Abstract
Animal models are essential for studying disease pathogenesis and to test the efficacy and safety of new vaccines and therapeutics. For most diseases, there is no single model that can recapitulate all features of the human condition, so it is vital to understand the advantages and disadvantages of each. The purpose of this review is to describe popular comparative animal models, including mice, ferrets, hamsters, and non-human primates (NHPs), that are being used to study clinical and pathological changes caused by influenza A virus infection with the aim to aid in appropriate model selection for disease modeling.
1. Introduction
The family Orthomyxoviridae encompasses four genera of influenza viruses: Alphainfluenzavirus (influenza A virus, IAV), Betainfluenzavirus (influenza B virus, IBV), Gammainfluenzavirus (influenza C virus, ICV), and Deltainfluenzavirus (influenza D virus, IDV) [1]. Influenza virus infections occur in people and animals worldwide and cause variable disease outcomes depending on the species affected and strain of virus. IAV and IAB infect humans and are responsible for seasonal influenza (flu) epidemics that result in 3–5 million severe illnesses and 290,000–650,000 deaths yearly worldwide [2]. Global flu pandemics, due to the introduction of new, antigenically distinct IAV strains in immunologically naïve populations, occur sporadically and cause increased morbidity and mortality [3]. IAV has a broad host range, infecting aquatic birds, domestic poultry, pigs, dogs, horses, bats, and people. Aquatic birds are considered the reservoir species and likely source of pandemic IAV in humans [4]. In contrast, IBV and ICV are primarily human pathogens, although IBV has been isolated from seals [5] and ICV from cattle [6], pigs [7], and dogs [8]. Infection with ICV is less common and mainly affects children [9]. IDV is a newly emerging virus detected in pigs [10] and cattle [11] with potential zoonotic risk [12].
Orthomyxoviruses are enveloped, segmented, single-stranded, negative-sense RNA viruses. There are either 8 (IAV and IBV) or 7 (ICV and IDV) genome segments (Figure 1). The envelope of IAV and IBV is studded with the transmembrane glycoproteins hemagglutinin (HA) and neuraminidase (NA), which are responsible for attachment/penetration and virion release, respectively. IAVs are classified into subtypes based on their HA and NA molecules, of which there are 18 (HA) and 11 (NA) currently identified. All but two subtypes of IAV are found in aquatic birds while only H1N1 and H3N2 currently circulate in humans [13]. HA and NA are immunodominant epitopes with HA serving as the major target of neutralizing antibodies [14]. Random point mutations in HA and NA, due to the error-prone RNA polymerase, cause slow and gradual antigenic changes (antigenic drift) whereas genetic reassortment via exchange of gene segments results in new, antigenically distinct strains of virus (antigenic shift). These strategies allow the virus to evade host immune responses and develop resistance to antivirals. The possibility of zoonotic transmission and emergence of new pathogenic influenza strains of pandemic potential poses a significant public health threat.
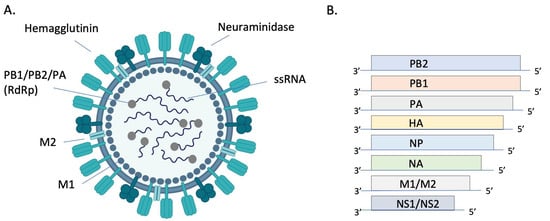
Figure 1.
Structure and genomic organization of influenza A virus. The virion (A) consists of 8 negative sense ssRNA segments complexed with the nucleoprotein (NP, not pictured) to form the helical ribonucleoprotein (RNP). Each segment is associated with an RNA-dependent RNA polymerase (RdRP) complex composed of basic protein 1 (PB1), basic protein 2 (PB2), and the acidic protein (PA). Envelope-associated proteins include hemagglutinin (HA), neuraminidase (NA), matrix protein (M1), and the integral membrane protein (M2). Genomic segments 1–7 encode for one of these structural proteins and the 8th segment encodes for nonstructural proteins (NS) (B). Image (A) created with BioRender.com.
In addition to the expected annual flu burden, pandemic influenza viruses have emerged every 10–40 years due to the reassortment of human IAVs with gene segments of avian and/or swine origin [15]. These novel viruses result in increased morbidity and mortality due to lack of preexisting immunity in human populations [16]. The 1918 H1N1 “Spanish flu” was the deadliest pandemic in history, causing over 40 million deaths worldwide [17]. In addition to the human influenza viruses causing seasonal and pandemic disease, people are sporadically infected with highly pathogenic avian influenza (HPAI) viruses of the H5 or H7 subtype, resulting in approximately 1000 deaths to date [18] and mortality rates of up to 60% [19].
Influenza viruses are transmitted by inhalation of droplets and/or aerosols and by direct contact with an infected individual or contaminated surface [20]. Prevention strategies include nonpharmaceutical interventions such as hand washing, use of hand sanitizer [21,22], social distancing, cough and sneeze hygiene, and cleaning of potentially contaminated surfaces [3]. Vaccination remains the most effective way of preventing disease. Efficacy of inactivated and live attenuated vaccines typically varies between 40–60% [23] depending on the age and health status of an individual, virulence of the season’s major strains of virus, and how well that season’s vaccine matches circulating strains [3]. Antivirals play an important role in combating disease, although drug resistance is a major challenge. Continued research efforts to develop universal influenza vaccines and novel antivirals are essential to combat this highly infectious and potentially fatal disease. The purpose of this review is to highlight the major pathologic features of select influenza animal models, including mice, ferrets, hamsters, and NHPs, with the ultimate goal of informing animal selection for disease modeling.
2. Human Disease and Clinical Symptoms caused by IAV and IBV
Most influenza infections are due to seasonal epidemics of IAV and IAB. Seasonal flu is typically an acute upper respiratory illness lasting 2 to 7 days [24]. Infection is often asymptomatic or a mild upper respiratory illness, although many people experience classic flu symptoms of varying severity, including fever, nonproductive cough, coryza, sore throat, fatigue, and myalgia [25]. While the acute illness typically abates within a week, coughing may continue for 2 or more weeks. Gastrointestinal symptoms such as vomiting, diarrhea, and abdominal pain can occur in severe disease and especially in children [26]. Infants, the elderly, and individuals with underlying health conditions are predisposed to severe infections and pneumonia is the most common complication leading to hospitalization. Other reported complications include myositis, myocarditis, encephalopathy, encephalitis, and Guillain-Barre syndrome [27]. Secondary bacterial pneumonia is more common than primary viral pneumonia [28,29]. Clinically, patients with secondary bacterial pneumonia will have an initial near recovery followed by recurrence of fever, cough, and dyspnea [27].
Pandemic flu causes a similar spectrum of disease ranging from mild to the classic flu-like illness described previously [30]. An unusual feature of the 1918 H1N1 pandemic was the disproportionate number of otherwise young, healthy adults affected, likely due to the unique virulence of this strain, dysregulated host immune responses, and lack of immune memory in the 25–35-year-old age group [17,31]. The 2009 H1N1 pandemic also caused more serious illness in children and adults under 65, although the overall case fatality rate was much lower than the 1918 pandemic [30]. Deaths during the 1918 H1N1 [28] and the 1957 H2N2 [32] pandemics can mostly be attributed to secondary bacterial infections with common upper respiratory tract pathogens. In contrast, bacterial coinfections were identified in only 26–38% of patients dying during the 2009 H1N1 pandemic with the majority of cases consisting of primary viral pneumonia and multiple organ failure [33,34].
Patients infected with zoonotic IAVs, such as HPAI H5N1 and H7N9 typically present with febrile flu symptoms and radiographic evidence of pneumonia following exposure to infected poultry [19,35]. Gastrointestinal symptoms are frequent in some reports [36,37]. Bacterial coinfections are uncommon in H5N1 infections [36,37,38] where death in severe cases occurs due to rapid respiratory failure and complications like acute respiratory distress syndrome and multiorgan failure [19].
3. Influenza Pathology
Influenza viruses enter host cells by binding of HA to sialic acid residues on glycoproteins and glycolipids. Binding affinity for specific sialic acid residues is an important determinant of host range, tissue tropism, and the potential for cross-species transmission (recently reviewed in ref [39]). In short, human influenza viruses preferentially bind to sialic acids with an α2,6 linkage to galactose (SAα2,6Gal) whereas avian influenza viruses preferentially bind to α2,3 linkages (SAα2,3Gal) [40]. In people, SAα2,6Gal is found predominantly in the upper airway and SAα2,3Gal mainly in the lower airway [41]. In contrast, birds express SAα2,3Gal predominantly in the upper airway and intestinal tract [42]. Thus, mutations that allow avian influenza viruses to bind to SAα2,6Gal in the upper respiratory tract of people are likely required for efficient human-to-human transmission via respiratory droplets and aerosols.
Influenza viruses replicate in the nucleus of respiratory and intestinal epithelial cells. Replication peaks at 48 h after infection and virus is shed for approximately 6–8 days [43]. Severity of disease is associated with viral replication in the lower respiratory tract [25]. While symptoms result from a combination of virus-mediated damage to epithelial cells and host immune responses (immunopathology), it is generally accepted that immunopathology plays the largest role in tissue damage [44]. Infected epithelial cells and innate immune cells release pro-inflammatory cytokines and chemokines that are important to control infection, but also lead to bystander damage to epithelial and endothelial cells. Excess neutrophil recruitment [45,46] and inflammatory cytokines such as IL-6, IL-8, IL-10, TNFα, CXCL10, IL-2R, GCSF, MCP1, and MIP1α are associated with disease severity and poor outcomes [35,47,48,49].
Because lung specimens are usually collected during autopsy, pathology is only documented in fatal cases of human IAV infection. Characteristic findings of viral pneumonia are similar between pandemic and non-pandemic years [25,32,34,50,51,52,53,54] so are described together. Grossly, the trachea and bronchi are hemorrhagic and often filled with blood-tinged, foamy fluid. The lungs are dark red and edematous, reflecting the underlying hemorrhagic bronchopneumonia that is frequently complicated by secondary bacterial infection [32,34,53]. Histologically, the trachea and bronchi have epithelial necrosis and desquamation in addition to submucosal edema, congestion, and hemorrhage. In the lower airways, there is necrotizing bronchiolitis and alveolitis, interstitial mononuclear inflammation, interstitial and alveolar edema, thrombi, hyaline membranes, and type II pneumocyte hyperplasia. These changes are consistent with the exudative phase of diffuse alveolar damage (DAD), which is the histologic hallmark of acute respiratory distress syndrome (ARDS) [55]. With time, epithelial regeneration, interstitial fibrosis, and bronchiolitis obliterans may develop. Secondary bacterial pneumonia consisting of overwhelming neutrophilic inflammation, extensive necrosis, and hemorrhage can obscure the underlying viral effects [25]. Hemophagocytosis is a prominent feature of some cases of fatal disease and is thought to be mediated by hypercytokinemia [33,51]. Hemophagocytosis was present in the lymph nodes of 18 of 36 (53%) pediatric patients dying of non-pandemic influenza [52] and in 25 of 41 (61%) patients dying of 2009 pH1N1 [33]. The clinical significance of hemophagocytosis in these infections is unclear.
While overall histologic findings are similar in fatal cases from pandemic and non-pandemic years (Table 1), antigen distribution appears to differ. In a study of 47 pediatric patients dying of seasonal influenza pneumonia from 2003–2004, antigen was detected primarily in the bronchial epithelial cells and mucous glands of the trachea, bronchi, and large bronchioles [52]. In contrast, antigen was mainly present in type I and II alveolar pneumocytes in patients dying of 2009 pandemic H1N1 (pH1N1) [33]. These findings likely reflect differences in tissue tropism based on receptor location and the variable course of disease at the time of sampling.
As the case numbers are significantly lower compared with seasonal and pandemic H1N1, there are fewer reports on the pathology of fatal avian H5N1 infection despite a case fatality rate of 56% [56]. Diffuse alveolar damage is the main histologic feature and most cases also display mild lymphocytic interstitial pneumonia, alveolar histiocytosis, hemorrhage, and type II pneumocyte hyperplasia [51,57,58,59,60,61,62,63,64]. Depending on the time course of disease, DAD may be exudative or organizing and fibrotic [62,63]. Extrapulmonary lesions including lymphoid depletion, hepatic necrosis, and acute tubular necrosis, are frequently reported [51,58,60,61,65]. Viral RNA can be detected outside of the respiratory tract in the spleen, liver, intestines [62,63,64], and cerebrospinal fluid [66] although extrapulmonary antigen is only rarely documented [61], suggesting that systemic spread may not be the culprit of multiorgan failure. In the respiratory tract, viral antigen and RNA are found most commonly in type I and II pneumocytes although they can also be present in macrophages, sloughed epithelial cells, non-ciliated and ciliated bronchiolar epithelium, and tracheal epithelium [57,58,60,61,62,63]. The predominant viral distribution in pneumocytes is consistent with the lower airway distribution of SAα2,3Gal, the receptor for avian influenza viruses [41]. Hemophagocytosis is a frequent finding in the lungs, lymph nodes, and spleen [51,57,58,60,61,63,64]. In severe cases, reactive hemophagocytic syndrome, consisting of pancytopenia, abnormal clotting times, and reduced liver function, is the most prominent finding [51,60]. A combination of high viral loads and an intense cytokine response appear central to the pathogenicity of H5N1 in people [57,67,68,69].

