Genetic Analysis of H5N1 High-Pathogenicity Avian Influenza Virus following a Mass Mortality Event in Wild Geese on the Solway Firth
Abstract
1. Introduction
2. Materials and Methods
2.1. Virological Investigation
2.2. Whole-Genome Sequencing and Phylogenetic Analysis
2.3. Serological Analysis
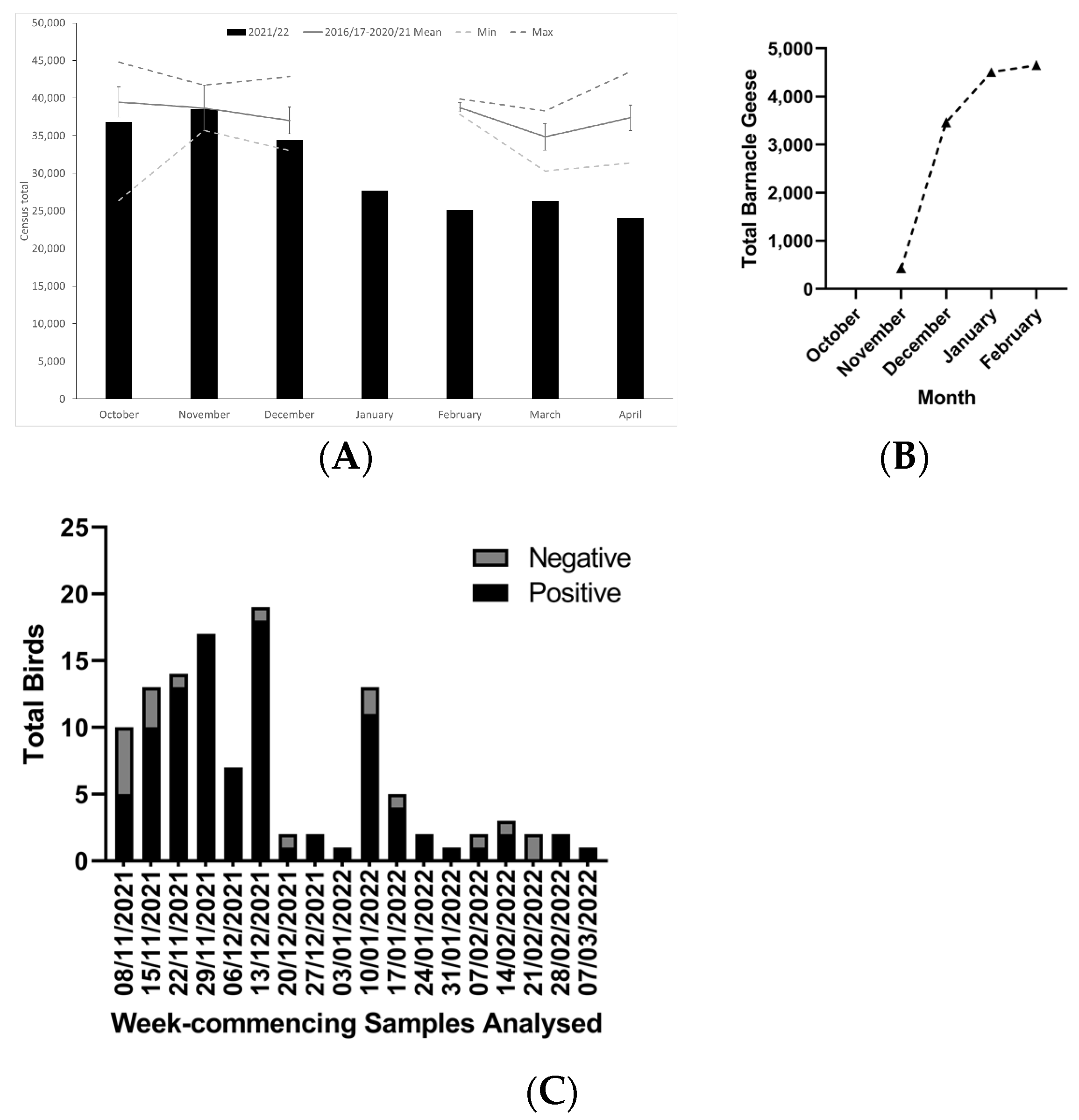
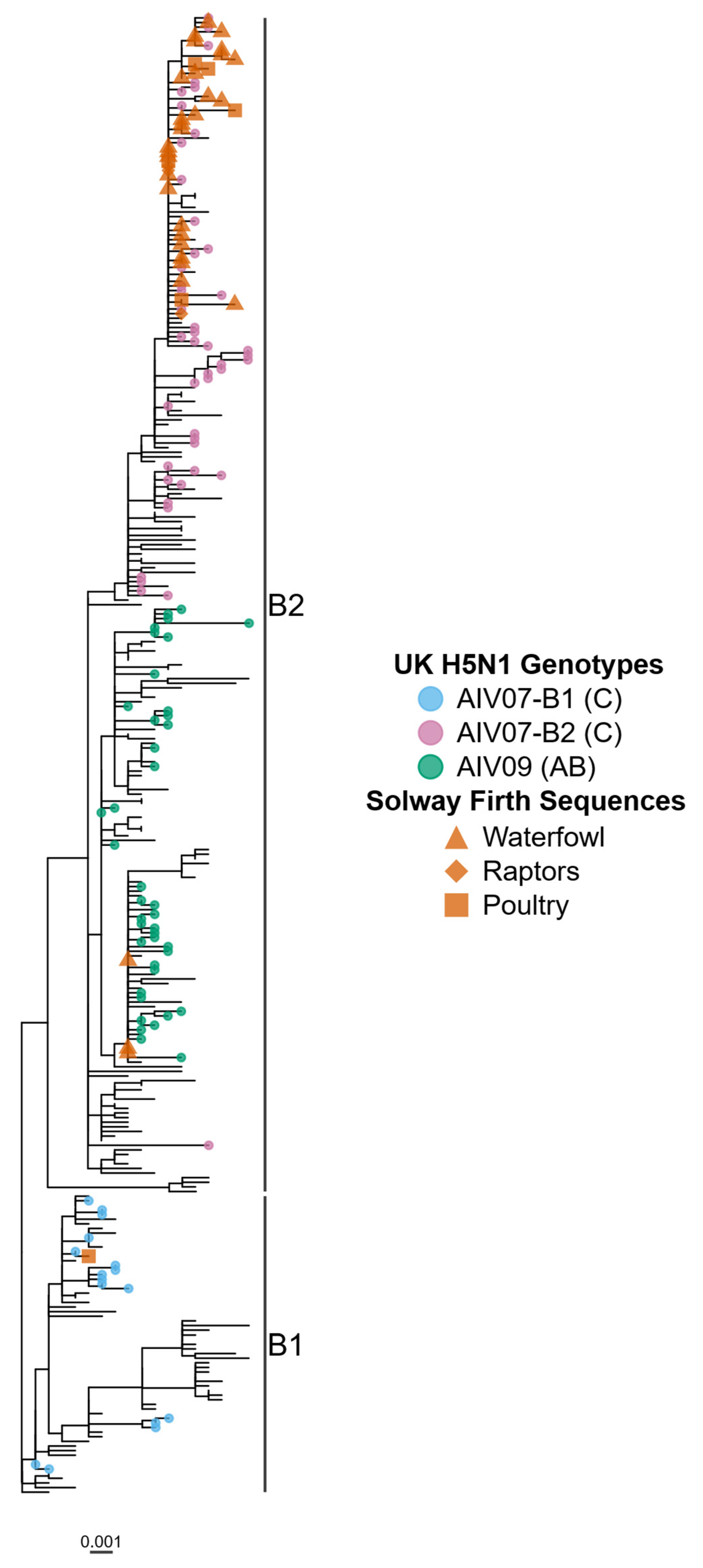
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Falchieri, M.; Reid, S.M.; Ross, C.S.; James, J.; Byrne, A.M.P.; Zamfir, M.; Brown, I.H.; Banyard, A.C.; Tyler, G.; Philip, E.; et al. Shift in HPAI Infection Dynamics Causes Significant Losses in Seabird Populations across Great Britain. Vet. Rec. 2022, 191, 294–296. [Google Scholar] [CrossRef]
- Banyard, A.C.; Lean, F.Z.X.; Robinson, C.; Howie, F.; Tyler, G.; Nisbet, C.; Seekings, J.; Meyer, S.; Whittard, E.; Ashpitel, H.F.; et al. Detection of Highly Pathogenic Avian Influenza Virus H5N1 Clade 2.3.4.4b in Great Skuas: A Species of Conservation Concern in Great Britain. Viruses 2022, 14, 212. [Google Scholar] [CrossRef]
- Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Mirinavičiūtė, G.; Niqueux, É.; Staubach, C.; Terregino, C.; Baldinelli, F.; Rusinà, A.; et al. Avian Influenza Overview June-September 2023. EFSA J. Eur. Food Saf. Auth. 2023, 21, e08328. [Google Scholar] [CrossRef]
- Animal and Plant Health Agency Highly Pathogenic Avian Influenza (HPAI) in the UK and Europe. Available online: https://assets.publishing.service.gov.uk/media/634d89e2e90e0731a8008917/HPAI_Europe__34_10_October_2022_FINAL__003_.pdf (accessed on 8 January 2024).
