Polyamine Metabolism for Drug Intervention in Trypanosomatids
Abstract
1. Introduction
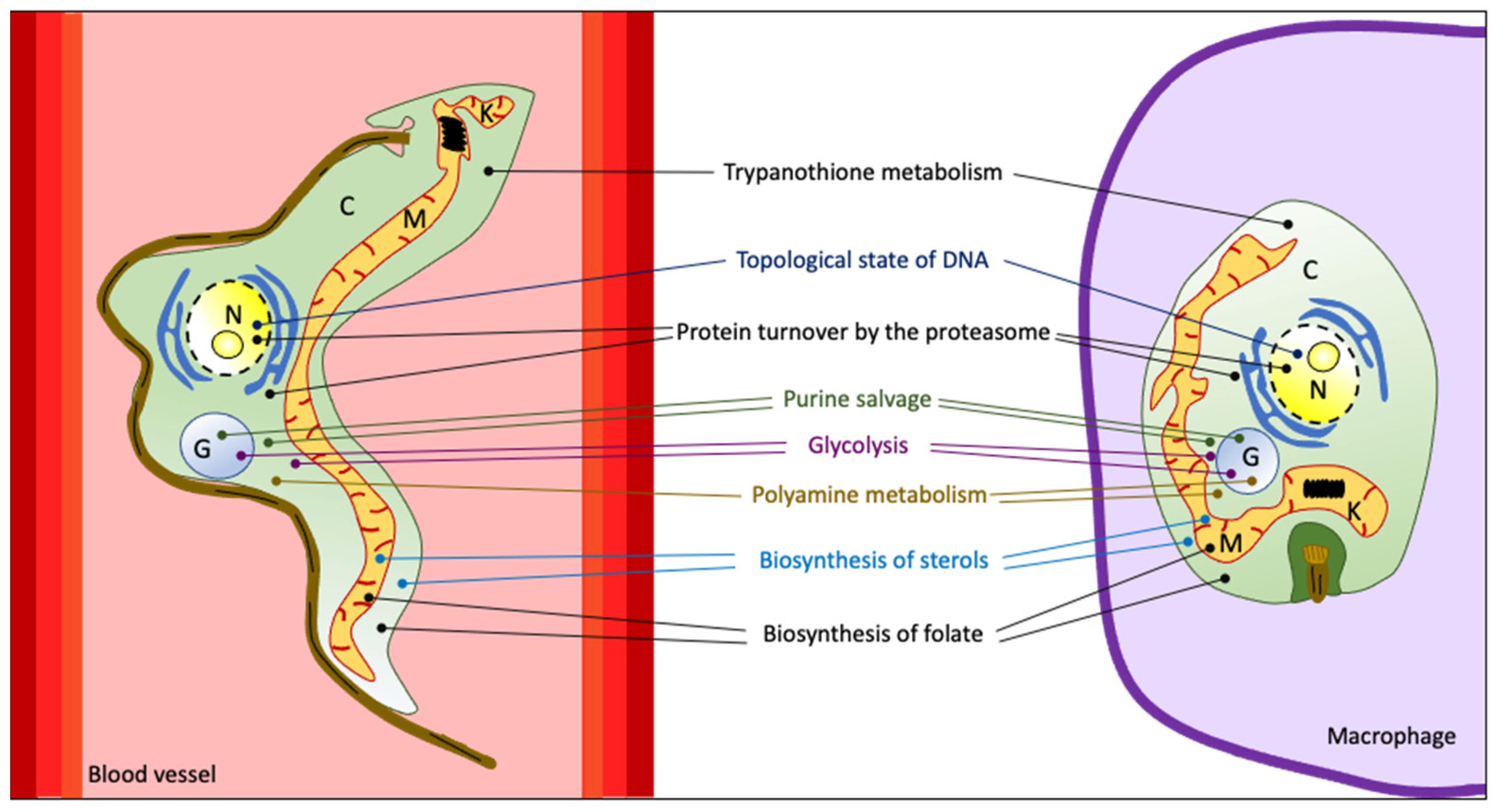
2. Druggable Targets in Trypanosomatids
2.1. Biosynthesis of Sterols
2.2. Glycolysis
2.3. Purine Salvage Pathway
2.4. Modification of the Topological State of DNA
2.5. Biosynthesis of Folate
2.6. Protein Turnover by the Proteasome
2.7. Polyamine and Redox Metabolism
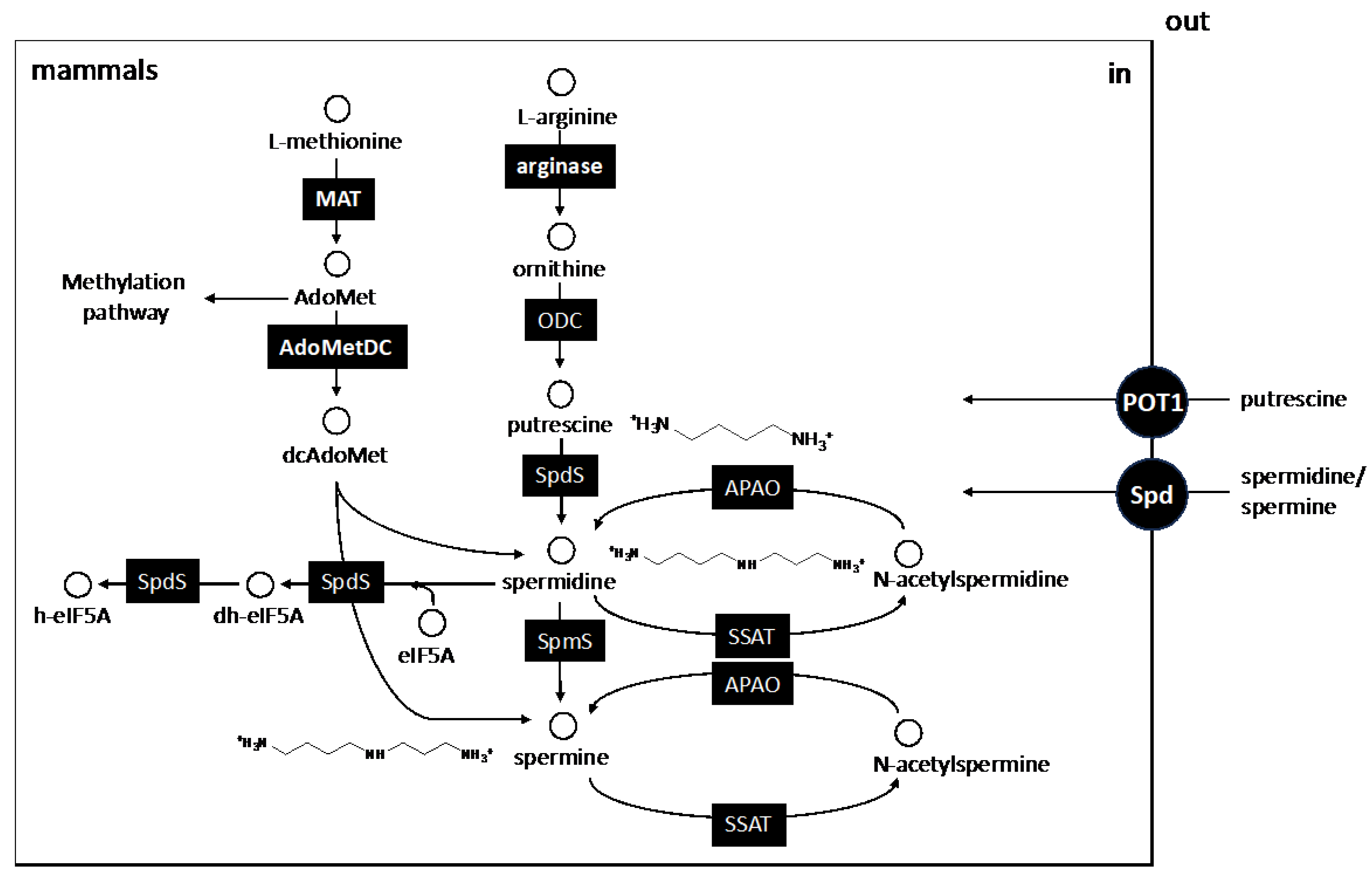
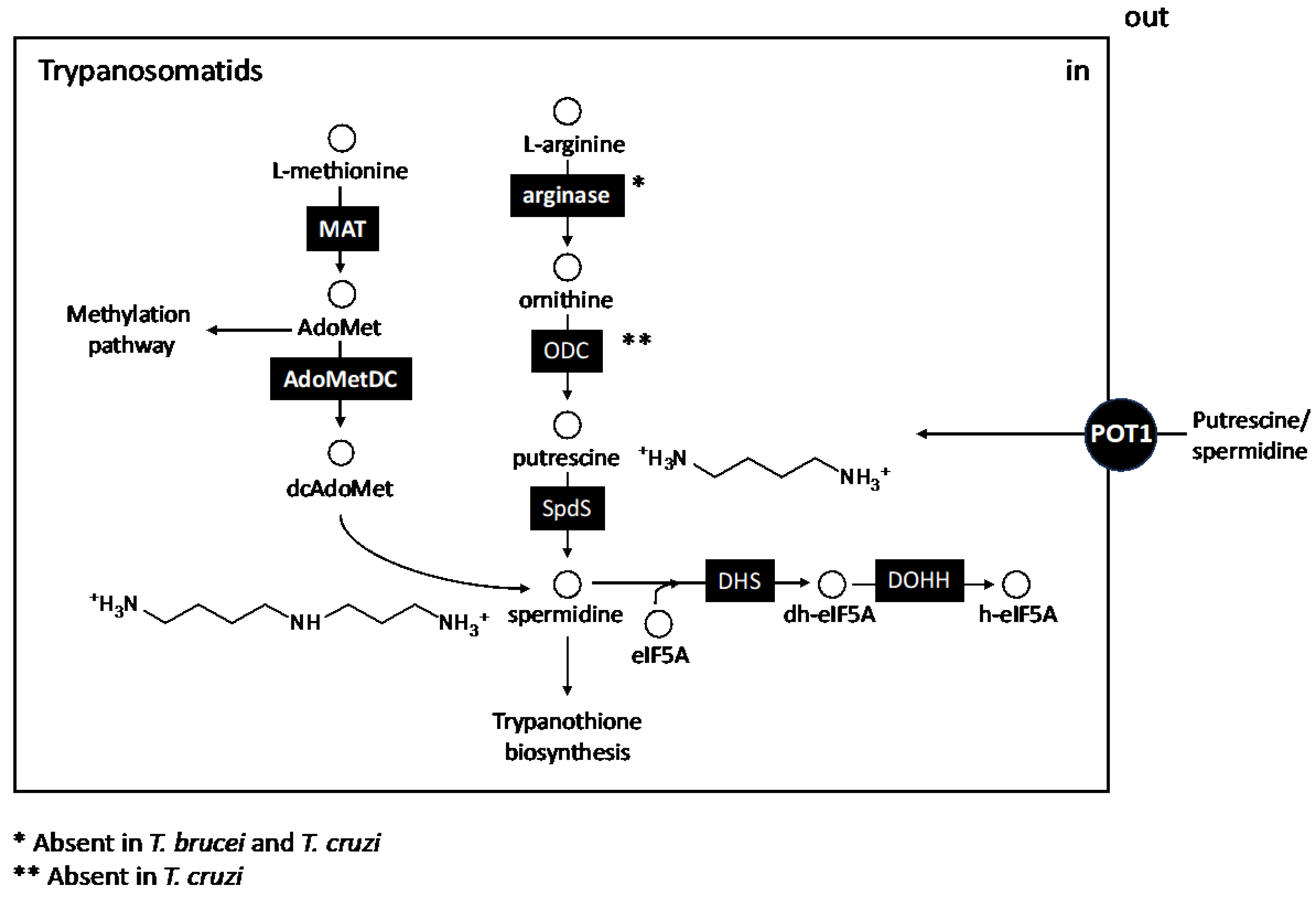
3. The Biosynthetic Core of Polyamines
4. Transport of Polyamines
5. Other Polyamine-Linked Pathways
5.1. S-Adenosilmethionine Synthesis: Transulfuration and Transmethylation Pathways
5.2. Synthesis of Hypusine
5.3. Parasite Arginase/Host NO Synthesis Interplay
5.4. Synthesis and Redox Metabolism of Trypanothione
6. Inhibitors of Polyamine Synthesis in Trypanosomatids
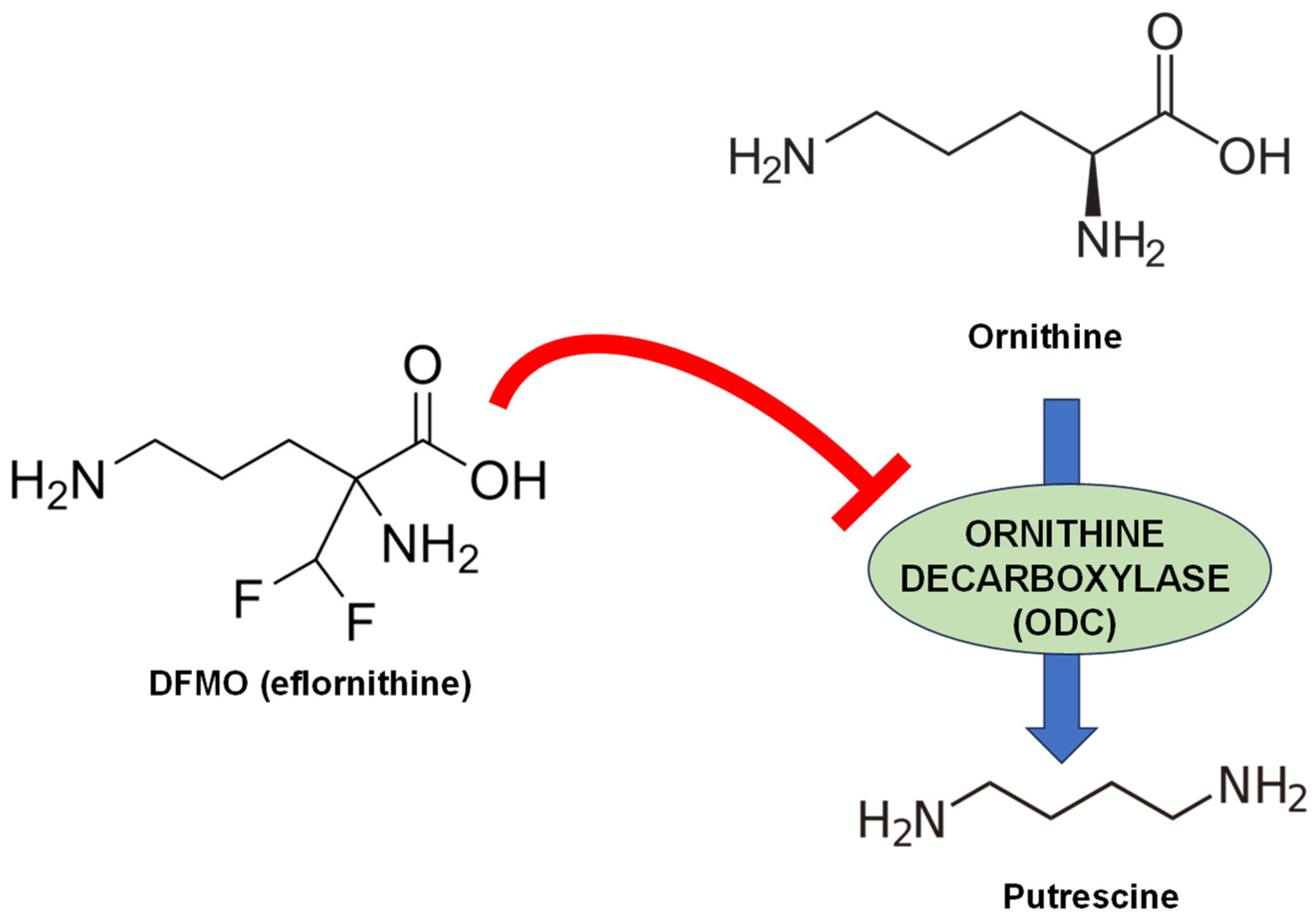
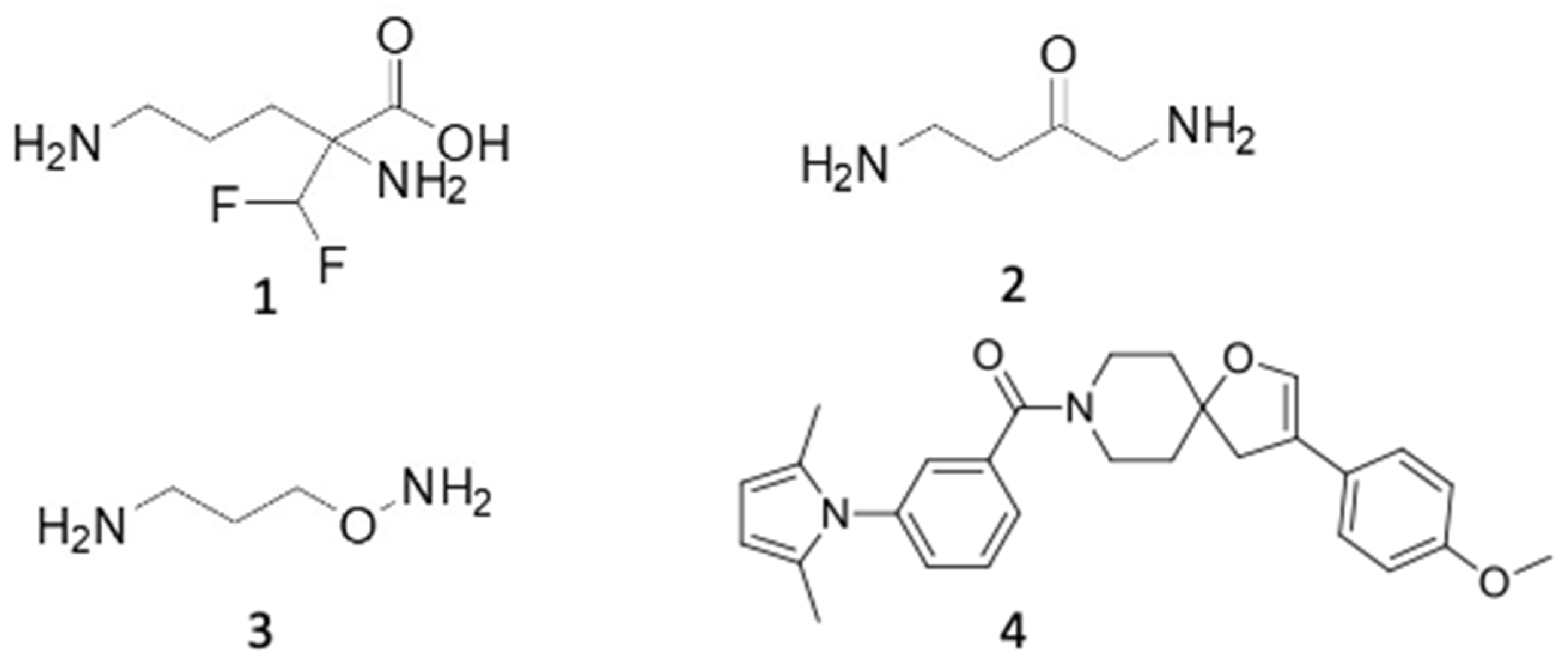
6.1. ODC Inhibitors
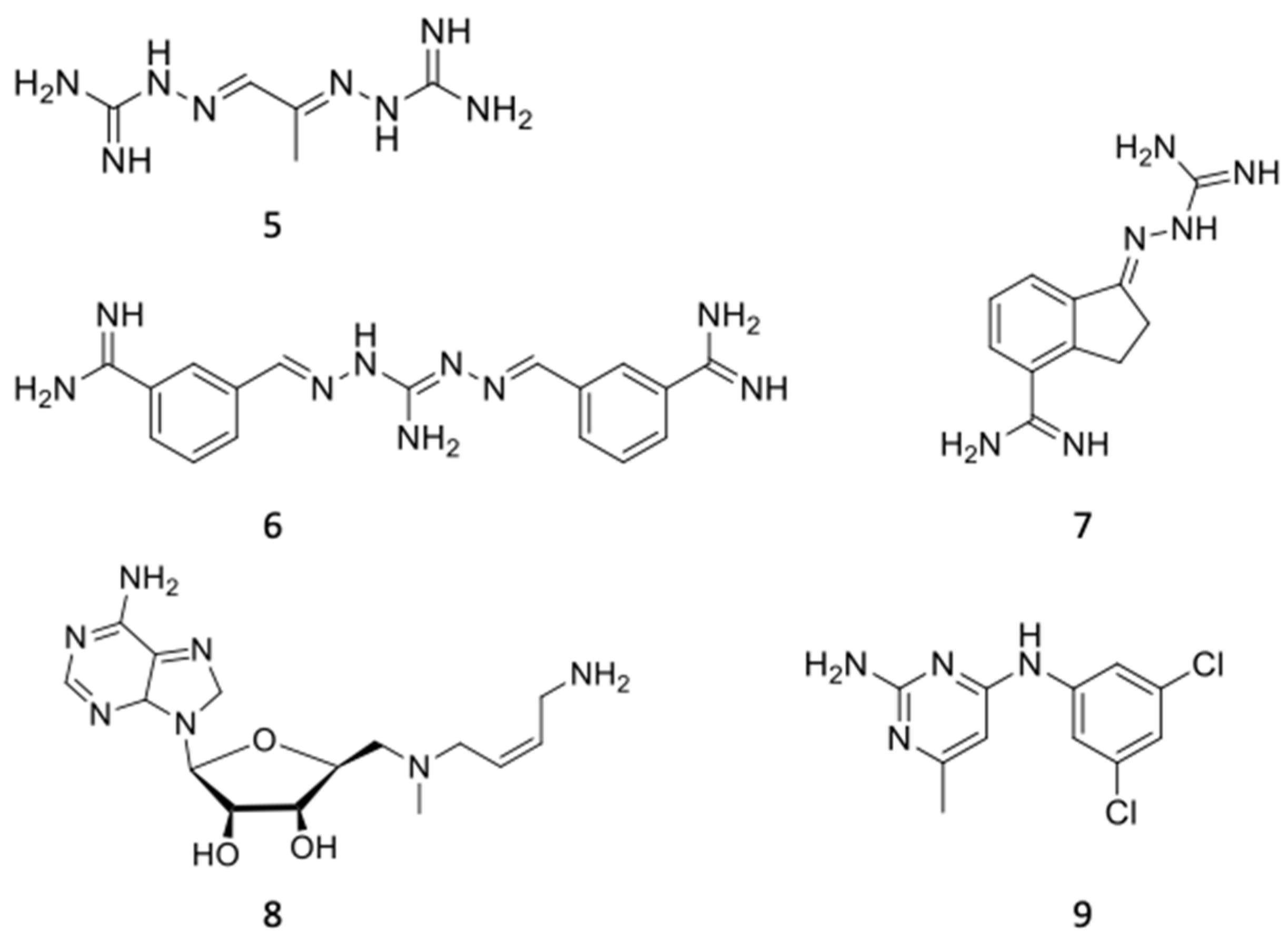
6.2. AdoMetDC Inhibitors
6.3. Inhibition of Spermidine Synthase
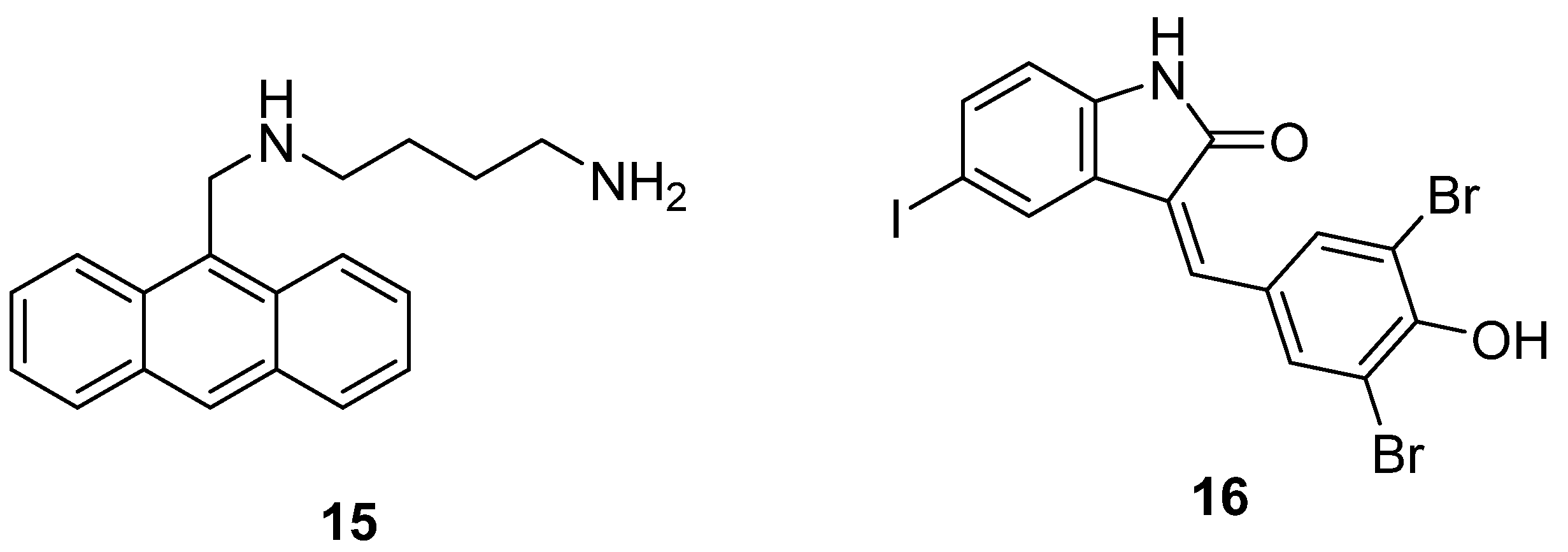
6.4. Inhibitors of Polyamine Uptake
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Booth, M. Climate change and the neglected tropical diseases. Adv. Parasitol. 2018, 100, 39–126. [Google Scholar] [PubMed]
- Tidman, R.; Abela-Ridder, B.; de Castañeda, R.R. The impact of climate change on neglected tropical diseases: A systematic review. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 147–168. [Google Scholar] [CrossRef] [PubMed]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Jamabo, M.; Mahlalela, M.; Edkins, A.L.; Boshoff, A. Tackling sleeping sickness: Current and promising therapeutics and treatment strategies. Int. J. Mol. Sci. 2023, 24, 12529. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, N.S.; Montgomery, S.P. Chagas Disease. Ann. Intern. Med. 2023, 176, ITC17–ITC32. [Google Scholar] [CrossRef]
- Gao, J.M.; Qian, Z.Y.; Hide, G.; Lai, D.H.; Lun, Z.R.; Wu, Z.D. Human African trypanosomiasis: The current situation in endemic regions and the risks for non-endemic regions from imported cases. Parasitology 2020, 147, 922–931. [Google Scholar] [CrossRef]
- WHO. Global Report on Neglected Tropical Diseases 2023; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2023.
- Hasker, E.; Hope, A.; Bottieau, E. Gambiense human African trypanosomiasis: The bumpy road to elimination. Curr. Opin. Infect. Dis. 2022, 35, 384–389. [Google Scholar] [CrossRef]
- Anonymous. Chagas disease in Latin America: An epidemiological update based on 2010 estimates. Wkly. Epidemiol. Rec. 2015, 90, 33–43. [Google Scholar]
- Messenger, L.A.; Miles, M.A.; Bern, C. Between a bug and a hard place: Trypanosoma cruzi genetic diversity and the clinical outcomes of Chagas disease. Expert. Rev. Anti Infect. Ther. 2015, 13, 995–1029. [Google Scholar] [CrossRef]
- Suárez, C.; Nolder, D.; García-Mingo, A.; Moore, D.A.J.; Chiodini, P.L. Diagnosis and clinical management of Chagas disease: An increasing challenge in non-endemic areas. Res. Rep. Trop. Med. 2022, 13, 25–40. [Google Scholar] [CrossRef]
- Caridha, D.; Vesely, B.; van Bocxlaer, K.; Arana, B.; Mowbray, C.E.; Rafati, S.; Uliana, S.; Reguera, R.M.; Kreishman-Deitrick, M.; Sciotti, R.; et al. Route map for the discovery and pre-clinical development of new drugs and treatments for cutaneous leishmaniasis. Int. J. Parasitol. Drugs Drug Resist. 2019, 11, 106–117. [Google Scholar] [CrossRef]
- Nuwangi, H.; Agampodi, T.C.; Price, H.P.; Shepherd, T.; Weerakoon, K.G.; Agampodi, S.B. The stigma associated with cutaneous leishmaniasis (CL) and mucocutaneous leishmaniasis (MCL): A protocol for a systematic review. PLoS ONE 2023, 18, e0285663. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, A.K.; Scorza, B.M.; Singh, O.P.; Rowton, E.; Lawyer, P.; Sundar, S.; Petersen, C.A. Domestic mammals as reservoirs for Leishmania donovani on the Indian subcontinent: Possibility and consequences on elimination. Transbound. Emerg. Dis. 2022, 69, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Pace, D. Leishmaniasis. J. Infect. 2014, 69 (Suppl. S1), S10–S18. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, V.K.; Tiwari, R.; Madhukar, P.; Rajneesh Kumar, S.; Gautam, V.; Engwerda, C.; Sundar, S.; Kumar, R. Post kala-azar dermal leishmaniasis in the Indian sub-continent: Challenges and strategies for elimination. Front. Immunol. 2023, 14, 1236952. [Google Scholar] [CrossRef] [PubMed]
- Lutje, V.; Probyn, K.; Seixas, J.; Bergman, H.; Villanueva, G. Chemotherapy for second-stage human African trypanosomiasis: Drugs in use. Cochrane Database Syst. Rev. 2021, 12, CD015374. [Google Scholar]
- García-Estrada, C.; Pérez-Pertejo, Y.; Domínguez-Asenjo, B.; Holanda, V.N.; Murugesan, S.; Martínez-Valladares, M.; Balaña-Fouce, R.; Reguera, R.M. Further investigations of nitroheterocyclic compounds as potential antikinetoplastid drug candidates. Biomolecules 2023, 13, 637. [Google Scholar] [CrossRef]
- Sundar, S.; Singh, A. Chemotherapeutics of visceral leishmaniasis: Present and future developments. Parasitology 2018, 145, 481–489. [Google Scholar] [CrossRef]
- Lago, A.S.D.; Nascimento, M.; Carvalho, A.M.; Lago, N.; Silva, J.; Queiroz, J.R.; Carvalho, L.P.; Schriefer, A.; Wilson, M.; Machado, P.; et al. The elderly responds to antimony therapy for cutaneous leishmaniasis similarly to young patients but have severe adverse reactions. Am. J. Trop. Med. Hyg. 2018, 98, 1317–1324. [Google Scholar] [CrossRef]
- Sundar, S.; More, D.K.; Singh, M.K.; Singh, V.P.; Sharma, S.; Makharia, A.; Kumar, P.C.; Murray, H.W. Failure of pentavalent antimony in visceral leishmaniasis in India: Report from the center of the Indian epidemic. Clin. Infect. Dis. 2000, 31, 1104–1107. [Google Scholar] [CrossRef]
- Chakravarty, J.; Sundar, S. Current and emerging medications for the treatment of leishmaniasis. Expert Opin. Pharmacother. 2019, 20, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Palić, S.; Beijnen, J.H.; Dorlo, T.P.C. An update on the clinical pharmacology of miltefosine in the treatment of leishmaniasis. Int. J. Antimicrob. Agents 2022, 59, 106459. [Google Scholar] [CrossRef] [PubMed]
- van Griensven, J.; Dorlo, T.P.; Diro, E.; Costa, C.; Burza, S. The status of combination therapy for visceral leishmaniasis: An updated review. Lancet Infect. Dis. 2023, 24, e36–e46. [Google Scholar] [CrossRef]
