using PCR targeting the groEL gene followed by the species identification of the pathogen through sequencing. As a result of this study, 1.3% (90 out of 7027 examined) of the animals were found to carry Anaplasma. The percentage of infected animals differed between regions, reaching a maximum of 4.46% in the southern region of Kazakhstan. Five types of Anaplasma were identified: A. centrale, A. marginale, A. ovis, Candidatus anaplasma mongolica, and Unknown Anaplasma.
Kazakhstan is located in the center of Eurasia, the republic’s area is 2724.9 thousand km2, and more than 80% of the country’s territory is occupied by dry steppes. The diversity of landscape and climatic conditions contributes to the existence of a wide variety of tick species, including more than 30 species of ticks belonging to the Ixodidae family, which are recognized as vectors of a number of tick-borne diseases [27]. Nevertheless, only four regions of southern Kazakhstan are recognized as disadvantaged by tick-borne diseases (TBDs) among cattle [18]. Much attention among TBDs in cattle is paid to the causative agent of teileriosis and babesiosis; there are few data on the spread of anaplasmosis in livestock in Kazakhstan. Our study detected the presence of Anaplasma spp. in cattle in 9 of the 17 regions of Kazakhstan. Importantly, we did not detect Anaplasma spp. in eight regions, suggesting that they might currently be free of Anaplasma spp. The southern regions of Kazakhstan are recognized as disadvantaged for blood-parasitic diseases; therefore, the detection of Anaplasma-infected animals in the Turkistan, Kyzylorda, Almaty, and Jetisu regions is to be expected. The only exception among southern regions is the Jambyl region, where the absence of Anaplasma-infected animals might be due to the geography. Samples for Anaplasma testing from this region were collected from localities situated in the mountains. It is known that the density of the main tick vectors is significantly lower in mountainous areas compared to steppe pastures. The detection of infected animals in the northern and northwestern regions of the country may indicate two independent pathways for the introduction of anaplasmosis into Kazakhstan. Most of the regions free of Anaplasma spp. are located in the interior of the country.
Of the 90 samples, 4 samples showed a clearly mixed profile, suggesting coinfection with more than one type of Anaplasma. Coinfection with various species of Anaplasma in domestic animals has previously been described in several studies. In Korea, coinfection with A. bovis and A. phagocytophilum reaches 16% [28], and in Algeria the coinfection of A. marginale and A. centrale was reported in 10% of cases [29]. The coinfection of various types of anaplasmas is of concern due to the possibility of recombination and the emergence of new variants that are dangerous to public health and livestock [30].
We showed here that A. centrale is currently the most common type of Anaplasma in cattle in Kazakhstan. It was detected in 44 of 7027 samples (0.63%). Infected animals were detected in southern (Turkistan region) and northern Kazakhstan (North Kazakhstan and Kostanay regions). The maximum percentage of infection (3.4%) was detected in the Turkistan region. The species A. centrale is found worldwide and can infect various species of domestic and wild ruminants [6,7]. The infection rate varies: in Tunisia and Algeria, A. centrale is detected in 15.1% and 39.4% of cattle. A rate of 14.4% and 18% was reported in Pakistan and Turkey, respectively [31,32]. In Chongqing province in southwestern China, A. centrale was detected in 7.83% of examined cattle, which was second only to A. bovis, reported in 8.41% of animals [33]. In Kyrgyzstan, the infection rate of A. centrale in cattle is 1.1% [34]. Anaplasma centrale causes mild infections in cattle, with the formation of immunity against A. marginale, which does not protect animals from infection, but excludes the severe course of infection [35]. Therefore, Anaplasma centrale is considered a naturally attenuated variant that has been used as a live vaccine for more than 100 years. It is currently widely used in South Africa, Israel, South America, and Australia [4]. In this regard, the high infection of vaccinated cattle with A. centrale is observed in these regions [36,37].