- Byrne, A.M.P.; James, J.; Mollett, B.C.; Meyer, S.M.; Lewis, T.; Czepiel, M.; Seekings, A.H.; Mahmood, S.; Thomas, S.S.; Ross, C.S.; et al. Investigating the Genetic Diversity of H5 Avian Influenza Viruses in the United Kingdom from 2020–2022. Microbiol. Spectr. 2023, 11, e0477622. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.H.; Madsen, J.; Nagy, S.; Lewis, M. AEWA International Single Species Management Plan for the Barnacle Goose (Branta Leucopsis): Russia/Germany & Netherlands Population, East Greenland/Scotland & Ireland Population, Svalbard/South-West Scotland Population. AEWA Tech. Ser. 2018, 63, 94. [Google Scholar]
- Griffin, L. Svalbard Barnacle Goose Distribution around the Solway Firth 2021–2022: Flock Counts from the Solway Goose Management Scheme Area. Final Report to NatureScot; Prepared by ECO-LG Ltd.: Dumfries, UK, 2022. [Google Scholar]
- Slomka, M.J.; Pavlidis, T.; Coward Vivien, V.J. J.; Voermans, J.; Koch, G.; Hanna, A.; Banks, J.; Brown, I.H. Validated RealTime Reverse Transcriptase PCR Methods for the Diagnosis and Pathotyping of Eurasian H7 Avian Influenza Viruses. Influenza Other Respi. Viruses 2009, 3, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Slomka, M.J.; Puranik, A.; Mahmood, S.; Thomas, S.S.; Seekings, A.H.; Byrne, A.M.P.; Núñez, A.; Bianco, C.; Mollett, B.C.; Watson, S.; et al. Ducks Are Susceptible to Infection with a Range of Doses of H5n8 Highly Pathogenic Avian Influenza Virus (2016, Clade 2.3.4.4b) and Are Largely Resistant to Virus-Specific Mortality, but Efficiently Transmit Infection to Contact Turkeys. Avian Dis. 2019, 63, 172–180. [Google Scholar] [CrossRef]
- Nagy, A.; Černíková, L.; Kunteová, K.; Dirbáková, Z.; Thomas, S.S.; Slomka, M.J.; Dán, Á.; Varga, T.; Máté, M.; Jiřincová, H.; et al. A Universal RT-QPCR Assay for “One Health” Detection of Influenza A Viruses. PLoS ONE 2021, 16, e0244669. [Google Scholar] [CrossRef]
- Slomka, M.J.; To, T.L.; Tong, H.H.; Coward, V.J.; Hanna, A.; Shell, W.; Pavlidis, T.; Densham, A.L.E.; Kargiolakis, G.; Arnold, M.E.; et al. Challenges for Accurate and Prompt Molecular Diagnosis of Clades of Highly Pathogenic Avian Influenza H5N1 Viruses Emerging in Vietnam. Avian Pathol. 2012, 41, 177–193. [Google Scholar] [CrossRef]
- James, J.; Slomka, M.J.; Reid, S.M.; Thomas, S.S.; Mahmood, S.; Byrne, A.M.P.; Cooper, J.; Russell, C.; Mollett, B.C.; Agyeman-Dua, E.; et al. Development and Application of Real-Time PCR Assays for Specific Detection of Contemporary Avian Influenza Virus Subtypes N5, N6, N7, N8, and N9. Avian Dis. 2018, 63, 209–218. [Google Scholar] [CrossRef]
- WOAH Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2021. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2021; World Organisation for Animal Health: Paris, France, 2021.