- Raj, S.; Sasidharan, S.; Balaji, S.N.; Saudagar, P. An overview of biochemically characterized drug targets in metabolic pathways of Leishmania parasite. Parasitol. Res. 2020, 119, 2025–2037. [Google Scholar] [CrossRef] [PubMed]
- Kourbeli, V.; Chontzopoulou, E.; Moschovou, K.; Pavlos, D.; Mavromoustakos, T.; Papanastasiou, I.P. An overview on target-based drug design against kinetoplastid protozoan infections: Human African trypanosomiasis, Chagas disease and leishmaniases. Molecules 2021, 26, 4629. [Google Scholar] [CrossRef]
- Jain, S.; Sahu, U.; Kumar, A.; Khare, P. Metabolic pathways of Leishmania parasite: Source of pertinent drug targets and potent drug candidates. Pharmaceutics 2022, 14, 1590. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.W.; McLeod, R.; Rice, D.W.; Ginger, M.; Chance, M.L.; Goad, L.J. Fatty acid and sterol metabolism: Potential antimicrobial targets in apicomplexan and trypanosomatid parasitic protozoa. Mol. Biochem. Parasitol. 2003, 126, 129–142. [Google Scholar] [CrossRef]
- Kumari, S.; Kumar, V.; Tiwari, R.K.; Ravidas, V.; Pandey, K.; Kumar, A. Amphotericin B: A drug of choice for Visceral Leishmaniasis. Acta Trop. 2022, 35, 106661. [Google Scholar] [CrossRef]
- de Souza, W.; Rodrigues, J.C. Sterol biosynthesis pathway as target for anti-trypanosomatid drugs. Interdiscip. Perspect. Infect. Dis. 2009, 2009, 642502. [Google Scholar] [CrossRef]
- Coppens, I.; Bastin, P.; Levade, T.; Courtoy, P.J. Activity, pharmacological inhibition and biological regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in Trypanosoma brucei. Mol. Biochem. Parasitol. 1995, 69, 29–40. [Google Scholar] [CrossRef]
- Dinesh, N.; Soumya, N.; Singh, S. Antileishmanial effect of mevastatin is due to interference with sterol metabolism. Parasitol. Res. 2015, 114, 3873–3883. [Google Scholar] [CrossRef]
- Araujo-Lima, C.F.; Peres, R.B.; Silva, P.B.; Batista, M.M.; Aiub, C.A.F.; Felzenszwalb, I.; Soeiro, M.N.C. Repurposing strategy of atorvastatin against Trypanosoma cruzi: In vitro monotherapy and combined therapy with benznidazole exhibit synergistic trypanocidal activity. Antimicrob. Agents Chemother. 2018, 62, e00979-18. [Google Scholar] [CrossRef] [PubMed]
- Magaraci, F.; Jiménez, C.J.; Rodrigues, C.; Rodrigues, J.C.; Braga, M.V.; Yardley, V.; de Luca-Fradley, K.; Croft, S.L.; de Souza, W.; Ruiz-Pérez, L.M.; et al. Azasterols as inhibitors of sterol 24-methyltransferase in Leishmania species and Trypanosoma cruzi. J. Med. Chem. 2003, 46, 4714–4727. [Google Scholar] [CrossRef]
- Leaver, D.J.; Patkar, P.; Singha, U.K.; Miller, M.B.; Haubrich, B.A.; Chaudhuri, M.; Nes, W.D. Fluorinated sterols are suicide inhibitors of ergosterol biosynthesis and growth in Trypanosoma brucei. Chem. Biol. 2015, 22, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Michels, P.A.; Bringaud, F.; Herman, M.; Hannaert, V. Metabolic functions of glycosomes in trypanosomatids. Biochim. Biophys. Acta 2006, 1763, 1463–1477. [Google Scholar] [CrossRef] [PubMed]
- Khanra, S.; Juin, S.K.; Jawed, J.J.; Ghosh, S.; Dutta, S.; Nabi, S.A.; Dash, J.; Dasgupta, D.; Majumdar, S.; Banerjee, R. In vivo experiments demonstrate the potent antileishmanial efficacy of repurposed suramin in visceral leishmaniasis. PLoS Negl. Trop. Dis. 2020, 14, e0008575. [Google Scholar] [CrossRef]
- Brimacombe, K.R.; Walsh, M.J.; Liu, L.; Vásquez-Valdivieso, M.G.; Morgan, H.P.; McNae, I.; Fothergill-Gilmore, L.A.; Michels, P.A.; Auld, D.S.; Simeonov, A.; et al. Identification of ML251, a potent inhibitor of T. brucei and T. cruzi phosphofructokinase. ACS Med. Chem. Lett. 2013, 5, 12–17. [Google Scholar] [CrossRef]
- Martinez-Oyanedel, J.; McNae, I.W.; Nowicki, M.W.; Keillor, J.W.; Michels, P.A.; Fothergill-Gilmore, L.A.; Walkinshaw, M.D. The first crystal structure of phosphofructokinase from a eukaryote: Trypanosoma brucei. J. Mol. Biol. 2007, 366, 1185–1198. [Google Scholar] [CrossRef]
- Coley, A.F.; Dodson, H.C.; Morris, M.T.; Morris, J.C. Glycolysis in the African trypanosome: Targeting enzymes and their subcellular compartments for therapeutic development. Mol. Biol. Int. 2011, 2011, 123702. [Google Scholar] [CrossRef]
- Sundar, S.; Singh, B. Emerging therapeutic targets for treatment of leishmaniasis. Expert. Opin. Ther. Targets 2018, 22, 467–486. [Google Scholar] [CrossRef]
- Gupta, R.; Kumar, V.; Kushawaha, P.K.; Tripathi, C.P.; Joshi, S.; Sahasrabuddhe, A.A.; Mitra, K.; Sundar, S.; Siddiqi, M.I.; Dube, A. Characterization of glycolytic enzymes--rAldolase and rEnolase of Leishmania donovani, identified as Th1 stimulatory proteins, for their immunogenicity and immunoprophylactic efficacies against experimental visceral leishmaniasis. PLoS ONE 2014, 9, e86073. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Yadav, N.K.; Rawat, K.; Tripathi, C.D.; Jaiswal, A.K.; Khare, P.; Tandon, R.; Baharia, R.K.; Das, S.; Gupta, R.; et al. Comparative analysis of cellular immune responses in treated leishmania patients and hamsters against recombinant th1 stimulatory proteins of Leishmania donovani. Front. Microbiol. 2016, 7, 312. [Google Scholar] [CrossRef] [PubMed]
- Boitz, J.M.; Ullman, B.; Jardim, A.; Carter, N.S. Purine salvage in Leishmania: Complex or simple by design? Trends Parasitol. 2012, 28, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Hofer, A. Targeting the nucleotide metabolism of Trypanosoma brucei and other trypanosomatids. FEMS Microbiol. Rev. 2023, 47, fuad020. [Google Scholar] [CrossRef] [PubMed]
- el Kouni, M.H. Potential chemotherapeutic targets in the purine metabolism of parasites. Pharmacol. Ther. 2003, 99, 283–309. [Google Scholar] [CrossRef]
- Farca, A.M.; Miniscalco, B.; Badino, P.; Odore, R.; Monticelli, P.; Trisciuoglio, A.; Ferroglio, E. Canine leishmaniosis: In vitro efficacy of miltefosine and marbofloxacin alone or in combination with allopurinol against clinical strains of Leishmania infantum. Parasitol. Res. 2012, 110, 2509–2513. [Google Scholar] [CrossRef]
- Croft, S.L.; Sundar, S.; Fairlamb, A.H. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 2006, 19, 111–126. [Google Scholar] [CrossRef]
- Champoux, J.J. DNA topoisomerases: Structure, function, and mechanism. Annu. Rev. Biochem. 2001, 70, 369–413. [Google Scholar] [CrossRef]
- Balaña-Fouce, R.; Alvarez-Velilla, R.; Fernández-Prada, C.; García-Estrada, C.; Reguera, R.M. Trypanosomatids topoisomerase re-visited. New structural findings and role in drug discovery. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 326–337. [Google Scholar] [CrossRef]
