Abstract
The aim of this study was to explore molecular measures of P. falciparum malaria burden (FOI and MOI) in the context of seasonal malaria chemoprevention. We analyzed malaria cases collected as part of a longitudinal cohort study. The cohort included P. falciparum-negative children aged 1.5 to 12, as confirmed by PCR 21 days after a radical cure using DHA-PQ or AS. Children were followed up for six months using active and passive case detection methods. At each visit, dried blood spots and blood smears were collected by finger prick, along with clinical data. Parasite DNA was extracted and analyzed by nested PCR for detection and genotyping of P. falciparum parasites. A total of 458 P. falciparum isolates collected during follow-up from October 2020 to March 2021 were genotyped. During the follow-up, children contracted 1.05 (95% IC [0.81–1.30]) new P. falciparum infections/child/time of exposure, and the MOI value was 3.00 (SD 1.60). Age is a protective factor (IRR: 0.74; 95% CI: 0.61, 0.90) against the occurrence of an episode of malaria, unlike an increase in MOI (IRR: 1.63; 95% CI: 1.04, 1.99), which is a favorable factor (p < 0.05). This study confirms the reduction in malaria transmission in our study area, probably due to the massive deployment of control tools.
1. Introduction
According to World Health Organization (WHO) estimates, there were 247 million cases of malaria worldwide in 2021 compared to 249 million in 2022, with most of this increase coming from countries in the WHO’s African Region. Twenty-nine countries accounted for 95% of global malaria cases, and four African countries accounted for almost half of all cases globally. The WHO African Region, with an estimated 233 million cases in 2022, accounted for about 94% of cases and just over half of all malaria deaths worldwide [1].
In Burkina Faso, malaria is a major public health problem. Data from WHO on the malaria burden in 2020 estimated more than 10 million suspected malaria cases and roughly 4000 deaths [1]. To address this problem, Burkina Faso has subscribed to several global initiatives such as the “Roll Back Malaria” initiative to fight the disease. To this end, several strategies have been implemented, the most important of which are: the adoption in 2005 of Artemisinin-based Combination Therapies as first-line treatment for uncomplicated malaria, the adoption of Intermittent Preventive Treatment with Sulfadoxine-Pyrimethamine for pregnant women [2,3], and the Chemoprevention of Seasonal Malaria in children aged 3 to 59 months in 2014 [4], as well as the massive distribution of long-lasting impregnated bed-nets.
Despite these malaria control strategies, the resistance of the parasite (Plasmodium) to antimalarial drugs adds to the epidemiological and economic burden of the scourge in many endemic countries [5]. Various factors, in particular the diversity and antigenic variation of the parasite responsible for the slow acquisition (several years) of protection against malaria, make malaria control complex [6].
Genetic diversity of malaria parasites is essential in understanding the mechanism underlying malaria pathology and determining parasite clones’ profiles in an infection for proper malaria control strategies such as malaria vaccine development, a crucial tool for malaria eradication. Indeed, the malaria vaccine development is complicated by the high genetic diversity of the parasite populations, which impede the setting of protective acquired immunity against malaria [7]. This genetic diversity within P. falciparum is a fundamental propriety by which the parasites escape the hosts’ immune responses, and it results from allelic polymorphism, recombination, chromosome rearrangements, and antigenic variation [8]. The mechanisms controlling this diversity within the parasite genome are many and complex. In several studies, according to malaria transmission intensity, individuals are often infected simultaneously by several parasite clones [9,10,11]. The described extensive polymorphisms are related to some key parasite genes coding antigens during the parasite’s asexual blood stage [12]. Indeed, polymorphisms of blocks 2 and 3 of the gene coding for the MSP1, MSP2, and GLURP, respectively, of P. falciparum have long been used as genetic markers for genotyping of parasite populations [13]. Several studies have demonstrated that the locus of the msp2 gene of P. falciparum is extremely polymorphic [14] and, therefore, most informative.
A previous study has shown that, for a better measurement of the outcomes of malaria control interventions, genotyping of P. falciparum parasites in longitudinal studies provides a robust approach to estimating the genomic metrics of transmission [13]. Two genetic parameters have been proposed for study of the effects of these interventions at the molecular level: the force of infection (FOI) and the multiplicity of infection (MOI). The FOI, defined as the number of new Plasmodium clone infections acquired over time [13], is an alternative measurement of malaria transmission. The multiplicity of infection (MOI) is defined as the number of simultaneous parasite clones per P. falciparum-positive host [13]. In endemic areas, multiple infections of P. falciparum clones are common, and the MOI varies with the degree of malaria endemicity [14].
At a potential vaccine trial site (southwest Burkina Faso, where malaria is endemic and seasonal), we performed a molecular characterization of highly polymorphic genetic MSP2 of P. falciparum malaria to explore the FOI and MOI with their influencing factors, which are valuable to inform the design of malaria vaccine trials.
2. Materials and Methods
2.1. Study Site
The study was conducted in an area covered by the Banfora Health District, located in the southwestern part of Burkina Faso (Figure 1). The population in the Cascades region was estimated in 2019 at 812,466 inhabitants, among whom 633,043 are residents of the administrative province of the Comoé [15]. This district covers a surface area of 15,405 km2. The Banfora Health District has intense seasonal malaria transmission over a six-month period following seasonal rains from May to November [16]. P. falciparum accounts for >90% of malaria cases [16]. With the WHO’s recommendation to introduce Seasonal Malaria Chemoprevention (SMC) since 2012 [17], children under 5 years in the study area have benefited from this intervention during the high-transmission season since 2014, as has the rest of the country [4,17].
Figure 1.
Map of Burkina Faso giving Banfora location (https://jumelage-pessac.org/ville/banfora) (accessed on 28 September 2024).