Despite the century-long history since the description of A. centrale by Arnold Theiler, the debate on the taxonomic position of the species continues [38]. An analysis of the 16S rRNA, groEL, and msp4 gene sequences, and the Msp1a/Msp1aS structure of A. marginale and A. centrale isolates from South Africa, groups A. centrale into a separate clade from A. marginale, which, with a combination of morphological differences (A. centrale forms smaller and more central morulae in erythrocytes), allows A. centrale to be considered a separate species [39]. The inclusion of additional sequences of the groEL and 16S rRNA genes in the phylogenetic analysis showed that the sequences of A. marginale and A. centrale are not clustered separately on the basis of both genes, requiring careful consideration of the taxonomic position of A. centrale [1]. In our study, A. centrale and A. marginale strains clustered separately, which is associated with the use of a fragment of the groEL gene c 171 of 574 nucleotides. Previously, Ben Said et al., by analyzing the complete sequence of the groEL gene among Anaplasma spp., found that two regions have a discriminating potential between A. centrale and A. marginale (region 1 between positions 1 and 546 and region 2 between positions 1059 and 1650) [40]. An interesting fact is the separate clustering of the groEL gene sequence of samples from southern and northern Kazakhstan, indicating independent introduction of the pathogen into these regions. The analysis of 16S rRNA did not allow us to differentiate A. centrale and A. marginale, since only one nucleotide distinguishes these species at position 156 A/G [41], which has not been sequenced using the primers we have proposed.
Anaplasma marginale turned out to be the second most widespread species, but was identified in more areas. There is also high genetic diversity: seven genotypes were identified, and while only one genotype combining eight samples had genetic analogues in the NCBI database, the rest formed a separate cluster and were unique to Kazakhstan. Our study showed a low prevalence of A. marginale among cattle in Kazakhstan; the total infection was 0.44%, while the highest number of positive animals was detected in the Almaty region with 1.2%. We found only one study on A. marginale in Kazakhstan. In that study, 256 samples from cattle were examined by light microscopy and 48.9% were found to be infected with A. marginale [19]. In that study, the authors examined more than ten thousand animals, but there is no information about the criteria for selecting material and forming a sample for microscopic examination, which makes it difficult to assess the true percentage of infection. The infection rate of cattle in neighboring countries differs. In Kyrgyzstan, for example, a PCR study found that the infection rate with A. marginale was 11.6%, while in four out of five regions the pathogen was not detected [42]. In southern Xinjiang (China), bordering Kazakhstan, the infection rate of Anaplasma spp. varies from 3.3% to 12.8%, while A. marginale has not been identified [43,44]. In Russia, there is a significant difference in the distribution of A. marginale in the regions, ranging from 8.3 to 71.1% [45,46].
Nine animal carriers of Candidatus Anaplasma mongolica were identified in four regions. The regions are located in the southern, central, and northern parts of Kazakhstan at a distance of more than 1000 km. Previously, this species was identified in ticks in Inner Mongolia (China) [47] and in ticks and blood samples of cattle in Mongolia, where the infection rate of cattle with this species was 31.8% [48]. Our data indicate a wider distribution area of this type of Anaplasma in Asia. The lack of information on the pathogenicity and severity of the disease caused by this pathogen requires additional research and increased observations.
The species A. ovis was identified in one animal in the Turkistan region (southern Kazakhstan). A. ovis causes anaplasmosis in sheep and goats and is much more host-specific than A. phagocytophilum [49]. However, A. ovis cannot be considered a strictly species-specific pathogen, as the number of A. ovis detections in other species has increased recently, most likely due to the development of molecular genetic methods for the species identification of Anaplasma [50]. Infection with A. ovis has been confirmed in wild ungulates: European roe deer (Capreolus capreolus) [51], big horn sheep (Ovis canadensis) and mule deer (Odocoileus hemionus) [52], and red deer (Cervus elaphus) and spotted deer (Cervus nippon) [53]. The infection of reindeer (Rangifer tarandus) in Mongolia reaches 80% [54]. Among domestic animals, in addition to the main hosts, sheep and goats, A. ovis has been found in camels in Iran and Tunisia [55,56] and cattle in China and Mongolia [43,57]. This is the first study confirming the presence of A. ovis among cattle in Kazakhstan. The detection of A. ovis in atypical species of domestic animals is observed in regions with a high incidence of sheep anaplasmosis. For example, in Iran, more than 50% of herds and 28% of sheep are infected with A. ovis [58]; in Tunisia, the infection rate of small ruminants reaches 80% [59]; and in Mongolia, the average is 70% of infected animals [60]. According to our unpublished data, the detection rate of A. ovis in sheep in the Turkistan region is indeed high and amounts to 42%, in agreement with these previous reports.
An unidentified species of Anaplasma spp. was identified in an animal from the Kyzylorda region. It is genetically closest to Candidatus Anaplasma camelii, with 90% groEL gene identity and 99.7% 16S rRNA identity with A. platys.