- Pommier, Y. Topoisomerase I inhibitors: Camptothecins and beyond. Nat. Rev. Cancer 2006, 6, 789–802. [Google Scholar] [CrossRef]
- Pommier, Y.; Leo, E.; Zhang, H.; Marchand, C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010, 17, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Reguera, R.M.; Elmahallawy, E.K.; García-Estrada, C.; Carbajo-Andrés, R.; Balaña-Fouce, R. DNA Topoisomerases of Leishmania parasites; druggable targets for drug discovery. Curr. Med. Chem. 2019, 26, 5900–5923. [Google Scholar] [CrossRef] [PubMed]
- Shulpekova, Y.; Nechaev, V.; Kardasheva, S.; Sedova, A.; Kurbatova, A.; Bueverova, E.; Kopylov, A.; Malsagova, K.; Dlamini, J.C.; Ivashkin, V. The concept of folic acid in health and disease. Molecules 2021, 26, 3731. [Google Scholar] [CrossRef] [PubMed]
- Vickers, T.J.; Beverley, S.M. Folate metabolic pathways in Leishmania. Essays Biochem. 2011, 51, 63–80. [Google Scholar] [PubMed]
- Tulloch, L.B.; Martini, V.P.; Iulek, J.; Huggan, J.K.; Lee, J.H.; Gibson, C.L.; Smith, T.K.; Suckling, C.J.; Hunter, W.N. Structure-based design of pteridine reductase inhibitors targeting African sleeping sickness and the leishmaniases. J. Med. Chem. 2010, 53, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Martínez, C.; Correa-Basurto, J.; Nieto-Meneses, R.; Márquez-Navarro, A.; Aguilar-Suárez, R.; Montero-Cortes, M.D.; Nogueda-Torres, B.; Suárez-Contreras, E.; Galindo-Sevilla, N.; Rojas-Rojas, Á.; et al. Design, synthesis and biological evaluation of quinazoline derivatives as anti-trypanosomatid and anti-plasmodial agents. Eur. J. Med. Chem. 2015, 96, 296–307. [Google Scholar] [CrossRef]
- Landi, G.; Linciano, P.; Borsari, C.; Bertolacini, C.P.; Moraes, C.B.; Cordeiro-da-Silva, A.; Gul, S.; Witt, G.; Kuzikov, M.; Costi, M.P.; et al. Structural insights into the development of cycloguanil derivatives as Trypanosoma brucei pteridine-reductase-1 inhibitors. ACS Infect. Dis. 2019, 5, 1105–1114. [Google Scholar] [CrossRef]
- Muñoz, C.; San Francisco, J.; Gutiérrez, B.; González, J. Role of the ubiquitin-proteasome systems in the biology and virulence of protozoan parasites. Biomed. Res. Int. 2015, 2015, 141526. [Google Scholar] [CrossRef]
- Bijlmakers, M.J. Ubiquitination and the proteasome as drug targets in trypanosomatid diseases. Front. Chem. 2021, 8, 630888. [Google Scholar] [CrossRef]
- Khare, S.; Nagle, A.S.; Biggart, A.; Lai, Y.H.; Liang, F.; Davis, L.C.; Barnes, S.W.; Mathison, C.J.; Myburgh, E.; Gao, M.Y.; et al. Proteasome inhibition for treatment of leishmaniasis, Chagas disease and sleeping sickness. Nature 2016, 537, 229–233. [Google Scholar] [CrossRef]
- Xie, S.C.; Dick, L.R.; Gould, A.; Brand, S.; Tilley, L. The proteasome as a target for protozoan parasites. Expert Opin. Ther. Targets 2019, 23, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Nagle, A.; Biggart, A.; Be, C.; Srinivas, H.; Liu, X.; Yeh, V.; Smith, J.; Lerario, I.; Xie, Y.; Chianelli, D.; et al. Discovery and characterization of clinical candidate LXE408 as a kinetoplastid-selective proteasome inhibitor for the treatment of leishmaniases. J. Med. Chem. 2020, 63, 10773–10781. [Google Scholar] [CrossRef]
- Carter, N.S.; Kawasaki, Y.; Nahata, S.S.; Elikaee, S.; Rajab, S.; Salam, L.; Alabdulal, M.Y.; Broessel, K.K.; Foroghi, F.; Abbas, A.; et al. Polyamine metabolism in leishmania parasites: A promising therapeutic target. Med. Sci. 2022, 10, 24. [Google Scholar] [CrossRef]
- Roberts, S.C.; Tancer, M.J.; Polinsky, M.R.; Gibson, K.M.; Heby, O.; Ullman, B. Arginase plays a pivotal role in polyamine precursor metabolism in Leishmania. Characterization of gene deletion mutants. J. Biol. Chem. 2004, 279, 23668–23678. [Google Scholar] [CrossRef] [PubMed]
- Balaña-Fouce, R.; Calvo-Álvarez, E.; Álvarez-Velilla, R.; Prada, C.F.; Pérez-Pertejo, Y.; Reguera, R.M. Role of trypanosomatid’s arginase in polyamine biosynthesis and pathogenesis. Mol. Biochem. Parasitol. 2012, 181, 85–93. [Google Scholar] [CrossRef]
- da Silva, M.F.; Floeter-Winter, L.M. Arginase in Leishmania. Subcell. Biochem. 2013, 74, 103–117. [Google Scholar]
- Roberts, S.C.; Paradis, D.; Perdeh, J.; Harrelson, J.; Jan, B.; Yates, P.; Ullman, B. The polyamine pathway of Leishmania donovani as a potential therapeutic target. FASEB J. 2016, 30, 1103.3. [Google Scholar] [CrossRef]
- Chawla, B.; Kumar, R.R.; Tyagi, N.; Subramanian, G.; Srinivasan, N.; Park, M.H.; Madhubala, R. A unique modification of the eukaryotic initiation factor 5A shows the presence of the complete hypusine pathway in Leishmania donovani. PLoS ONE 2012, 7, e33138. [Google Scholar] [CrossRef]
- Muxel, S.M.; Aoki, J.I.; Fernandes, J.C.R.; Laranjeira-Silva, M.F.; Zampieri, R.A.; Acuña, S.M.; Müller, K.E.; Vanderlinde, R.H.; Floeter-Winter, L.M. Arginine and polyamines fate in Leishmania infection. Front. Microbiol. 2018, 8, 2682. [Google Scholar] [CrossRef]
- Pegg, A.E. Introduction to the Thematic Minireview Series: Sixty plus years of polyamine research. J. Biol. Chem. 2018, 293, 18681–18692. [Google Scholar] [CrossRef]
- Phillips, M.A. Polyamines in protozoan pathogens. J. Biol. Chem. 2018, 293, 18746–18756. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.; Roos, D.; Morada, M.; Zhu, G.; Keithly, J.S.; Feagin, J.E.; Wu, G.; Yarlett, N. Divergent polyamine metabolism in the Apicomplexa. Microbiology 2007, 153, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.J.; Ghaffar, A.; Abdalal, S.; Perrin, B.; Aly, A.S. Plasmodium AdoMetDC/ODC bifunctional enzyme is essential for male sexual stage development and mosquito transmission. Biol. Open 2016, 5, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.F.; Cerqueira, N.M.; Fernandes, P.A.; Ramos, M.J. Mechanism of formation of the internal aldimine in pyridoxal 5’-phosphate-dependent enzymes. J. Am. Chem. Soc. 2011, 133, 15496–15505. [Google Scholar] [CrossRef] [PubMed]
- Ghoda, L.; Sidney, D.; Macrae, M.; Coffino, P. Structural elements of ornithine decarboxylase required for intracellular degradation and polyamine-dependent regulation. Mol. Cell Biol. 1992, 12, 2178–2185. [Google Scholar]
- Coleman, C.S.; Stanley, B.A.; Viswanath, R.; Pegg, A.E. Rapid exchange of subunits of mammalian ornithine decarboxylase. J. Biol. Chem. 1994, 269, 3155–3158. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Matsufuji, S.; Hayashi, S.; Tanahashi, N.; Tanaka, K. Degradation of ornithine decarboxylase by the 26S proteasome. Biochem. Biophys. Res. Commun. 2000, 267, 1–6. [Google Scholar] [CrossRef]
