Abstract
An emerging multidrug-resistant pathogenic yeast called Candida auris has a high potential to spread quickly among hospitalized patients and immunodeficient patients causing nosocomial outbreaks. It has the potential to cause pandemic outbreaks in about 45 nations with high mortality rates. Additionally, the fungus has become resistant to decontamination techniques and can survive for weeks in a hospital environment. Nanoparticles might be a good substitute to treat illnesses brought on by this newly discovered pathogen. Nanoparticles have become a trend and hot topic in recent years to combat this fatal fungus. This review gives a general insight into the epidemiology of C. auris and infection. It discusses the current conventional therapy and mechanism of resistance development. Furthermore, it focuses on nanoparticles, their different types, and up-to-date trials to evaluate the promising efficacy of nanoparticles with respect to C. auris.
1. Introduction
Fungi are eukaryotic organisms that can be found anywhere. They can be found indoors on surfaces and in the air, on people’s skin, inside the body, and outdoors, for example, in soil and on plants. Although there are countless varieties of fungi, only a few of them can truly cause danger to people. Fungal infections are a significant threat to public health as they are associated with life-threatening mycoses and mortality [1,2]. Fungal infections are one of the most common causes of death globally, affecting more than 300 million individuals and resulting in over 2 million deaths annually. In addition, the challenge of mycoses is exacerbated when new pathogenic fungi appear due to their capacity to withstand the few available antifungal drugs, significantly decreasing the efficacy of treatments [3,4,5,6,7]. As seen from this angle, Candida auris (C. auris) infections have grown to pose a serious hazard to human health worldwide as it is difficult to be diagnosed by conventional laboratory techniques, and some strains are resistant to all kinds of antifungal drugs that are frequently utilized to combat Candida infections [8,9].
Some fungi are commensal organisms that reside on the skin and in the digestive tract; if they leave their normal environment, they are thought to pose a risk of developing various fungal infections. For instance, the danger of infection spreading has increased due to a dramatic rise in the usage of antibiotics, chemotherapeutic treatments, and immunosuppressive medications [10,11]. Due to the increase in the use of invasive medical devices and procedures (such as catheters and hematopoietic transplantation), these commensal fungi also have a greater potential to enter tissues and blood and cause invasive diseases [12,13]. Additionally, recent substantial health problems unrelated to mycoses, such as seasonal influenza outbreaks and the SARS-CoV-2 pandemic, have exacerbated the intensity of the population’s diseases and susceptibility to secondary fungal infections [14,15,16].
When a patient fails to respond or no longer responds to a treatment when it is administered at the advised dosage, therapeutic failure and the development of resistance occur. Several factors lead to therapeutic failure, some related to the patient and others related to the drug. For instance, poor compliance, co-infection, cavitary lesions and abscesses near the site of infection, and obesity may relate to the patient [17]. In addition, immunocompromised patients receiving immunosuppressive drugs are more vulnerable to treatment failure, because the drug is not accompanied by a robust immune response in the fight against infection [18]. Other factors may relate to drugs, such as non-linear pharmacokinetics, drug–drug interactions, selectivity, toxicity, and spectrum of activity [19].
There are only five classes of drugs available for treating fungal infections. Since fungi are eukaryotic cells, such as mammalian cells, it is difficult to identify specific therapeutic targets against them. These previous issues drive modern research to nanoparticles, as carriers or adjuvants, to improve the efficacy and performance of current medication [20,21]. This review will provide insights into current trends in nanoparticles and their mechanisms to combat multidrug resistance (MDR) fungal infection.
2. Fungal Infections
Fungal infection can be categorized according to the affected site of infection, into superficial, cutaneous, subcutaneous, mucosal, and systemic infection. The main three pathogenic fungi in humans are Candida, Aspergillus, and Cryptococcus which account for 90% of fatalities in either immunocompetent or immunodeficient people. Pneumocystis, Coccidioides, and Histoplasma are three other pathogenic fungi that can seriously harm tissues and even kill people [22]. The species of the infected fungus and the state of the host’s immune system have a significant impact on the type of infection [23]. For example, nearly one billion people have superficial fungal infections, which are among the most prevalent fungal illnesses [24]. Conversely, invasive fungal infections are the most dangerous. These are brought on by inhaling or injecting fungal spores, or by an imbalance of the host’s commensal fungi [25,26].
Candida species (spp.) are commensal fungi found on the human skin, mucosa, or intestinal tract; their growth and proliferation are highly restricted in people with a healthy immune system. According to previous studies, the most common pathogenic Candida spp. that cause human infections are Candida parapsilosis, Candida albicans, Candida krusei, Candida glabrata, and Candida tropicalis. Recent research demonstrates that C. auris has spread around the world as an MDR fungal infection that significantly increases patient death [27,28]. Furthermore, the CDC’s data show that C. auris most closely mimics infectious, MDR bacteria, such as methicillin-resistant Staphylococcus aureus (MRSA) [9].
Cryptococcus spp. cause cryptococcosis which is a widespread invasive fungal infection that poses serious therapeutic difficulties and high fatality rates [29,30]. However, due to advances in molecular science and studies on epidemics, C. gattii was recognized as a separate species in 2002 [31,32,33]. Cryptococcal infection can result in pneumonia in immunodeficient patients and is brought on by the inhalation of cryptococcal spores into the lungs. However, in immunocompetent hosts, the infection may be latent without any symptoms. Unfortunately, this cryptococcal infection may spread to any organs, including the brain, and cause lethal cryptococcal meningitis [34,35].
Aspergillus is a saprophytic fungus that grows in soil and has over 200 species. Aspergillus spp. is widespread and frequently isolated from cultures of the respiratory tracts of asymptomatic individuals. Conversely, invasive aspergillosis leads to chronic obstructive pulmonary disease (COPD) [36,37]. The most frequent species associated with invasive infection, especially in an immunodeficient patient, is A. fumigatus A. flavus, A. niger, and A. terreus also cause invasive infections [38].
Candida auris Infection
The Candida species, a diploid fungus, is regarded as an opportunistic pathogen that can harm people’s health and cause fatal illnesses. Candidiasis is ranked as the fourth nosocomial infection with a high mortality rate ranging from 35 up to 100% in immunodeficient patients [8,39]. C. auris is a newly discovered pathogenic fungus that was first discovered in Japan in 2009 [40]. Infections with C. auris have been documented in 45 nations in Far East Asia, the Middle East, Africa, Europe, North America and South America, demonstrating its worrying rapid evolution worldwide [9]. Moreover, many clinical reports demonstrated that C. auris outbreaks are linked to the COVID-19 pandemic [41,42,43,44,45,46].
C. auris has been divided into four major separate genetic clades, the South Asian, East Asian, South African, and South American clades, based on geographic origin and genomic data gained by whole genome sequencing and the first isolated locations [28]. Recently, it was revealed that a fifth clade came from Iran [47]. Although C. auris is most frequently found on human skin, multiple investigations have shown that the organism may also be isolated from the mucosae of the mouth, esophagus, and gut [28]. Horton et al. reported that C. auris produced biofilms on pig skin and in synthetic sweat media that mimicked the physiological circumstances of the axilla [48]. Acquiring infection from contact with soiled surfaces is significantly more troubling, where C. auris biofilms have been proven to withstand artificial dehydration [48]. Similarly to other significant Candida infections, C. auris primarily affects a wide range of vulnerable people, including those with a deficient immune system, a chronic illness such as uncontrolled diabetes, or those taking immunosuppressive medications [49]. Currently, it is documented that C. auris can infect people and cause a wide range of illnesses, including fungemia, wound infections, urinary tract infections, meningitis, myocarditis, skin abscesses, and bone infections [50,51].
According to the most recent systematic review and meta-analysis study by Chen et al. [52] over 4733 cases of C. auris were documented in around 33 countries, with most cases in South Africa, the USA, India, Spain, the UK, South Korea, Colombia, and Pakistan. The majority of cases were identified between 2013 and 2019, peaking in 2016 and then declining after that. Clades I and III were the most common, with more cases documented and an expanded geographical range. Furthermore, 32% of the patients had bloodstream infections which differed based on the clades. The fluconazole, amphotericin B, caspofungin, anidulafungin, and micafungin resistances in C. auris were 91, 12, 12, 1.1, and 1%, respectively. The total mortality rate of C. auris infections was 39%. Moreover, subgroup analysis revealed that the mortality rate was lower in Europe (20%) and greater in those with bloodstream infections (45%) [52].