Table 1.
Summary of clinical and pathologic findings in human and laboratory animal influenza infections.
Table 1.
Summary of clinical and pathologic findings in human and laboratory animal influenza infections.
| Common Animal Strain/Species | Virus Strain | Clinical Signs | Microscopic Pathology | References | |
|---|---|---|---|---|---|
| Humans | N/A | Seasonal IAV | Varying degrees of fever, non-productive cough, dyspnea, coryza, fatigue, and myalgia (classic flu symptoms); vomiting and diarrhea in severe cases | Necrotizing tracheobronchitis and bronchointerstitial pneumonia with thrombi, edema, hemorrhage, hyaline membranes, and type II pneumocyte hyperplasia (diffuse alveolar damage) | [25,26,50] |
| 2009 pH1N1 | Mild to severe flu symptoms | Same as seasonal IAV | [30,34,50,52] | ||
| 1918 H1N1 | Mild to severe flu symptoms | Same as seasonal IAV | [17,25,53] | ||
| HPAI H5N1 and H7N9 | Mild to severe flu symptoms with history of contact with live poultry | Same as seasonal IAV Extrapulmonary necrotic lesions are common and hemophagocytosis may be the most prominent lesion | [19,35,36,37,50,51,70,71] | ||
| Mouse | BALB/c C57BL/6 DBA/2J A/J | PR8 | Dyspnea, ruffled fur, weight loss, and anorexia | Interstitial pneumonia, suppurative bronchiolitis and alveolitis, hyaline membranes, and alveolar edema | [72,73,74,75] |
| BALB/c C57BL/6 | 2009 pH1N1 | Variable weight loss (dose and strain dependent) | Histiocytic to neutrophilic bronchitis, bronchiolitis and alveolitis with varying epithelial necrosis (strain dependent) | [76,77,78,79] | |
| BALB/c | 1918 H1N1 | Weight loss and death | Interstitial pneumonia, suppurative bronchiolitis and alveolitis, hyaline membranes, and alveolar edema | [76,80,81] | |
| BALB/c DBA/2J | HPAI H5N1 | Dyspnea, ruffled fur, weight loss, and anorexia | Interstitial pneumonia, suppurative bronchiolitis and alveolitis, hyaline membranes, and alveolar edema Encephalitis and myocardial necrosis | [81,82,83,84,85,86,87,88] | |
| C57BL/6 BALB/c | H7N9 | Weight loss, ruffled fur, hunching | Bronchiolitis, patchy interstitial pneumonia, and varying amounts of bronchiolar and alveolar epithelial necrosis | [89,90,91,92] | |
| BALB/c | LPAI | Variable weight loss, ruffled fur, and hunching (strain dependent) | Necrotizing bronchitis and bronchiolitis with peribronchial pneumonia | [93,94,95,96,97] | |
| Hamster | Golden Syrian hamster | Seasonal IAV | Mild weight loss and temperature changes | None or mild necrotizing rhinitis and bronchopneumonia with perivascular cuffing | [98,99,100,101,102] |
| 2009 pH1N1 | Mild weight loss | Necrotizing rhinitis, bronchiolitis, perivasculitis, edema, and mild interstitial pneumonia | [101,103,104,105] | ||
| HPAI H5N1 | Not reported | Intranasal route: Bronchiolitis and bronchopneumonia Intragastric route: Interstitial pneumonia | [106] | ||
| Ferret | Mustela putorius furo | Seasonal IAV | Asymptomatic or mild lethargy with sneezing, nasal discharge, and mild weight loss | Conventional intranasal model: Rhinitis and mild bronchiolitis and pneumoniaHigh dose intratracheal model: Moderate rhinitis and severe necrotizing bronchointerstitial pneumonia with edema | [107,108,109,110,111,112] |
| 2009 pH1N1 | Lethargy, anorexia, dyspnea, and elevated body temperature | Necrotizing rhinotracheitis, bronchitis, and bronchiolitis with varying degrees of interstitial pneumonia and diffuse alveolar damage | [77,110,111,112] | ||
| 1918 H1N1 | Weight loss, sneezing, dyspnea, lethargy, and death | Necrotizing rhinitis, bronchiolitis and bronchointerstitial pneumonia with edema | [113,114,115] | ||
| HPAI H5N1 | Lethargy, anorexia, dyspnea, nasal discharge, sneezing, weight loss, elevated body temperature, diarrhea, and neurologic signs (ataxia, torticollis, and hind limb paresis) | Severe necrotizing bronchointerstitial pneumonia with diffuse alveolar damage Meningoencephalitis | [111,116,117,118,119] | ||
| LPAI | Transiently elevated body temperature and weight loss; occasional dyspnea and lethargy | Suppurative rhinitis | [91,120,121] | ||
| NHP | Cynomolgus macaques (most common), rhesus macaques, and common marmosets | Seasonal IAV | Asymptomatic or mild lethargy | Mild bronchointerstitial pneumonia and peribronchiolitis | [122,123] |
| 2009 pH1N1 | Asymptomatic or mildly elevated body temperature and lethargy; tachypnea, dyspnea, and nasal discharge reported for some strains of virus | Suppurative rhinitis in mild cases; necrotizing bronchopneumonia with edema and hyaline membranes in severe cases (strain dependent) | [124,125,126,127,128,129] | ||
| 1918 H1N1 | Anorexia, lethargy, cough, nasal discharge, and tachypnea | Necrotizing bronchointerstitial pneumonia with hemorrhage, edema, and hyaline membranes | [123,130,131] | ||
| HPAI H5N1 | Anorexia, lethargy, cough, tachypnea, elevated body temperature, diarrhea, and thrombocytopenia | Necrotizing bronchointerstitial pneumonia with hemorrhage, edema, hyaline membranes, and type II pneumocyte hyperplasia Lymphoid necrosis and renal tubular necrosis | [130,132,133,134] |
4. Comparative Pathology of Animal Models for Influenza A
4.1. Mouse Models
Mice (Mus musculus) are the most common laboratory animal model of influenza. Benefits of using mice include their cost efficiency and the abundant immunologic and genetic tools available. Laboratory mice, however, are not susceptible to infection with many wild-type human influenza viruses but are readily infected with mouse adapted strains generated via serial passage through mouse lung [135,136]. The most common mouse strains used for influenza research are C57BL/6 and BALB/c, although DBA/2J and A/J mice are more susceptible to disease [72,73]. Susceptible inbred strains have a large deletion or nonsense mutation in the interferon inducible Mx1 gene and thus fail to express the influenza restriction factor Mx1 [137]. Wild mice, in contrast, resist infection with even mouse adapted strains due to a functional Mx1 gene [138]. Pathogenesis studies in C57BL/6 and BALB/c mice must therefore be interpreted with caution, especially regarding innate anti-influenza immunity.
The commonly used laboratory adapted influenza A/Puerto Rico/8/34 strain (PR8) is lethal in C57BL/6 [72] and BALB/c mice [74,75]. Clinical signs include tachypnea, ruffled fur, weight loss, and anorexia. Grossly, the lungs are dark red and consolidated [74]. Infected mice have characteristic viral pneumonia consisting of interstitial inflammation and neutrophilic bronchiolar exudates [72,74]. Examples of histologic lesions are shown in Figure 2. Fukushi et al. [74] compared the pulmonary lesions of BALB/c mice surviving infection with those that died or were humanely euthanized and found that dead mice had characteristic lesions of DAD, including hyaline membranes, edema, and alveolar collapse, whereas as survivors did not. As previously described, DAD is the typical histologic finding in fatal cases of influenza viral pneumonia in people (Table 1), so the PR8 mouse model can be used to test vaccines and therapeutics [75,139] and to investigate pathogenesis of severe disease [140,141] with the notable caveat that the virus is adapted to mice.
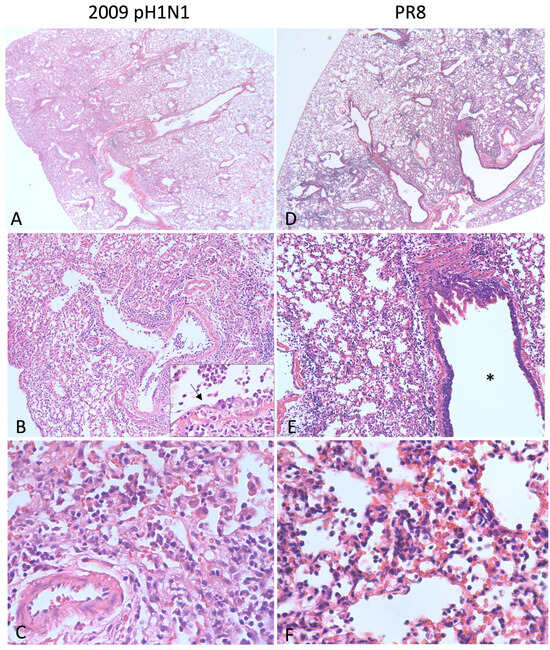
Figure 2.
Lung pathology in mice infected with influenza. (A–C) BALB/c mouse inoculated with 1000 plaque forming units (pfu) of 2009 pH1N1 intranasally and euthanized on day 5. At low magnification (A), approximately 30% of the lung is consolidated and hypercellular. There is degeneration, necrosis, and loss of the bronchiolar epithelium (B) and a neutrophilic exudate (inset, arrow = degenerating epithelium). Alveolar septa are expanded by fibrin, edema, hemorrhage, neutrophils, lymphocytes, and plasma cells and alveolar spaces are filled with fibrin and degenerate neutrophils (C). (D–F) Tropomyosin receptor kinase A-knockin mouse (C57BL/6 background) inoculated with 250 pfu PR8 and euthanized on day 5. Similar to 2009 pH1N1, approximately 30% of the lung is consolidated (D). The inflammation affects the alveoli, and the airway (*) is spared (E). Alveolar septa are thickened due to congestion and the accumulation of neutrophils, lymphocytes, and macrophages. Alveolar spaces contain neutrophils, necrotic debris, and fibrin (F).
Mice are susceptible to certain human IAV strains without prior adaptation. The reconstructed 1918 H1N1 influenza virus is lethal in BALB/c mice where it causes necrotizing bronchitis, bronchiolitis, and neutrophilic alveolitis with alveolar edema and hemorrhage [80] similar to patients infected in 1918 [53]. By infecting mice with the fully reconstructed recombinant 1918 H1N1 virus or other recombinant viruses containing one or more 1918 H1N1 genes, Tumpey et al. [80] showed that the HA and polymerase genes likely played a large role in the unusual lethality of the pandemic. The 2009 pH1N1 virus also replicates efficiently in mouse lungs without prior adaptation. Weight loss and pathology are both dose- and strain-dependent and generally non-lethal [76,77,78,79]. The histologic lesions of 2009 pH1N1 (strain California/4/09) are shown in Figure 2A–C and include necrotizing bronchiolitis and interstitial pneumonia. Pneumonia due to the mouse-adapted H1N1 virus PR8 is similar in this case, although the airways are relatively spared (Figure 2D–F).
Because mice mainly express SAα2,3Gal rather than SAα2,6Gal on airway epithelial cells and type II pneumocytes [142], they are readily infected with avian IAVs. Avian IAVs attach predominantly to ciliated and non-ciliated cells in the trachea with lesser attachment in the lower respiratory tract of birds [143]. This pattern is opposite to avian IAV attachment patterns in humans, where virus mainly attaches to alveolar macrophages, type II pneumocytes, and nonciliated bronchiolar epithelial cells of the lower respiratory tract [143,144]. Common laboratory mouse strains are susceptible to the non-adapted HPAI strains H5N1 [82,83,84,85,86] and H7N9 [89,90] as well as some strains of low pathogenicity avian influenza (LPAI) [93]. Mice infected with HPAI H5N1 exhibit rapid weight loss and clinical signs such as hunched posture, ruffled fur, inappetence, and dyspnea within 24 h of infection [83,84,86]. Clinical signs, lesions, and lethality are dependent on the virus strain, with strains originating from the Hong Kong outbreak in 1997 causing the most severe disease [82,86]. In lethal infections, there is degeneration and necrosis of epithelial cells lining the nasal cavity, trachea, bronchi, and bronchioles with associated intraluminal fibrinosuppurative inflammation. Alveolitis typically occurs in a peribronchial pattern and consists of pneumocyte necrosis and accumulations of fibrin, erythrocytes, neutrophils, and increased alveolar macrophages in alveolar spaces [86]. Antigen is found in respiratory epithelial cells of the nasal cavity, bronchi, and bronchioles, and within sloughed necrotic cells [82,84,86]. In addition to pneumonia, some have shown cardiomyocyte necrosis [83] and encephalitis [84,87] with accompanying influenza viral antigen, indicating systemic infection. The neurovirulent influenza mouse model is particularly relevant because encephalitis is a known, although uncommon, complication of H5N1 infection [66,145]. H7N9 is generally less pathogenic than H5N1 in mice, causing weight loss, multifocal peribronchial interstitial pneumonia, and variable necrosis of bronchiolar and alveolar epithelia [89,90,91,92].