2.2. Study Population, Design and Period
Data for this study were obtained from a longitudinal cohort study, aiming primarily to assess the incidence of clinical malaria in children aged 1.5 to 12 and to identify the most effective antimalarial drug to use for a pre-vaccination radical cure strategy. Male or female children aged 1.5 to 12 years and living permanently in the study area were included in the cohort. They were malaria-free as confirmed by a negative PCR 21 days after a malaria radical cure using either artesunate (AS) alone or dihydroartemisinin-piperaquine (DHA-PQ), and their parents or guardians provided written informed consent. Children were followed for up to six months from October 2020 to April 2021 using both active and passive case detection methods to ensure the capture of a high proportion of malaria infections in the cohort. The active case detection consisted of fortnightly visits to the child at home. During each visit, clinical data were collected; then, malaria smears and dried blood spots were prepared using a finger prick. Between two active visits, parents were encouraged to take their child to the local health facility whenever he/she felt unwell. During these passive visits, the same procedures as for the active visits were conducted. We took advantage of this cohort study to describe P. falciparum genetic diversity, targeting the most polymorphic region of msp2 gene. All the malaria infection cases that occurred during the active and passive follow-up periods were used to characterize msp2 allelic families. In addition to the MOI, the longitudinal cohort follow-up was appropriate to estimate the FOI.
2.3. Sample Collection
Dried blood spots on Whatman filter papers (Sigma, Pittsburg, PA, USA) were labeled, then air-dried and placed individually in plastic bags containing a desiccant, thus protecting them from humidity. These filter papers were used for the analysis of P. falciparum’s presence by PCR. Thick and thin blood smears were prepared on the slide for malaria detection by microscopy. Samples were collected through a finger prick.
2.4. Malaria Parasites Density by Microscopy
Thick and thin blood films were air-dried and stained with 6% Giemsa. Each slide was read by two laboratory technicians. Asexual and sexual parasites were counted separately and species differentiated. Malaria parasites were counted against 200 white blood cells (WBC). A slide was declared negative only after reading against 100 microscopic fields without observation of a malaria parasite. Parasite densities were calculated assuming an average of 8000 white blood cells/µL of blood. The arithmetic mean of the two readings was used as the final parasite density. In the event of a discrepancy over the presence or absence of malaria parasites between the two readers, or if parasite density estimates differed by more than 30%, the slide was re-examined by a third laboratory technician. The arithmetic mean of the two most concordant results (out of the three readings) was considered as the final parasite density.
2.5. DNA Extraction by Methanol Method
DNA was extracted using the ethanol method. For that, three pieces of filter paper were soaked in methanol (100 µL) for 15 min at room temperature. After 15 min of action, the methanol was transferred from the tube and the pieces were left to dry completely under vacuum or in the open air for 1–2 h. After drying, 100 µL of sterile distilled water was added to each tube. The tubes were then heated in a water bath for 15 min at 95–100 °C. During the incubation phase, the tube was vortexed every 5 min to extract plasmodial DNA. The DNA extracts were used directly.
2.6. Molecular Analysis
The P. falciparum species was identified by a nested PCR amplification from two PCR reactions. This is a nested PCR method targeting the 18 s rRNA small sub-unit gene. Products obtained after the first PCR were amplified using specific primers for P. falciparum. The sequence of the primers and the protocol of PCR are described in detail elsewhere [18]. Primary and secondary PCRs were carried out in a final volume of 20 μL containing 4 μL of Master Mix (5x FIREPol®, 1.25 μL of dNTP, 0.8 μL of 25 mM MgCl2, 0.1 μL of One Taq® DNA polymerase) and 1 μL of Template DNA or primary PCR product under the following conditions: 95 °C for 5 min, 58 °C for 2 min, and 72 °C for 2 min, followed by cycles (24 for primary PCR and 30 cycles for secondary PCR) of 94° C for 1 min, 58 °C for 2 min, and 72 °C for 5 min. After amplification, samples were subjected to electrophoretic migration on a 1.5% agarose (Sigma Aldrich Chemie GMBH, Taufkirchen, Germany) gel and 3% GelRed® (Nucleic Acid Stain) in Tris Borate Ethylene-Diamine-Tetra-Acetic. Migration was performed at 100 V for 90 min. Fragment size was determined using the molecular weight marker. UV electrophoresis development was carried out using an image documentation system (Axygen, Corning, NY, USA) coupled to a computer, enabling the exact size of DNA bands to be estimated. The expected fragment length was 205 base pairs (bp) for P. falciparum [18].
msp2 (central region) was genotyped using a nested PCR. Briefly, products obtained after the first PCR were amplified using specific primers of the two distinct allelic families for msp2 (FC27 and 3D7) in accordance with the recommended genotyping protocol [19]. The sequence of the primers and the protocol of PCR were previously described in detail by Snounou et al. [20]. Both primary and secondary PCRs for msp2 were carried out in a final volume of 25 μL containing 4 μL of Master Mix (5x FIREPol®, 1.25 μL of dNTP, 0.8 μL of 25 mM MgCl2, 0.1 μL of One Taq® DNA polymerase) and 1 μL of Template DNA or primary PCR product under the following conditions for primary and secondary PCR (30 cycles): initial denaturation at 95 °C for 5 min, extension at 94 °C for 1 min, annealing at 58 °C for 2 min for the primary and 61 °C for 2 min for the secondary amplification, extension at 72 °C for 2 min, and final elongation at 72 °C for 10 min for the primary and 72 °C for 5 min for the secondary amplification. All PCRs were performed in an Applied Biosystems 2720 thermal cycler (Applied Biosystems, Walthman, MA, USA). As with P. falciparum identification, the band lengths of the different allelic families were detected after migration on an agarose gel. Using the molecular weight marker (100 bp), we determined the lengths of the different fragments.