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Larsson, A. AliView: A Fast and Lightweight Alignment Viewer and Editor for Large Datasets. Bioinformatics 2014, 30, 3276–3278. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R.; Teeling, E. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast Model Selection for Accurate Phylogenetic Estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.T.; Chernomor, O.; Von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- Lean, F.Z.X.; Leblond, A.L.; Byrne, A.M.P.; Mollett, B.; James, J.; Watson, S.; Hurley, S.; Brookes, S.M.; Weber, A.; Núñez, A. Subclinical Hepatitis E Virus Infection in Laboratory Ferrets in the UK. J. Gen. Virol. 2022, 103, 001803. [Google Scholar] [CrossRef]
- Griffin, L. Svalbard Barnacle Goose Distribution around the Solway Firth 2016–2017: Flock Counts from the Solway Goose Management Scheme Area; ECO-LG Ltd.: Dumfries, UK, 2017. [Google Scholar]
- Lewis, N.S.; Banyard, A.C.; Whittard, E.; Karibayev, T.; Al Kafagi, T.; Chvala, I.; Byrne, A.; Meruyert, S.; King, J.; Harder, T.; et al. Emergence and Spread of Novel H5N8, H5N5 and H5N1 Clade 2.3.4.4 Highly Pathogenic Avian Influenza in 2020. Emerg. Microbes Infect. 2021, 10, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Pohlmann, A.; King, J.; Fusaro, A.; Zecchin, B.; Banyard, A.C.; Brown, I.H.; Byrne, A.M.P.; Beerens, N.; Liang, Y.; Heutink, R.; et al. Has Epizootic Become Enzootic? Evidence for a Fundamental Change in the Infection Dynamics of Highly Pathogenic Avian Influenza in Europe. 2021. mBio 2022, 13, e00609-22. [Google Scholar] [CrossRef]
- Lean, F.Z.X.; Núñez, A.; Banyard, A.C.; Reid, S.M.; Brown, I.H.; Hansen, R.D.E. Gross Pathology Associated with Highly Pathogenic Avian Influenza H5N8 and H5N1 in Naturally Infected Birds in the UK (2020–2021). Vet Rec. 2021, 190, e731. [Google Scholar] [CrossRef]
- Caliendo, V.; Leijten, L.; van de Bildt, M.W.G.; Poen, M.J.; Kok, A.; Bestebroer, T.; Richard, M.; Fouchier, R.A.M.; Kuiken, T. Long-Term Protective Effect of Serial Infections with H5N8 Highly Pathogenic Avian Influenza Virus in Wild Ducks. J. Virol. 2022, 96, e0123322. [Google Scholar] [CrossRef]
- Lane, J.V.; Jeglinski, J.W.E.; Avery-Gomm, S.; Ballstaedt, E.; Banyard, A.C.; Barychka, T.; Brown, I.H.; Brugger, B.; Burt, T.V.; Careen, N.; et al. High Pathogenicity Avian Influenza (H5N1) in Northern Gannets: Global Spread, Clinical Signs, and Demographic Consequences. IBIS-Int. J. Avian Sci. 2023. [Google Scholar] [CrossRef]
- Pohlmann, A.; Stejskal, O.; King, J.; Bouwhuis, S.; Packmor, F.; Ballstaedt, E.; Hälterlein, B.; Hennig, V.; Stacker, L.; Graaf, A.; et al. Mass Mortality among Colony-Breeding Seabirds in the German Wadden Sea in 2022 Due to Distinct Genotypes of HPAIV H5N1 Clade 2.3.4.4b. J. Gen. Virol. 2023, 104, 001834. [Google Scholar] [CrossRef] [PubMed]
- Rijks, J.M.; Leopold, M.F.; Kühn, S.; in ’t Veld, R.; Schenk, F.; Brenninkmeijer, A.; Lilipaly, S.J.; Ballmann, M.Z.; Kelder, L.; de Jong, J.W.; et al. Mass Mortality Caused by Highly Pathogenic Influenza A(H5N1) Virus in Sandwich Terns, the Netherlands, 2022. Emerg. Infect. Dis. 2022, 28, 2538–2542. [Google Scholar] [CrossRef] [PubMed]
- Stanbury, A.; Eaton, M.; Aebischer, N.; Balmer, D.; Brown, A.; Douse, A.; Lindley, P.; McCulloch, N.; Noble, D.; Win, I. The Status of Our Bird Populations: The Fifth Birds of Conservation Concern in the United Kingdom, Channel Islands and Isle of Man and Second IUCN Red List Assessment of Extinction Risk for Great Britain. Br. Birds 2021, 114, 723–747. [Google Scholar]
- Duff, P.; Holmes, P.; Aegerter, J.; Man, C.; Fullick, E.; Reid, S.; Lean, F.; Núñez, A.; Hansen, R.; Tye, J.; et al. Investigations Associated with the 2020/21 Highly Pathogenic Avian Influenza Epizootic in Wild Birds in Great Britain. Vet. Rec. 2021, 189, 356–358. [Google Scholar] [CrossRef]
- British Trust for Ornithology. Ringing and Nest Recording Report. Available online: https://app.bto.org/ring/countyrec/results2022/longevity.htm (accessed on 8 January 2024).