Mice are an efficient and low-cost influenza model that has been indispensable for the discovery of new vaccines (reviewed in ref [146]) and therapeutics [147,148,149] and gaining insight into disease pathogenesis (Table 2) [80,84,87,141]. A major drawback of this model is that mice are not naturally susceptible to many strains of human IAV. They are, however, susceptible to mouse-adapted and certain highly pathogenic human and avian IAV strains. Additionally, transmission is generally inefficient in this species [150,151]. Additional animal models described in the following sections serve to fill these gaps.

Table 2.
Benefits, drawbacks, and main applications of animal models of influenza.
4.2. Ferret Model
Ferrets (Mustela putorius furo) were the first animal experimentally infected with influenza virus [160] and are still the one of the best models of infection [161]. They are naturally susceptible to seasonal human influenza isolates [107,108], 1918 and 2009 pandemic H1N1 influenza [84,117,120,121], LPAI [91,120], HPAI [116,117,118] and other animal-derived influenza viruses [121,162]. Ferrets exhibit upper respiratory symptoms similar to humans such as sneezing, nasal discharge, lethargy, and fever [163] and transmit virus via respiratory droplets [116,155,164]. Patterns of transmission between ferrets are similar to those in humans, so transmission studies can help predict the potential of a novel influenza virus to cause a pandemic [154]. The distribution of sialic acids in the respiratory tract is similar between ferrets and humans [165,166], so, like in people, human influenza viruses attach to ciliated epithelial cells in the trachea and bronchi and type II pneumocytes in ferrets [143].
The severity of disease depends on dose, route of inoculation, and strain of IAV. With human seasonal IAVs, the conventional intranasal challenge model of 1 × 106 median tissue culture infectious dose (TCID50) predominantly causes rhinitis and bronchiolitis [110,112] and mild, localized bronchointerstitial pneumonia [109]. Intratracheal inoculation is used to reliably induce lower respiratory tract inflammation [111,167,168]. High intratracheal doses (1 × 109 TCID50) of seasonal influenza strains are required to cause reproducible necrotizing bronchointerstitial pneumonia [168]. In comparison, the 2009 pH1N1 virus causes diffuse alveolar damage and necrotizing pneumonia at standard challenge doses [111]. Ferrets inoculated intraocularly have similar antigen distribution and inflammation compared to those inoculated intranasally [169], representing another route of mucosal exposure [170].
Ferrets are also highly susceptible to infection with select agent IAVs, including HPAI H5N1 [111,119,167] and the reconstructed 1918 H1N1 influenza virus [113,114,115]. Tumpey et al. [113] used the ferret model to show the importance of HA receptor specificity in the transmission of pandemic viruses; reconstructed 1918 H1N1 viruses with mutations causing a change in binding preference from SAα2,6Gal to SAα2,3Gal lost the ability to be transmitted between ferrets. The viral RNA polymerase complex was identified by Watanabe et al. [115] as an essential virulence factor of 1918 H1N1 influenza in ferrets. These studies illustrate the importance of this model in understanding the pathogenesis of influenza viruses with a particular focus on those with pandemic potential.
HPAI H5N1 and 2009 pH1N1 infections in ferrets causes a range of clinical signs including lethargy, inappetence, dyspnea, sneezing, increased body temperature, and weight loss [111,119,167,171]. Seasonal IAV infections are often asymptomatic or may result in mildly reduced activity levels [111,119]. Of these strains, HPAI H5N1 causes the most severe clinical disease and pathology [111,119]. Extrarespiratory disease like diarrhea [119,171] and neurologic signs (e.g., ataxia, torticollis, and hind limb paresis) [119,167,171] are also common with H5N1 infection. The infection is often lethal, especially at high intratracheal doses [111]. Zitzow et al. [119] infected ferrets with two strains of H5N1 and found that both replicated at high titers in the lungs and nasal turbinates. Virus was also detected in the brain, spleen, intestine, and fecal swabs of some animals, consistent with systemic infection seen in people infected with H5N1 [69,172]. Grossly, infected animals have dark red, consolidated lungs [119,167] and histologic lesions are characterized by severe necrotizing bronchointerstitial pneumonia and diffuse alveolar damage [111,119,171]. Brain lesions consist of nonsuppurative and necrotizing meningoencephalitis with glial nodules and neuronophagia [119,167,171]. The route of inoculation plays a role in the severity of central nervous system and pulmonary lesions. Bodewes et al. [167] found that all ferrets inoculated intranasally with H5N1 had necrotizing encephalitis while fewer than half had mild to moderate bronchointerstitial pneumonia. In comparison, ferrets inoculated intratracheally with a lower dose were more ill and all had severe pneumonia, no neurologic symptoms, and five times greater viral titers in the lungs. They hypothesized that direct extension of virus from the nasal cavity to the olfactory bulb during intranasal inoculation led to viral encephalitis.
As shown here, ferrets are an invaluable tool for dissecting influenza virus transmission and pathogenesis (Table 2), especially in the face of potential future pandemic viruses. The anatomy and physiology of the ferret respiratory tract is similar to humans [173] and they develop pulmonary lesions that accurately reflect human disease (Table 1). Drawbacks of this model are increased costs, lack of inbred strains, and fewer available reagents when compared to mice [160].
4.3. Hamster Model
The Syrian (Golden) hamster (Mesocricetus auratus), like the ferret, is naturally susceptible to IBV [174] and many strains of IAV, including seasonal viruses [98,99,100,101], pandemic 2009 H1N1 [101,103,104], and avian H5N1 [106]. They are small, inexpensive, and are simpler to manage when compared to ferrets. Hamsters were first used to study immune responses to infection and immunization [100,175,176,177], although other small animal models subsequently became more popular. There has recently been renewed interest in the hamster influenza model, especially as a model for coronavirus and IAV coinfection [103,104,105,152].
IAV in hamsters is largely an upper respiratory tract infection. Virus is readily retrieved from nasal washes of infected animals [100,101,175,177] with titers peaking at 3–4 days post-infection (dpi) and clearing by 7 dpi [100,101]. Clinical signs that can be observed in ferrets, like nasal discharge, lethargy, and sneezing, have not been reported in hamsters regardless of the IAV strain [101,102,103,177]. Mild weight loss may occur without other observable signs of infection following inoculation with pH1N1 [101,104] and seasonal H1N1 strains [102]. Descriptions of influenza pathology in hamsters are limited and vary depending on the study and virus strain. Iwatsuki-Horimoto et al. [101] compared the nasal pathology associated with seasonal H3N2, 2009 pH1N1, and IBV strains. Necrotizing rhinitis concentrated in the olfactory epithelium was identified in pH1N1-infected hamsters, but no lesions were identified in H3N2- or IBV-infected hamsters. Antigen (IAV NP) was detected in the upper respiratory tract of some hamsters infected with H3N2 and in both the upper and lower respiratory tracts of hamsters infected with pH1N1 and IBV. In hamsters infected with 2009 pH1N1, pulmonary lesions vary from bronchiolitis with luminal exudates, perivasculitis, with edema [103] to mononuclear interstitial pneumonia that may be very mild (Table 1) [104,105]. Antigen (IAV NP) is present in nasal epithelial cells in hamsters infected with H3N2 [101] and in nasal, tracheal, bronchiolar, and bronchial epithelial cells but infrequently in alveolar pneumocytes in hamsters infected with pH1N1 [101,103]. This contrasts with fatal pH1N1 infection in people where antigen is largely located in pneumocytes [33]. Paterson et al. [102] infected hamsters with a recent seasonal strain of H1N1 that caused more weight loss and respiratory lesions compared to other studies using the 2009 pH1N1 strain. These hamsters had necrotizing rhinitis, laryngitis, and tracheitis with acute, necrotizing bronchopneumonia and perivascular cuffing. Lastly, hamsters are susceptible to avian H5N1 infection via intranasal and intragastric routes where they develop bronchopneumonia and interstitial pneumonia, respectively [106].
Hamsters are a cost-efficient and easy-to-manage small animal model of influenza infection that do not require viral adaptation. Like ferrets, hamsters can transmit influenza [101,178] and develop comparable lesions and immune responses [102,106]. Additionally, hamsters have both SAα2,3Gal and SAα2,6Gal in the upper respiratory tract and, like people, SAα2,3Gal in the lower respiratory tract, supporting their use as a model for the human disease. A major drawback is that they do not develop clinical signs and lesion severity is variable, which could preclude their use in testing the efficacy of antivirals and vaccines.
4.4. Non-Human Primate Models
Non-human primates (NHPs) are popular animal models due to their close genetic relationship with humans. As they share common anatomic and immunologic features, they are generally excellent models for viral respiratory diseases, including influenza. Their use, however, is limited by ethical concerns, high husbandry costs, and lack of availability compared to rodents and small mammals. NHPs are naturally [179] and experimentally susceptible to human [123,126,180] and avian [180,181] IAVs.
Rhesus (Macaca mulatta) and cynomolgus (Macaca fascicularis) macaques are the most frequently used NHP in influenza research, although other species including African green monkeys (Chlorocebus sebaeus), pig-tailed macaques (Macaca nemestrina), squirrel monkeys (Saimiri spp.), and common marmosets (Callithrix jacchus) have also been studied [182]. When infected with seasonal strains, NHPs are typically asymptomatic or have mild disease [122,123,180]. Similarly, 2009 pH1N1 is often a subclinical infection in NHPs [124,125,126] although varying degrees of respiratory illness can occur [127,128,129], which likely depends on the viral strain, species of NHP, and route of infection. Cynomolgus are generally preferred over rhesus macaques as they have higher and more consistent viral titers in the respiratory tract and increased pulmonary pathology [129,183]. This difference can likely be attributed to the greater number of SAa2,6Gal receptors in the trachea and bronchioles in cynomolgus compared to rhesus macaques [129].
Clinical signs in macaques infected with 1918 H1N1 or avian IAVs include fever, anorexia, lethargy, tachypnea, and cough that is often fatal [123,132,133]. Diarrhea and thrombocytopenia, which have been reported in people [36], may also occur in H5N1 infected macaques [132]. H5N1 and 1918 H1N1 antigen can be detected as early as 12 h post infection in bronchiolar and alveolar epithelial cells [130] with histologic lesions that include hemorrhage, edema, and necrotizing bronchiolitis and alveolitis starting by at least 24 h after infection (Table 1) [123,130,132]. Interstitial inflammation is composed of neutrophils and macrophages [132,134]; hyaline membranes and extra-pulmonary necrotic lesions are occasionally reported [134]. In contrast to studies showing severe and often lethal disease due to 1918 H1N1 in macaques [123,130,131], it was recently reported that infection even with high doses causes minimal disease in rhesus macaques and dose-dependent, non-lethal disease in cynomolgus macaques [183]. These discrepancies could be due to differences in study design, genetics, or euthanasia criteria. Regardless, cynomolgus macaques are, to date, the best NHP model for these deadly influenza virus infections.
Due to lack of accessibility, ethical concerns, and high costs associated with housing and husbandry, NHPs are used less often than small animal models. Because they are genetically and anatomically more similar to humans, however, they are a valuable tool for examining pathologic and immunologic responses to influenza (Table 2) [123,124,125,130,132,156,157].
5. Conclusions
Influenza viruses have caused multiple pandemics in the last century, including the deadliest recorded pandemic of any etiology in 1918. While zoonotic influenza strains have had limited human-to-human transmission to date, a virus that gains the ability to be efficiently transmitted between people would be a serious global threat. Great strides have been made in developing antivirals and vaccines, although a universal influenza vaccine remains elusive. In this review, we have described the major clinical and pathologic findings of four popular animal models—mice, ferrets, hamsters, and NHPs—that are summarized in Table 1 to aid researchers in selecting the most relevant disease model.