2.7. Data Management and Statistical Analysis
Data were directly recorded on android tablets, double-checked by the investigators, and transferred to the database. Controls and edit checks were included in the data capture system to ensure data quality.
The mean MOI was calculated by dividing the total number of alleles detected by the total number of positive samples. The FOI was calculated from the number of new P. falciparum infections, expressed as the number of new infections per unit time, and determined by counting all new msp2 genotypes not present in preceding visits [13]. Multiclonal infections were defined as infections with more than one allele of the msp2 gene. Molecular data were generated and recorded on an Excel sheet, checked for consistency, and merged with the rest of the dataset. Data were cleaned before being exported for analysis using R version 3.5.1. Descriptive statistics (means and proportions) were calculated with their 95% confidence intervals. As part of the analysis of the different predictive factors (a model with several independent variables), a multivariate analysis was carried out to assess their impact. A clinical malaria episode was defined as objective fever (axillary temperature ≥ 37.5 °C/tympanic ≥ 38 °C or forehead temperature ≥ 37.5 °C using a non-contact infrared thermometer (Microlife, Ängelholm, Sweden)) and parasitemia of >2500 parasites/µL by microscopy.
The Pearson chi-square test was used for the comparison of proportions and frequencies or the Fisher exact test for the comparison of proportions when the theoretical number was less than 5. The Student test was used for comparison of means. The p-values were reported, with differences considered significant at p < 0.05.
3. Results
3.1. Baseline Characteristics of Participants
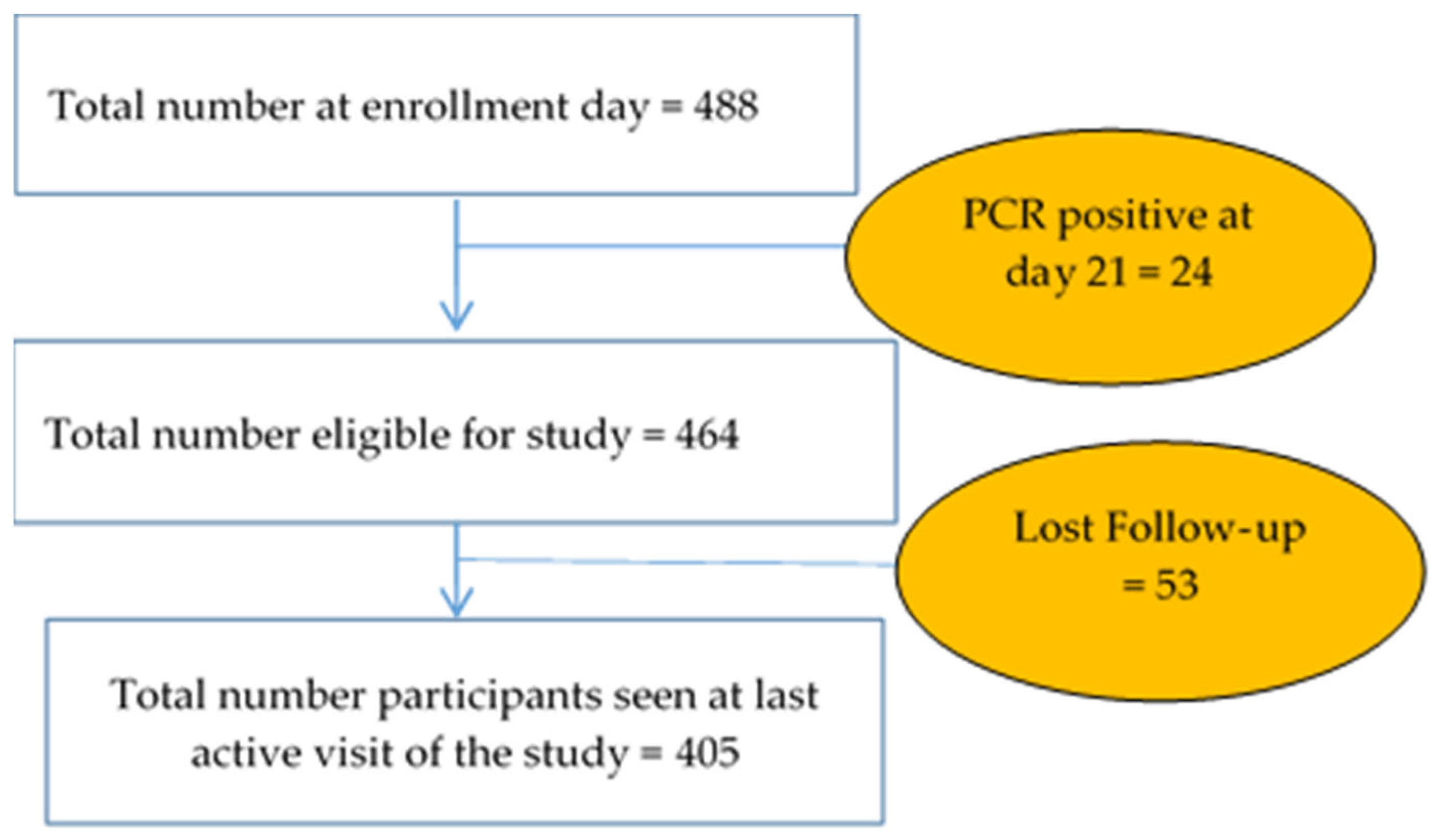
For a total of 488 children enrolled, 464 (95.08%) were PCR-negative on Day 21 after anti-malarial treatment for radical cure. A total of 405 (87.28%) subjects completed the study (Figure 2).