In general, our study indicates a low infection rate of cattle with anaplasmosis in Kazakhstan. Perhaps this is due to the fact that, for centuries, the territory of Kazakhstan was dominated by nomadic livestock breeding, where the main animal species were sheep, horses, and camels [61]. The transition to a sedentary lifestyle in the early 20th century increased the number of cattle [62].
5. Conclusions
This is the first study using molecular genetic methods to investigate the species diversity and prevalence of Anaplasma spp. in privately owned cattle in Kazakhstan. The results of this study showed a low level of infection of cattle with anaplasmosis, and a high species diversity of circulating Anaplasma spp. This view of the current situation of Anaplasma spp. will help to monitor the epidemiological situation of the infection, and hopefully help detect emerging trends sufficiently early to allow for the implementation of countermeasures such as vaccination and the anti-tick treatment of animals and pastures.
The limitation of our study is the lack of information on the clinical manifestation of anaplasmosis in Kazakhstan. Further studies on the health impact and species diversity of Anaplasma in cattle and ticks in Central Asia will help clarify the pathogenicity and range of Candidatus Anaplasma mongolica and uncharacterized Anaplasma species.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens13100894/s1, Figure S1: A phylogenetic tree based on the analysis of a fragment of the nucleotide sequence of the groEL gene in 86 samples obtained in this study; Table S1: Characteristics of PCR-positive Anaplasma spp. samples.
Author Contributions
Conceptualization, K.M. and A.S.; methodology, K.M. and A.S.; validation, A.O., M.K. and A.K.; formal analysis, K.M. and A.S.; investigation, K.M.; resources, K.M.; data curation, A.S., K.M., A.O. and G.V.; writing—original draft preparation, M.K. A.O., K.M., A.K., E.S., M.B., G.V. and A.S.; writing—review and editing, A.S., K.M. and G.V.; visualization, A.S., A.O. and G.V.; project administration, K.M.; funding acquisition, K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and High Education of the Republic of Kazakhstan, grant number AP14869821 “Development of a PCR test system for determining the species diversity of Anaplasma spp. among cattle and small ruminants in Kazakhstan”.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of the National Center for Biotechnology (protocol code 1, dated 4 April 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data from this project are publicly available from NCBI, GenBank Ac#: PQ038044-PQ038058, PQ133423-PQ133438, PQ141665.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rar, V.; Tkachev, S.; Tikunova, N. Genetic diversity of Anaplasma bacteria: Twenty years later. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2021, 91, 104833. [Google Scholar] [CrossRef] [PubMed]
- Atif, F.A. Alpha proteobacteria of genus Anaplasma (Rickettsiales: Anaplasmataceae): Epidemiology and characteristics of Anaplasma species related to veterinary and public health importance. Parasitology 2016, 143, 659–685. [Google Scholar] [CrossRef]
- Matei, I.A.; Estrada-Peña, A.; Cutler, S.J.; Vayssier-Taussat, M.; Varela-Castro, L.; Potkonjak, A.; Zeller, H.; Mihalca, A.D. A review on the eco-epidemiology and clinical management of human granulocytic anaplasmosis and its agent in Europe. Parasites Vectors 2019, 12, 599. [Google Scholar] [CrossRef]