Mice are a convenient and cost-effective option, although they are not susceptible to most human IAV strains and lack an important innate antiviral effector [137]. They are, however, susceptible to avian IAVs and 1918 H1N1 and, like people, develop necrotizing interstitial pneumonia [80,86]. The mouse adapted IAV strain PR8 elicits many histologic features of severe influenza pneumonia in people, including alveolitis, hyaline membrane formation, and alveolar edema that characterize diffuse alveolar damage, so is a convenient system in which to test vaccines and therapeutics. Of the four species discussed here, ferrets display symptoms, transmission dynamics, and pathologic features that are most similar to people. Unlike mice, they are naturally susceptible to human IAVs and exhibit upper respiratory symptoms like sneezing and nasal discharge [163]. Using high dose intratracheal inoculation, severe bronchointerstitial pneumonia can be induced with seasonal IAV strains, which is a unique feature of the ferret model [111,112,167]. In comparison, hamsters are susceptible to seasonal and pandemic IAV strains but are asymptomatic [101,102,103,177]. NHPs are also asymptomatic when infected with seasonal IAV [122,123,180] but have been particularly valuable in studying pandemic H1N1 and avian strains as they develop pulmonary pathology, including the formation of hyaline membranes, that is very similar to people [123,130,132]. However, their use is generally limited due to high costs, lack of availability, and ethical concerns. The prominent hemophagocytosis seen in both H1N1 and H5N1 infections has yet to be replicated in these animal models. In general, influenza pneumonia is more severe in people than in laboratory animals. A great deal of information has been learned about influenza viruses using these models and they will continue to be essential for the development of effective therapeutic and vaccine strategies to combat potential emerging viruses.
Author Contributions
Writing—original draft preparation, N.M.K.; writing—review and editing, Y.L. and H.L.; supervision—Y.L. and H.L. All authors have read and agreed to the published version of the manuscript.
Funding
NK was supported by NIH T32 Comparative Medicine and Pathology Postdoctoral Fellowship OD010993.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shi, M.; Lin, X.-D.; Chen, X.; Tian, J.-H.; Chen, L.-J.; Li, K.; Wang, W.; Eden, J.-S.; Shen, J.-J.; Liu, L.; et al. The Evolutionary History of Vertebrate RNA Viruses. Nature 2018, 556, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Influenza (Seasonal). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 18 September 2023).
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Primer 2018, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and Ecology of Influenza A Viruses. Microbiol. Rev. 1992, 56, 152–179. [Google Scholar] [CrossRef] [PubMed]
- Osterhaus, A.D.M.E.; Rimmelzwaan, G.F.; Martina, B.E.E.; Bestebroer, T.M.; Fouchier, R.A.M. Influenza B Virus in Seals. Science 2000, 288, 1051–1053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Porter, E.; Lohman, M.; Lu, N.; Peddireddi, L.; Hanzlicek, G.; Marthaler, D.; Liu, X.; Bai, J. Influenza C Virus in Cattle with Respiratory Disease, United States, 2016–2018. Emerg. Infect. Dis. 2018, 24, 1926–1929. [Google Scholar] [CrossRef]
- Guo, Y.J.; Jin, F.G.; Wang, P.; Wang, M.; Zhu, J.M. Isolation of Influenza C Virus from Pigs and Experimental Infection of Pigs with Influenza C Virus. J. Gen. Virol. 1983, 64 Pt 1, 177–182. [Google Scholar] [CrossRef]
- Manuguerra, J.C.; Hannoun, C. Natural Infection of Dogs by Influenza C Virus. Res. Virol. 1992, 143, 199–204. [Google Scholar] [CrossRef]
- Thielen, B.K.; Friedlander, H.; Bistodeau, S.; Shu, B.; Lynch, B.; Martin, K.; Bye, E.; Como-Sabetti, K.; Boxrud, D.; Strain, A.K.; et al. Detection of Influenza C Viruses Among Outpatients and Patients Hospitalized for Severe Acute Respiratory Infection, Minnesota, 2013–2016. Clin. Infect. Dis. 2018, 66, 1092–1098. [Google Scholar] [CrossRef]
- Hause, B.M.; Ducatez, M.; Collin, E.A.; Ran, Z.; Liu, R.; Sheng, Z.; Armien, A.; Kaplan, B.; Chakravarty, S.; Hoppe, A.D.; et al. Isolation of a Novel Swine Influenza Virus from Oklahoma in 2011 Which Is Distantly Related to Human Influenza C Viruses. PLoS Pathog. 2013, 9, e1003176. [Google Scholar] [CrossRef]
- Hause, B.M.; Collin, E.A.; Liu, R.; Huang, B.; Sheng, Z.; Lu, W.; Wang, D.; Nelson, E.A.; Li, F. Characterization of a Novel Influenza Virus in Cattle and Swine: Proposal for a New Genus in the Orthomyxoviridae Family. mBio 2014, 5, e00031-14. [Google Scholar] [CrossRef]
- White, S.K.; Ma, W.; McDaniel, C.J.; Gray, G.C.; Lednicky, J.A. Serologic Evidence of Exposure to Influenza D Virus among Persons with Occupational Contact with Cattle. J. Clin. Virol. 2016, 81, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Influenza Type A Viruses|Avian Influenza (Flu). Available online: https://www.cdc.gov/flu/avianflu/influenza-a-virus-subtypes.htm (accessed on 18 September 2023).
- Brandenburg, B.; Koudstaal, W.; Goudsmit, J.; Klaren, V.; Tang, C.; Bujny, M.V.; Korse, H.J.W.M.; Kwaks, T.; Otterstrom, J.J.; Juraszek, J.; et al. Mechanisms of Hemagglutinin Targeted Influenza Virus Neutralization. PLoS ONE 2013, 8, e80034. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.G.; Govorkova, E.A. Continuing Challenges in Influenza. Ann. N. Y. Acad. Sci. 2014, 1323, 115–139. [Google Scholar] [CrossRef] [PubMed]
- Palese, P. Influenza: Old and New Threats. Nat. Med. 2004, 10, S82–S87. [Google Scholar] [CrossRef] [PubMed]
- Palese, P.; Tumpey, T.M.; Garcia-Sastre, A. What Can We Learn from Reconstructing the Extinct 1918 Pandemic Influenza Virus? Immunity 2006, 24, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zeng, X.; Cui, P.; Yan, C.; Chen, H. Alarming Situation of Emerging H5 and H7 Avian Influenza and Effective Control Strategies. Emerg. Microbes Infect. 2022, 12, 2155072. [Google Scholar] [CrossRef] [PubMed]
- Gambotto, A.; Barratt-Boyes, S.M.; De Jong, M.D.; Neumann, G.; Kawaoka, Y. Human Infection with Highly Pathogenic H5N1 Influenza Virus. Lancet 2008, 371, 1464–1475. [Google Scholar] [CrossRef]
- Killingley, B.; Nguyen-Van-Tam, J. Routes of Influenza Transmission. Influenza Other Respir. Viruses 2013, 7, 42–51. [Google Scholar] [CrossRef]
- Stebbins, S.; Cummings, D.A.T.; Stark, J.H.; Vukotich, C.; Mitruka, K.; Thompson, W.; Rinaldo, C.; Roth, L.; Wagner, M.; Wisniewski, S.R.; et al. Reduction in the Incidence of Influenza A but Not Influenza B Associated with Use of Hand Sanitizer and Cough Hygiene in Schools: A Randomized Controlled Trial. Pediatr. Infect. Dis. J. 2011, 30, 921–926. [Google Scholar] [CrossRef]
- Torner, N.; Soldevila, N.; Garcia, J.J.; Launes, C.; Godoy, P.; Castilla, J.; Domínguez, A.; CIBERESP Cases and Controls in Pandemic Influenza Working Group, Spain. Effectiveness of Non-Pharmaceutical Measures in Preventing Pediatric Influenza: A Case-Control Study. BMC Public Health 2015, 15, 543. [Google Scholar] [CrossRef]
- CDC Seasonal Flu Vaccine Effectiveness Studies|CDC. Available online: https://www.cdc.gov/flu/vaccines-work/effectiveness-studies.htm (accessed on 18 September 2023).
- Influenza—Vaccine Preventable Diseases Surveillance Manual|CDC. Available online: https://www.cdc.gov/vaccines/pubs/surv-manual/chpt06-influenza.html (accessed on 18 September 2023).
- Taubenberger, J.K.; Morens, D.M. The Pathology of Influenza Virus Infections. Annu. Rev. Pathol. 2008, 3, 499–522. [Google Scholar] [CrossRef] [PubMed]
- Minodier, L.; Charrel, R.N.; Ceccaldi, P.-E.; Van der Werf, S.; Blanchon, T.; Hanslik, T.; Falchi, A. Prevalence of Gastrointestinal Symptoms in Patients with Influenza, Clinical Significance, and Pathophysiology of Human Influenza Viruses in Faecal Samples: What Do We Know? Virol. J. 2015, 12, 215. [Google Scholar] [CrossRef] [PubMed]
- Rothberg, M.B.; Haessler, S.D.; Brown, R.B. Complications of Viral Influenza. Am. J. Med. 2008, 121, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Morens, D.M.; Taubenberger, J.K.; Fauci, A.S. Predominant Role of Bacterial Pneumonia as a Cause of Death in Pandemic Influenza: Implications for Pandemic Influenza Preparedness. J. Infect. Dis. 2008, 198, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Dunning, J.; Thwaites, R.S.; Openshaw, P.J.M. Seasonal and Pandemic Influenza: 100 Years of Progress, Still Much to Learn. Mucosal Immunol. 2020, 13, 566–573. [Google Scholar] [CrossRef]
- Writing Committee of the WHO Consultation on Clinical Aspects of Pandemic (H1N1) 2009 Influenza; Bautista, E.; Chotpitayasunondh, T.; Gao, Z.; Harper, S.A.; Shaw, M.; Uyeki, T.M.; Zaki, S.R.; Hayden, F.G.; Hui, D.S.; et al. Clinical Aspects of Pandemic 2009 Influenza A (H1N1) Virus Infection. N. Engl. J. Med. 2010, 362, 1708–1719. [Google Scholar] [CrossRef] [PubMed]
- McAuley, J.L.; Kedzierska, K.; Brown, L.E.; Shanks, G.D. Host Immunological Factors Enhancing Mortality of Young Adults during the 1918 Influenza Pandemic. Front. Immunol. 2015, 6, 419. [Google Scholar] [CrossRef]
- Hers, J.F.P.; Masurel, N.; Mulder, J. Bacteriology and histopathology of the respiratory tract and lungs in fatal Asian influenza. Lancet 1958, 272, 1141–1143. [Google Scholar] [CrossRef]
- Shieh, W.-J.; Blau, D.M.; Denison, A.M.; DeLeon-Carnes, M.; Adem, P.; Bhatnagar, J.; Sumner, J.; Liu, L.; Patel, M.; Batten, B.; et al. 2009 Pandemic Influenza A (H1N1). Am. J. Pathol. 2010, 177, 166–175. [Google Scholar] [CrossRef]
- Mauad, T.; Hajjar, L.A.; Callegari, G.D.; Da Silva, L.F.F.; Schout, D.; Galas, F.R.B.G.; Alves, V.A.F.; Malheiros, D.M.A.C.; Auler, J.O.C.; Ferreira, A.F.; et al. Lung Pathology in Fatal Novel Human Influenza A (H1N1) Infection. Am. J. Respir. Crit. Care Med. 2010, 181, 72–79. [Google Scholar] [CrossRef]