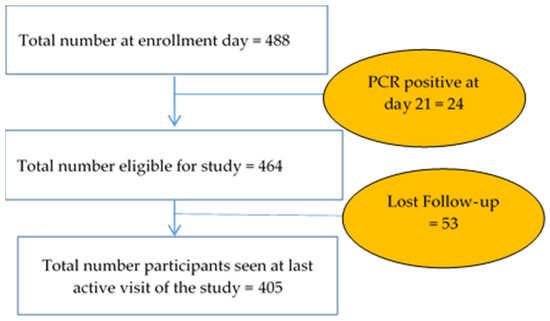
Figure 2.
Follow-up profile.
Children aged 1.5 to 5 years accounted for 41.16% (191) of the sample, and 58.84% (273) were older (aged 5 to 12 years). Both genders were equally represented, with 229 (49.35%) males and 235 (50.65%) females. As shown in Table 1, insecticide-treated mosquito nets (ITN) alone as a means of protection were used by less than a quarter of the study population (20.30%). The combination of insecticide-treated mosquito nets and other malaria prevention methods (mosquito repellent cream or spray, coils, traditional or herbal preventive methods) was more widely used (77.32%). However, there were patients who did not use any method of protection.

Table 1.
Characteristics of the samples.
3.2. Incidence of P. falciparum Infection by PCR during the Study
A 6-month follow-up was carried out, combining active (with a bi-weekly sampling) and passive surveillance. Thus, the cumulative incidence of P. falciparum infection was 44.11% [39.55–48.76], with a significant difference (p = 0.049) according to age (38.32% [31.40–45.73] for subjects under five years and 48.01% [42.02–54.06] for the elder group).
3.3. FOI and MOI in Study Participants
This study successfully identified msp2 allelic families (3D7 and FC27) in 87.99% of P. falciparum isolates, with the 3D7 family (82.63%) exhibiting a significantly higher frequency (p = 0.001) compared to the FC27 family (73.20%). The MOI value was 3.00 (SD 1.60), with a slight decrease with age (3.15 (SD 1.67) for subjects aged 1.5 to 5 years and 2.95 (SD 1.58) for subjects aged over 5 years), but with no statistically significant difference (p = 0.16). The FOI was 1.05 (95% IC [0.81–1.30]) new P. falciparum infections per child per time of exposition. Subjects under 5 years had an FOI of 0.71 (95% IC [0.36–1.04]) new P. falciparum infections per child per time of exposition versus 1.28 (95% IC [0.94–1.62]) new P. falciparum infections per child per time of exposition for subjects over 5 years. This difference between age groups was statistically significant (p = 0.023).
The prevalence of multiple infections (polyclonality) was close to 80% in all cases. However, this parameter did not seem to be affected by age group (p = 0.53), with 84.44% for <5 years and 80.83% in children ≥5 years.
3.4. Risks Factors of Clinical Episode
In total, 21 children developed a clinical episode during the follow-up period. The results in Table 2 show that, with the unadjusted model, only MOI and age were significantly associated with the occurrence of clinical malaria episodes. The same observations were valid in the adjusted model.

Table 2.
Predictors of time to onset of clinical episodes.
3.5. Predictive Factors of MOI and FOI
3.5.1. Predictive Factors of MOI
Multivariate analysis (Table 3) of different factors showed that only polyclonality (IRR: 2.962; 95% CI: [2.17–4.03), parasitemia (IRR: 1.239; 95% CI: [1.06–1.45]), FOI (IRR: 1.168; 95% CI: [1.12–1.22]), and rainy months (IRR: 1.680; 95% CI: [1.38–2.04) were predictive factors given the adjusted results (p ˂ 0.05).

Table 3.
Predictive factors for MOI.
3.5.2. Predictive Factors of New Clones’ Acquisition (FOI)
The summary of our findings is presented in Table 4. In multivariate Poisson regression, pre-existing polyclonality (IRR: 2.46; 95% CI: [1.62–3.76]), MOI (IRR: 1.27; 95% CI: [1.22–1.34]), and study month (IRR: 2.09; 95% CI: [1.67–2.63]) were found to significantly influence the acquisition of new clones.

Table 4.
Predictive factors for FOI.
4. Discussion
In this study, we explored molecular measures of FOI and MOI using the msp2 gene in children aged from 1.5 to 12 years after they had received a radical cure consisting of antimalarial drugs to eliminate existing P. falciparum parasites.
The high diversity of the msp2 gene is due to an allele-specific core region that includes repeats of varying lengths. The alleles are grouped into two distinct families, the 3D7 family (Indochina) and the FC27 family [21,22], based on the dimorphic structure of the non-repeat variable region [21]. These features make msp2 a suitable genetic marker for genotyping P. falciparum infections and provide an informational tool for enumerating multiple simultaneous infections in a blood sample and for distinguishing individual alleles [23,24]. Better knowledge of P. falciparum genetic diversity could improve our understanding of malaria’s pathological mechanisms, acquired immunity processes, spread, and the genetic background of drug resistance and transmission conditions [25].
An increase in the age of participants was associated with a reduced risk of occurrence of a clinical episode. This is in line with previous observations that acquisition of premunition increases with age and, therefore, older children are expected to be at a lower risk of malaria episodes. The results of previous studies have reported that school-aged children (aged over five years) are the main reservoir of malaria parasites [26,27,28]. In addition, the malaria control methods introduced in Burkina Faso targeting young children, especially under-fives, may have contributed to better protection of the younger children in this study.