- Aubry, P.; Geale, D.W. A review of bovine anaplasmosis. Transbound. Emerg. Dis. 2011, 58, 1–30. [Google Scholar] [CrossRef]
- Salinas-Estrella, E.; Amaro-Estrada, I.; Cobaxin-Cárdenas, M.E.; Preciado de la Torre, J.F.; Rodríguez, S.D. Bovine Anaplasmosis: Will there ever be an almighty effective vaccine? Front. Vet. Sci. 2022, 9, 946545. [Google Scholar] [CrossRef] [PubMed]
- Khumalo, Z.T.; Catanese, H.N.; Liesching, N.; Hove, P.; Collins, N.E.; Chaisi, M.E.; Gebremedhin, A.H.; Oosthuizen, M.C.; Brayton, K.A. Characterization of Anaplasma marginale subsp. centrale Strains by Use of msp1aS Genotyping Reveals a Wildlife Reservoir. J. Clin. Microbiol. 2016, 54, 2503–2512. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yoshikawa, Y.; Kawamori, F.; Ikegaya, A.; Ohtake, M.; Ohashi, M.; Shimada, M.; Takada, A.; Iwai, K.; Ohashi, N. A Molecular and Serological Survey of Rickettsiales Bacteria in Wild Sika Deer (Cervus nippon nippon) in Shizuoka Prefecture, Japan: High Prevalence of Anaplasma Species. Jpn. J. Infect. Dis. 2015, 68, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Chien, N.T.H.; Nguyen, T.L.; Bui, K.L.; Nguyen, T.V.; Le, T.H. Anaplasma marginale and A. platys Characterized from Dairy and Indigenous Cattle and Dogs in Northern Vietnam. Korean J. Parasitol. 2019, 57, 43–47. [Google Scholar] [CrossRef]
- Makgabo, S.M.; Brayton, K.A.; Oosthuizen, M.C.; Collins, N.E. Unravelling the diversity of Anaplasma species circulating in selected African wildlife hosts by targeted 16S microbiome analysis. Curr. Res. Microb. Sci. 2023, 5, 100198. [Google Scholar] [CrossRef]
- Priyanka, M.; Dhanalakshmi, H.; Rakesh, R.L.; Thimmareddy, P.M.; Narayana Bhat, M. Monocytic anaplasmosis in a cow: A case report. J. Parasit. Dis. Off. Organ Indian Soc. Parasitol. 2017, 41, 687–688. [Google Scholar] [CrossRef]
- Al-Saadi, M.; Al-Sallami, D.; Alsultan, A. Molecular identification of Anaplasma platys in cattle by nested PCR. Iran. J. Microbiol. 2023, 15, 433. [Google Scholar] [CrossRef] [PubMed]
- Han, X.-Y.; Du, L.-F.; Lin, Z.-T.; Li, C.; Xiong, T.; Zhu, W.-J.; Ye, R.-Z.; Wang, N.; Wang, Y.-F.; Gao, W.-Y.J.E.M.; et al. Genomic characters of Anaplasma bovis and genetic diversity in China. Emerg. Microbes Infect. 2024, 13, 2323153. [Google Scholar] [CrossRef]
- Park, J.H.; Han, D.G.; Ryu, J.H.; Chae, J.B.; Chae, J.S.; Yu, D.H.; Park, B.K.; Kim, H.C.; Choi, K.S. Molecular detection of Anaplasma bovis in Holstein cattle in the Republic of Korea. Acta Vet. Scand. 2018, 60, 15. [Google Scholar] [CrossRef]
- Teshale, S.; Geysen, D.; Ameni, G.; Dorny, P.; Berkvens, D.J.P. Survey of Anaplasma phagocytophilum and Anaplasma sp.‘Omatjenne’infection in cattle in Africa with special reference to Ethiopia. Parasites Vectors 2018, 11, 162. [Google Scholar] [CrossRef]
- Sharma, A.; Singla, L.D.; Kaur, P.; Bal, M.S. PCR and ELISA vis-à-vis microscopy for detection of bovine anaplasmosis: A study on associated risk of an upcoming problem in North India. Sci. World J. 2015, 2015, 352519. [Google Scholar] [CrossRef]
- Silaghi, C.; Santos, A.S.; Gomes, J.; Christova, I.; Matei, I.A.; Walder, G.; Domingos, A.; Bell-Sakyi, L.; Sprong, H.; Von Loewenich, F.D.J.V.-B.; et al. Guidelines for the direct detection of Anaplasma spp. in diagnosis and epidemiological studies. Vector-Borne Zoonotic Dis. 2017, 17, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.-P.; Wang, X.; Li, Y.-N.; Xu, G.; Wang, Y.-H.; Zhou, E.-M. GroEL gene typing and genetic diversity of Anaplasma bovis in ticks in Shaanxi, China. Infect. Genet. Evol. 2019, 74, 103927. [Google Scholar] [CrossRef] [PubMed]