- Burri, C.; Brun, R. Eflornithine for the treatment of human African trypanosomiasis. Parasitol. Res. 2003, 90 (Suppl. S1), S49–S52. [Google Scholar] [CrossRef]
- Iten, M.; Matovu, E.; Brun, R.; Kaminsky, R. Innate lack of susceptibility of Ugandan Trypanosoma brucei rhodesiense to DL-alpha-difluoromethylornithine (DFMO). Trop. Med. Parasitol. 1995, 46, 190–194. [Google Scholar]
- Iten, M.; Mett, H.; Evans, A.; Enyaru, J.C.; Brun, R.; Kaminsky, R. Alterations in ornithine decarboxylase characteristics account for tolerance of Trypanosoma brucei rhodesiense to D, L-alpha-difluoromethylornithine. Antimicrob. Agents Chemother. 1997, 41, 1922–1925. [Google Scholar] [CrossRef]
- Reguera, R.M.; Balaña-Fouce, R.; Cubria, J.C.; Bujidos, M.L.; Ordoñez, D. Fluorinated analogues of L-ornithine are powerful inhibitors of ornithine decarboxylase and cell growth of Leishmania infantum promastigotes. Life Sci. 1995, 56, 223–230. [Google Scholar] [CrossRef]
- Boitz, J.M.; Yates, P.A.; Kline, C.; Gaur, U.; Wilson, M.E.; Ullman, B.; Roberts, S.C. Leishmania donovani ornithine decarboxylase is indispensable for parasite survival in the mammalian host. Infect. Immun. 2009, 77, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Ekstrom, J.L.; Mathews, I.I.; Stanley, B.A.; Pegg, A.E.; Ealick, S.E. The crystal structure of human S-adenosylmethionine decarboxylase at 2.25 A resolution reveals a novel fold. Structure 1999, 7, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Willert, E.K.; Fitzpatrick, R.; Phillips, M.A. Allosteric regulation of an essential trypanosome polyamine biosynthetic enzyme by a catalytically dead homolog. Proc. Natl. Acad. Sci. USA 2007, 104, 8275–8280. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; McCloskey, D.E.; Phillips, M.A. RNA interference-mediated silencing of ornithine decarboxylase and spermidine synthase genes in Trypanosoma brucei provides insight into regulation of polyamine biosynthesis. Eukaryot. Cell 2009, 8, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Willert, E.K.; Phillips, M.A. Regulated expression of an essential allosteric activator of polyamine biosynthesis in African trypanosomes. PLoS Pathog. 2008, 4, e1000183. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Nguyen, S.; Kim, S.H.; Volkov, O.A.; Tu, B.P.; Phillips, M.A. Product feedback regulation implicated in translational control of the Trypanosoma brucei S-adenosylmethionine decarboxylase regulatory subunit prozyme. Mol. Microbiol. 2013, 88, 846–861. [Google Scholar] [CrossRef]
- Velez, N.; Brautigam, C.A.; Phillips, M.A. Trypanosoma brucei S-adenosylmethionine decarboxylase N-terminus is essential for allosteric activation by the regulatory subunit prozyme. J. Biol. Chem. 2013, 288, 5232–5240. [Google Scholar] [CrossRef]
- Volkov, O.A.; Kinch, L.; Ariagno, C.; Deng, X.; Zhong, S.; Grishin, N.; Tomchick, D.R.; Chen, Z.; Phillips, M.A. Relief of autoinhibition by conformational switch explains enzyme activation by a catalytically dead paralog. Elife 2016, 5, e20198. [Google Scholar] [CrossRef]
- Patel, M.M.; Volkov, O.A.; Leija, C.; Lemoff, A.; Phillips, M.A. A dual regulatory circuit consisting of S-adenosylmethionine decarboxylase protein and its reaction product controls expression of the paralogous activator prozyme in Trypanosoma brucei. PLoS Pathog. 2018, 14, e1007404. [Google Scholar] [CrossRef]
- Wu, H.; Min, J.; Ikeguchi, Y.; Zeng, H.; Dong, A.; Loppnau, P.; Pegg, A.E.; Plotnikov, A.N. Structure and mechanism of spermidine synthases. Biochemistry 2007, 46, 8331–8339. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.C.; Kaur, H.; Blessington, B.; Kelly, J.M.; Wilkinson, S.R. Validation of spermidine synthase as a drug target in African trypanosomes. Biochem. J. 2008, 409, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, C.; Olenyik, T.; Roberts, S.C.; Ullman, B. Spermidine synthase is required for virulence of Leishmania donovani. Infect. Immun. 2011, 79, 2764–2769. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Agnihotri, P.; Srivastava, V.K.; Pratap, J.V. Novel protein-protein interaction between spermidine synthase and S-adenosylmethionine decarboxylase from Leishmania donovani. Biochem. Biophys. Res. Commun. 2015, 456, 637–642. [Google Scholar] [CrossRef]
- Abdulhussein, A.A.; Wallace, H.M. Polyamines and membrane transporters. Amino Acids 2014, 46, 655–660. [Google Scholar] [CrossRef]
- Sakata, K.; Kashiwagi, K.; Igarashi, K. Properties of a polyamine transporter regulated by antizyme. Biochem. J. 2000, 347, 297–303. [Google Scholar] [CrossRef]
- Reguera, R.M.; Tekwani, B.L.; Balaña-Fouce, R. Polyamine transport in parasites: A potential target for new antiparasitic drug development. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005, 140, 151–164. [Google Scholar] [CrossRef]
- Carrillo, C.; Canepa, G.E.; Algranati, I.D.; Pereira, C.A. Molecular and functional characterization of a spermidine transporter (TcPAT12) from Trypanosoma cruzi. Biochem. Biophys. Res. Commun. 2006, 344, 936–940. [Google Scholar] [CrossRef]
- Hasne, M.P.; Coppens, I.; Soysa, R.; Ullman, B. A high-affinity putrescine-cadaverine transporter from Trypanosoma cruzi. Mol. Microbiol. 2010, 76, 78–91. [Google Scholar] [CrossRef]
- Reigada, C.; Sayé, M.; Vera, E.V.; Balcazar, D.; Fraccaroli, L.; Carrillo, C.; Miranda, M.R.; Pereira, C.A. Trypanosoma cruzi polyamine transporter: Its role on parasite growth and survival under stress conditions. J. Membr. Biol. 2016, 249, 475–481. [Google Scholar] [CrossRef]
- Llanos, M.A.; Alberca, L.N.; Ruiz, M.D.; Sbaraglini, M.L.; Miranda, C.; Pino-Martínez, A.; Fraccaroli, L.; Carrillo, C.; Alba Soto, C.D.; Gavernet, L.; et al. A combined ligand and target-based virtual screening strategy to repurpose drugs as putrescine uptake inhibitors with trypanocidal activity. J. Comput. Aided Mol. Des. 2023, 37, 75–90. [Google Scholar] [CrossRef]
- Henriques, C.; Miller, M.P.; Catanho, M.; De Carvalho, T.M.U.; Krieger, M.A.; Probst, C.M.; De Souza, W.; Degrave, W.; Amara, S.G. Identification and functional characterization of a novel arginine/ornithine transporter, a member of a cationic amino acid transporter subfamily in the Trypanosoma cruzi genome. Parasites Vectors 2015, 8, 346. [Google Scholar] [CrossRef] [PubMed]