| Town | Country | Date Collected | Species | Sample ID | GISAID Ref | Age |
|---|---|---|---|---|---|---|
| Carlisle | England | 8 November 2021 | Barnacle Goose | A/barnacle_goose/England/292151/2021 | EPI_ISL_13369744 | Immature |
| Caerlaverock | Scotland | 9 November 2021 | Whooper Swan | A/Whooper_swan/Scotland/056219/2021 | EPI_ISL_9029962 | Unknown |
| Caerlaverock | Scotland | 12 November 2021 | Barnacle Goose | A/barnacle_goose/Scotland/292583/2021 | EPI_ISL_13369753 | Adult |
| Annan | Scotland | 16 November 2021 | Barnacle Goose | A/Barnacle_goose/Scotland/058494/2021 | EPI_ISL_13453561 | Adult |
| Caerlaverock | Scotland | 16 November 2021 | Common Kestrel | A/Kestrel/Scotland/058512/2021 | EPI_ISL_13369761 | Immature |
| Castle Douglas | Scotland | 16 November 2021 | Pink-footed goose | A/pink-footed_goose/Scotland/058488/2021 | EPI_ISL_13369760 | Adult |
| Caerlaverock | Scotland | 17 November 2021 | Barnacle Goose | A/barnacle_goose/England/061307/2021 | EPI_ISL_13369762 | Adult |
| Ruthwell | Scotland | 19 November 2021 | Barnacle Goose | A/barnacle_goose/Scotland/062003/2021 | EPI_ISL_13369765 | Adult |
| Annan | Scotland | 19 November 2021 | Barnacle Goose | A/barnacle_goose/Scotland/062007/2021 | EPI_ISL_13369766 | Adult |
| Silloth | England | 26 November 2021 | Barnacle Goose | A/barnacle_goose/England/293824/2021 | EPI_ISL_13369779 | Adult |
| Carlisle | England | 26 November 2021 | Barnacle Goose | A/barnacle_goose/England/293809/2021 | EPI_ISL_13369780 | Adult |
| Kirkcudbright | Scotland | 30 November 2021 | Barnacle Goose | A/barnacle_goose/Scotland/064368/2021 | EPI_ISL_13370417 | Adult |
| Kirkcolm | Scotland | 1 December 2021 | Common Buzzard | A/common_buzzard/Scotland/294149/2021 | EPI_ISL_13370418 | Adult |
| Allerdale | England | 1 December 2021 | Mute Swan | A/Mute_Swan/England/294146/2021 | EPI_ISL_13370506 | Adult |
| Silloth | England | 4 December 2021 | Barnacle Goose | A/barnacle_goose/England/294327/2021 | EPI_ISL_13370517 | Adult |
| Annan | Scotland | 4 December 2021 | Barnacle Goose | A/Barnacle_goose/Scotland/075760/2021 | EPI_ISL_13486748 | Adult |
| Caerlaverock | Scotland | 4 December 2021 | Barnacle Goose | A/Barnacle_goose/Scotland/075827/2021 | EPI_ISL_13486760 | Adult |
| Annan | Scotland | 4 December 2021 | Mute Swan | A/Mute_swan/Scotland/075821/2021 | EPI_ISL_13486881 | Adult |
| Annan | Scotland | 6 December 2021 | Barnacle Goose | A/barnacle_goose/Scotland/072143/2021 | EPI_ISL_13370522 | Adult |
| Allerdale | England | 9 December 2021 | Barnacle Goose | A/barnacle_goose/England/294620/2021 | EPI_ISL_13370561 | Adult |
| Caerlaverock | Scotland | 10 December 2021 | Barnacle Goose | A/Barnacle_goose/Scotland/075563/2021 | EPI_ISL_13486738 | Adult |
| Caerlaverock | Scotland | 11 December 2021 | Barnacle Goose | A/Barnacle_goose/Scotland/072168/2021 | EPI_ISL_13486729 | Adult |