- Gao, H.-N.; Lu, H.-Z.; Cao, B.; Du, B.; Shang, H.; Gan, J.-H.; Lu, S.-H.; Yang, Y.-D.; Fang, Q.; Shen, Y.-Z.; et al. Clinical Findings in 111 Cases of Influenza A (H7N9) Virus Infection. N. Engl. J. Med. 2013, 368, 2277–2285. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Nguyen, T.L.; Nguyen, T.D.; Luong, T.S.; Pham, P.M.; Van Vinh Chau, N.; Pham, T.S.; Vo, C.D.; Le, T.Q.M.; Ngo, T.T.; et al. Avian Influenza A (H5N1) in 10 Patients in Vietnam. N. Engl. J. Med. 2004, 350, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Yuen, K.; Chan, P.; Peiris, M.; Tsang, D.; Que, T.; Shortridge, K.; Cheung, P.; To, W.; Ho, E.; Sung, R.; et al. Clinical Features and Rapid Viral Diagnosis of Human Disease Associated with Avian Influenza A H5N1 Virus. Lancet 1998, 351, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Oner, A.F.; Bay, A.; Arslan, S.; Akdeniz, H.; Sahin, H.A.; Cesur, Y.; Epcacan, S.; Yilmaz, N.; Deger, I.; Kizilyildiz, B.; et al. Avian Influenza A (H5N1) Infection in Eastern Turkey in 2006. N. Engl. J. Med. 2006, 355, 2179–2185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Pu, J. Influence of Host Sialic Acid Receptors Structure on the Host Specificity of Influenza Viruses. Viruses 2022, 14, 2141. [Google Scholar] [CrossRef] [PubMed]
- Rogers, G.N.; Paulson, J.C.; Daniels, R.S.; Skehel, J.J.; Wilson, I.A.; Wiley, D.C. Single Amino Acid Substitutions in Influenza Haemagglutinin Change Receptor Binding Specificity. Nature 1983, 304, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Shinya, K.; Ebina, M.; Yamada, S.; Ono, M.; Kasai, N.; Kawaoka, Y. Influenza Virus Receptors in the Human Airway. Nature 2006, 440, 435–436. [Google Scholar] [CrossRef]
- Pillai, S.P.; Lee, C.W. Species and Age Related Differences in the Type and Distribution of Influenza Virus Receptors in Different Tissues of Chickens, Ducks and Turkeys. Virol. J. 2010, 7, 5. [Google Scholar] [CrossRef]
- Knipe, D.M.; Howley, P. Fields Virology; Wolters Kluwer Health: Philadelphia, PA, USA, 2013; ISBN 978-1-4698-7422-7. [Google Scholar]
- Boyd, D.F.; Wilson, T.L.; Thomas, P.G. Chapter Seven—One Hundred Years of (Influenza) Immunopathology. In Advances in Virus Research; Carr, J.P., Roossinck, M.J., Eds.; Immunopathology; Academic Press: Cambridge, MA, USA, 2020; Volume 107, pp. 247–284. [Google Scholar]
- Tang, B.M.; Shojaei, M.; Teoh, S.; Meyers, A.; Ho, J.; Ball, T.B.; Keynan, Y.; Pisipati, A.; Kumar, A.; Eisen, D.P.; et al. Neutrophils-Related Host Factors Associated with Severe Disease and Fatality in Patients with Influenza Infection. Nat. Commun. 2019, 10, 3422. [Google Scholar] [CrossRef]
- Guan, W.; Yang, Z.; Wu, N.C.; Lee, H.H.Y.; Li, Y.; Jiang, W.; Shen, L.; Wu, D.C.; Chen, R.; Zhong, N.; et al. Clinical Correlations of Transcriptional Profile in Patients Infected with Avian Influenza H7N9 Virus. J. Infect. Dis. 2018, 218, 1238–1248. [Google Scholar] [CrossRef]
- Fiore-Gartland, A.; Panoskaltsis-Mortari, A.; Agan, A.A.; Mistry, A.J.; Thomas, P.G.; Matthay, M.A.; PALISI PICFlu Investigators; Hertz, T.; Randolph, A.G. Cytokine Profiles of Severe Influenza Virus-Related Complications in Children. Front. Immunol. 2017, 8, 1423. [Google Scholar] [CrossRef] [PubMed]
- Bradley-Stewart, A.; Jolly, L.; Adamson, W.; Gunson, R.; Frew-Gillespie, C.; Templeton, K.; Aitken, C.; Carman, W.; Cameron, S.; McSharry, C. Cytokine Responses in Patients with Mild or Severe Influenza A(H1N1)Pdm09. J. Clin. Virol. 2013, 58, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Oshansky, C.M.; Gartland, A.J.; Wong, S.-S.; Jeevan, T.; Wang, D.; Roddam, P.L.; Caniza, M.A.; Hertz, T.; Devincenzo, J.P.; Webby, R.J.; et al. Mucosal Immune Responses Predict Clinical Outcomes during Influenza Infection Independently of Age and Viral Load. Am. J. Respir. Crit. Care Med. 2014, 189, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Guarner, J.; Falcón-Escobedo, R. Comparison of the Pathology Caused by H1N1, H5N1, and H3N2 Influenza Viruses. Arch. Med. Res. 2009, 40, 655–661. [Google Scholar] [CrossRef] [PubMed]
- To, K.-F.; Chan, P.K.S.; Chan, K.-F.; Lee, W.-K.; Lam, W.-Y.; Wong, K.-F.; Tang, N.L.S.; Tsang, D.N.C.; Sung, R.Y.T.; Buckley, T.A.; et al. Pathology of Fatal Human Infection Associated with Avian Influenza A H5N1 Virus. J. Med. Virol. 2001, 63, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Guarner, J.; Paddock, C.D.; Shieh, W.-J.; Packard, M.M.; Patel, M.; Montague, J.L.; Uyeki, T.M.; Bhat, N.; Balish, A.; Lindstrom, S.; et al. Histopathologic and Immunohistochemical Features of Fatal Influenza Virus Infection in Children during the 2003-2004 Season. Clin. Infect. Dis. 2006, 43, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Lucke, B.; Kime, E. PATHOLOGIC ANATOMY AND BACTERIOLOGY OF INFLUENZA: EPIDEMIC OF AUTUMN, 1918. Arch. Intern. Med. 1919, 24, 154–237. [Google Scholar] [CrossRef]
- Lindsay, M.I.; Herrmann, E.C.; Morrow, G.W.; Brown, A.L. Hong Kong Influenza: Clinical, Microbiologic, and Pathologic Features in 127 Cases. JAMA 1970, 214, 1825–1832. [Google Scholar] [CrossRef]
- The ARDS Definition Task Force. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Avian Influenza Weekly Update Number 924. Available online: https://cdn.who.int/media/docs/default-source/wpro---documents/emergency/surveillance/avian-influenza/ai_20231203.pdf?sfvrsn=5f006f99_123 (accessed on 6 December 2023).
- Peiris, J.S.M.; Yu, W.C.; Leung, C.W.; Cheung, C.Y.; Ng, W.F.; Nicholls, J.M.; Ng, T.K.; Chan, K.H.; Lai, S.T.; Lim, W.L.; et al. Re-Emergence of Fatal Human Influenza A Subtype H5N1 Disease. Lancet 2004, 363, 617–619. [Google Scholar] [CrossRef]
- Ungchusak, K.; Auewarakul, P.; Dowell, S.F.; Kitphati, R.; Auwanit, W.; Puthavathana, P.; Uiprasertkul, M.; Boonnak, K.; Pittayawonganon, C.; Cox, N.J.; et al. Probable Person-to-Person Transmission of Avian Influenza A (H5N1). N. Engl. J. Med. 2005, 352, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Chotpitayasunondh, T.; Ungchusak, K.; Hanshaoworakul, W.; Chunsuthiwat, S.; Sawanpanyalert, P.; Kijphati, R.; Lochindarat, S.; Srisan, P.; Suwan, P.; Osotthanakorn, Y.; et al. Human Disease from Influenza A (H5N1), Thailand, 2004. Emerg. Infect. Dis. 2005, 11, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.K.S. Outbreak of Avian Influenza A(H5N1) Virus Infection in Hong Kong in 1997. Clin. Infect. Dis. 2002, 34 (Suppl. S2), S58–S64. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Xie, Z.; Gao, Z.; Liu, J.; Korteweg, C.; Ye, J.; Lau, L.T.; Lu, J.; Gao, Z.; Zhang, B.; et al. H5N1 Infection of the Respiratory Tract and beyond: A Molecular Pathology Study. Lancet 2007, 370, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Uiprasertkul, M.; Puthavathana, P.; Sangsiriwut, K.; Pooruk, P.; Srisook, K.; Peiris, M.; Nicholls, J.M.; Chokephaibulkit, K.; Vanprapar, N.; Auewarakul, P. Influenza A H5N1 Replication Sites in Humans. Emerg. Infect. Dis. 2005, 11, 1036–1041. [Google Scholar] [CrossRef]
- Uiprasertkul, M.; Kitphati, R.; Puthavathana, P.; Kriwong, R.; Kongchanagul, A.; Ungchusak, K.; Angkasekwinai, S.; Chokephaibulkit, K.; Srisook, K.; Vanprapar, N.; et al. Apoptosis and Pathogenesis of Avian Influenza A (H5N1) Virus in Humans. Emerg. Infect. Dis. 2007, 13, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Chokephaibulkit, K.; Uiprasertkul, M.; Puthavathana, P.; Chearskul, P.; Auewarakul, P.; Dowell, S.F.; Vanprapar, N. A Child with Avian Influenza A (H5N1) Infection. Pediatr. Infect. Dis. J. 2005, 24, 162–166. [Google Scholar] [CrossRef]
- Ku, A.S.; Chan, L.T. The First Case of H5N1 Avian Influenza Infection in a Human with Complications of Adult Respiratory Distress Syndrome and Reye’s Syndrome. J. Paediatr. Child Health 1999, 35, 207–209. [Google Scholar] [CrossRef]
- De Jong, M.D.; Cam, B.V.; Qui, P.T.; Hien, V.M.; Thanh, T.T.; Hue, N.B.; Beld, M.; Phuong, L.T.; Khanh, T.H.; Chau, N.V.V.; et al. Fatal Avian Influenza A (H5N1) in a Child Presenting with Diarrhea Followed by Coma. N. Engl. J. Med. 2005, 352, 686–691. [Google Scholar] [CrossRef]
- De Jong, M.D.; Simmons, C.P.; Thanh, T.T.; Hien, V.M.; Smith, G.J.D.; Chau, T.N.B.; Hoang, D.M.; Van Vinh Chau, N.; Khanh, T.H.; Dong, V.C.; et al. Fatal Outcome of Human Influenza A (H5N1) Is Associated with High Viral Load and Hypercytokinemia. Nat. Med. 2006, 12, 1203–1207. [Google Scholar] [CrossRef]
- Cheung, C.Y.; Poon, L.L.M.; Lau, A.S.; Luk, W.; Lau, Y.L.; Shortridge, K.F.; Gordon, S.; Guan, Y.; Peiris, J.S.M. Induction of Proinflammatory Cytokines in Human Macrophages by Influenza A (H5N1) Viruses: A Mechanism for the Unusual Severity of Human Disease? Lancet 2002, 360, 1831–1837. [Google Scholar] [CrossRef] [PubMed]
- Korteweg, C.; Gu, J. Pathology, Molecular Biology, and Pathogenesis of Avian Influenza A (H5N1) Infection in Humans. Am. J. Pathol. 2008, 172, 1155–1170. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wang, Z.; Chen, Y.; Ding, W.; Jia, H.; Chan, J.F.-W.; To, K.K.-W.; Chen, H.; Yang, Y.; Liang, W.; et al. Clinical, Virological, and Histopathological Manifestations of Fatal Human Infections by Avian Influenza A(H7N9) Virus. Clin. Infect. Dis. 2013, 57, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-B.; Li, H.-Y.; Liu, J.-F.; Lan, C.-Q.; Lin, Q.-H.; Chen, S.-X.; Zhang, H.-Y.; Wang, X.-H.; Lin, X.; Pan, J.-G.; et al. Histopathological Findings in a Critically Ill Patient with Avian Influenza A (H7N9). J. Thorac. Dis. 2015, 7, E672–E677. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, B.; Błazejewska, P.; Hessmann, M.; Bruder, D.; Geffers, R.; Mauel, S.; Gruber, A.D.; Schughart, K. Host Genetic Background Strongly Influences the Response to Influenza a Virus Infections. PLoS ONE 2009, 4, e4857. [Google Scholar] [CrossRef]
- Pica, N.; Iyer, A.; Ramos, I.; Bouvier, N.M.; Fernandez-Sesma, A.; García-Sastre, A.; Lowen, A.C.; Palese, P.; Steel, J. The DBA.2 Mouse Is Susceptible to Disease Following Infection with a Broad, but Limited, Range of Influenza A and B Viruses. J. Virol. 2011, 85, 12825–12829. [Google Scholar] [CrossRef] [PubMed]