- Macedo, J.P.; Currier, R.B.; Wirdnam, C.; Horn, D.; Alsford, S.; Rentsch, D. Ornithine uptake and the modulation of drug sensitivity in Trypanosoma brucei. FASEB J. 2017, 31, 4649–4660. [Google Scholar] [CrossRef] [PubMed]
- Reguera, R.; Fouce, R.B.; Cubria, J.C.; Bujidos, M.L.A.; Ordoñez, D. Putrescine uptake inhibition by aromatic diamidines in Leishmania infantum promastigotes. Biochem. Pharmacol. 1994, 47, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Hasne, M.P.; Ullman, B. Identification and characterization of a polyamine permease from the protozoan parasite Leishmania major. J. Biol. Chem. 2005, 280, 15188–15194. [Google Scholar] [CrossRef] [PubMed]
- Mato, J.M.; Corrales, F.J.; Lu, S.C.; Avila, M.A. S-Adenosylmethionine: A control switch that regulates liver function. FASEB J. 2002, 16, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Reguera, R.M.; Balaña-Fouce, R.; Pérez-Pertejo, Y.; Fernández, F.J.; García-Estrada, C.; Cubría, J.C.; Ordóñez, C.; Ordóñez, D. Cloning expression and characterization of methionine adenosyltransferase in Leishmania infantum promastigotes. J. Biol. Chem. 2002, 277, 3158–3167. [Google Scholar] [CrossRef]
- Pérez-Pertejo, Y.; Reguera, R.M.; Villa, H.; García-Estrada, C.; Balaña-Fouce, R.; Pajares, M.A.; Ordóñez, D. Leishmania donovani methionine adenosyltransferase. Role of cysteine residues in the recombinant enzyme. Eur. J. Biochem. 2003, 270, 28–35. [Google Scholar] [CrossRef]
- Pérez-Pertejo, Y.; Reguera, R.M.; García-Estrada, C.; Balaña-Fouce, R.; Ordóñez, D. Mutational analysis of methionine adenosyltransferase from Leishmania donovani. Eur. J. Biochem. 2004, 271, 2791–2798. [Google Scholar] [CrossRef]
- Pérez-Pertejo, Y.; Reguera, R.M.; Ordóñez, D.; Balaña-Fouce, R. Characterization of a methionine adenosyltransferase over-expressing strain in the trypanosomatid Leishmania donovani. Biochim. Biophys. Acta 2006, 1760, 10–19. [Google Scholar] [CrossRef]
- Pérez-Pertejo, Y.; Alvarez-Velilla, R.; Estrada, C.G.; Balaña-Fouce, R.; Reguera, R.M. Leishmania donovani: Proteasome-mediated down-regulation of methionine adenosyltransferase. Parasitology 2011, 138, 1082–1092. [Google Scholar] [CrossRef]
- Park, M.H.; Nishimura, K.; Zanelli, C.F.; Valentini, S.R. Functional significance of eIF5A and its hypusine modification in eukaryotes. Amino Acids 2010, 38, 491–500. [Google Scholar] [CrossRef]
- Umland, T.C.; Wolff, E.C.; Park, M.H.; Davies, D.R. A new crystal structure of deoxyhypusine synthase reveals the configuration of the active enzyme and of an enzyme-NAD-inhibitor ternary complex. J. Biol. Chem. 2004, 279, 28697–28705. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.; Jones, D.C.; Wyllie, S.; Fairlamb, A.H.; Phillips, M.A. Allosteric activation of trypanosomatid deoxyhypusine synthase by a catalytically dead paralog. J. Biol. Chem. 2013, 288, 15256–15267. [Google Scholar] [CrossRef]
- Chawla, B.; Jhingran, A.; Singh, S.; Tyagi, N.; Park, M.H.; Srinivasan, N.; Roberts, S.C.; Madhubala, R. Identification and characterization of a novel deoxyhypusine synthase in Leishmania donovani. J. Biol. Chem. 2010, 285, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Afanador, G.A.; Tomchick, D.R.; Phillips, M.A. Trypanosomatid deoxyhypusine synthase activity is dependent on shared active-site complementation between pseudoenzyme paralogs. Structure 2018, 26, 1499–1512. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.F.; Klippel, A.H.; Ramos, P.Z.; Santiago, A.D.S.; Valentini, S.R.; Bengtson, M.H.; Massirer, K.B.; Bilsland, E.; Couñago, R.M.; Zanelli, C.F. Structural features and development of an assay platform of the parasite target deoxyhypusine synthase of Brugia malayi and Leishmania major. PLoS Negl. Trop. Dis. 2020, 14, e0008762. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Aravind, L.; Wolff, E.C.; Kaevel, J.; Kim, Y.S.; Park, M.H. Molecular cloning, expression, and structural prediction of deoxyhypusine hydroxylase: A HEAT-repeat-containing metalloenzyme. Proc. Natl. Acad. Sci. USA 2006, 103, 51–56. [Google Scholar] [CrossRef]
- Katiki, M.; Sharma, M.; Neetu, N.; Rentala, M.; Kumar, P. Biophysical and modeling-based approach for the identification of inhibitors against DOHH from Leishmania donovani. Brief. Funct. Genom. 2023, 22, 217–226. [Google Scholar] [CrossRef]
- Shaked-Mishan, P.; Suter-Grotemeyer, M.; Yoel-Almagor, T.; Holland, N.; Zilberstein, D.; Rentsch, D. A novel high-affinity arginine transporter from the human parasitic protozoan Leishmania donovani. Mol. Microbiol. 2006, 60, 30–38. [Google Scholar] [CrossRef]
- Carrillo, C.; Canepa, G.E.; Giacometti, A.; Bouvier, L.A.; Miranda, M.R.; de los Milagros Camara, M.; Pereira, C.A. Trypanosoma cruzi amino acid transporter Tcaaap411 mediates arginine uptake in yeasts. FEMS Microbiol. Lett. 2010, 306, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.A.; Alonso, G.D.; Paveto, M.C.; Flawia, M.M.; Torres, H.N. L-arginine uptake and L-phosphoarginine synthesis in Trypanosoma cruzi. J. Eukaryot. Microbiol. 1999, 46, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Darlyuk, I.; Goldman, A.; Roberts, S.C.; Ullman, B.; Rentsch, D.; Zilberstein, D. Arginine homeostasis and transport in the human pathogen Leishmania donovani. J. Biol. Chem. 2009, 284, 19800–19807. [Google Scholar] [CrossRef]
- Hai, Y.; Kerkhoven, E.J.; Barrett, M.P.; Christianson, D.W. Crystal structure of an arginase-like protein from Trypanosoma brucei that evolved without a binuclear manganese cluster. Biochemistry 2015, 54, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Vincent, I.M.; Creek, D.J.; Burgess, K.; Woods, D.J.; Burchmore, R.J.; Barrett, M.P. Untargeted metabolomics reveals a lack of synergy between nifurtimox and eflornithine against Trypanosoma brucei. PLoS Negl. Trop. Dis. 2012, 6, e1618. [Google Scholar] [CrossRef]
- Gaur, U.; Roberts, S.C.; Dalvi, R.P.; Corraliza, I.; Ullman, B.; Wilson, M.E. An effect of parasite-encoded arginase on the outcome of murine cutaneous leishmaniasis. J. Immunol. 2007, 179, 8446–8453. [Google Scholar] [CrossRef]
- Reguera, R.M.; Balaña-Fouce, R.; Showalter, M.; Hickerson, S.; Beverley, S.M. Leishmania major lacking arginase (ARG) are auxotrophic for polyamines but retain infectivity to susceptible BALB/c mice. Mol. Biochem. Parasitol. 2009, 165, 48–56. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.F.; Zampieri, R.A.; Muxel, S.M.; Beverley, S.M.; Floeter-Winter, L.M. Leishmania amazonensis arginase compartmentalization in the glycosome is important for parasite infectivity. PLoS ONE 2012, 7, e34022. [Google Scholar] [CrossRef]