C. auris shares virulence features with the majority of other Candida spp., including C. albicans, C. tropicalis, and C. parapsilosis, which belong to the CTG clade, or species that translate the CTG codon into serine rather than leucine. [53]. These traits include biofilm formation, yeast-to-hyphae transition, and phenotypic switching [54,55,56].
3. Current Conventional Medications
There are roughly five classes of conventional antifungal drugs that can be used for topical and systemic antifungal therapies, including azoles, polyenes, echinocandins, allylamines and pyrimidine analogs (Figure 1) [57,58]. Polyenes have been identified as being produced by Streptomyces spp., in which they play a role as a natural defense mechanism. This class includes amphotericin B and nystatin. They work by attaching to the ergosterol present in the fungal cell membrane, generating holes there, and increasing ion permeability. This alters the ion gradient inside and outside the cell membrane, loss of cell integrity, and ultimately results in fungal cell death [59,60]. The most effective polyene for invasive fungal infections is amphotericin B, which works by generating an extra-membranous fungicidal sterol sponge that impairs membrane integrity [61]. Another way by which amphotericin B acts is by the accumulation of reactive oxygen species (ROS), which in turn disrupt the mitochondria, proteins, DNA, and membranes [62,63].
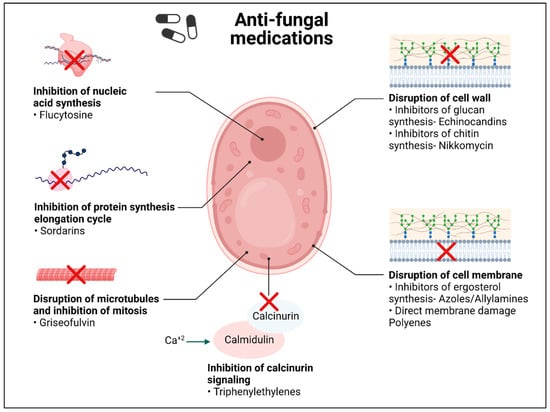
Figure 1.
Current conventional anti-fungal drugs and their mechanism of action. (Created with BioRender).
The azole class includes triazole and imidazole [64]. This class acts by blocking the ergosterol synthesis pathway. They bind to and inhibit the lanosterol 14-α-demethylase enzyme that is responsible for the rate-limiting step in the conversion of lanosterol to ergosterol [65,66]. Lanosterol 14-α-demethylase is produced by the genes ERG11 in yeast and Cyp51 in mold [67].
The echinocandin class mostly includes caspofungin, micafungin, and anidulafungin. They target the β-1,3 glucan synthase and interfere with the integrity of the fungal cell wall [68,69]. The FKS family of genes encodes the 1,3-d glucan synthase enzyme [69]. Although the safety profiles of these antifungal drugs are good, the lipid side chains limit their oral absorption. They are effective against both planktonic cells and biofilm-forming cells (sessile cells). Similarly, aspergillosis has been treated with this class [70].
Allylamines work by blocking the squalene epoxidase that converts squalene into lanosterol, thus inhibiting the formation of ergosterol and thereby inhibiting fungal growth. They have a broad spectrum of activity and low toxicity [71,72].
The antimetabolite, 5-flucytosine (5-FC), is the fifth antifungal class. They enter the fungal cell via cytosine permeases, where it is deaminated to 5-fluorouracil. This prevents the synthesis of both nucleic acids (DNA and RNA), which therefore prevents the synthesis of proteins [73]. Moreover, 5-FC can penetrate the blood–brain barrier to treat fungal infections of the central nervous system [74].
The main challenge for current conventional drugs is to combat and overcome MDR fungi such as C. auris, as it is naturally resistant to one or more kinds of commercially available antifungals. Fluconazole is extremely resistant to most C. auris isolates, but the minimum inhibitory concentration (MIC) analysis also revealed that certain strains are also resistant to all kinds of antifungal medications [27]. The best method of combating C. auris is not yet established. Echinocandins are recommended as the first line of treatment since they are effective against most isolates in the US [75,76]. Additionally, isavuconazole was discovered to be effective against a range of C. auris isolates despite their resistance to azoles [75].
4. Resistance of C. auris to Conventional Antifungals
C. auris is a recent trend, unlike other candida spp., due to its persistent resistance has subsequently evolved to be MDR-resistant. Furthermore, C. auris generates chronic and fatal infections with poor prognoses, especially in susceptible people [77]. According to a study by Osei Sekyere, nearly half of all C. auris isolates from various studies exhibited resistance to fluconazole (44.29%), the most commonly used azole antifungal, followed by amphotericin B (15.46%), voriconazole (12.67%), caspofungin (3.48%), and flucytosine (1.95%). Fortunately, it seems that the yeast still responds to echinocandin, so this can be used as the first line of treatment [78].
C. auris uses a variety of different molecular strategies to bypass the effects of antifungals (Figure 2). Briefly, C. auris develops azole resistance by overexpressing or developing a point mutation of the ERG11 gene, which encodes the lanosterol-14-α-demethylase enzyme, preventing azoles from binding their target. Additionally, C. auris can reduce the internal concentration of antifungals by overexpressing the MDR-1 gene, which encodes the major facilitator superfamily (MFS) drug exporter pump, and the CDR-1 gene, which encodes the ATP-binding cassette (ABC) drug exporter pump [79,80,81].
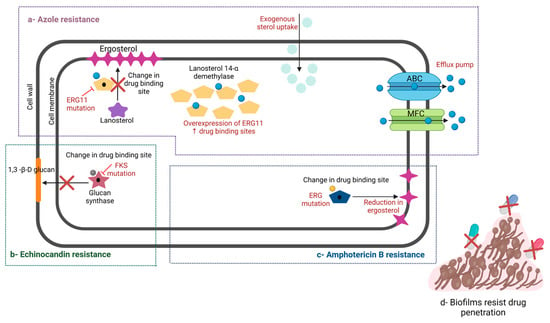
Figure 2.
Development of resistance in C. auris against conventional medication. (a) C. auris develops resistance to azoles through overexpression and point mutation in ERG11 gene, which encodes the lanosterol-14-α-demethylase enzyme. (b) C. auris develops resistance to echinocandins through mutation or substitution in FKS-1 gene, which encodes the β-1,3 glucan synthase enzyme. (c) C. auris develops resistance to polyenes through mutation in ERG and so impairs ergosterol biosynthesis. (d) C. auris also can build biofilm and develop resistance to almost all antifungal classes. (Created with BioRender).
Echinocandin resistance is developed through mutation or substitution in the FKS-1 gene that encodes β-1,3 glucan synthase enzyme, which is a critical component in the fungal cell wall [80,82,83]. Furthermore, resistance to amphotericin B is developed through mutation in ERG11 gene, thereby impairing ergosterol biosynthesis [84]. Amino acid substitution in the FUR-1 gene (F211I), which is involved in 5-FU metabolism, leads to the development of 5-FC resistance [83].
C. auris also can build biofilm and develop resistance to almost all antifungal classes. For instance, in a previous study, Sherry et al. demonstrated that sessile C. auris cells have higher MICs for several antifungals than planktonic cells [55]. Unfortunately, the biofilm not only increases the resistance and virulence of inside fungal communities but also enhances the upregulation of the ABC and MSF exporter pumps by 2 to 4 folds [55,81].
It is essential to find new strategies for fighting C. auris because of the widespread antifungal resistance and high rates of morbidity and mortality. Nanoparticles appear to offer a promising replacement for resistant drugs. In addition, nanoparticles can be used with antifungal medications to create a powerful synergistic impact that can effectively combat MDR C. auris.
5. Nanoparticles (NPs) and Nanotechnology (NT) to Combat MDR C. auris
To effectively treat fungal infections and circumvent the fungal multi-resistance to existing medications, the creation of drug delivery systems based on nanoparticles (NPs) is a potential substitute for creating novel pharmaceutical formulations [85,86]. NPs can be constructed from lipids, polymers, or metals [87,88,89]. They offer many advantages over conventional drugs [90,91]. They are more targeted to the site of infection, possess a larger surface area, possess fewer toxic effects and side effects, and rarely develop resistance [92,93,94,95,96]. NPs can be divided into three categories: organic, inorganic, or polymeric. Carbon nanoparticles are further categorized based on their size, shape, chemical composition, and physical characteristics [97,98,99]. Organic NPs are biodegradable, non-toxic, and sensitive to heat and light. Examples include polymers [100,101,102,103], liposomes [104,105], micelles [106]. and dendrimers [107,108]. This type of NP is the first choice in the biomedical field, especially for medication delivery [97,98]. Inorganic NPs can be constructed from metal or metal oxide [109]. Metal NPs commonly include Ag [87,110,111,112], Au [113], Cu [114,115,116,117], Si [118], and Se [119,120]. However, metal oxide NPs are produced when the characteristics of the metal particles are altered in the presence of oxygen, boosting their reactivity and effectiveness. Metal oxide NPs commonly include NO [121], ZnO [122,123,124], CuO [125], TiO2 [126], and Fe2O3 [127]. Moreover, carbon NPs may include black carbon, carbon nanotubes, carbon nanofibers, and graphene [97,98].