| Caerlaverock | Scotland | 11 December 2021 | Whooper Swan | A/Whooper_swan/Scotland/072171/2021 | EPI_ISL_13486975 | Adult |
| Dumfries | Scotland | 13 December 2021 | Barnacle Goose | A/Barnacle_goose/Scotland/072140/2021 | EPI_ISL_13453562 | Adult |
| Annan | Scotland | 13 December 2021 | Barnacle Goose | A/Barnacle_goose/Scotland/072152/2021 | EPI_ISL_13453563 | Adult |
| Thornhill | Scotland | 15 December 2021 | Common Buzzard | A/Common_buzzard/Scotland/073099/2021 | EPI_ISL_13486819 | Adult |
| Dumfries | Scotland | 17 December 2021 | Barnacle Goose | A/Barnacle_goose/Scotland/109090/2021 | EPI_ISL_13486785 | Adult |
| Dumfries | Scotland | 17 December 2021 | Barnacle Goose | A/Barnacle_goose/Scotland/109105/2021 | EPI_ISL_13486796 | Adult |
| Ruthwell | Scotland | 27 December 2021 | Barnacle Goose | A/Barnacle_goose/Scotland/003169/2021 | EPI_ISL_13486688 | Adult |
| Dumfries | Scotland | 30 December 2021 | Barnacle Goose | A/Barnacle_goose/Scotland/076711/2021 | EPI_ISL_13486772 | Adult |
| Clarencefield | Scotland | 31 December 2021 | Barnacle Goose | A/Barnacle_goose/Scotland/003588/2022 | EPI_ISL_13486713 | Adult |
| Port Logan | Scotland | 2 January 2022 | Barnacle Goose | A/Barnacle_goose/Scotland/003579/2022 | EPI_ISL_13486700 | Adult |
| Troqueer | Scotland | 8 January 2022 | Pink-footed goose | A/pink-footed_goose/Scotland/003617/2022 | EPI_ISL_13782461 | Adult |
| Species | Number Tested | Positive | % Positivity | Number of Samples Submitted for WGS |
|---|---|---|---|---|
| Barnacle Goose (Branta leucopsis) | 43 | 41 | 95.35 | 24 |
| Mute Swan (Cygnus olor) | 20 | 17 | 85 | 2 |
| Common Buzzard (Buteo buteo) | 12 | 11 | 91.67 | 2 |
| Whooper Swan (Cygnus cygnus) | 7 | 7 | 100 | 2 |
| Pink-footed Goose (Anser brachyrhynchus) | 6 | 5 | 83.33 | 2 |
| Greylag Goose (Anser anser) | 5 | 5 | 100 | |
| Pheasant (Phasianus colchicus) | 3 | 2 | 66.67 | |
| Common Starling (Sturnus vulgaris) | 3 | 0 | 0 | |
| Canada goose (Branta canadensis) | 2 | 2 | 100 | |
| Indian runner duck (Anas platyrhynchos domesticus) | 2 | 0 | 0 | |
| Kestrel (Falco tinnunculus) | 2 | 1 | 50 | 1 |
| Hen Harrier (Circus cyaneus) | 1 | 0 | 0 | |
| Herring Gull (Larus argentatus) | 1 | 0 | 0 | |
| Eurasian Sparrowhawk (Accipiter nisus) | 1 | 0 | 0 | |
| Tawny Owl (Strix aluco) | 1 | 0 | 0 | |
| Goose unspecified | 5 | 5 | 100 | |
| Gull unspecified | 1 | 1 | 100 | |
| Swan unspecified | 1 | 1 | 100 | |
| Grand Total | 116 | 98 | 33 |
| Sample Number | H5N1 | H5N8 | H5N3 | Sex | |||
|---|---|---|---|---|---|---|---|
| Titre | Result | Titre | Result | Titre | Result | ||
| 1 | <2 | Negative | 8 | Negative | 128 | Positive | F |
| 2 | 8 | Negative | 16 | Positive | 32 | Positive | F |
| 3 | 16 | Positive | 32 | Positive | 64 | Positive | F |
| 4 | 128 | Positive | 64 | Positive | 256 | Positive | M |