- Kuibagarov, M.; Makhamed, R.; Zhylkibayev, A.; Berdikulov, M.; Abdrakhmanov, S.; Kozhabayev, M.; Akhmetollayev, I.; Mukanov, K.; Ryskeldina, A.; Ramankulov, Y.; et al. Theileria and Babesia infection in cattle—First molecular survey in Kazakhstan. Ticks Tick-Borne Dis. 2023, 14, 102078. [Google Scholar] [CrossRef]
- Shabdarbayeva, G.S.; Abdybekova, A.M. Identification of foci of blood-parasitic diseases of ruminants in the south of Kazakhstan. Eurasian Union Sci. 2016, 33, 17–21. [Google Scholar]
- Boom, R.; Sol, C.J.; Salimans, M.M.; Jansen, C.L.; Wertheim-van Dillen, P.M.; van der Noordaa, J. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 1990, 28, 495–503. [Google Scholar] [CrossRef]
- Berdimuratova, K.; Amirgazin, A.; Kuibagarov, M.; Lutsay, V.; Mukanov, K.; Shevtsov, A. Optimization of PCR Purification Using Silica-Coated Magnetic Beads. Eurasian J. Appl. Biotechnol. 2020, 1, 11. [Google Scholar] [CrossRef]
- Burland, T.G. DNASTAR’s Lasergene sequence analysis software. Methods Mol. Biol. 2000, 132, 71–91. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Reller, M.E.; Dumler, J.S. Ehrlichia, Anaplasma, and Related Intracellular Bacteria. In Manual of Clinical Microbiology; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 1135–1149. [Google Scholar]
- Railey, A.F.; Marsh, T.L. Economic benefits of diagnostic testing in livestock: Anaplasmosis in cattle. Front. Vet. Sci. 2021, 8, 626420. [Google Scholar] [CrossRef]
- Perfilyeva, Y.V.; Shapiyeva, Z.Z.; Ostapchuk, Y.O.; Berdygulova, Z.A.; Bissenbay, A.O.; Kulemin, M.V.; Ismagulova, G.A.; Skiba, Y.A.; Sayakova, Z.Z.; Mamadaliyev, S.M.; et al. Tick-borne pathogens and their vectors in Kazakhstan—A review. Ticks Tick-Borne Dis. 2020, 11, 101498. [Google Scholar] [CrossRef] [PubMed]
- Miranda, E.A.; Han, S.W.; Cho, Y.K.; Choi, K.S.; Chae, J.S. Co-Infection with Anaplasma Species and Novel Genetic Variants Detected in Cattle and Goats in the Republic of Korea. Pathogens 2021, 10, 28. [Google Scholar] [CrossRef]
- Rjeibi, M.R.; Ayadi, O.; Rekik, M.; Gharbi, M. Molecular survey and genetic characterization of Anaplasma centrale, A. marginale and A. bovis in cattle from Algeria. Transbound. Emerg. Dis. 2018, 65, 456–464. [Google Scholar] [CrossRef]
- Qi, Y.; Ai, L.; Zhu, C.; Lu, Y.; Lv, R.; Mao, Y.; Lu, N.; Tan, W. Co-existence of Multiple Anaplasma Species and Variants in Ticks Feeding on Hedgehogs or Cattle Poses Potential Threats of Anaplasmosis to Humans and Livestock in Eastern China. Front. Microbiol. 2022, 13, 913650. [Google Scholar] [CrossRef]
- Ashraf, S.; Parveen, A.; Asif, M.; Alanazi, A.D.; Alouffi, A.; Muhammad Awais, M.; Khan, A.; Aktas, M.; Ozubek, S.; Iqbal, F. First report regarding molecular epidemiology and novel variant identification of Anaplasma centrale in cattle from Pakistan. Saudi J. Biol. Sci. 2021, 28, 6488–6494. [Google Scholar] [CrossRef]
- Aktas, M.; Özübek, S. Bovine anaplasmosis in Turkey: First laboratory confirmed clinical cases caused by Anaplasma phagocytophilum. Vet. Microbiol. 2015, 178, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, K.; Sun, Y.; Shi, J.; Li, H.; Chen, Y.; Yang, H.; Li, X.; Wu, B.; Li, X.J.P.o. Molecular epidemiology and risk factors of Anaplasma spp., Babesia spp. and Theileria spp. infection in cattle in Chongqing, China. PloS ONE 2019, 14, e0215585. [Google Scholar] [CrossRef]