5.1. Metallic NPs
5.1.1. Silver Nanoparticles (AgNPs)
AgNPs are now recognized to have a strong anti-C. albicans biofilm action. Previous studies have proven that silver nanoparticles are effective against MDR pathogens and nosocomial infections [128,129,130,131]. Roberto et al. demonstrated that AgNPs can exert promising antifungal activity against MDR C. auris, whether present in planktonic form or sessile in biofilm [87]. In their study, they tested different strains from different clades and proved that AgNPs exhibited strong action against the fully developed and preformed biofilm of C. auris, regardless of their clade. Additionally, they found that AgNPs have a powerful effect on preventing the production of biofilm by the various C. auris strains. Moreover, AgNPs may affect the structure of biofilm in some strains.
In a parallel study, Lara et al. proved the inhibitory effect of AgNPs against the ability of C. auris to develop biofilm on medical surfaces such as silicon elastomer catheters and elastic bandage fibers. They synthesized pure and round AgNPs with a size range of 1 to 3 nm and discovered their dose-related activity against C. auris with an altered and disrupted cell wall. Additionally, they showed that elastic bandage wraps maintained the fungicidal action of AgNPs even after numerous washings, demonstrating their long-lasting antifungal potency and efficiency [129].
Sheeanana et al. developed a new coating surface system, consisting of a copper sheet coated with a cluster of AgNPs, through an ion exchange reaction and a reduction reaction [132]. This developed surface passed 1 to 7 days of tests for pathogenic C. auris. Following the prolonged exposure intervals, it was discovered that more than 90% of the C. auris were no longer viable.
In the most recent study, Reem et al. proved the promising activity of AgNPs to combat C. auris growth and biofilm formation [133]. In their study, they test the susceptibility of eight isolates of C. auris against AgNPs and showed that over 80% of biofilm development was prevented at a comparatively high AgNPs concentration (6.25 g/mL). In contrast, Malik et al. synthesized chemically-stable AgNPs (CC-AgNPs) with a green synthesis method using Cynara cardunculus extract as a reducing and capping agent. They tested the potency of their AgNP system against C. auris MRL6057 and found that CC-AgNPs can combat C. auris through direct inhibition of the cell cycle and arrest the cells in the G2/M phase [134].
5.1.2. Bismuth Nanoparticles (BiNPs)
Vazquez-MunozIn et al. (at 2020) recognized the antibacterial characteristics of elemental BiNPs, especially their anti-candidal activity, particularly against C. albicans [135]. In the same year, they derived another study and proved that BiNPs also have potent activity against different strains of C. auris [136]. In a later study, they found a significant anti-C. auris activity of BiNPs with a MIC ranging from (1 to 4 µg/mL), regardless of their clades. However, BiNPs seemed to have a moderate inhibitory effect on biofilm. Despite this lower activity, BiNPs can alter the biofilm structure and, in some cases, the cell morphology of the cells within biofilms.
5.1.3. Trimetallic NPs
Majid Kamli et al. developed a novel trimetallic NP system (Ag-Cu-Co), using Salvia officinalis leaves [137]. According to their investigation, C. auris cells exposed to these trimetallic NPs experienced cell cycle arrest in the G2/M phase, a breakdown of the mitochondrial membrane, the release of an apoptotic marker, and apoptosis at an MIC ranging from 0.39 to 0.78 µg/mL. In addition, compared to their monometallic competitors, Ag-Cu-Co trimetallic NPs have stronger antibacterial characteristics. This is because of the synergistic impact of the Ag, Cu, and Co present in the as-synthesized nanoparticles.
5.2. Metal Oxide NPs
Levi Cleare et al. created a novel N-acetylcysteine S-nitrosothiol NP (NAC-SNO-NP) system that promotes a prolonged release of nitric oxide (NO) [121]. Using this NP model, they want to mimic the natural NO which is considered an important component in the innate immune system and possesses cytotoxic activity against a variety of pathogens [138,139,140]. They demonstrated that this NP system can perfectly reduce the growth of C. auris and decrease the development of biofilm by more than 70% at 10 mg/mL [121]. Notably, the NP architecture itself exhibited an intrinsic inhibition of C. auris, demonstrating that the antifungal activity was a combined consequence of the NP itself and the released NO. In another study, Vargas-Cruz et al. prepared a nitroglycerin–citrate–ethanol (NiCE) catheter lock solution and evaluated its efficiency in eradicating C. auris biofilms in central line lumens by converting nitroglycerin into NO [141]. Additionally, they compared the effect of the NiCE catheter lock solution with widely accepted antifungal drugs, such as caspofungin, micafungin, voriconazole, liposomal amphotericin B, and others, and proved that NiCE possesses a superior effect in eradicating C. auris biofilms [141].
Moreover, Sherin Philip et al. synthesized iron oxide (Fe2O3) NPs that were stabilized by supramolecular β-cyclodextrin and evaluated their activity in combatting C. auris. They showed that Fe2O3 NPs can inhibit C. auris with an MIC of around 500 µg/mL [142].
5.3. Nanofibrous Membrane
Liu et al. generated a novel form of polylactic acid-hypocretin A (PLA-HA) nanofibrous membrane. They conducted in vitro and in vivo studies to evaluate the PLA-HA-based antimicrobial photodynamic therapy (aPDT) effects in combatting C. auris infection [143]. aPDT is a novel antimicrobial strategy that uses a non-toxic photosensitizer (PS) and appropriate light sources to stimulate the generation of reactive oxygen species (ROS), which can destroy pathogenic microbes [144,145]. Hypocrellin A (HA) is a natural lipid-soluble pigment that belongs to the perylenequinonoid class and is considered a novel form of PS [146]. Liu and his colleagues provided evidence that PLA-HA is an effective antifungal agent for treating superficial C. auris infections. They concluded that this is because intracellular ROS generation causes yeast cells to die [143].
5.4. NPs Loaded with Commercially Available Antifungal Drugs
In a recent novel study, Henry et al. synthesized chitosan-(poly lactide co-glycolide) NPs (C-PLGA NPs) as a nanocarrier system and loaded it with fluconazole. The sustained drug release from this nanocarrier system is pH-dependent. For instance, at a pH of 7.0, 34% of the release happened and at a pH of 4, 83% of the release happened [147]. Moreover, they evaluate the efficacy of C-PLGA-loaded NPs versus MDR C. auris and demonstrated that this nano-formulation significantly increases the antifungal activity up to 64-fold compared to conventional fluconazole [147]. Fayed et al. synthesized zinc oxide NPs loaded with caspofungin and demonstrated that these loaded NPs can prevent the phenotypic changes in C. auris that lead to the development of caspofungin resistance [148].
In another study, Gabriel Davi et al. developed a nano-emulsion system and loaded it with amphotericin B. Additionally, they tested its antifungal potency against C. auris using an in vivo model of Galleria mellonella and proved the significant activity of this loaded nano-emulsion system compared to free amphotericin B [149].
In a similar manner, the same team conducted another trial using micafungin-loaded nano-emulsion and test its in vitro and in vivo efficacy and toxicity using Galleria mellonella model [150].
5.5. NPs Loaded with Natural Drugs
Essential oils (EOs) are an effective option for treating fungi and acting as a modulator of fungal biofilms [151,152]. De Alteriis et al. encapsulated Lavandula angustifolia EOs, extracted from a lavender plant, in liposomes and investigated their effectiveness against C. auris persister-derived biofilm. They concluded that this loaded liposome could combat both primary and persister C. auris biofilm through the production of ROS that may affect the expression of certain genes involved in biofilms [153].