- Fukushi, M.; Ito, T.; Oka, T.; Kitazawa, T.; Miyoshi-Akiyama, T.; Kirikae, T.; Yamashita, M.; Kudo, K. Serial Histopathological Examination of the Lungs of Mice Infected with Influenza A Virus PR8 Strain. PLoS ONE 2011, 6, e21207. [Google Scholar] [CrossRef]
- Chen, J.; Fang, F.; Li, X.; Chang, H.; Chen, Z. Protection against Influenza Virus Infection in BALB/c Mice Immunized with a Single Dose of Neuraminidase-Expressing DNAs by Electroporation. Vaccine 2005, 23, 4322–4328. [Google Scholar] [CrossRef]
- Belser, J.A.; Wadford, D.A.; Pappas, C.; Gustin, K.M.; Maines, T.R.; Pearce, M.B.; Zeng, H.; Swayne, D.E.; Pantin-Jackwood, M.; Katz, J.M.; et al. Pathogenesis of Pandemic Influenza A (H1N1) and Triple-Reassortant Swine Influenza A (H1) Viruses in Mice. J. Virol. 2010, 84, 4194–4203. [Google Scholar] [CrossRef]
- Maines, T.R.; Jayaraman, A.; Belser, J.A.; Wadford, D.A.; Pappas, C.; Zeng, H.; Gustin, K.M.; Pearce, M.B.; Viswanathan, K.; Shriver, Z.H.; et al. Transmission and Pathogenesis of Swine-Origin 2009 A(H1N1) Influenza Viruses in Ferrets and Mice. Science 2009, 325, 484–487. [Google Scholar] [CrossRef]
- Manicassamy, B.; Medina, R.A.; Hai, R.; Tsibane, T.; Stertz, S.; Nistal-Villán, E.; Palese, P.; Basler, C.F.; García-Sastre, A. Protection of Mice against Lethal Challenge with 2009 H1N1 Influenza A Virus by 1918-like and Classical Swine H1N1 Based Vaccines. PLoS Pathog. 2010, 6, e1000745. [Google Scholar] [CrossRef] [PubMed]
- Groves, H.T.; McDonald, J.U.; Langat, P.; Kinnear, E.; Kellam, P.; McCauley, J.; Ellis, J.; Thompson, C.; Elderfield, R.; Parker, L.; et al. Mouse Models of Influenza Infection with Circulating Strains to Test Seasonal Vaccine Efficacy. Front. Immunol. 2018, 9, 126. [Google Scholar] [CrossRef] [PubMed]
- Tumpey, T.M.; Basler, C.F.; Aguilar, P.V.; Zeng, H.; Solórzano, A.; Swayne, D.E.; Cox, N.J.; Katz, J.M.; Taubenberger, J.K.; Palese, P.; et al. Characterization of the Reconstructed 1918 Spanish Influenza Pandemic Virus. Science 2005, 310, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Tumpey, T.M.; Szretter, K.J.; Van Hoeven, N.; Katz, J.M.; Kochs, G.; Haller, O.; García-Sastre, A.; Staeheli, P. The Mx1 Gene Protects Mice against the Pandemic 1918 and Highly Lethal Human H5N1 Influenza Viruses. J. Virol. 2007, 81, 10818–10821. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Tumpey, T.M.; Morken, T.; Zaki, S.R.; Cox, N.J.; Katz, J.M. A Mouse Model for the Evaluation of Pathogenesis and Immunity to Influenza A (H5N1) Viruses Isolated from Humans. J. Virol. 1999, 73, 5903–5911. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Watanabe, S.; Ito, T.; Goto, H.; Wells, K.; McGregor, M.; Cooley, A.J.; Kawaoka, Y. Biological Heterogeneity, Including Systemic Replication in Mice, of H5N1 Influenza A Virus Isolates from Humans in Hong Kong. J. Virol. 1999, 73, 3184–3189. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Itamura, S.; Iwasaki, T.; Kurata, T.; Tashiro, M. Characterization of Human Influenza A (H5N1) Virus Infection in Mice: Neuro-, Pneumo- and Adipotropic Infection. J. Gen. 2000, 81, 2503–2510. [Google Scholar] [CrossRef]
- Gubareva, L.V.; McCullers, J.A.; Bethell, R.C.; Webster, R.G. Characterization of Influenza A/HongKong/156/97 (H5N1) Virus in a Mouse Model and Protective Effect of Zanamivir on H5N1 Infection in Mice. J. Infect. Dis. 1998, 178, 1592–1596. [Google Scholar] [CrossRef]
- Dybing, J.K.; Schultz-Cherry, S.; Swayne, D.E.; Suarez, D.L.; Perdue, M.L. Distinct Pathogenesis of Hong Kong-Origin H5N1 Viruses in Mice Compared to That of Other Highly Pathogenic H5 Avian Influenza Viruses. J. Virol. 2000, 74, 1443–1450. [Google Scholar] [CrossRef]
- Tanaka, H.; Park, C.-H.; Ninomiya, A.; Ozaki, H.; Takada, A.; Umemura, T.; Kida, H. Neurotropism of the 1997 Hong Kong H5N1 Influenza Virus in Mice. Vet. Microbiol. 2003, 95, 1–13. [Google Scholar] [CrossRef]
- Boon, A.C.M.; Finkelstein, D.; Zheng, M.; Liao, G.; Allard, J.; Klumpp, K.; Webster, R.; Peltz, G.; Webby, R.J. H5N1 Influenza Virus Pathogenesis in Genetically Diverse Mice Is Mediated at the Level of Viral Load. mBio 2011, 2, e00171-11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Liu, C.; Kou, Z.; Gao, T.; Pan, T.; Wu, X.; Yu, H.; Guo, Y.; Zeng, Y.; Du, L.; et al. Differences in the Pathogenicity and Inflammatory Responses Induced by Avian Influenza A/H7N9 Virus Infection in BALB/c and C57BL/6 Mouse Models. PLoS ONE 2014, 9, e92987. [Google Scholar] [CrossRef] [PubMed]
- Mok, C.K.P.; Lee, H.H.Y.; Chan, M.C.W.; Sia, S.F.; Lestra, M.; Nicholls, J.M.; Zhu, H.; Guan, Y.; Peiris, J.M.S. Pathogenicity of the Novel A/H7N9 Influenza Virus in Mice. mBio 2013, 4, e00362-13. [Google Scholar] [CrossRef] [PubMed]
- Belser, J.A.; Gustin, K.M.; Pearce, M.B.; Maines, T.R.; Zeng, H.; Pappas, C.; Sun, X.; Carney, P.J.; Villanueva, J.M.; Stevens, J.; et al. Pathogenesis and Transmission of Avian Influenza A (H7N9) Virus in Ferrets and Mice. Nature 2013, 501, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Meliopoulos, V.A.; Karlsson, E.A.; Kercher, L.; Cline, T.; Freiden, P.; Duan, S.; Vogel, P.; Webby, R.J.; Guan, Y.; Peiris, M.; et al. Human H7N9 and H5N1 Influenza Viruses Differ in Induction of Cytokines and Tissue Tropism. J. Virol. 2014, 88, 12982–12991. [Google Scholar] [CrossRef]
- Lee, Y.-N.; Lee, D.-H.; Cheon, S.-H.; Park, Y.-R.; Baek, Y.-G.; Si, Y.-J.; Kye, S.-J.; Lee, E.-K.; Heo, G.-B.; Bae, Y.-C.; et al. Genetic Characteristics and Pathogenesis of H5 Low Pathogenic Avian Influenza Viruses from Wild Birds and Domestic Ducks in South Korea. Sci. Rep. 2020, 10, 12151. [Google Scholar] [CrossRef] [PubMed]
- Belser, J.A.; Lu, X.; Maines, T.R.; Smith, C.; Li, Y.; Donis, R.O.; Katz, J.M.; Tumpey, T.M. Pathogenesis of Avian Influenza (H7) Virus Infection in Mice and Ferrets: Enhanced Virulence of Eurasian H7N7 Viruses Isolated from Humans. J. Virol. 2007, 81, 11139–11147. [Google Scholar] [CrossRef]
- Driskell, E.A.; Jones, C.A.; Stallknecht, D.E.; Howerth, E.W.; Tompkins, S.M. Avian Influenza Virus Isolates from Wild Birds Replicate and Cause Disease in a Mouse Model of Infection. Virology 2010, 399, 280–289. [Google Scholar] [CrossRef]
- Gillim-Ross, L.; Santos, C.; Chen, Z.; Aspelund, A.; Yang, C.-F.; Ye, D.; Jin, H.; Kemble, G.; Subbarao, K. Avian Influenza H6 Viruses Productively Infect and Cause Illness in Mice and Ferrets. J. Virol. 2008, 82, 10854–10863. [Google Scholar] [CrossRef]
- Joseph, T.; McAuliffe, J.; Lu, B.; Jin, H.; Kemble, G.; Subbarao, K. Evaluation of Replication and Pathogenicity of Avian Influenza a H7 Subtype Viruses in a Mouse Model. J. Virol. 2007, 81, 10558–10566. [Google Scholar] [CrossRef]
- Heath, A.W.; Addison, C.; Ali, M.; Teale, D.; Potter, C.W. In Vivo and in Vitro Hamster Models in the Assessment of Virulence of Recombinant Influenza Viruses. Antiviral Res. 1983, 3, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.J.; Teh, C.Z.; Jennings, R.; Potter, C.W. Transmissibility of Influenza Viruses in Hamsters. Arch. Virol. 1982, 72, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Jennings, R.; Denton, M.D.; Potter, C.W. The Hamster as an Experimental Animal for the Study of Influenza. I. The Role of Antibody in Protection. Med. Microbiol. Immunol. 1976, 162, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Iwatsuki-Horimoto, K.; Nakajima, N.; Ichiko, Y.; Sakai-Tagawa, Y.; Noda, T.; Hasegawa, H.; Kawaoka, Y. Syrian Hamster as an Animal Model for the Study of Human Influenza Virus Infection. J. Virol. 2018, 92, e01693-17. [Google Scholar] [CrossRef] [PubMed]
- Paterson, J.; Ryan, K.A.; Morley, D.; Jones, N.J.; Yeates, P.; Hall, Y.; Whittaker, C.J.; Salguero, F.J.; Marriott, A.C. Infection with Seasonal H1N1 Influenza Results in Comparable Disease Kinetics and Host Immune Responses in Ferrets and Golden Syrian Hamsters. Pathogens 2023, 12, 668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.J.; Lee, A.C.-Y.; Chan, J.F.-W.; Liu, F.; Li, C.; Chen, Y.; Chu, H.; Lau, S.-Y.; Wang, P.; Chan, C.C.-S.; et al. Coinfection by Severe Acute Respiratory Syndrome Coronavirus 2 and Influenza A(H1N1)Pdm09 Virus Enhances the Severity of Pneumonia in Golden Syrian Hamsters. Clin. Infect. Dis. 2021, 72, e978–e992. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Kang, J.-A.; Lyoo, K.-S.; Le, T.B.; Yeo, Y.H.; Wong, S.-S.; Na, W.; Song, D.; Webby, R.J.; Zanin, M.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 and Influenza A Virus Co-Infection Alters Viral Tropism and Haematological Composition in Syrian Hamsters. Transbound. Emerg. Dis. 2022, 69, e3297–e3304. [Google Scholar] [CrossRef] [PubMed]
- Oishi, K.; Horiuchi, S.; Minkoff, J.M.; Ten Oever, B.R. The Host Response to Influenza A Virus Interferes with SARS-CoV-2 Replication during Coinfection. J. Virol. 2022, 96, e0076522. [Google Scholar] [CrossRef]
- Shinya, K.; Makino, A.; Tanaka, H.; Hatta, M.; Watanabe, T.; Le, M.Q.; Imai, H.; Kawaoka, Y. Systemic Dissemination of H5N1 Influenza A Viruses in Ferrets and Hamsters after Direct Intragastric Inoculation. J. Virol. 2011, 85, 4673–4678. [Google Scholar] [CrossRef]
- Pappas, C.; Yang, H.; Carney, P.J.; Pearce, M.B.; Katz, J.M.; Stevens, J.; Tumpey, T.M. Assessment of Transmission, Pathogenesis and Adaptation of H2 Subtype Influenza Viruses in Ferrets. Virology 2015, 477, 61–71. [Google Scholar] [CrossRef]
- Huang, S.S.H.; Banner, D.; Fang, Y.; Ng, D.C.K.; Kanagasabai, T.; Kelvin, D.J.; Kelvin, A.A. Comparative Analyses of Pandemic H1N1 and Seasonal H1N1, H3N2, and Influenza B Infections Depict Distinct Clinical Pictures in Ferrets. PLoS ONE 2011, 6, e27512. [Google Scholar] [CrossRef] [PubMed]
- Svitek, N.; Rudd, P.A.; Obojes, K.; Pillet, S.; Von Messling, V. Severe Seasonal Influenza in Ferrets Correlates with Reduced Interferon and Increased IL-6 Induction. Virology 2008, 376, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Munster, V.J.; De Wit, E.; Van den Brand, J.M.A.; Herfst, S.; Schrauwen, E.J.A.; Bestebroer, T.M.; Van de Vijver, D.; Boucher, C.A.; Koopmans, M.; Rimmelzwaan, G.F.; et al. Pathogenesis and Transmission of Swine-Origin 2009 A(H1N1) Influenza Virus in Ferrets. Science 2009, 325, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Van den Brand, J.M.A.; Stittelaar, K.J.; Van Amerongen, G.; Rimmelzwaan, G.F.; Simon, J.; De Wit, E.; Munster, V.; Bestebroer, T.; Fouchier, R.A.M.; Kuiken, T.; et al. Severity of Pneumonia Due to New H1N1 Influenza Virus in Ferrets Is Intermediate between That Due to Seasonal H1N1 Virus and Highly Pathogenic Avian Influenza H5N1 Virus. J. Infect. Dis. 2010, 201, 993–999. [Google Scholar] [CrossRef]