The mean value of the MOI obtained in this study could be explained by the climatic and environmental conditions and the humidity of the area, which favor the maintenance of high and perennial transmission in this part of Burkina Faso. It was close to that found in 2020 in Burkina Faso [29], relatively higher than the values previously found in the country [30,31,32], and largely different from those found by Tadele et al. in Ethiopia in 2022 [33]. In our study, all participants received a radical cure to clear all P. falciparum parasites before their enrolment and were divided into two groups; the under-five-years subjects who were under SMC and the non-SMC over-five-years children had similar multiclonal infections. This would suggest that SMC did not alter the infection trend. In addition, studies have shown that when chemoprophylaxis is used, malaria may be atypical and those infected may have symptoms. Previous studies on the variation in MOI with age have suggested that this influence on multiplicity of infection may be strongly affected by malaria’s endemicity [34,35]. Studies have also shown that MOI is age-dependent in areas of intense and perennial malaria transmission, but not in areas of meso-endemic malaria [34,35]. A comparable trend of a positive association between multiplicity and age was observed in a previous study by Soulama et al. in Burkina Faso [31], but Arzika et al. [36] did not observe an age-dependent pattern in Niger.
A high prevalence of multiclonal infections was observed in this study, which could be due to high parasite pressure consecutive to continuous and intense malaria transmission, suggesting efficient genetic recombination of the parasite population within the female anopheles [37,38]. It could also result from multiple independent inoculations of single-parasite clones [39]. Simultaneous infections of a large number of individuals with several different parasite genotypes in areas of high transmission intensity have been attributed to multiple inoculations of single clones or single inoculation of multiple clones (co-transmission) that may have undergone crossover and recombination in the female anopheles [40,41]. In a study conducted by Soulama et al., the data showed that older subjects were more often infected by several different clones [28] in contrast to Sondo et al., where the MOI decreased significantly in older hosts [26]. Previous studies have suggested that high genetic diversity in the parasite population may lead to a progressive selection of more virulent strains, which in turn may lead to the emergence and proliferation of drug-resistant parasites [42,43].
The FOI is particularly useful as an outcome measure in trials or surveillance measuring the incidence of P. falciparum malaria, such as our study. A longitudinal study conducted over 69 weeks in Papua New Guinea on children aged 0.9 to 3.2 years found higher FOI values. Our lower values may be explained by the fact that in our study, samples were collected outside the main malaria transmission season. Collection between the months of June and September (peak of rainy season) would probably have shown higher values. An increase in FOI with age was observed, confirming previous entomological data showing that the number of infectious bites increased with body size [44] and that FOI increased with age [45]. Our results could be explained by the fact that malaria vectors are known to bite easily outdoors and in the early hours of the night, so older children, being more independent (and possibly unprotected), may be at greater risk of being bitten outdoors. Indeed, in a previous study conducted in Banfora, the results indicated that vector bites in the early evening were observed in our study area and that this was the period during which it was common to find active communities in the peri-domestic environment [46]. This increase in FOI with age in our study could alter assumptions that children under five are the most infected and raise the question of reorienting the target audience for SMC coverage.
In this study, older children appear to be more exposed to clinical cases of malaria. A study with opposite results showed that repeated exposure to P. falciparum infections in previous years could confer immunity. In this study, cases of P. falciparum malaria observed in a population exposed to a high level of transmission showed that the acquisition of antimalarial immunity with age was accompanied by a reduction in the duration and frequency [47].
The limitation of this study could be that the genetic diversity was incorrectly estimated due to the detection limit of the PCR technique. Thus, our results could be explained by a possible underestimation of the exact number of alleles due to limitations of the analytical techniques used in genotyping in contrast to other studies, such as the one conducted by Ibrar Ullah et al., because it is difficult to distinguish fragments with length differences less than 20 bp [48] or with the use of the amplicon sequencing [49,50]. In addition, this study was not designed to answer a question related to alleles.
5. Conclusions
Multiple infections were reported in the study subjects, the majority of whom were aged 1.5 to 12 years. Older age and the MOI appear to be protective factors against the occurrence of clinical malaria episodes. The polyclonality and rainy months (coinciding with the high transmission season) are predictive factors associated with MOI and with the acquisition of new clones. The results show that FOI and MOI are age dependent, with the youngest children being more affected. This justifies the current malaria control strategies targeting children under five years of age. Extending the control strategy to children above five years of age will probably lead to better control of malaria in endemic countries.
Author Contributions
Conceptualization, A.O., A.B.T. and S.B.S. investigation, E.S.B., L.K., A.D., D.O., I.N., A.O. and A.B.T. formal analysis, E.S.B.; funding acquisition, S.B.S.; methodology, E.S.B., A.B.T. and S.B.S.; resources, S.B.S.; supervision, S.B.S. writing—original draft, E.S.B.; writing—review and editing, E.S.B., L.K., A.D., D.O., I.N., A.O., A.B.T. and S.B.S. All authors have read and agreed to the published version of the manuscript.
Funding
The study is part of the MIMVaC-Africa (Multilateral Initiative to foster the clinical development of effective Malaria Vaccine candidates in Africa) project funded by European & Developing Countries Clinical Trials Partnership (EDCTP2) supported by the European Union (grant number RIA2018SV-2310). The views and opinions of authors expressed herein do not necessarily state or reflect those of EDCTP 2.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethical Committee for Biomedical Research of the Ministry of Health, Burkina Faso before its implementation (DELIBERATION N02020-6-101 of 10 June 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent was obtained from the parents or guardians of all participating children before enrolment. Confidentiality of the information collected the from participants was warranted by assigning identification numbers to subjects.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank the MIMVaC-Africa Consortium members for their contribution to the efficient management of the consortium which is the framework of this study. Authors wish to thank community members, field supervisors, GRAS staff, and the participants in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- WHO (World Health Organization). World Malaria Report. 2023. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2023 (accessed on 12 December 2023).
- EIPBF, Enquête sur les Indicateurs du Paludisme. 2017–2018. Available online: https://dhsprogram.com/pubs/pdf/MIS32/MIS32.pdf (accessed on 12 December 2023).