5.6. Nanotechnology (NT) for Diagnosis of C. auris
Luis et al. constructed a novel system for the selective and sensitive detection of C. auris in clinical samples using a nanoporous anodic alumina (NAA) biosensor that had been encapsulated with oligonucleotides. The NAA support is firstly packed with rhodamine B, a fluorescent reporter dye, and then capped with a variety of oligonucleotide sequences that precisely hybridize with distinct regions of the C. auris genome. Therefore, the capping oligonucleotide prevents dye release by obstructing pores. In the presence of C. auris genomic DNA, the capping oligonucleotide is displaced (due to favorable oligonucleotide–DNA hybridization), uncapping the pores and permitting dye transportation. Through this system, C. auris can be detected at concentrations as low as 6 CFU/mL, making it possible to diagnose clinical samples in just one hour without the need for DNA extraction or amplification procedures first [154].
6. Expected Mechanisms of NPs to Combat MDR C. auris
The exact mechanism of action of NPs is not known; however, many reports and studies suggest the general mechanisms of free NPs or loaded NPs to exert their antimicrobial activity. In general, the size, shape, and coating agents of NPs have a significant impact on their antifungal activity. Firstly, NPs interact with the outer surface of fungi and form aggregates, leading to the formation of pits in the cell wall. As a result, a decrease in membrane permeability and loss of membrane fluidity may occur, resulting in a disruption of energy transmission and cell death (Figure 3). Formed pits let NPs enter the fungal cell. Once entered, they lead to the accumulation of ROS that trigger and enhance apoptosis. ROS can disrupt macromolecules in the cell, resulting in lipid peroxidation, protein modification, enzyme inhibition, inhibition of the electron transport chain, RNA or DNA damage, and therefore cell death [155,156,157].
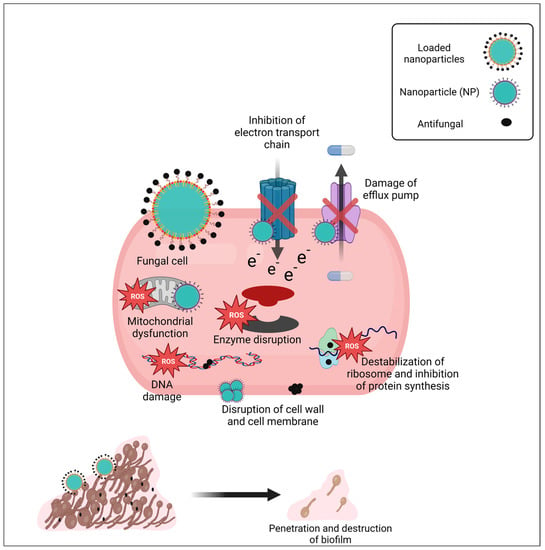
Figure 3.
Expected mechanism of free and loaded NPs for combatting C. auris infection. In the case of free NPs, they accumulate at the outer surface of the cell, disrupt the cell wall, and form pits through which they enter the cell and then disrupt the cell membrane, resulting in a decrease in membrane permeability, and a loss of membrane fluidity may occur, resulting in a disruption of energy transmission and cell death. Once NPs enter through formed pits, they bind to and disrupt vital cell components and interrupt significant intracellular signaling pathways. On the other hand, NPs increase oxidative stress, which leads to an accumulation of ROS, which have the capacity to disrupt macromolecules in the cell, resulting in lipid peroxidation, protein modification, enzyme inhibition, inhibition of electron transport chain, and RNA or DNA damage, thereby promoting cell death. In case of loaded NPs, they carry antifungal drugs and facilitate their transport to its specific targets inside the fungal cell. Hence, loaded NPs possess a synergistic activity, transport antifungal drugs to the target site, provide a large surface area of both NPs and antifungal drugs, leading to more toxic action on fungal cells, and combat MDR pathogens, such as C. auris. Moreover, C. auris tends to form biofilm and become resistant to conventional antifungals. Loaded NPs have the capacity to the penetrate extracellular matrix of biofilm, transport antifungal agents inside the biofilm, and exert their fungicidal effect. (Created with BioRender).
NPs may bind to and disrupt vital cell components. Furthermore, they may interrupt significant intracellular signaling pathways [137,156]. They can penetrate the biofilm structure and may change the cell morphology or disrupt sessile organisms within biofilm [87].
Specifically to AgNPs and splatted Ag+, the essential functions of fungal cells are considerably changed by the modulation of the transcriptome, epigenome, and metabolome. Moreover, they may cause the down-regulation of the genes involved in the tricarboxylic acid cycle, redox metabolism, ergosterol production, and lipid metabolism causing structural alterations, primarily at the level of biological membranes [158,159,160].
Synergistic activity may occur when NPs are loaded with antifungal drugs. In this case, they act by dual mechanisms: they transport antifungal drugs to the target site, provide a large surface area of both NPs and antifungal drugs, leading to more toxic action on fungal cells, and combat MDR pathogens [147,161] (Figure 3).
7. Conclusions and Future Perspectives
C. auris is a newly emerged fungus and may cause outbreaks of nosocomial infection. C. auris infections have become a severe threat to human health across the world because they are difficult to identify using normal laboratory approaches and certain strains are resistant to all antifungal classes. Thus, alternative therapies that are both safer and more effective are urgently needed. Moreover, the increase in MDR fungal infections and the scarcity of clinically effective antifungal drugs signals the need for the development of new antifungal approaches to manage these issues in the context of a future that is already challenging.
NPs seem to be a promising approach to combatting and overcoming MDR fungi, such as C. auris. Although recent studies demonstrated the promising effect of NPs on combatting C. auris infection, the applications of NPs will not be ready until future studies emphasize their pharmacokinetic and pharmacodynamic profiles, physicochemical interactions, toxicities, and specific mechanisms of action.
Author Contributions
Conceptualization, H.F.H., Y.N.R., I.M.S.A.-K. and N.H.A.E.; literature search, data analysis, curation and visualization, H.F.H., Y.N.R., I.M.S.A.-K. and N.H.A.E.; writing—original draft preparation, H.F.H., Y.N.R., I.M.S.A.-K., N.H.A.E., L.S. and B.B.; writing—review and editing, H.F.H., Y.N.R., I.M.S.A.-K., N.H.A.E., L.S. and B.B.; funding acquisition, H.F.H. and B.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- CDC. Types of Fungal Diseases. 2019. Available online: https://www.cdc.gov/fungal/diseases/index.html (accessed on 4 April 2023).
- Algammal, A.M.; Elsayed, M.E.; Hashem, H.R.; Ramadan, H.; Sheraba, N.S.; El-Diasty, E.M.; Abbas, S.M.; Hetta, H.F. Molecular and HPLC-based approaches for detection of aflatoxin B 1 and ochratoxin A released from toxigenic Aspergillus species in processed meat. BMC Microbiol. 2021, 21, 82. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Perlin, D.S.; Muldoon, E.G.; Colombo, A.L.; Chakrabarti, A.; Richardson, M.D.; Sorrell, T.C. Delivering on Antimicrobial Resistance Agenda Not Possible without Improving Fungal Diagnostic Capabilities. Emerg. Infect. Dis. 2017, 23, 177–183. [Google Scholar] [CrossRef] [PubMed]
- GAFFI. Fungal Disease Frequency. 2021. Available online: https://gaffi.org/why/fungal-disease-frequency/ (accessed on 4 April 2023).
- Farghly Youssif, S.; Abdelrady, M.M.; Thabet, A.A.; Abdelhamed, M.A.; Gad, M.O.A.; Abu-Elfatth, A.M.; Saied, G.M.; Goda, I.; Algammal, A.M.; Batiha, G.E.-S. COVID-19 associated mucormycosis in Assiut University Hospitals: A multidisciplinary dilemma. Sci. Rep. 2022, 12, 10494. [Google Scholar] [CrossRef]
- Ezeonuegbu, B.A.; Abdullahi, M.D.; Whong, C.M.; Sohunago, J.W.; Kassem, H.S.; Yaro, C.A.; Hetta, H.F.; Mostafa-Hedeab, G.; Zouganelis, G.D.; Batiha, G.E.-S. Characterization and phylogeny of fungi isolated from industrial wastewater using multiple genes. Sci. Rep. 2022, 12, 2094. [Google Scholar] [CrossRef] [PubMed]
- Abd Ellah, N.H.; Abdel-Aleem, J.A.; Abdo, M.N.; Abou-Ghadir, O.F.; Zahran, K.M.; Hetta, H.F. Efficacy of ketoconazole gel-flakes in treatment of vaginal candidiasis: Formulation, in vitro and clinical evaluation. Int. J. Pharm. 2019, 567, 118472. [Google Scholar] [CrossRef] [PubMed]
- Rial, R.C.; de Freitas, O.N.; Nazário, C.E.D.; Viana, L.H. Biodiesel from soybean oil using Porcine pancreas lipase immobilized on a new support: p-nitrobenzyl cellulose xanthate. Renew. Energy 2020, 149, 970–979. [Google Scholar] [CrossRef]
- CDC. Candida auris. 21 March 2022. Available online: https://www.cdc.gov/fungal/candida-auris/index.html (accessed on 4 April 2023).