- Altay, K.; Erol, U.; Sahin, O.F.; Aytmirzakizi, A.J.T.; Diseases, T.-b. First molecular detection of Anaplasma species in cattle from Kyrgyzstan; molecular identification of human pathogenic novel genotype Anaplasma capra and Anaplasma phagocytophilum related strain. Ticks Tick-Borne Dis. 2022, 13, 101861. [Google Scholar] [CrossRef] [PubMed]
- De Wall, D.T. Anaplasmosis control and diagnosis in South Africa. Ann. N. Y. Acad. Sci. 2000, 916, 474–483. [Google Scholar] [CrossRef]
- Molad, T.; Mazuz, M.L.; Fleiderovitz, L.; Fish, L.; Savitsky, I.; Krigel, Y.; Leibovitz, B.; Molloy, J.; Jongejan, F.; Shkap, V. Molecular and serological detection of A. centrale- and A. marginale-infected cattle grazing within an endemic area. Vet. Microbiol. 2006, 113, 55–62. [Google Scholar] [CrossRef]
- Shkap, V.; Molad, T.; Fish, L.; Palmer, G.H. Detection of the Anaplasma centralevaccine strain and specific differentiation from Anaplasma marginale in vaccinated and infected cattle. Parasitol. Res. 2002, 88, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Khumalo, Z.T.H.; Brayton, K.A.; Collins, N.E.; Chaisi, M.E.; Quan, M.; Oosthuizen, M.C. Evidence confirming the phylogenetic position of Anaplasma centrale (ex Theiler 1911) Ristic and Kreier 1984. Int. J. Syst. Evol. Microbiol. 2018, 68, 2682–2691. [Google Scholar] [CrossRef]
- Hove, P.; Khumalo, Z.T.H.; Chaisi, M.E.; Oosthuizen, M.C.; Brayton, K.A.; Collins, N.E. Detection and Characterisation of Anaplasma marginale and A. centrale in South Africa. Vet. Sci. 2018, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Ben Said, M.; Belkahia, H.; Selmi, R.; Messadi, L. Computational selection of minimum length groESL operon required for Anaplasma species attribution and strain diversity analysis. Mol. Cell. Probes 2019, 48, 101467. [Google Scholar] [CrossRef]
- Caudill, M.T.; Brayton, K.A. The Use and Limitations of the 16S rRNA Sequence for Species Classification of Anaplasma Samples. Microorganisms 2022, 10, 605. [Google Scholar] [CrossRef]
- Zhyldyz, A.; Aitakin, K.; Atabek, B.; Elmurat, J.; Rysbek, N.; Jailobek, O.; Ahedor, B.; Otgonsuren, D.; Mumbi, N.N.M.; Guswanto, A.; et al. An epidemiological survey of vector-borne pathogens infecting cattle in Kyrgyzstan. Parasitol. Int. 2023, 97, 102791. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Jiang, Y.; Tao, D.; Zhao, A.; Qi, M.; Ning, C. Molecular detection of Anaplasma spp. in dairy cattle in southern Xinjiang, China. Vet. Parasitol. Reg. Stud. Rep. 2020, 20, 100406. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Liu, Z.; Liu, J.; Niu, Q.; Ren, Q.; Chen, Z.; Guan, G.; Luo, J.; Yin, H. Molecular detection and characterization of Anaplasma spp. in sheep and cattle from Xinjiang, northwest China. Parasites Vectors 2015, 8, 108. [Google Scholar] [CrossRef]
- Arkhipova, A.L.; Tagmazyan, P.P.; Kureev, N.; Brigida, A.V.; Kovalchuk, S. Distribution of bovine anaplasmosis in Russian Federation. Vet. Korml. 2019, 2, 23–25. [Google Scholar] [CrossRef]
- Rahr, V.A.; Marchenko, V.A.; Biryukov, I.V. On epizootology of anaplasmosis of ruminants in the south of west siberia. Bull. Altai State Agric. Univ. 2019, 7, 109–115. [Google Scholar]
- Tian, J.; Liu, J.; Zhao, H.; Chen, X.; Geng, X.; Lu, M.; Li, K. Molecular surveillance reveals a potential hotspot of tick-borne disease in Yakeshi City, Inner Mongolia. BMC Microbiol. 2023, 23, 359. [Google Scholar] [CrossRef]
- Fischer, T.; Myalkhaa, M.; Krücken, J.; Battsetseg, G.; Batsukh, Z.; Baumann, M.P.O.; Clausen, P.H.; Nijhof, A.M. Molecular detection of tick-borne pathogens in bovine blood and ticks from Khentii, Mongolia. Transbound. Emerg. Dis. 2020, 67 (Suppl. 2), 111–118. [Google Scholar] [CrossRef]