- WHO (World Health Organization). World Malaria Report 2011. Available online: https://www.who.int/publications/i/item/9789241564403 (accessed on 12 December 2023).
- Sermé, L.; Bicaba, A.; Ly, A.; Bila, A.; Druetz, T.; Haddad, S. Comprendre le Succès de L’implantation et L’expansion de la Chimioprophylaxie Saisonnière du Paludisme au Burkina Faso. 2018. IDRC Research Results/Innovating for Maternal and Child Health in Africa (IMCHA). Available online: http://hdl.handle.net/10625/57545 (accessed on 12 December 2023).
- Sakihama, N.; Nakamura, M.; Palanca, A.A.; Argubano, R.A.; Realon, E.P.; Larracas, A.L.; Espina, R.L.; Tanabe, K. Allelic diversity in the merozoite surface protein 1 gene of Plasmodium falciparum on Palawan Island, the Philippines. Parasitol. Int. 2007, 56, 185–194. [Google Scholar] [CrossRef]
- Sondo, P. Etude de la Relation Entre le Polymorphisme Génétique de Plasmodium falciparum et les Signes du Paludisme à Nanoro, Burkina Faso 2014. Available online: https://beep.ird.fr/collect/upb/index/assoc/INSSA-2014-SON-ETU/INSSA-2014-SON-ETU.pdf (accessed on 12 December 2023).
- Eisen, D.; Billman-Jacobe, H.; Marshall, V.F.; Fryauff, D.; Coppel, R.L. Temporal Variation of the Merozoite Surface Protein-2 Gene of Plasmodium falciparum. Infect. Immun. 1998, 66, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Kemp, D.J.; Cowman, A.F.; Walliker, D. Genetic Diversity in Plasmodium falciparum. Adv. Parasitol. 1990, 29, 75–149. [Google Scholar] [CrossRef] [PubMed]
- Viriyakosol, S.; Siripoon, N.; Petcharapirat, C.; Petcharapirat, P.; Jarra, W.; Thaithong, S.; Brown, K.N.; Snounou, G. Genotyping of Plasmodium falciparum isolates by the polymerase chain reaction and potential uses in epidemiological studies. Bull. World Health Organ. 1995, 73, 85–95. [Google Scholar]
- Felger, I.; Tavul, L.; Kabintik, S.; Marshall, V.; Genton, B.; Alpers, M.; Beck, H. Plasmodium falciparum: Extensive Polymorphism in Merozoite Surface Antigen 2 Alleles in an Area with Endemic Malaria in Papua New Guinea. Exp. Parasitol. 1994, 79, 106–116. [Google Scholar] [CrossRef]
- Premji, Z.; Ndayanga, P.; Shiff, C.; Minjas, J.; Lubega, P.; MacLeod, J. Community based studies on childhood mortality in a malaria holoendemic area on the Tanzanian coast. Acta Trop. 1997, 63, 101–109. [Google Scholar] [CrossRef]
- Bolad, A.; Berzins, K. Antigenic Diversity of Plasmodium falciparum and Antibody-Mediated Parasite Neutralization. Scand. J. Immunol. 2000, 52, 233–239. [Google Scholar] [CrossRef]
- Mueller, I.; Schoepflin, S.; Smith, T.A.; Benton, K.L.; Bretscher, M.T.; Lin, E.; Kiniboro, B.; Zimmerman, P.A.; Speed, T.P.; Siba, P.; et al. Force of infection is key to understanding the epidemiology of Plasmodium falciparum malaria in Papua New Guinean children. Proc. Natl. Acad. Sci. USA 2012, 109, 10030–10035. [Google Scholar] [CrossRef]
- Arnot, D. Clone multiplicity of Plasmodium falciparum infections in individuals exposed to variable levels of disease transmission. Trans. R. Soc. Trop. Med. Hyg. 1998, 92, 580–585. [Google Scholar] [CrossRef]
- INSD (Institut National de la Statistique et de la Démographie). Résultats Cinquième Recensement Général de la Population et de L’habitation: Monographie de la Région des Cascades; Institut National de la Statistique et de la Démographie: Ouagadougou, Burkina Faso, 2022; 192p, Available online: http://www.cns.bf/IMG/pdf/monographie_des_cascades_5e_rgph.pdf (accessed on 12 December 2023).
- Tiono, A.B.; Kangoye, D.T.; Rehman, A.M.; Kargougou, D.G.; Kaboré, Y.; Diarra, A.; Ouedraogo, E.; Nébié, I.; Ouédraogo, A.; Okech, B.; et al. Malaria Incidence in Children in South-West Burkina Faso: Comparison of Active and Passive Case Detection Methods. PLoS ONE 2014, 9, e86936. [Google Scholar] [CrossRef]
- WHO (World Health Organization). WHO Policy Recommendation: Seasonal Malaria Chemoprevention (SMC) for Plasmodium falciparum Malaria Control in Highly Seasonal Transmission Areas of the Sahel Sub-Region in Africa; World Health Organization: Geneva, Switzerland, 2012; 4p, Available online: https://iris.who.int/bitstream/handle/10665/337978/WHO-HTM-GMP-2012.02-eng.pdf (accessed on 12 December 2023).
- Snounou, G.; Viriyakosol, S.; Zhu, X.P.; Jarra, W.; Pinheiro, L.; Rosario, V.E.D.; Thaithong, S.; Brown, K. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol. Biochem. Parasitol. 1993, 61, 315–320. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Methods and Techniques for Clinical Trials on Antimalarial Drug Efficacy: Genotyping to Identify Parasite Populations. In Amsterdam The Netherlands Geneva: Medicines for Malaria Venture Informal consultation.; World Health Organization: Geneva, Switzerland, 2007; 54p, Available online: https://iris.who.int/handle/10665/43824 (accessed on 12 December 2023).