- Casadevall, A. Fungal Diseases in the 21st Century: The Near and Far Horizons. Pathog. Immun. 2018, 3, 183–196. [Google Scholar] [CrossRef]
- Webb, B.J.; Ferraro, J.P.; Rea, S.; Kaufusi, S.; Goodman, B.E.; Spalding, J. Epidemiology and Clinical Features of Invasive Fungal Infection in a US Health Care Network. Open Forum Infect. Dis. 2018, 5, ofy187. [Google Scholar] [CrossRef]
- Richardson, M.D. Changing patterns and trends in systemic fungal infections. J. Antimicrob. Chemother. 2005, 56 (Suppl. S1), i5–i11. [Google Scholar] [CrossRef]
- Singh, N. Trends in the epidemiology of opportunistic fungal infections: Predisposing factors and the impact of antimicrobial use practices. Clin. Infect. Dis. 2001, 33, 1692–1696. [Google Scholar] [CrossRef]
- Rijnders, B.J.A.; Schauwvlieghe, A.F.A.D.; Wauters, J. Influenza-Associated Pulmonary Aspergillosis: A Local or Global Lethal Combination? Clin. Infect. Dis. 2020, 71, 1764–1767. [Google Scholar] [CrossRef]
- Lamoth, F. Invasive aspergillosis in coronavirus disease 2019: A practical approach for clinicians. Curr. Opin. Infect. Dis. 2022, 35, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Hoenigl, M.; Seidel, D.; Carvalho, A.; Rudramurthy, S.M.; Arastehfar, A.; Gangneux, J.P.; Nasir, N.; Bonifaz, A.; Araiza, J.; Klimko, N.; et al. The emergence of COVID-19 associated mucormycosis: A review of cases from 18 countries. Lancet Microbe 2022, 3, e543–e552. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ami, R.; Kontoyiannis, D.P. Resistance to Antifungal Drugs. Infect. Dis. Clin. N. Am. 2021, 35, 279–311. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Netea, M.G. Exciting developments in the immunology of fungal infections. Cell Host Microbe 2012, 11, 422–424. [Google Scholar] [CrossRef][Green Version]
- Perfect, J.R. The antifungal pipeline: A reality check. Nat. Rev. Drug Discov. 2017, 16, 603–616. [Google Scholar] [CrossRef]
- Cruz-Luna, A.R.; Cruz-Martínez, H.; Vásquez-López, A.; Medina, D.I. Metal Nanoparticles as Novel Antifungal Agents for Sustainable Agriculture: Current Advances and Future Directions. J. Fungi 2021, 7, 1033. [Google Scholar] [CrossRef]
- Mussin, J.E.; Roldán, M.V.; Rojas, F.; Sosa, M.; Pellegri, N.; Giusiano, G. Antifungal activity of silver nanoparticles in combination with ketoconazole against Malassezia furfur. AMB Express 2019, 9, 131. [Google Scholar] [CrossRef]
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv113. [Google Scholar] [CrossRef]
- Köhler, J.R.; Hube, B.; Puccia, R.; Casadevall, A.; Perfect, J.R. Fungi that Infect Humans. Microbiol. Spectr. 2017, 5, 813–843. [Google Scholar] [CrossRef]
- Denning, D.W.; Kneale, M.; Sobel, J.D.; Rautemaa-Richardson, R. Global burden of recurrent vulvovaginal candidiasis: A systematic review. Lancet Infect. Dis. 2018, 18, e339–e347. [Google Scholar] [CrossRef] [PubMed]
- von Lilienfeld-Toal, M.; Wagener, J.; Einsele, H.; Cornely, O.A.; Kurzai, O. Invasive Fungal Infection. Dtsch. Arztebl. Int. 2019, 116, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Tyler, M.A.; Lam, K.; Marino, M.J.; Yao, W.C.; Schmale, I.; Citardi, M.J.; Luong, A.U. Revisiting the controversy: The role of fungi in chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2021, 11, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Spivak, E.S.; Hanson, K.E. Candida auris: An Emerging Fungal Pathogen. J. Clin. Microbiol. 2018, 24–56. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef]
- Springer, D.J.; Billmyre, R.B.; Filler, E.E.; Voelz, K.; Pursall, R.; Mieczkowski, P.A.; Larsen, R.A.; Dietrich, F.S.; May, R.C.; Filler, S.G.; et al. Cryptococcus gattii VGIII isolates causing infections in HIV/AIDS patients in Southern California: Identification of the local environmental source as arboreal. PLoS Pathog. 2014, 10, e1004285. [Google Scholar] [CrossRef]
- Baddley, J.W.; Schain, D.C.; Gupte, A.A.; Lodhi, S.A.; Kayler, L.K.; Frade, J.P.; Lockhart, S.R.; Chiller, T.; Bynon, J.S., Jr.; Bower, W.A. Transmission of Cryptococcus neoformans by Organ Transplantation. Clin. Infect. Dis. 2011, 52, e94–e98. [Google Scholar] [CrossRef]
- Engelthaler, D.M.; Casadevall, A. On the Emergence of Cryptococcus gattii in the Pacific Northwest: Ballast Tanks, Tsunamis, and Black Swans. mBio 2019, 10, e02193-19. [Google Scholar] [CrossRef]
- Stephen, C.; Lester, S.; Black, W.; Fyfe, M.; Raverty, S. Multispecies outbreak of cryptococcosis on southern Vancouver Island, British Columbia. Can. Vet. J. 2002, 43, 792–794. [Google Scholar]
- Byrnes, E.J., 3rd; Bildfell, R.J.; Frank, S.A.; Mitchell, T.G.; Marr, K.A.; Heitman, J. Molecular evidence that the range of the Vancouver Island outbreak of Cryptococcus gattii infection has expanded into the Pacific Northwest in the United States. J. Infect. Dis. 2009, 199, 1081–1086. [Google Scholar] [CrossRef]
- Hurt, W.J.; Harrison, T.S.; Molloy, S.F.; Bicanic, T.A. Combination Therapy for HIV-Associated Cryptococcal Meningitis—A Success Story. J. Fungi 2021, 7, 1098. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.M.; Montrief, T.; Ramzy, M.; Koyfman, A.; Long, B. Cryptococcal meningitis: A review for emergency clinicians. Intern. Emerg. Med. 2021, 16, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Cadena, J.; Thompson, G.R., 3rd; Patterson, T.F. Aspergillosis: Epidemiology, Diagnosis, and Treatment. Infect. Dis. Clin. N. Am. 2021, 35, 415–434. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Muthu, V.; Sehgal, I.S.; Dhooria, S.; Prasad, K.T.; Aggarwal, A.N. Allergic Bronchopulmonary Aspergillosis. Clin. Chest Med. 2022, 43, 99–125. [Google Scholar] [CrossRef]
- Segal, B.H. Aspergillosis. N. Engl. J. Med. 2009, 360, 1870–1884. [Google Scholar] [CrossRef]
- Mba, I.E.; Nweze, E.I. The use of nanoparticles as alternative therapeutic agents against Candida infections: An up-to-date overview and future perspectives. World J. Microbiol. Biotechnol. 2020, 36, 163. [Google Scholar] [CrossRef]
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef]
- Prestel, C.; Anderson, E.; Forsberg, K.; Lyman, M.; de Perio, M.A.; Kuhar, D.; Edwards, K.; Rivera, M.; Shugart, A.; Walters, M.; et al. Candida auris Outbreak in a COVID-19 Specialty Care Unit—Florida, July–August 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 56–57. [Google Scholar] [CrossRef]
- Rodriguez, J.Y.; Le Pape, P.; Lopez, O.; Esquea, K.; Labiosa, A.L.; Alvarez-Moreno, C. Candida auris: A Latent Threat to Critically Ill Patients with Coronavirus Disease 2019. Clin. Infect. Dis. 2021, 73, e2836–e2837. [Google Scholar] [CrossRef]