- Boitz, J.M.; Gilroy, C.A.; Olenyik, T.D.; Paradis, D.; Perdeh, J.; Dearman, K.; Davis, M.J.; Yates, P.A.; Li, Y.; Riscoe, M.K.; et al. Arginase is essential for survival of Leishmania donovani promastigotes but not intracellular amastigotes. Infect. Immun. 2016, 85, e00554-16. [Google Scholar] [CrossRef]
- Acuña, S.M.; Aoki, J.I.; Laranjeira-Silva, M.F.; Zampieri, R.A.; Fernandes, J.C.R.; Muxel, S.M.; Floeter-Winter, L.M. Arginase expression modulates nitric oxide production in Leishmania (Leishmania) amazonensis. PLoS ONE 2017, 12, e0187186. [Google Scholar] [CrossRef]
- Fairlamb, A.H.; Blackburn, P.; Ulrich, P.; Chait, B.T.; Cerami, A. Trypanothione: A novel bis(glutathionyl)spermidine cofactor for glutathione reductase in trypanosomatids. Science 1985, 227, 1485–1487. [Google Scholar] [CrossRef] [PubMed]
- Fairlamb, A.H.; Cerami, A. Metabolism and functions of trypanothione in the Kinetoplastida. Annu. Rev. Microbiol. 1992, 46, 695–729. [Google Scholar] [CrossRef] [PubMed]
- Saudagar, P.; Dubey, V.K. Cloning, expression, characterization and inhibition studies on trypanothione synthetase, a drug target enzyme, from Leishmania donovani. Biol. Chem. 2011, 392, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Dumas, C.; Ouellette, M.; Tovar, J.; Cunningham, M.L.; Fairlamb, A.H.; Tamar, S.; Olivier, M.; Papadopoulou, B. Disruption of the trypanothione reductase gene of Leishmania decreases its ability to survive oxidative stress in macrophages. EMBO J. 1997, 16, 2590–2598. [Google Scholar] [CrossRef] [PubMed]
- Piñeyro, M.D.; Arias, D.; Parodi-Talice, A.; Guerrero, S.; Robello, C. Trypanothione metabolism as drug target for trypanosomatids. Curr. Pharm. Des. 2021, 27, 1834–1846. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, A.; Colotti, G.; Exertier, C.; Liuzzi, A.; Seghetti, F.; Salerno, A.; Caciolla, J.; Ilari, A. Innovative approach for a classic target: Fragment screening on trypanothione reductase reveals new opportunities for drug design. Front. Mol. Biosci. 2022, 9, 900882. [Google Scholar] [CrossRef] [PubMed]
- Colotti, G.; Saccoliti, F.; Gramiccia, M.; Di Muccio, T.; Prakash, J.; Yadav, S.; Dubey, V.K.; Vistoli, G.; Battista, T.; Mocci, S.; et al. Structure-guided approach to identify a novel class of anti-leishmaniasis diaryl sulfide compounds targeting the trypanothione metabolism. Amino Acids 2020, 52, 247–259. [Google Scholar] [CrossRef]
- Arias, D.G.; Herrera, F.E.; Garay, A.S.; Rodrigues, D.; Forastieri, P.S.; Luna, L.E.; Bürgi, M.D.; Prieto, C.; Iglesias, A.A.; Cravero, R.M.; et al. Rational design of nitrofuran derivatives: Synthesis and valuation as inhibitors of Trypanosoma cruzi trypanothione reductase. Eur. J. Med. Chem. 2017, 125, 1088–1097. [Google Scholar] [CrossRef]
- Melcón-Fernández, E.; Galli, G.; García-Estrada, C.; Balaña-Fouce, R.; Reguera, R.M.; Pérez-Pertejo, Y. Miltefosine and nifuratel combination: A promising therapy for the treatment of Leishmania donovani visceral leishmaniasis. Int. J. Mol. Sci. 2023, 24, 1635. [Google Scholar] [CrossRef]
- Matadamas-Martínez, F.; Hernández-Campos, A.; Téllez-Valencia, A.; Vázquez-Raygoza, A.; Comparán-Alarcón, S.; Yépez-Mulia, L.; Castillo, R. Leishmania mexicana trypanothione reductase inhibitors: Computational and biological studies. Molecules 2019, 24, 3216. [Google Scholar] [CrossRef]
- Madia, V.N.; Ialongo, D.; Patacchini, E.; Exertier, C.; Antonelli, L.; Colotti, G.; Messore, A.; Tudino, V.; Saccoliti, F.; Scipione, L.; et al. Inhibition of Leishmania infantum trypanothione reductase by new aminopropanone derivatives interacting with the NADPH binding site. Molecules 2023, 28, 338. [Google Scholar] [CrossRef] [PubMed]
- Fauro, R.; Lo Presti, S.; Bazan, C.; Baez, A.; Strauss, M.; Triquell, F.; Cremonezzi, D.; Negrete, O.S.; Willhuber, G.C.; Paglini-Oliva, P.; et al. Use of clomipramine as chemotherapy of the chronic phase of Chagas disease. Parasitology 2013, 140, 917–927. [Google Scholar] [CrossRef]
- Turcano, L.; Battista, T.; De Haro, E.T.; Missineo, A.; Alli, C.; Paonessa, G.; Colotti, G.; Harper, S.; Fiorillo, A.; Ilari, A.; et al. Spiro-containing derivatives show antiparasitic activity against Trypanosoma brucei through inhibition of the trypanothione reductase enzyme. PLoS Negl. Trop. Dis. 2020, 14, e0008339. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, A.; Benítez, D.; Korn, R.S.; Ferreira, V.C.; Barrera, E.; Carrión, F.; Pritsch, O.; Pantano, S.; Kunick, C.; de Oliveira, C.I.; et al. Mechanistic and biological characterisation of novel N5-substituted paullones targeting the biosynthesis of trypanothione in Leishmania. J. Enzyme Inhib. Med. Chem. 2020, 35, 1345–1358. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.N.; Park, K.P.; Benítez, D.; Comini, M.A.; Shum, D.; No, J.H. Discovery of novel Leishmania major trypanothione synthetase inhibitors by high-throughput screening. Biochem. Biophys. Res. Commun. 2022, 637, 308–313. [Google Scholar] [CrossRef]
- Benítez, D.; Franco, J.; Sardi, F.; Leyva, A.; Durán, R.; Choi, G.; Yang, G.; Kim, T.; Kim, N.; Heo, J.; et al. Drug-like molecules with anti-trypanothione synthetase activity identified by high throughput screening. J. Enzyme Inhib. Med. Chem. 2022, 37, 912–929. [Google Scholar] [CrossRef]
- Tetaud, E.; Giroud, C.; Prescott, A.R.; Parkin, D.W.; Baltz, D.; Biteau, N.; Baltz, T.; Fairlamb, A.H. Molecular characterisation of mitochondrial and cytosolic trypanothione-dependent tryparedoxin peroxidases in Trypanosoma brucei. Mol. Biochem. Parasitol. 2001, 116, 171–183. [Google Scholar] [CrossRef]
- Ilari, A.; Fiorillo, A.; Genovese, I.; Colotti, G. Polyamine-trypanothione pathway: An update. Future Med. Chem. 2017, 9, 61–77. [Google Scholar] [CrossRef]
- Casero, R.A., Jr.; Murray Stewart, T.; Pegg, A.E. Polyamine metabolism and cancer: Treatments, challenges and opportunities. Nat. Rev. Cancer 2018, 18, 681–695. [Google Scholar] [CrossRef]
- Von Hoff, D.D. MGBG: Teaching an old drug, new tricks. Ann. Oncol. 1994, 5, 487–493. [Google Scholar] [CrossRef]