- Bauer, B.U.; Răileanu, C.; Tauchmann, O.; Fischer, S.; Ambros, C.; Silaghi, C.; Ganter, M. Anaplasma phagocytophilum and Anaplasma ovis-Emerging Pathogens in the German Sheep Population. Pathogens 2021, 10, 1298. [Google Scholar] [CrossRef] [PubMed]
- Renneker, S.; Abdo, J.; Salih, D.E.; Karagenç, T.; Bilgiç, H.; Torina, A.; Oliva, A.G.; Campos, J.; Kullmann, B.; Ahmed, J.; et al. Can Anaplasma ovis in small ruminants be neglected any longer? Transbound. Emerg. Dis. 2013, 60 (Suppl. 2), 105–112. [Google Scholar] [CrossRef]
- de la Fuente, J.; Ruiz-Fons, F.; Naranjo, V.; Torina, A.; Rodríguez, O.; Gortázar, C. Evidence of Anaplasma infections in European roe deer (Capreolus capreolus) from southern Spain. Res. Vet. Sci. 2008, 84, 382–386. [Google Scholar] [CrossRef]
- de la Fuente, J.; Atkinson, M.W.; Naranjo, V.; Fernández de Mera, I.G.; Mangold, A.J.; Keating, K.A.; Kocan, K.M. Sequence analysis of the msp4 gene of Anaplasma ovis strains. Vet. Microbiol. 2007, 119, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Z.; Liu, Z.; Liu, J.; Yang, J.; Li, Q.; Li, Y.; Luo, J.; Yin, H. Molecular Survey of Anaplasma and Ehrlichia of Red Deer and Sika Deer in Gansu, China in 2013. Transbound. Emerg. Dis. 2016, 63, e228–e236. [Google Scholar] [CrossRef] [PubMed]
- Haigh, J.C.; Gerwing, V.; Erdenebaatar, J.; Hill, J.E. A novel clinical syndrome and detection of Anaplasma ovis in Mongolian reindeer (Rangifer tarandus). J. Wildl. Dis. 2008, 44, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Noaman, V. Molecular Detection of Novel Genetic Variants Associated to Anaplasma ovis among Dromedary Camels in Iran. Arch. Razi Inst. 2018, 73, 11–18. [Google Scholar] [CrossRef]
- Selmi, R.; Ben Said, M.; Dhibi, M.; Ben Yahia, H.; Abdelaali, H.; Messadi, L. Genetic diversity of groEL and msp4 sequences of Anaplasma ovis infecting camels from Tunisia. Parasitol. Int. 2020, 74, 101980. [Google Scholar] [CrossRef]
- Ochirkhuu, N.; Konnai, S.; Odbileg, R.; Murata, S.; Ohashi, K. Molecular Epidemiological Survey and Genetic Characterization of Anaplasma Species in Mongolian Livestock. Vector Borne Zoonotic Dis. 2017, 17, 539–549. [Google Scholar] [CrossRef]
- Noaman, V.; Sazmand, A. Anaplasma ovis infection in sheep from Iran: Molecular prevalence, associated risk factors, and spatial clustering. Trop. Anim. Health Prod. 2021, 54, 6. [Google Scholar] [CrossRef]
- M’Ghirbi, Y.; Oporto, B.; Hurtado, A.; Bouattour, A. First Molecular Evidence for the Presence of Anaplasma phagocytophilum in Naturally Infected Small Ruminants in Tunisia, and Confirmation of Anaplasma ovis Endemicity. Pathogens 2022, 11, 315. [Google Scholar] [CrossRef]
- Enkhtaivan, B.; Narantsatsral, S.; Davaasuren, B.; Otgonsuren, D.; Amgalanbaatar, T.; Uuganbayar, E.; Zoljargal, M.; Myagmarsuren, P.; Suganuma, K.; Molefe, N.I.; et al. Molecular detection of Anaplasma ovis in small ruminants and ixodid ticks from Mongolia. Parasitol. Int. 2019, 69, 47–53. [Google Scholar] [CrossRef]
- Kuketova, A.A. Development of cattle breeding of the Kazakh steppe in the XVIII-XIX centuries in the works of Russian travelers: Historiographic analysis (on the example of Semirechye). Bull. Karaganda Univ. Hist. Philos. Ser. 2020, 100, 50–56. [Google Scholar]
- Mutaliyeva, A.; Yesbolova, A.; Dyrka, S.; Saparbayev, M.; Kazanbayeva, Z.; Balabekova, D.; Orazova, B. Formation and History of the Agrarian Economy of Kazakhstan: The State of Development Today. Acad. J. Interdiscip. Stud. 2023, 12, 401. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).