- Magnasco, L.; Mikulska, M.; Giacobbe, D.R.; Taramasso, L.; Vena, A.; Dentone, C.; Dettori, S.; Tutino, S.; Labate, L.; Di Pilato, V.; et al. Spread of Carbapenem-Resistant Gram-Negatives and Candida auris during the COVID-19 Pandemic in Critically Ill Patients: One Step Back in Antimicrobial Stewardship? Microorganisms 2021, 9, 95. [Google Scholar] [CrossRef]
- Villanueva-Lozano, H.; Treviño-Rangel, R.J.; González, G.M.; Ramírez-Elizondo, M.T.; Lara-Medrano, R.; Aleman-Bocanegra, M.C.; Guajardo-Lara, C.E.; Gaona-Chávez, N.; Castilleja-Leal, F.; Torre-Amione, G.; et al. Outbreak of Candida auris infection in a COVID-19 hospital in Mexico. Clin. Microbiol. Infect. 2021, 27, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Sharma, G.; Singla, S.; Garg, S. Case Report: Secondary Hemophagocytic Lymphohistiocytosis (sHLH) and Candida auris Fungemia in Post-acute COVID-19 Syndrome: A Clinical Challenge. Front. Med. 2022, 9, 835421. [Google Scholar] [CrossRef]
- Vaseghi, N.; Sharifisooraki, J.; Khodadadi, H.; Nami, S.; Safari, F.; Ahangarkani, F.; Meis, J.F.; Badali, H.; Morovati, H. Global prevalence and subgroup analyses of coronavirus disease (COVID-19) associated Candida auris infections (CACa): A systematic review and meta-analysis. Mycoses 2022, 65, 683–703. [Google Scholar] [CrossRef]
- Chow, N.A.; de Groot, T.; Badali, H.; Abastabar, M.; Chiller, T.M.; Meis, J.F. Potential Fifth Clade of Candida auris, Iran, 2018. Emerg. Infect. Dis. 2019, 25, 1780–1781. [Google Scholar] [CrossRef]
- Horton, M.V.; Johnson, C.J.; Kernien, J.F.; Patel, T.D.; Lam, B.C.; Cheong, J.Z.A.; Meudt, J.J.; Shanmuganayagam, D.; Kalan, L.R.; Nett, J.E. Candida auris Forms High-Burden Biofilms in Skin Niche Conditions and on Porcine Skin. mSphere 2020, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Ademe, M.; Girma, F. Candida auris: From Multidrug Resistance to Pan-Resistant Strains. Infect. Drug Resist. 2020, 13, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Voss, A.; Meis, J.F. Multidrug-resistant Candida auris: ‘New kid on the block’ in hospital-associated infections? J. Hosp. Infect. 2016, 94, 209–212. [Google Scholar] [CrossRef]
- Hata, D.J.; Humphries, R.; Lockhart, S.R. Candida auris: An Emerging Yeast Pathogen Posing Distinct Challenges for Laboratory Diagnostics, Treatment, and Infection Prevention. Arch. Pathol. Lab. Med. 2020, 144, 107–114. [Google Scholar] [CrossRef]
- Chen, J.; Tian, S.; Han, X.; Chu, Y.; Wang, Q.; Zhou, B.; Shang, H. Is the superbug fungus really so scary? A systematic review and meta-analysis of global epidemiology and mortality of Candida auris. BMC Infect. Dis. 2020, 20, 827. [Google Scholar] [CrossRef]
- Santos, M.A.; Gomes, A.C.; Santos, M.C.; Carreto, L.C.; Moura, G.R. The genetic code of the fungal CTG clade. Comptes Rendus Biol. 2011, 334, 607–611. [Google Scholar] [CrossRef]
- Yue, H.; Bing, J.; Zheng, Q.; Zhang, Y.; Hu, T.; Du, H.; Wang, H.; Huang, G. Filamentation in Candida auris, an emerging fungal pathogen of humans: Passage through the mammalian body induces a heritable phenotypic switch. Emerg. Microbes Infect. 2018, 7, 188. [Google Scholar] [CrossRef] [PubMed]
- Sherry, L.; Ramage, G.; Kean, R.; Borman, A.; Johnson, E.M.; Richardson, M.D.; Rautemaa-Richardson, R. Biofilm-Forming Capability of Highly Virulent, Multidrug-Resistant Candida auris. Emerg. Infect. Dis. 2017, 23, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Mba, I.E.; Nweze, E.I. Mechanism of Candida pathogenesis: Revisiting the vital drivers. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1797–1819. [Google Scholar] [CrossRef] [PubMed]
- Fuentefria, A.M.; Pippi, B.; Dalla Lana, D.F.; Donato, K.K.; de Andrade, S.F. Antifungals discovery: An insight into new strategies to combat antifungal resistance. Lett. Appl. Microbiol. 2018, 66, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Hokken, M.W.; Zwaan, B.; Melchers, W.; Verweij, P. Facilitators of adaptation and antifungal resistance mechanisms in clinically relevant fungi. Fungal Genet. Biol. 2019, 132, 103254. [Google Scholar] [CrossRef] [PubMed]
- Matsumori, N.; Sawada, Y.; Murata, M. Mycosamine orientation of amphotericin B controlling interaction with ergosterol: Sterol-dependent activity of conformation-restricted derivatives with an amino-carbonyl bridge. J. Am. Chem. Soc. 2005, 127, 10667–10675. [Google Scholar] [CrossRef]
- Houšť, J.; Spížek, J.; Havlíček, V. Antifungal drugs. Metabolites 2020, 10, 106. [Google Scholar] [CrossRef]
- Anderson, T.M.; Clay, M.C.; Cioffi, A.G.; Diaz, K.A.; Hisao, G.S.; Tuttle, M.D.; Nieuwkoop, A.J.; Comellas, G.; Maryum, N.; Wang, S.; et al. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat. Chem. Biol. 2014, 10, 400–406. [Google Scholar] [CrossRef]
- Mesa-Arango, A.C.; Scorzoni, L.; Zaragoza, O. It only takes one to do many jobs: Amphotericin B as antifungal and immunomodulatory drug. Front. Microbiol. 2012, 3, 286. [Google Scholar] [CrossRef]
- Wang, X.; Mohammad, I.S.; Fan, L.; Zhao, Z.; Nurunnabi, M.; Sallam, M.A.; Wu, J.; Chen, Z.; Yin, L.; He, W. Delivery strategies of amphotericin B for invasive fungal infections. Acta Pharm. Sin. B 2021, 11, 2585–2604. [Google Scholar] [CrossRef]
- Kelemen, H.; Orgovan, G.; Szekely-Szentmiklosi, B. The pharmaceutical chemistry of azole antifungals. Acta Pharm. Hung. 2016, 86, 85–98. [Google Scholar]
- Paul, S.; Shaw, D.; Joshi, H.; Singh, S.; Chakrabarti, A.; Rudramurthy, S.M.; Ghosh, A.K. Mechanisms of azole antifungal resistance in clinical isolates of Candida tropicalis. PLoS ONE 2022, 17, e0269721. [Google Scholar] [CrossRef] [PubMed]
- Kazeminejad, Z.; Marzi, M.; Shiroudi, A.; Kouhpayeh, S.A.; Farjam, M.; Zarenezhad, E. Novel 1, 2, 4-Triazoles as Antifungal Agents. BioMed Res. Int. 2022, 2022, 4584846. [Google Scholar] [CrossRef]
- Pristov, K.; Ghannoum, M. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W. Echinocandin antifungal drugs. Lancet 2003, 362, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Szymański, M.; Chmielewska, S.; Czyżewska, U.; Malinowska, M.; Tylicki, A. Echinocandins—Structure, mechanism of action and use in antifungal therapy. J. Enzym. Inhib. Med. Chem. 2022, 37, 876–894. [Google Scholar] [CrossRef] [PubMed]
- Simitsopoulou, M.; Peshkova, P.; Tasina, E.; Katragkou, A.; Kyrpitzi, D.; Velegraki, A.; Walsh, T.J.; Roilides, E. Species-specific and drug-specific differences in susceptibility of Candida biofilms to echinocandins: Characterization of less common bloodstream isolates. Antimicrob. Agents Chemother. 2013, 57, 2562–2570. [Google Scholar] [CrossRef] [PubMed]
- Maxfield, L.; Preuss, C.V.; Bermudez, R. Terbinafine. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Ryder, N.S. Squalene epoxidase as a target for the allylamines. Biochem. Soc. Trans. 1991, 19, 774–777. [Google Scholar] [CrossRef]