- Van den Brand, J.M.A.; Stittelaar, K.J.; Van Amerongen, G.; Reperant, L.; De Waal, L.; Osterhaus, A.D.M.E.; Kuiken, T. Comparison of Temporal and Spatial Dynamics of Seasonal H3N2, Pandemic H1N1 and Highly Pathogenic Avian Influenza H5N1 Virus Infections in Ferrets. PLoS ONE 2012, 7, e42343. [Google Scholar] [CrossRef] [PubMed]
- Tumpey, T.M.; Maines, T.R.; Van Hoeven, N.; Glaser, L.; Solórzano, A.; Pappas, C.; Cox, N.J.; Swayne, D.E.; Palese, P.; Katz, J.M.; et al. A Two-Amino Acid Change in the Hemagglutinin of the 1918 Influenza Virus Abolishes Transmission. Science 2007, 315, 655–659. [Google Scholar] [CrossRef]
- De Wit, E.; Siegers, J.Y.; Cronin, J.M.; Weatherman, S.; Van den Brand, J.M.; Leijten, L.M.; Van Run, P.; Begeman, L.; Van den Ham, H.-J.; Andeweg, A.C.; et al. 1918 H1N1 Influenza Virus Replicates and Induces Proinflammatory Cytokine Responses in Extrarespiratory Tissues of Ferrets. J. Infect. Dis. 2018, 217, 1237–1246. [Google Scholar] [CrossRef]
- Watanabe, T.; Watanabe, S.; Shinya, K.; Kim, J.H.; Hatta, M.; Kawaoka, Y. Viral RNA Polymerase Complex Promotes Optimal Growth of 1918 Virus in the Lower Respiratory Tract of Ferrets. Proc. Natl. Acad. Sci. USA 2009, 106, 588–592. [Google Scholar] [CrossRef]
- Maines, T.R.; Chen, L.-M.; Matsuoka, Y.; Chen, H.; Rowe, T.; Ortin, J.; Falcón, A.; Hien, N.T.; Mai, L.Q.; Sedyaningsih, E.R.; et al. Lack of Transmission of H5N1 Avian–Human Reassortant Influenza Viruses in a Ferret Model. Proc. Natl. Acad. Sci. USA 2006, 103, 12121–12126. [Google Scholar] [CrossRef]
- Imai, M.; Watanabe, T.; Kiso, M.; Nakajima, N.; Yamayoshi, S.; Iwatsuki-Horimoto, K.; Hatta, M.; Yamada, S.; Ito, M.; Sakai-Tagawa, Y.; et al. A Highly Pathogenic Avian H7N9 Influenza Virus Isolated from A Human Is Lethal in Some Ferrets Infected via Respiratory Droplets. Cell Host Microbe 2017, 22, 615–626.e8. [Google Scholar] [CrossRef]
- Pearce, M.B.; Pappas, C.; Gustin, K.M.; Davis, C.T.; Pantin-Jackwood, M.J.; Swayne, D.E.; Maines, T.R.; Belser, J.A.; Tumpey, T.M. Enhanced Virulence of Clade 2.3.2.1 Highly Pathogenic Avian Influenza A H5N1 Viruses in Ferrets. Virology 2017, 502, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Zitzow, L.A.; Rowe, T.; Morken, T.; Shieh, W.-J.; Zaki, S.; Katz, J.M. Pathogenesis of Avian Influenza A (H5N1) Viruses in Ferrets. J. Virol. 2002, 76, 4420–4429. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.; Schrauwen, E.J.A.; De Graaf, M.; Bestebroer, T.M.; Spronken, M.I.J.; Van Boheemen, S.; De Meulder, D.; Lexmond, P.; Linster, M.; Herfst, S.; et al. Limited Airborne Transmission of H7N9 Influenza A Virus between Ferrets. Nature 2013, 501, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Belser, J.A.; Pulit-Penaloza, J.A.; Sun, X.; Brock, N.; Pappas, C.; Creager, H.M.; Zeng, H.; Tumpey, T.M.; Maines, T.R. A Novel A(H7N2) Influenza Virus Isolated from a Veterinarian Caring for Cats in a New York City Animal Shelter Causes Mild Disease and Transmits Poorly in the Ferret Model. J. Virol. 2017, 91, e00672-17. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.D.; Olsen, R.J.; LaCasse, R.A.; Safronetz, D.; Ashraf, M.; Porter, A.R.; Braughton, K.R.; Feldmann, F.; Clifton, D.R.; Kash, J.C.; et al. Seasonal H3N2 Influenza A Virus Fails to Enhance Staphylococcus Aureus Co-Infection in a Non-Human Primate Respiratory Tract Infection Model. Virulence 2013, 4, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Kobasa, D.; Jones, S.M.; Shinya, K.; Kash, J.C.; Copps, J.; Ebihara, H.; Hatta, Y.; Hyun Kim, J.; Halfmann, P.; Hatta, M.; et al. Aberrant Innate Immune Response in Lethal Infection of Macaques with the 1918 Influenza Virus. Nature 2007, 445, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Skinner, J.A.; Zurawski, S.M.; Sugimoto, C.; Vinet-Oliphant, H.; Vinod, P.; Xue, Y.; Russell-Lodrigue, K.; Albrecht, R.A.; García-Sastre, A.; Salazar, A.M.; et al. Immunologic Characterization of a Rhesus Macaque H1N1 Challenge Model for Candidate Influenza Virus Vaccine Assessment. Clin. Vaccine Immunol. CVI 2014, 21, 1668–1680. [Google Scholar] [CrossRef] [PubMed]
- Josset, L.; Engelmann, F.; Haberthur, K.; Kelly, S.; Park, B.; Kawoaka, Y.; García-Sastre, A.; Katze, M.G.; Messaoudi, I. Increased Viral Loads and Exacerbated Innate Host Responses in Aged Macaques Infected with the 2009 Pandemic H1N1 Influenza A Virus. J. Virol. 2012, 86, 11115–11127. [Google Scholar] [CrossRef]
- Marriott, A.C.; Dennis, M.; Kane, J.A.; Gooch, K.E.; Hatch, G.; Sharpe, S.; Prevosto, C.; Leeming, G.; Zekeng, E.-G.; Staples, K.J.; et al. Influenza A Virus Challenge Models in Cynomolgus Macaques Using the Authentic Inhaled Aerosol and Intra-Nasal Routes of Infection. PLoS ONE 2016, 11, e0157887. [Google Scholar] [CrossRef]
- Safronetz, D.; Rockx, B.; Feldmann, F.; Belisle, S.E.; Palermo, R.E.; Brining, D.; Gardner, D.; Proll, S.C.; Marzi, A.; Tsuda, Y.; et al. Pandemic Swine-Origin H1N1 Influenza A Virus Isolates Show Heterogeneous Virulence in Macaques. J. Virol. 2011, 85, 1214–1223. [Google Scholar] [CrossRef]
- Moncla, L.H.; Ross, T.M.; Dinis, J.M.; Weinfurter, J.T.; Mortimer, T.D.; Schultz-Darken, N.; Brunner, K.; Capuano, S.V., III; Boettcher, C.; Post, J.; et al. A Novel Nonhuman Primate Model for Influenza Transmission. PLoS ONE 2013, 8, e78750. [Google Scholar] [CrossRef] [PubMed]
- Mooij, P.; Koopman, G.; Mortier, D.; Van Heteren, M.; Oostermeijer, H.; Fagrouch, Z.; De Laat, R.; Kobinger, G.; Li, Y.; Remarque, E.J.; et al. Pandemic Swine-Origin H1N1 Influenza Virus Replicates to Higher Levels and Induces More Fever and Acute Inflammatory Cytokines in Cynomolgus versus Rhesus Monkeys and Can Replicate in Common Marmosets. PLoS ONE 2015, 10, e0126132. [Google Scholar] [CrossRef] [PubMed]
- Cillóniz, C.; Shinya, K.; Peng, X.; Korth, M.J.; Proll, S.C.; Aicher, L.D.; Carter, V.S.; Chang, J.H.; Kobasa, D.; Feldmann, F.; et al. Lethal Influenza Virus Infection in Macaques Is Associated with Early Dysregulation of Inflammatory Related Genes. PLoS Pathog. 2009, 5, e1000604. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, F.; Kobasa, D.; Embury-Hyatt, C.; Grolla, A.; Taylor, T.; Kiso, M.; Kakugawa, S.; Gren, J.; Jones, S.M.; Kawaoka, Y.; et al. Oseltamivir Is Effective against 1918 Influenza Virus Infection of Macaques but Vulnerable to Escape. mBio 2019, 10, e02059-19. [Google Scholar] [CrossRef] [PubMed]
- Baskin, C.R.; Bielefeldt-Ohmann, H.; Tumpey, T.M.; Sabourin, P.J.; Long, J.P.; García-Sastre, A.; Tolnay, A.-E.; Albrecht, R.; Pyles, J.A.; Olson, P.H.; et al. Early and Sustained Innate Immune Response Defines Pathology and Death in Nonhuman Primates Infected by Highly Pathogenic Influenza Virus. Proc. Natl. Acad. Sci. USA 2009, 106, 3455–3460. [Google Scholar] [CrossRef] [PubMed]
- Rimmelzwaan, G.F.; Kuiken, T.; Van Amerongen, G.; Bestebroer, T.M.; Fouchier, R.A.; Osterhaus, A.D. Pathogenesis of Influenza A (H5N1) Virus Infection in a Primate Model. J. Virol. 2001, 75, 6687–6691. [Google Scholar] [CrossRef] [PubMed]
- Kuiken, T.; Rimmelzwaan, G.F.; Van Amerongen, G.; Osterhaus, A.D.M.E. Pathology of Human Influenza A (H5N1) Virus Infection in Cynomolgus Macaques (Macaca Fascicularis). Vet. Pathol. 2003, 40, 304–310. [Google Scholar] [CrossRef]
- Hirst, G.K. Studies on the mechanism of adaptation of influenza virus to mice. J. Exp. Med. 1947, 86, 357–366. [Google Scholar] [CrossRef]
- Wyde, P.R.; Couch, R.B.; Mackler, B.F.; Cate, T.R.; Levy, B.M. Effects of Low- and High-Passage Influenza Virus Infection in Normal and Nude Mice. Infect. Immun. 1977, 15, 221–229. [Google Scholar] [CrossRef]
- Staeheli, P.; Grob, R.; Meier, E.; Sutcliffe, J.G.; Haller, O. Influenza Virus-Susceptible Mice Carry Mx Genes with a Large Deletion or a Nonsense Mutation. Mol. Cell. Biol. 1988, 8, 4518–4523. [Google Scholar] [CrossRef]
- Haller, O.; Acklin, M.; Staeheli, P. Influenza Virus Resistance of Wild Mice: Wild-Type and Mutant Mx Alleles Occur at Comparable Frequencies. J. Interferon Res. 1987, 7, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Dhanwani, R.; Zhou, Y.; Huang, Q.; Verma, V.; Dileepan, M.; Ly, H.; Liang, Y. A Novel Live Pichinde Virus-Based Vaccine Vector Induces Enhanced Humoral and Cellular Immunity after a Booster Dose. J. Virol. 2016, 90, 2551–2560. [Google Scholar] [CrossRef] [PubMed]
- Mozdzanowska, K.; Maiese, K.; Gerhard, W. Th Cell-Deficient Mice Control Influenza Virus Infection More Effectively Than Th- and B Cell-Deficient Mice: Evidence for a Th-Independent Contribution by B Cells to Virus Clearance1. J. Immunol. 2000, 164, 2635–2643. [Google Scholar] [CrossRef] [PubMed]
- Kamal, R.P.; Katz, J.M.; York, I.A. Molecular Determinants of Influenza Virus Pathogenesis in Mice. Curr. Top. Microbiol. Immunol. 2014, 385, 243–274. [Google Scholar] [CrossRef] [PubMed]
- Ibricevic, A.; Pekosz, A.; Walter, M.J.; Newby, C.; Battaile, J.T.; Brown, E.G.; Holtzman, M.J.; Brody, S.L. Influenza Virus Receptor Specificity and Cell Tropism in Mouse and Human Airway Epithelial Cells. J. Virol. 2006, 80, 7469–7480. [Google Scholar] [CrossRef] [PubMed]
- Van Riel, D.; Munster, V.J.; De Wit, E.; Rimmelzwaan, G.F.; Fouchier, R.A.M.; Osterhaus, A.D.M.E.; Kuiken, T. Human and Avian Influenza Viruses Target Different Cells in the Lower Respiratory Tract of Humans and Other Mammals. Am. J. Pathol. 2007, 171, 1215–1223. [Google Scholar] [CrossRef]