- LoGiudice, N.; Le, L.; Abuan, I.; Leizorek, Y.; Roberts, S.C. Alpha-Difluoromethylornithine, an Irreversible Inhibitor of Polyamine Biosynthesis, as a Therapeutic Strategy against Hyperproliferative and Infectious Diseases. Med. Sci. 2018, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Bacchi, C.J.; Nathan, H.C.; Hutner, S.H.; McCann, P.P.; Sjoerdsma, A. Polyamine metabolism: A potential therapeutic target in trypanosomes. Science 1980, 210, 332–334. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E.; Feith, D.J. Polyamines and neoplastic growth. Biochem. Soc. Trans. 2007, 35 Pt 2, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Meyskens, F.L., Jr.; Gerner, E.W. Development of difluoromethylornithine (DFMO) as a chemoprevention agent. Clin. Cancer Res. 1999, 5, 945–951. [Google Scholar] [PubMed]
- Raj, K.P.; Zell, J.A.; Rock, C.L.; McLaren, C.E.; Zoumas-Morse, C.; Gerner, E.W.; Meyskens, F.L. Role of dietary polyamines in a phase III clinical trial of difluoromethylornithine (DFMO) and sulindac for prevention of sporadic colorectal adenomas. Br. J. Cancer 2013, 108, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Tangella, A.V.; Gajre, A.S.; Chirumamilla, P.C.; Rathhan, P.V. Difluoromethylornithine (DFMO) and neuroblastoma: A review. Cureus 2023, 15, e37680. [Google Scholar] [CrossRef] [PubMed]
- Fairlamb, A.H.; Henderson, G.B.; Bacchi, C.J.; Cerami, A. In vivo effects of difluoromethylornithine on trypanothione and polyamine levels in bloodstream forms of Trypanosoma brucei. Mol. Biochem. Parasitol. 1987, 24, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Yun, O.; Priotto, G.; Tong, J.; Flevaud, L.; Chappuis, F. NECT is next: Implementing the new drug combination therapy for Trypanosoma brucei gambiense sleeping sickness. PLoS Negl. Trop. Dis. 2010, 4, e720. [Google Scholar] [CrossRef]
- Lutje, V.; Seixas, J.; Kennedy, A. Chemotherapy for second-stage human African trypanosomiasis. Cochrane Database Syst. Rev. 2013, 2013, CD006201. [Google Scholar] [CrossRef]
- Heby, O.; Persson, L.; Rentala, M. Targeting the polyamine biosynthetic enzymes: A promising approach to therapy of African sleeping sickness, Chagas’ disease, and leishmaniasis. Amino Acids 2007, 33, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Roberts, S.C.; Jardim, A.; Carter, N.S.; Shih, S.; Ariyanayagam, M.; Fairlamb, A.H.; Ullman, B. Ornithine decarboxylase gene deletion mutants of Leishmania donovani. J. Biol. Chem. 1999, 274, 3781–3788. [Google Scholar] [CrossRef] [PubMed]
- Gradoni, L.; Iorio, M.A.; Gramiccia, M.; Orsini, S. In vivo effect of eflornithine (DFMO) and some related compounds on Leishmania infantum preliminary communication. Farmaco 1989, 44, 1157–1166. [Google Scholar] [PubMed]
- Singh, S.; Mukherjee, A.; Khomutov, A.R.; Persson, L.; Heby, O.; Chatterjee, M.; Madhubala, R. Antileishmanial effect of 3-aminooxy-1-aminopropane is due to polyamine depletion. Antimicrob. Agents Chemother. 2007, 51, 528–534. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vannier-Santos, M.A.; Menezes, D.; Oliveira, M.F.; de Mello, F.G. The putrescine analogue 1,4-diamino-2-butanone affects polyamine synthesis, transport, ultrastructure and intracellular survival in Leishmania amazonensis. Microbiology 2008, 154 Pt 10, 3104–3111. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Singh, S.; Dubey, V.K. Novel inhibitors of ornithine decarboxylase of leishmania parasite (LdODC): The parasite resists LdODC inhibition by overexpression of spermidine synthase. Chem. Biol. Drug Des. 2016, 87, 352–360. [Google Scholar] [CrossRef]
- Hu, X.; Washington, S.; Verderame, M.F.; Demers, L.M.; Mauger, D.; Manni, A. Biological activity of the S-adenosylmethionine decarboxylase inhibitor SAM486A in human breast cancer cells in vitro and in vivo. Int. J. Oncol. 2004, 25, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.C.; Scott, J.; Gasteier, J.E.; Jiang, Y.; Brooks, B.; Jardim, A.; Carter, N.S.; Heby, O.; Ullman, B. S-adenosylmethionine decarboxylase from Leishmania donovani. Molecular, genetic, and biochemical characterization of null mutants and overproducers. J. Biol. Chem. 2002, 277, 5902–5909. [Google Scholar] [CrossRef]
- Jennings, F.W.; Ulrich, P.; Cerami, A. Chemotherapy of trypanosomiasis: The use of guanylhydrazone compounds in the treatment of experimental murine trypanosomiasis. Trop. Med. Parasitol. 1987, 38, 181–186. [Google Scholar]
- Brun, R.; Bühler, Y.; Sandmeier, U.; Kaminsky, R.; Bacchi, C.J.; Rattendi, D.; Lane, S.; Croft, S.L.; Snowdon, D.; Yardley, V.; et al. In vitro trypanocidal activities of new S-adenosylmethionine decarboxylase inhibitors. Antimicrob. Agents Chemother. 1996, 40, 1442–1447. [Google Scholar] [CrossRef]
- Balaña-Fouce, R.; Garzón Pulido, T.; Ordóñez-Escudero, D.; Garrido-Pertierra, A. Inhibition of diamine oxidase and S-adenosylmethionine decarboxylase by diminacene aceturate (berenil). Biochem. Pharmacol. 1986, 35, 1597–1600. [Google Scholar] [CrossRef]
- Calonge, M.; Johnson, R.; Balaña-Fouce, R.; Ordóñez, D. Effects of cationic diamidines on polyamine content and uptake on Leishmania infantum in vitro cultures. Biochem. Pharmacol. 1996, 52, 835–841. [Google Scholar] [CrossRef]
- Bacchi, C.J.; Brun, R.; Croft, S.L.; Alicea, K.; Bühler, Y. In vivo trypanocidal activities of new S-adenosylmethionine decarboxylase inhibitors. Antimicrob. Agents Chemother. 1996, 40, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, R.; Kapoor, P.; Madhubala, R. Antileishmanial effect of a potent S-adenosylmethionine decarboxylase inhibitor: CGP 40215A. Pharmacol. Res. 1996, 33, 67–70. [Google Scholar] [CrossRef]
- Gillingwater, K.; Kunz, C.; Braghiroli, C.; Boykin, D.W.; Tidwell, R.R.; Brun, R. In vitro, ex vivo, and in vivo activities of diamidines against Trypanosoma congolense and Trypanosoma vivax. Antimicrob. Agents Chemother. 2017, 61, e02356-16. [Google Scholar] [CrossRef]
- Bitonti, A.J.; Byers, T.L.; Bush, T.L.; Casara, P.J.; Bacchi, C.J.; Clarkson, A.B.; McCann, P.P.; Sjoerdsma, A. Cure of Trypanosoma brucei brucei and Trypanosoma brucei rhodesiense infections in mice with an irreversible inhibitor of S-adenosylmethionine decarboxylase. Antimicrob. Agents Chemother. 1990, 34, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Bacchi, C.J.; Nathan, H.C.; Yarlett, N.; Goldberg, B.; McCann, P.P.; Bitonti, A.J.; Sjoerdsma, A. Cure of murine Trypanosoma brucei rhodesiense infections with an S-adenosylmethionine decarboxylase inhibitor. Antimicrob. Agents Chemother. 1992, 36, 2736–2740. [Google Scholar] [CrossRef]