- Delma, F.Z.; Al-Hatmi, A.M.S.; Brüggemann, R.J.M.; Melchers, W.J.G.; de Hoog, S.; Verweij, P.E.; Buil, J.B. Molecular Mechanisms of 5-Fluorocytosine Resistance in Yeasts and Filamentous Fungi. J. Fungi 2021, 7, 909. [Google Scholar] [CrossRef]
- Stott, K.E.; Loyse, A.; Jarvis, J.N.; Alufandika, M.; Harrison, T.S.; Mwandumba, H.C.; Day, J.N.; Lalloo, D.G.; Bicanic, T.; Perfect, J.R.; et al. Cryptococcal meningoencephalitis: Time for action. Lancet Infect. Dis. 2021, 21, e259–e271. [Google Scholar] [CrossRef]
- Larkin, E.; Hager, C.; Chandra, J.; Mukherjee, P.K.; Retuerto, M.; Salem, I.; Long, L.; Isham, N.; Kovanda, L.; Borroto-Esoda, K.; et al. The Emerging Pathogen Candida auris: Growth Phenotype, Virulence Factors, Activity of Antifungals, and Effect of SCY-078, a Novel Glucan Synthesis Inhibitor, on Growth Morphology and Biofilm Formation. Antimicrob. Agents Chemother. 2017, 24–61. [Google Scholar] [CrossRef] [PubMed]
- de Cássia Orlandi Sardi, J.; Silva, D.R.; Soares Mendes-Giannini, M.J.; Rosalen, P.L. Candida auris: Epidemiology, risk factors, virulence, resistance, and therapeutic options. Microb. Pathog. 2018, 125, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Cortegiani, A.; Misseri, G.; Fasciana, T.; Giammanco, A.; Giarratano, A.; Chowdhary, A. Epidemiology, clinical characteristics, resistance, and treatment of infections by Candida auris. J. Intensive Care 2018, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Osei Sekyere, J. Candida auris: A systematic review and meta-analysis of current updates on an emerging multidrug-resistant pathogen. MicrobiologyOpen 2018, 7, e00578. [Google Scholar] [CrossRef]
- Muñoz, J.F.; Gade, L.; Chow, N.A.; Loparev, V.N.; Juieng, P.; Berkow, E.L.; Farrer, R.A.; Litvintseva, A.P.; Cuomo, C.A. Genomic insights into multidrug-resistance, mating and virulence in Candida auris and related emerging species. Nat. Commun. 2018, 9, 5346. [Google Scholar] [CrossRef]
- Chowdhary, A.; Prakash, A.; Sharma, C.; Kordalewska, M.; Kumar, A.; Sarma, S.; Tarai, B.; Singh, A.; Upadhyaya, G.; Upadhyay, S.; et al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009-17) in India: Role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J. Antimicrob. Chemother. 2018, 73, 891–899. [Google Scholar] [CrossRef]
- Chaabane, F.; Graf, A.; Jequier, L.; Coste, A.T. Review on Antifungal Resistance Mechanisms in the Emerging Pathogen Candida auris. Front. Microbiol. 2019, 10, 2788. [Google Scholar] [CrossRef]
- Kordalewska, M.; Lee, A.; Park, S.; Berrio, I.; Chowdhary, A.; Zhao, Y.; Perlin, D.S. Understanding Echinocandin Resistance in the Emerging Pathogen Candida auris. Antimicrob. Agents Chemother. 2018, 25–62. [Google Scholar] [CrossRef]
- Rhodes, J.; Abdolrasouli, A.; Farrer, R.A.; Cuomo, C.A.; Aanensen, D.M.; Armstrong-James, D.; Fisher, M.C.; Schelenz, S. Genomic epidemiology of the UK outbreak of the emerging human fungal pathogen Candida auris. Emerg. Microbes Infect. 2018, 7, 43. [Google Scholar] [CrossRef]
- Escandón, P.; Chow, N.A.; Caceres, D.H.; Gade, L.; Berkow, E.L.; Armstrong, P.; Rivera, S.; Misas, E.; Duarte, C.; Moulton-Meissner, H.; et al. Molecular Epidemiology of Candida auris in Colombia Reveals a Highly Related, Countrywide Colonization with Regional Patterns in Amphotericin B Resistance. Clin. Infect. Dis. 2019, 68, 15–21. [Google Scholar] [CrossRef]
- Sardi Jde, C.; Pitangui Nde, S.; Rodríguez-Arellanes, G.; Taylor, M.L.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J. Highlights in pathogenic fungal biofilms. Rev. Iberoam. Micol. 2014, 31, 22–29. [Google Scholar] [CrossRef]
- Abdellatif, A.A.; Tawfeek, H.M.; Abdelfattah, A.; Batiha, G.E.-S.; Hetta, H.F. Recent updates in COVID-19 with emphasis on inhalation therapeutics: Nanostructured and targeting systems. J. Drug Deliv. Sci. Technol. 2021, 63, 102435. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Munoz, R.; Lopez, F.D.; Lopez-Ribot, J.L. Silver Nanoantibiotics Display Strong Antifungal Activity against the Emergent Multidrug-Resistant Yeast Candida auris under Both Planktonic and Biofilm Growing Conditions. Front. Microbiol. 2020, 11, 1673. [Google Scholar] [CrossRef]
- Vazquez-Rodriguez, A.; Vasto-Anzaldo, X.G.; Leon-Buitimea, A.; Zarate, X.; Morones-Ramirez, J.R. Antibacterial and Antibiofilm Activity of Biosynthesized Silver Nanoparticles Coated with Exopolysaccharides Obtained from Rhodotorula mucilaginosa. IEEE Trans. Nanobiosci. 2020, 19, 498–503. [Google Scholar] [CrossRef]
- Garza-Cervantes, J.A.; Escárcega-González, C.E.; Barriga Castro, E.D.; Mendiola-Garza, G.; Marichal-Cancino, B.A.; López-Vázquez, M.A.; Morones-Ramirez, J.R. Antimicrobial and antibiofilm activity of biopolymer-Ni, Zn nanoparticle biocomposites synthesized using R. mucilaginosa UANL-001L exopolysaccharide as a capping agent. Int. J. Nanomed. 2019, 14, 2557–2571. [Google Scholar] [CrossRef] [PubMed]
- Abd Ellah, N.H.; Ahmed, E.A.; Abd-Ellatief, R.B.; Ali, M.F.; Zahran, A.M.; Hetta, H.F. Metoclopramide nanoparticles modulate immune response in a diabetic rat model: Association with regulatory T cells and proinflammatory cytokines. Int. J. Nanomed. 2019, 14, 2383–2395. [Google Scholar] [CrossRef] [PubMed]
- Abo-Shama, U.H.; El-Gendy, H.; Mousa, W.S.; Hamouda, R.A.; Yousuf, W.E.; Hetta, H.F.; Abdeen, E.E. Synergistic and antagonistic effects of metal nanoparticles in combination with antibiotics against some reference strains of pathogenic microorganisms. Infect. Drug Resist. 2020, 13, 351–362. [Google Scholar] [CrossRef]
- Escárcega-González, C.E.; Garza-Cervantes, J.A.; Vázquez-Rodríguez, A.; Montelongo-Peralta, L.Z.; Treviño-González, M.T.; Díaz Barriga Castro, E.; Saucedo-Salazar, E.M.; Chávez Morales, R.M.; Regalado Soto, D.I.; Treviño González, F.M.; et al. In vivo antimicrobial activity of silver nanoparticles produced via a green chemistry synthesis using Acacia rigidula as a reducing and capping agent. Int. J. Nanomed. 2018, 13, 2349–2363. [Google Scholar] [CrossRef]
- Sousa, F.; Ferreira, D.; Reis, S.; Costa, P. Current Insights on Antifungal Therapy: Novel Nanotechnology Approaches for Drug Delivery Systems and New Drugs from Natural Sources. Pharmaceuticals 2020, 13, 248. [Google Scholar] [CrossRef]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef]
- Abd Ellah, N.H.; Gad, S.F.; Muhammad, K.; E Batiha, G.; Hetta, H.F. Nanomedicine as a promising approach for diagnosis, treatment and prophylaxis against COVID-19. Nanomedicine 2020, 15, 2085–2102. [Google Scholar] [CrossRef] [PubMed]
- Abd Ellah, N.H.; Tawfeek, H.M.; John, J.; Hetta, H.F. Nanomedicine as a future therapeutic approach for Hepatitis C virus. Nanomedicine 2019, 14, 1471–1491. [Google Scholar] [CrossRef] [PubMed]