- Van Riel, D.; Den Bakker, M.A.; Leijten, L.M.E.; Chutinimitkul, S.; Munster, V.J.; De Wit, E.; Rimmelzwaan, G.F.; Fouchier, R.A.M.; Osterhaus, A.D.M.E.; Kuiken, T. Seasonal and Pandemic Human Influenza Viruses Attach Better to Human Upper Respiratory Tract Epithelium than Avian Influenza Viruses. Am. J. Pathol. 2010, 176, 1614–1618. [Google Scholar] [CrossRef]
- Meijer, W.J.; Linn, F.H.H.; Wensing, A.M.J.; Leavis, H.L.; Van Riel, D.; GeurtsvanKessel, C.H.; Wattjes, M.P.; Murk, J.-L. Acute Influenza Virus-Associated Encephalitis and Encephalopathy in Adults: A Challenging Diagnosis. JMM Case Rep. 2016, 3, e005076. [Google Scholar] [CrossRef]
- Roubidoux, E.K.; Schultz-Cherry, S. Animal Models Utilized for the Development of Influenza Virus Vaccines. Vaccines 2021, 9, 787. [Google Scholar] [CrossRef]
- Fukao, K.; Ando, Y.; Noshi, T.; Kitano, M.; Noda, T.; Kawai, M.; Yoshida, R.; Sato, A.; Shishido, T.; Naito, A. Baloxavir Marboxil, a Novel Cap-Dependent Endonuclease Inhibitor Potently Suppresses Influenza Virus Replication and Represents Therapeutic Effects in Both Immunocompetent and Immunocompromised Mouse Models. PLoS ONE 2019, 14, e0217307. [Google Scholar] [CrossRef]
- Honce, R.; Jones, J.; Meliopoulos, V.A.; Livingston, B.; Sharp, B.; Estrada, L.D.; Wang, L.; Caulfield, W.; Freeman, B.; Govorkova, E.; et al. Efficacy of Oseltamivir Treatment in Influenza Virus-Infected Obese Mice. mBio 2023, 14, e00887-23. [Google Scholar] [CrossRef] [PubMed]
- Ison, M.G.; Mishin, V.P.; Braciale, T.J.; Hayden, F.G.; Gubareva, L.V. Comparative Activities of Oseltamivir and A-322278 in Immunocompetent and Immunocompromised Murine Models of Influenza Virus Infection. J. Infect. Dis. 2006, 193, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Lowen, A.C.; Mubareka, S.; Tumpey, T.M.; García-Sastre, A.; Palese, P. The Guinea Pig as a Transmission Model for Human Influenza Viruses. Proc. Natl. Acad. Sci. USA 2006, 103, 9988–9992. [Google Scholar] [CrossRef] [PubMed]
- Schulman, J.L.; Kilbourne, E.D. Experimental transmission of influenza virus infection in mice. II. Some factors affecting the incidence of transmitted infection. J. Exp. Med. 1963, 118, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Fong Legaspi, S.L.; Schwartzman, L.M.; Gygli, S.M.; Sheng, Z.-M.; Freeman, A.D.; Matthews, L.M.; Xiao, Y.; Ramuta, M.D.; Batchenkova, N.A.; et al. An Inactivated Multivalent Influenza A Virus Vaccine Is Broadly Protective in Mice and Ferrets. Sci. Transl. Med. 2022, 14, eabo2167. [Google Scholar] [CrossRef]
- Kinoshita, T.; Watanabe, K.; Sakurai, Y.; Nishi, K.; Yoshikawa, R.; Yasuda, J. Co-Infection of SARS-CoV-2 and Influenza Virus Causes More Severe and Prolonged Pneumonia in Hamsters. Sci. Rep. 2021, 11, 21259. [Google Scholar] [CrossRef]
- Buhnerkempe, M.G.; Gostic, K.; Park, M.; Ahsan, P.; Belser, J.A.; Lloyd-Smith, J.O. Mapping Influenza Transmission in the Ferret Model to Transmission in Humans. eLife 2015, 4, e07969. [Google Scholar] [CrossRef]
- Herlocher, M.L.; Elias, S.; Truscon, R.; Harrison, S.; Mindell, D.; Simon, C.; Monto, A.S. Ferrets as a Transmission Model for Influenza: Sequence Changes in HA1 of Type A (H3N2) Virus. J. Infect. Dis. 2001, 184, 542–546. [Google Scholar] [CrossRef]
- Weinfurter, J.T.; Brunner, K.; Capuano, S.V.; Li, C.; Broman, K.W.; Kawaoka, Y.; Friedrich, T.C. Cross-Reactive T Cells Are Involved in Rapid Clearance of 2009 Pandemic H1N1 Influenza Virus in Nonhuman Primates. PLoS Pathog. 2011, 7, e1002381. [Google Scholar] [CrossRef]
- McCarthy, K.R.; Von Holle, T.A.; Sutherland, L.L.; Oguin, T.H.; Sempowski, G.D.; Harrison, S.C.; Moody, M.A. Differential Immune Imprinting by Influenza Virus Vaccination and Infection in Nonhuman Primates. Proc. Natl. Acad. Sci. USA 2021, 118, e2026752118. [Google Scholar] [CrossRef]
- Carroll, T.D.; Matzinger, S.R.; Barry, P.A.; McChesney, M.B.; Fairman, J.; Miller, C.J. Efficacy of Influenza Vaccination of Elderly Rhesus Macaques Is Dramatically Improved by Addition of a Cationic Lipid/DNA Adjuvant. J. Infect. Dis. 2014, 209, 24–33. [Google Scholar] [CrossRef]
- Rimmelzwaan, G.F.; Baars, M.; Van Beek, R.; Van Amerongen, G.; Lövgren-Bengtsson, K.; Claas, E.C.; Osterhaus, A.D. Induction of Protective Immunity against Influenza Virus in a Macaque Model: Comparison of Conventional and Iscom Vaccines. J. Gen. Virol. 1997, 78 Pt 4, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.; Andrewes, C.H.; Laidlaw, P.P. A virus obtained from influenza patients. Lancet 1933, 222, 66–68. [Google Scholar] [CrossRef]
- Maher, J.A.; DeStefano, J. The Ferret: An Animal Model to Study Influenza Virus. Lab Anim. 2004, 33, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Baz, M.; Paskel, M.; Matsuoka, Y.; Zengel, J.; Cheng, X.; Jin, H.; Subbarao, K. Replication and Immunogenicity of Swine, Equine, and Avian H3 Subtype Influenza Viruses in Mice and Ferrets. J. Virol. 2013, 87, 6901–6910. [Google Scholar] [CrossRef] [PubMed]
- Belser, J.A.; Eckert, A.M.; Tumpey, T.M.; Maines, T.R. Complexities in Ferret Influenza Virus Pathogenesis and Transmission Models. Microbiol. Mol. Biol. Rev. 2016, 80, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Andrewes, C.H.; Glover, R.E. Spread of Infection from the Respiratory Tract of the Ferret. I. Transmission of Influenza A Virus. Br. J. Exp. Pathol. 1941, 22, 91–97. [Google Scholar]
- Jayaraman, A.; Chandrasekaran, A.; Viswanathan, K.; Raman, R.; Fox, J.G.; Sasisekharan, R. Decoding the Distribution of Glycan Receptors for Human-Adapted Influenza A Viruses in Ferret Respiratory Tract. PLoS ONE 2012, 7, e27517. [Google Scholar] [CrossRef]
- Jia, N.; Barclay, W.S.; Roberts, K.; Yen, H.-L.; Chan, R.W.Y.; Lam, A.K.Y.; Air, G.; Peiris, J.S.M.; Dell, A.; Nicholls, J.M.; et al. Glycomic Characterization of Respiratory Tract Tissues of Ferrets: Implications for Its Use in Influenza Virus Infection Studies. J. Biol. Chem. 2014, 289, 28489–28504. [Google Scholar] [CrossRef]
- Bodewes, R.; Kreijtz, J.H.C.M.; Van Amerongen, G.; Fouchier, R.A.M.; Osterhaus, A.D.M.E.; Rimmelzwaan, G.F.; Kuiken, T. Pathogenesis of Influenza A/H5N1 Virus Infection in Ferrets Differs between Intranasal and Intratracheal Routes of Inoculation. Am. J. Pathol. 2011, 179, 30–36. [Google Scholar] [CrossRef]
- Van den Brand, J.M.A.; Stittelaar, K.J.; Leijten, L.M.E.; Van Amerongen, G.; Simon, J.H.; Osterhaus, A.D.M.E.; Kuiken, T. Modification of the Ferret Model for Pneumonia from Seasonal Human Influenza A Virus Infection. Vet. Pathol. 2012, 49, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Gary, J.M.; Ritter, J.M.; Sun, X.; Maines, T.R.; Belser, J.A. Influenza A Virus Infection and Pathology in Nasal and Periocular Tissues after Ocular Inoculation in Ferrets. Vet. Pathol. 2022, 59, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Prather, K.A.; Sznitman, J.; Jimenez, J.L.; Lakdawala, S.S.; Tufekci, Z.; Marr, L.C. Airborne Transmission of Respiratory Viruses. Science 2021, 373, eabd9149. [Google Scholar] [CrossRef] [PubMed]
- Govorkova, E.A.; Rehg, J.E.; Krauss, S.; Yen, H.-L.; Guan, Y.; Peiris, M.; Nguyen, T.D.; Hanh, T.H.; Puthavathana, P.; Long, H.T.; et al. Lethality to Ferrets of H5N1 Influenza Viruses Isolated from Humans and Poultry in 2004. J. Virol. 2005, 79, 2191–2198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, J.; Huang, K.; Li, K.-S.; Yuen, K.-Y.; Guan, Y.; Chen, H.; Ng, W.F. Systemic Infection of Avian Influenza A Virus H5N1 Subtype in Humans. Hum. Pathol. 2009, 40, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Delaney, C.A.; Orosz, S.E. Ferret Respiratory System: Clinical Anatomy, Physiology, and Disease. Vet. Clin. N. Am. Exot. Anim. Pract. 2011, 14, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Reeve, P.; Pibermann, M.; Gerendas, B. Studies with Some Influenza B Viruses in Cell Cultures, Hamsters and Hamster Tracheal Organ Cultures. Med. Microbiol. Immunol. 1981, 169, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.; Chanock, V.; Chanock, R.M. Temperature-Sensitive Mutants of Influenza Virus. I. Behavior in Tissue Culture and in Experimental Animals. J. Infect. Dis. 1971, 123, 145–157. [Google Scholar] [CrossRef]
- Jennings, R.; Potter, C.W.; McLaren, C. Effect of Preinfection and Preimmunization on the Serum Antibody Response to Subsequent Immunization with Heterotypic Influenza Vaccines. J. Immunol. Baltim. Md 1950 1974, 113, 1834–1843. [Google Scholar] [CrossRef]
- Potter, C.W.; Jennings, R. The Hamster as a Model System for the Study of Influenza Vaccines. Postgrad. Med. J. 1976, 52, 345–351. [Google Scholar] [CrossRef]
- Bouvier, N.M.; Lowen, A.C. Animal Models for Influenza Virus Pathogenesis and Transmission. Viruses 2010, 2, 1530–1563. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, E.A.; Engel, G.A.; Feeroz, M.M.; San, S.; Rompis, A.; Lee, B.P.Y.-H.; Shaw, E.; Oh, G.; Schillaci, M.A.; Grant, R.; et al. Influenza Virus Infection in Nonhuman Primates. Emerg. Infect. Dis. 2012, 18, 1672–1675. [Google Scholar] [CrossRef] [PubMed]
- Rimmelzwaan, G.F.; Baars, M.; Van Amerongen, G.; Van Beek, R.; Osterhaus, A.D. A Single Dose of an ISCOM Influenza Vaccine Induces Long-Lasting Protective Immunity against Homologous Challenge Infection but Fails to Protect Cynomolgus Macaques against Distant Drift Variants of Influenza A (H3N2) Viruses. Vaccine 2001, 20, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, S.; Iwatsuki-Horimoto, K.; Kiso, M.; Nakajima, N.; Gregg, R.W.; Katsura, H.; Tomita, Y.; Maemura, T.; Da Silva Lopes, T.J.; Watanabe, T.; et al. Pathogenesis of Influenza A(H7N9) Virus in Aged Nonhuman Primates. J. Infect. Dis. 2020, 222, 1155–1164. [Google Scholar] [CrossRef]
- Davis, A.S.; Taubenberger, J.K.; Bray, M. The Use of Nonhuman Primates in Research on Seasonal, Pandemic and Avian Influenza, 1893–2014. Antiviral Res. 2015, 117, 75–98. [Google Scholar] [CrossRef]
- Chan, M.; Tiwary, M.; Wu, H.L.; Tailor, N.; Vendramelli, R.; Audet, J.; Warner, B.M.; Tierney, K.; Albietz, A.; Truong, T.; et al. Pandemic 1918 Influenza Virus Does Not Cause Lethal Infection in Rhesus or Cynomolgus Macaques. J. Virol. 2022, 96, e00728-22. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).