| 5 | <2 | Negative | 2 | Negative | 32 | Positive | F |
| 6 | 2 | Negative | 8 | Negative | 512 | Positive | F |
| 7 | 2 | Negative | 8 | Negative | 64 | Positive | F |
| 8 | 2 | Negative | 32 | Positive | 256 | Positive | F |
| 9 | 16 | Positive | 8 | Negative | 4 | Negative | M |
| 10 | 8 | Negative | 8 | Negative | 512 | Positive | M |
| 11 | 128 | Positive | 32 | Positive | 512 | Positive | F |
| 12 | <2 | Negative | 16 | Positive | 32 | Positive | F |
| 13 | IS | ND | IS | ND | IS | ND | M |
| 14 | IS | ND | IS | ND | IS | ND | M |
| 15 | <2 | Negative | <2 | Negative | <2 | Negative | M |
| 16 | <2 | Negative | <2 | Negative | 256 | Positive | M |
| 17 | <2 | Negative | 32 | Positive | 256 | Positive | F |
| 18 | 4 | Negative | 32 | Positive | 64 | Positive | M |
| 19 | 2 | Negative | 8 | Negative | 8 | Negative | F |
| 20 | <2 | Negative | <2 | Negative | 32 | Positive | F |
| 21 | <2 | Negative | <2 | Negative | <2 | Negative | M |
| 22 | <2 | Negative | <2 | Negative | <2 | Negative | M |
| 23 | 8 | Negative | 32 | Positive | 128 | Positive | M |
| 24 | <2 | Negative | 8 | Negative | <2 | Negative | F |
| 25 | 64 | Positive | 64 | Positive | 128 | Positive | M |
| 26 | <2 | Negative | <2 | Negative | <2 | Negative | M |
| 27 | 32 | Positive | 16 | Positive | 64 | Positive | M |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ross, C.S.; Byrne, A.M.P.; Mahmood, S.; Thomas, S.; Reid, S.; Freath, L.; Griffin, L.R.; Falchieri, M.; Holmes, P.; Goldsmith, N.; et al. Genetic Analysis of H5N1 High-Pathogenicity Avian Influenza Virus following a Mass Mortality Event in Wild Geese on the Solway Firth. Pathogens 2024, 13, 83. https://doi.org/10.3390/pathogens13010083
Ross CS, Byrne AMP, Mahmood S, Thomas S, Reid S, Freath L, Griffin LR, Falchieri M, Holmes P, Goldsmith N, et al. Genetic Analysis of H5N1 High-Pathogenicity Avian Influenza Virus following a Mass Mortality Event in Wild Geese on the Solway Firth. Pathogens. 2024; 13(1):83. https://doi.org/10.3390/pathogens13010083
Chicago/Turabian StyleRoss, Craig S., Alexander M. P. Byrne, Sahar Mahmood, Saumya Thomas, Scott Reid, Lorna Freath, Larry R. Griffin, Marco Falchieri, Paul Holmes, Nick Goldsmith, and et al. 2024. "Genetic Analysis of H5N1 High-Pathogenicity Avian Influenza Virus following a Mass Mortality Event in Wild Geese on the Solway Firth" Pathogens 13, no. 1: 83. https://doi.org/10.3390/pathogens13010083
APA StyleRoss, C. S., Byrne, A. M. P., Mahmood, S., Thomas, S., Reid, S., Freath, L., Griffin, L. R., Falchieri, M., Holmes, P., Goldsmith, N., Shaw, J. M., MacGugan, A., Aegerter, J., Hansen, R., Brown, I. H., & Banyard, A. C. (2024). Genetic Analysis of H5N1 High-Pathogenicity Avian Influenza Virus following a Mass Mortality Event in Wild Geese on the Solway Firth. Pathogens, 13(1), 83. https://doi.org/10.3390/pathogens13010083