- Brockway, A.J.; Volkov, O.A.; Cosner, C.C.; MacMillan, K.S.; Wring, S.A.; Richardson, T.E.; Peel, M.; Phillips, M.A.; De Brabander, J.K. Synthesis and evaluation of analogs of 5’-(((Z)-4-amino-2-butenyl)-methylamino)-5’-deoxyadenosine (MDL 73811, or AbeAdo)—An inhibitor of S-adenosylmethionine decarboxylase with antitrypanosomal activity. Bioorg. Med. Chem. 2017, 25, 5433–5440. [Google Scholar] [CrossRef]
- Bacchi, C.J.; Barker, R.H., Jr.; Rodriguez, A.; Hirth, B.; Rattendi, D.; Yarlett, N.; Hendrick, C.L.; Sybertz, E. Trypanocidal activity of 8-methyl-5’-{[(Z)-4-aminobut-2-enyl]-(methylamino)}adenosine (Genz-644131), an adenosylmethionine decarboxylase inhibitor. Antimicrob. Agents Chemother. 2009, 53, 3269–3272. [Google Scholar] [CrossRef] [PubMed]
- Bacchi, C.J.; Sanabria, K.; Spiess, A.J.; Vargas, M.; Marasco, C.J., Jr.; Jimenez, L.M.; Goldberg, B.; Sufrin, J.R. In vivo efficacies of 5’-methylthioadenosine analogs as trypanocides. Antimicrob. Agents Chemother. 1997, 41, 2108–2112. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, Q.; Zhou, Y.; Liu, S. Repurposing clinical drugs as AdoMetDC inhibitors using the SCAR strategy. Front. Pharmacol. 2020, 11, 248. [Google Scholar] [CrossRef]
- Volkov, O.A.; Cosner, C.C.; Brockway, A.J.; Kramer, M.; Booker, M.; Zhong, S.; Ketcherside, A.; Wei, S.; Longgood, J.; McCoy, M.; et al. Identification of Trypanosoma brucei AdoMetDC inhibitors using a high-throughput mass spectrometry-based assay. ACS Infect. Dis. 2017, 3, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Volkov, O.A.; Brockway, A.J.; Wring, S.A.; Peel, M.; Chen, Z.; Phillips, M.A.; De Brabander, J.K. Species-selective pyrimidineamine inhibitors of Trypanosoma brucei S-adenosylmethionine decarboxylase. J. Med. Chem. 2018, 61, 1182–1203. [Google Scholar] [CrossRef]
- Bitonti, A.J.; Kelly, S.E.; McCann, P.P. Characterization of spermidine synthase from Trypanosoma brucei brucei. Mol. Biochem. Parasitol. 1984, 13, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.C.; Jiang, Y.; Gasteier, J.; Frydman, B.; Marton, L.J.; Heby, O.; Ullman, B. Leishmania donovani polyamine biosynthetic enzyme overproducers as tools to investigate the mode of action of cytotoxic polyamine analogs. Antimicrob. Agents Chemother. 2007, 51, 438–445. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Amano, Y.; Namatame, I.; Tateishi, Y.; Honboh, K.; Tanabe, E.; Niimi, T.; Sakashita, H. Structural insights into the novel inhibition mechanism of Trypanosoma cruzi spermidine synthase. Acta Crystallogr. D Biol. Crystallogr. 2015, 71, 1879–1889. [Google Scholar] [CrossRef]
- Zou, Y.; Wu, Z.; Sirisoma, N.; Woster, P.M.; Casero, R.A., Jr.; Weiss, L.M.; Rattendi, D.; Lane, S.; Bacchi, C.J. Novel alkylpolyamine analogues that possess both antitrypanosomal and antimicrosporidial activity. Bioorg. Med. Chem. Lett. 2001, 11, 1613–1617. [Google Scholar] [CrossRef]
- Labadie, G.R.; Choi, S.R.; Avery, M.A. Diamine derivatives with antiparasitic activities. Bioorg. Med. Chem. Lett. 2004, 14, 615–619. [Google Scholar] [CrossRef]
- Yamanaka, C.N.; Giordani, R.B.; Rezende, C.O., Jr.; Eger, I.; Kessler, R.L.; Tonini, M.L.; de Moraes, M.H.; Araújo, D.P.; Zuanazzi, J.A.; de Almeida, M.V.; et al. Assessment of leishmanicidal and trypanocidal activities of aliphatic diamine derivatives. Chem. Biol. Drug Des. 2013, 82, 697–704. [Google Scholar] [CrossRef]
- Alberca, L.N.; Sbaraglini, M.L.; Balcazar, D.; Fraccaroli, L.; Carrillo, C.; Medeiros, A.; Benitez, D.; Comini, M.; Talevi, A. Discovery of novel polyamine analogs with anti-protozoal activity by computer guided drug repositioning. J. Comput. Aided Mol. Des. 2016, 30, 305–321. [Google Scholar] [CrossRef]
- Reigada, C.; Valera-Vera, E.A.; Sayé, M.; Errasti, A.E.; Avila, C.C.; Miranda, M.R.; Pereira, C.A. Trypanocidal effect of isotretinoin through the inhibition of polyamine and amino acid transporters in Trypanosoma cruzi. PLoS Negl. Trop. Dis. 2017, 11, e0005472. [Google Scholar] [CrossRef]
- Reigada, C.; Sayé, M.; Phanstiel, O., 4th; Valera-Vera, E.; Miranda, M.R.; Pereira, C.A. Identification of Trypanosoma cruz polyamine transport inhibitors by computational drug repurposing. Front. Med. 2019, 6, 256. [Google Scholar] [CrossRef] [PubMed]
- Reigada, C.; Phanstiel, O., 4th; Miranda, M.R.; Pereira, C.A. Targeting polyamine transport in Trypanosoma cruzi. Eur. J. Med. Chem. 2018, 147, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaite, A.; Madan, M.; Pandey, V.; Altomare, D.A.; Phanstiel, O., 4th. The discovery of indolone GW5074 during a comprehensive search for non-polyamine-based polyamine transport inhibitors. Int. J. Biochem. Cell Biol. 2021, 138, 106038. [Google Scholar] [CrossRef] [PubMed]
- Gerlin, L.; Baroukh, C.; Genin, S. Polyamines: Double agents in disease and plant immunity. Trends Plant Sci. 2021, 26, 1061–1071. [Google Scholar] [CrossRef]
- Szeliga, M.; Albrecht, J. Roles of nitric oxide and polyamines in brain tumor growth. Adv. Med. Sci. 2021, 66, 199–205. [Google Scholar] [CrossRef]
- Hamouda, N.N.; Van den Haute, C.; Vanhoutte, R.; Sannerud, R.; Azfar, M.; Mayer, R.; Calabuig, A.C.; Swinnen, J.V.; Agostinis, P.; Baekelandt, V.; et al. ATP13A3 is a major component of the enigmatic mammalian polyamine transport system. J. Biol. Chem. 2021, 296, 100182. [Google Scholar] [CrossRef]
- Mu, J.; Xue, C.; Fu, L.; Yu, Z.; Nie, M.; Wu, M.; Chen, X.; Liu, K.; Bu, R.; Huang, Y.; et al. Conformational cycle of human polyamine transporter ATP13A2. Nat. Commun. 2023, 14, 1978. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Pertejo, Y.; García-Estrada, C.; Martínez-Valladares, M.; Murugesan, S.; Reguera, R.M.; Balaña-Fouce, R. Polyamine Metabolism for Drug Intervention in Trypanosomatids. Pathogens 2024, 13, 79. https://doi.org/10.3390/pathogens13010079
Pérez-Pertejo Y, García-Estrada C, Martínez-Valladares M, Murugesan S, Reguera RM, Balaña-Fouce R. Polyamine Metabolism for Drug Intervention in Trypanosomatids. Pathogens. 2024; 13(1):79. https://doi.org/10.3390/pathogens13010079
Chicago/Turabian StylePérez-Pertejo, Yolanda, Carlos García-Estrada, María Martínez-Valladares, Sankaranarayanan Murugesan, Rosa M. Reguera, and Rafael Balaña-Fouce. 2024. "Polyamine Metabolism for Drug Intervention in Trypanosomatids" Pathogens 13, no. 1: 79. https://doi.org/10.3390/pathogens13010079
APA StylePérez-Pertejo, Y., García-Estrada, C., Martínez-Valladares, M., Murugesan, S., Reguera, R. M., & Balaña-Fouce, R. (2024). Polyamine Metabolism for Drug Intervention in Trypanosomatids. Pathogens, 13(1), 79. https://doi.org/10.3390/pathogens13010079