- Snounou, G.; Zhu, X.; Siripoon, N.; Jarra, W.; Thaithong, S.; Brown, K.N.; Viriyakosol, S. Biased distribution of msp1 and msp2 allelic variants in Plasmodium falciparum populations in Thailand. Trans. R. Soc. Trop. Med. Hyg. 1999, 93, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Smythe, J.A.; Peterson, M.; Coppel, R.L.; Saul, A.J.; Kemp, D.J.; Anders, R.F. Structural diversity in the 45-kilodalton merozoite surface antigen of Plasmodium falciparum. Mol. Biochem. Parasitol. 1990, 39, 227–234. [Google Scholar] [CrossRef]
- Tanabe, K.; Mackay, M.; Goman, M.; Scaife, J.G. Allelic dimorphism in a surface antigen gene of the malaria parasite Plasmodium falciparum. J. Mol. Biol. 1987, 195, 273–287. [Google Scholar] [CrossRef]
- Ntoumi, F.; Contamin, H.; Rogier, C.; Bonnefoy, S.; Trape, J.-F.; Mercereau-Puijalon, O. Age-Dependent Carriage of Multiple Plasmodium falciparum Merozoite Surface Antigen-2 Alleles in Asymptomatic Malaria Infections. Am. J. Trop. Med. Hyg. 1995, 52, 81–88. [Google Scholar] [CrossRef]
- Felger, I.; Irion, A.; Steiger, S.; Beck, H.-P. 2. Genotypes of merozoite surface protein 2 of Plasmodium falciparum in Tanzania. Trans. R. Soc. Trop. Med. Hyg. 1999, 93, 3–9. [Google Scholar] [CrossRef]
- Ojurongbe, O.; Fagbenro-Beyioku, A.F.; A Adeyeba, O.; Kun, J.F. Allelic diversity of merozoite surface protein 2 gene of P falciparum among children in Osogbo, Nigeria. West Indian Med. J. 2011, 60, 19–23. [Google Scholar]
- Walldorf, J.A.; Cohee, L.M.; Coalson, J.E.; Bauleni, A.; Nkanaunena, K.; Kapito-Tembo, A.; Seydel, K.B.; Ali, D.; Mathanga, D.; Taylor, T.E.; et al. School-Age Children Are a Reservoir of Malaria Infection in Malawi. PLoS ONE 2015, 10, e0134061. [Google Scholar] [CrossRef]
- Cohee, L.M.; Opondo, C.; E Clarke, S.; E Halliday, K.; Cano, J.; Shipper, A.G.; Barger-Kamate, B.; Djimde, A.; Diarra, S.; Dokras, A.; et al. Preventive malaria treatment among school-aged children in sub-Saharan Africa: A systematic review and meta-analyses. Lancet Glob. Health 2020, 8, e1499–e1511. [Google Scholar] [CrossRef]
- Nankabirwa, J.; Brooker, S.J.; Clarke, S.E.; Fernando, D.; Gitonga, C.W.; Schellenberg, D.; Greenwood, B. Malaria in school-age children in Africa: An increasingly important challenge. Trop. Med. Int. Health 2014, 19, 1294–1309. [Google Scholar] [CrossRef]
- Sondo, P.; Derra, K.; Rouamba, T.; Diallo, S.N.; Taconet, P.; Kazienga, A.; Ilboudo, H.; Tahita, M.C.; Valéa, I.; Sorgho, H.; et al. Determinants of Plasmodium falciparum multiplicity of infection and genetic diversity in Burkina Faso. Parasites Vectors 2020, 13, 427. [Google Scholar] [CrossRef] [PubMed]
- Badoum, E.S.; Bougouma, E.C.; Sombie, S.; Sermé, S.S.; Yaro, J.B.; Diarra, A.; Nébié, I.; Ouedraogo, A.; Tiono, A.B.; Soulama, I.; et al. Relationship between human genetic factors and Plasmodium falciparum genetic diversity of msp1, msp2 and glurp in a malaria endemic area of Burkina Faso. Biomed. Genet. Genom. 2019, 4, 10-15761. [Google Scholar] [CrossRef]
- Soulama, I.; Nébié, I.; Ouédraogo, A.; Gansane, A.; Diarra, A.; Tiono, A.B.; Bougouma, E.C.; Konaté, A.T.; Kabré, G.B.; Taylor, W.R.; et al. Plasmodium falciparum genotypes diversity in symptomatic malaria of children living in an urban and a rural setting in Burkina Faso. Malar. J. 2009, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Somé, A.F.; Bazié, T.; Zongo, I.; Yerbanga, R.S.; Nikiéma, F.; Neya, C.; Taho, L.K.; Ouédraogo, J.-B. Plasmodium falciparum msp1 and msp2 genetic diversity and allele frequencies in parasites isolated from symptomatic malaria patients in Bobo-Dioulasso, Burkina Faso. Parasites Vectors 2018, 11, 328. [Google Scholar] [CrossRef]
- Tadele, G.; Jaiteh, F.K.; Oboh, M.; Oriero, E.; Dugassa, S.; Amambua-Ngwa, A.; Golassa, L. Low genetic diversity of Plasmodium falciparum merozoite surface protein 1 and 2 and multiplicity of infections in western Ethiopia following effective malaria interventions. Malar. J. 2022, 21, 383. [Google Scholar] [CrossRef]
- Konaté, L.; Zwetyenga, J.; Rogier, C.; Bischoff, E.; Fontenille, D.; Tall, A.; Spiegel, A.; Trape, J.-F.; Mercereau-Puijalon, O. 5. Variation of Plasmodium falciparum msp1 block 2 and msp2 allele prevalence and of infection complexity in two neighbouring Senegalese villages with different transmission conditions. Trans. R. Soc. Trop. Med. Hyg. 1999, 93, 21–28. [Google Scholar] [CrossRef]
- Zwetyenga, J.; Rogier, C.; Trape, J.F.; Snounou, G.; Fontenille, D.; Tall, A.; Mercereau-Puijalon, O. No influence of age on infection complexity and allelic distribution in Plasmodium falciparum infections in Ndiop, a Senegalese village with seasonal, mesoendemic malaria. Am. J. Trop. Med. Hyg. 1998, 59, 726–735. [Google Scholar] [CrossRef]
- Arzika, I.; Lamine, M.M.; Mahamadou, A.; Zamanka, H.; Laminou, I.M. Etude du polymorphisme genetique des souches de Plasmodium falciparum au Niger. Rev. CAMES SANTE 5 2017, 7, 42–48. [Google Scholar]
- Miles, A.; Iqbal, Z.; Vauterin, P.; Pearson, R.; Campino, S.; Theron, M.; Gould, K.; Mead, D.; Drury, E.; O’Brien, J.; et al. Indels, structural variation, and recombination drive genomic diversity in Plasmodium falciparum. Genome Res. 2016, 26, 1288–1299. [Google Scholar] [CrossRef]
- Funwei, R.I.; Thomas, B.N.; Falade, C.O.; Ojurongbe, O. Extensive diversity in the allelic frequency of Plasmodium falciparum merozoite surface proteins and glutamate-rich protein in rural and urban settings of southwestern Nigeria. Malar. J. 2018, 17, 1. [Google Scholar] [CrossRef]
- Chang, H.-H.; Childs, L.M.; Buckee, C.O. Variation in infection length and superinfection enhance selection efficiency in the human malaria parasite. Sci. Rep. 2016, 6, 26370. [Google Scholar] [CrossRef]
- Vafa, M.; Troye-Blomberg, M.; Anchang, J.; Garcia, A.; Migot-Nabias, F. Multiplicity of Plasmodium falciparum infection in asymptomatic children in Senegal: Relation to transmission, age and erythrocyte variants. Malar. J. 2008, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Ranford-Cartwright, L.C.; Balfe, P.; Carter, R.; Walliker, D. Frequency of cross-fertilization in the human malaria parasite Plasmodium falciparum. Parasitology 1993, 107, 11–18. [Google Scholar] [CrossRef]
- Hastings, I.M.; Watkins, W.M. Intensity of malaria transmission and the evolution of drug resistance. Acta Trop. 2005, 94, 218–229. [Google Scholar] [CrossRef]
- Patgiri, S.J.; Sarma, K.; Sarmah, N.; Bhattacharyya, N.; Sarma, D.K.; Nirmolia, T.; Bhattacharyya, D.R.; Mohapatra, P.K.; Bansal, D.; Bharti, P.K.; et al. Characterization of drug resistance and genetic diversity of Plasmodium falciparum parasites from Tripura, Northeast India. Sci. Rep. 2019, 9, 13704. [Google Scholar] [CrossRef]
- Port, G.R.; Boreham, P.F.L.; Bryan, J.H. The relationship of host size to feeding by mosquitoes of the Anopheles gambiae Giles complex (Diptera: Culicidae). Bull. Entomol. Res. 1980, 70, 133–144. [Google Scholar] [CrossRef]
- Smith, T.; Killeen, G.F.; Maire, N.; Dietz, K.; Molineaux, L.; Vounatsou, P.; Tanner, M. Relationship between the entomologic inoculation rate and the force of infection for Plasmodium falciparum malaria. Am. J. Trop. Med. Hyg. 2006, 75, 11–18. [Google Scholar] [CrossRef]
- Yaro, J.B.; Ouedraogo, A.; Ouedraogo, Z.A.; Diarra, A.; Lankouande, M.; Agboraw, E.; Worrall, E.; Toe, K.H.; Sanou, A.; Guelbeogo, W.M.; et al. A cohort study to identify risk factors for Plasmodium falciparum infection in Burkinabe children: Implications for other high burden high impact countries. Malar. J. 2020, 19, 371. [Google Scholar] [CrossRef]
- Rogier, C.; Henry, M.C.; Spiegel, A. Diagnostic des accès palustres en zone d’endémie: Bases théoriques et implications pratiques. Med. Trop. 2001, 61, 27–46. Available online: https://www.jle.com/en/MedSanteTrop/2001/61.1/027-046 Diagnostic des accès palustres en zone d’endémie bases théoriques et implications pratiques (Rogier).pdf (accessed on 12 December 2023).
- Ullah, I.; Khan, A.; Israr, M.; Shah, M.; Shams, S.; Khan, W.; Shah, M.; Siraj, M.; Akbar, K.; Naz, T.; et al. Genomic miscellany and allelic frequencies of Plasmodium falciparum msp-1, msp-2 and glurp in parasite isolates. PLoS ONE 2022, 17, e0264654. [Google Scholar] [CrossRef]
- Boyce, R.M.; Hathaway, N.; Fulton, T.; Reyes, R.; Matte, M.; Ntaro, M.; Mulogo, E.; Waltmann, A.; Bailey, J.A.; Siedner, M.J.; et al. Reuse of malaria rapid diagnostic tests for amplicon deep sequencing to estimate Plasmodium falciparum transmission intensity in western Uganda. Sci. Rep. 2018, 8, 10159. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.T.; Hathaway, N.J.; Saunders, D.L.; Lon, C.; Balasubramanian, S.; Kharabora, O.; Gosi, P.; Sriwichai, S.; Kartchner, L.; Chuor, C.M.; et al. Using Amplicon Deep Sequencing to Detect Genetic Signatures of Plasmodium vivax Relapse. J. Infect. Dis. 2015, 212, 999–1008. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).