- Ealia, S.A.M.; Saravanakumar, M. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032019. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Wasef, L.; Nassar, A.M.; El-Sayed, Y.S.; Samak, D.; Noreldin, A.; Elshony, N.; Saleh, H.; Elewa, Y.H.; Hassan, S.M.; Saati, A.A. The potential ameliorative impacts of cerium oxide nanoparticles against fipronil-induced hepatic steatosis. Sci. Rep. 2021, 11, 1310. [Google Scholar] [CrossRef]
- Sánchez, A.; Mejía, S.P.; Orozco, J. Recent Advances in Polymeric Nanoparticle-Encapsulated Drugs against Intracellular Infections. Molecules 2020, 25, 3760. [Google Scholar] [CrossRef]
- Bahlool, A.Z.; Fattah, S.; O’Sullivan, A.; Cavanagh, B.; MacLoughlin, R.; Keane, J.; O’Sullivan, M.P.; Cryan, S.A. Development of Inhalable ATRA-Loaded PLGA Nanoparticles as Host-Directed Immunotherapy against Tuberculosis. Pharmaceutics 2022, 14, 1745. [Google Scholar] [CrossRef]
- Abd El-Aziz, F.E.-Z.A.; Hetta, H.F.; Abdelhamid, B.N.; Abd Ellah, N.H. Antibacterial and wound-healing potential of PLGA/spidroin nanoparticles: A study on earthworms as a human skin model. Nanomedicine 2022, 17, 353–365. [Google Scholar] [CrossRef]
- Hetta, H.F.; Ahmed, E.A.; Hemdan, A.G.; El-Deek, H.E.; Abd-Elregal, S.; Abd Ellah, N.H. Modulation of rifampicin-induced hepatotoxicity using poly (lactic-co-glycolic acid) nanoparticles: A study on rat and cell culture models. Nanomedicine 2020, 15, 1375–1390. [Google Scholar] [CrossRef]
- Zylberberg, C.; Matosevic, S. Pharmaceutical liposomal drug delivery: A review of new delivery systems and a look at the regulatory landscape. Drug Deliv. 2016, 23, 3319–3329. [Google Scholar] [CrossRef]
- Lemière, J.; Carvalho, K.; Sykes, C. Cell-sized liposomes that mimic cell motility and the cell cortex. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 128, pp. 271–285. [Google Scholar]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Sherje, A.P.; Jadhav, M.; Dravyakar, B.R.; Kadam, D. Dendrimers: A versatile nanocarrier for drug delivery and targeting. Int. J. Pharm. 2018, 548, 707–720. [Google Scholar] [CrossRef]
- Mlynarczyk, D.T.; Dlugaszewska, J.; Kaluzna-Mlynarczyk, A.; Goslinski, T. Dendrimers against fungi—A state of the art review. J. Control. Release 2021, 330, 599–617. [Google Scholar] [CrossRef]
- Saleh, H.; Nassar, A.M.; Noreldin, A.E.; Samak, D.; Elshony, N.; Wasef, L.; Elewa, Y.H.; Hassan, S.M.; Saati, A.A.; Hetta, H.F. Chemo-protective potential of cerium oxide nanoparticles against fipronil-induced oxidative stress, apoptosis, inflammation and reproductive dysfunction in male white albino rats. Molecules 2020, 25, 3479. [Google Scholar] [CrossRef]
- Haidari, H.; Bright, R.; Kopecki, Z.; Zilm, P.S.; Garg, S.; Cowin, A.J.; Vasilev, K.; Goswami, N. Polycationic Silver Nanoclusters Comprising Nanoreservoirs of Ag+ Ions with High Antimicrobial and Antibiofilm Activity. ACS Appl. Mater. Interfaces 2021, 14, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, S.; Vahidi, M.; Malekabad, E.S.; Izadi, A.; Khatami, M.; Dadashi, A. In Vitro Antifungal Activity of Green Synthesized Silver Nanoparticles in Comparison to Conventional Antifungal Drugs against Trichophyton Interdigitale, Trichophyton Rubrum and Epidermophyton Floccosum. Infect. Disord. Drug Targets 2021, 21, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, V.K.; Yadav, N.; Rai, N.K.; Ellah, N.H.A.; Bohara, R.A.; Rehan, I.F.; Marraiki, N.; Batiha, G.E.-S.; Hetta, H.F.; Singh, M. Pleurotus sajor-caju-mediated synthesis of silver and gold nanoparticles active against colon cancer cell lines: A new era of herbonanoceutics. Molecules 2020, 25, 3091. [Google Scholar] [CrossRef]
- Ahmad, T.; Wani, I.A.; Lone, I.H.; Ganguly, A.; Manzoor, N.; Ahmad, A.; Ahmed, J.; Al-Shihri, A.S. Antifungal activity of gold nanoparticles prepared by solvothermal method. Mater. Res. Bull. 2013, 48, 12–20. [Google Scholar] [CrossRef]
- Ameh, T.; Gibb, M.; Stevens, D.; Pradhan, S.H.; Braswell, E.; Sayes, C.M. Silver and Copper Nanoparticles Induce Oxidative Stress in Bacteria and Mammalian Cells. Nanomaterials 2022, 12, 2402. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Laclette, E.; Blaz, J.; Pérez-Torres, C.A.; Villafán, E.; Lamelas, A.; Rosas-Saito, G.; Ibarra-Juárez, L.A.; García-Ávila, C.J.; Martínez-Enriquez, A.I.; Pariona, N. Antifungal Effect of Copper Nanoparticles against Fusarium kuroshium, an Obligate Symbiont of Euwallacea kuroshio Ambrosia Beetle. J. Fungi 2022, 8, 347. [Google Scholar] [CrossRef]
- Al-Kadmy, I.M.; Aziz, S.N.; Rheima, A.M.; Abid, S.A.; Suhail, A.; Hamzah, I.H.; Naji, E.N.; Besinis, A.; Hetta, H.F. Anti-capsular activity of CuO nanoparticles against Acinetobacter baumannii produce efflux pump. Microb. Pathog. 2023, 181, 106184. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.N.; Al-Kadmy, I.M.; Rheima, A.M.; Al-Sallami, K.J.; Abd Ellah, N.H.; El-Saber Batiha, G.; El-Bouseary, M.M.; Algammal, A.M.; Hetta, H.F. Binary CuO\CoO nanoparticles inhibit biofilm formation and reduce the expression of papC and fimH genes in multidrug-resistant Klebsiella oxytoca. Mol. Biol. Rep. 2023, 50, 5969–5976. [Google Scholar] [CrossRef] [PubMed]
- Paulo, C.S.; Vidal, M.; Ferreira, L.S. Antifungal nanoparticles and surfaces. Biomacromolecules 2010, 11, 2810–2817. [Google Scholar] [CrossRef] [PubMed]
- Parsameher, N.; Rezaei, S.; Khodavasiy, S.; Salari, S.; Hadizade, S.; Kord, M.; Ayatollahi Mousavi, S.A. Effect of biogenic selenium nanoparticles on ERG11 and CDR1 gene expression in both fluconazole-resistant and -susceptible Candida albicans isolates. Curr. Med. Mycol. 2017, 3, 16–20. [Google Scholar] [CrossRef]
- Bafghi, M.H.; Nazari, R.; Darroudi, M.; Zargar, M.; Zarrinfar, H. The effect of biosynthesized selenium nanoparticles on the expression of CYP51A and HSP90 antifungal resistance genes in Aspergillus fumigatus and Aspergillus flavus. Biotechnol. Prog. 2022, 38, e3206. [Google Scholar] [CrossRef]
- Cleare, L.G.; Li, K.L.; Abuzeid, W.M.; Nacharaju, P.; Friedman, J.M.; Nosanchuk, J.D. NO Candida auris: Nitric Oxide in Nanotherapeutics to Combat Emerging Fungal Pathogen Candida auris. J. Fungi 2020, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- Sohail, Y.; Raza, N.; Shakeel, N.; Raza, H.; Manzoor, S.; Yasmin, G.; Iqbal, A.; Manzoor, S.; Albaqami, M.D.; Mohammad Wabaidur, S. Polyaniline-coated nanoparticles of zinc oxide and copper oxide as antifungal agents against Aspergillus parasiticus. Front. Plant Sci. 2022, 13, 925451. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Ghaemi, E.; Noroozi, A.; Niknejad, F. Zinc Oxide Nanoparticles Inhibition of Initial Adhesion and ALS1 and ALS3 Gene Expression in Candida albicans Strains from Urinary Tract Infections. Mycopathologia 2019, 184, 261–271. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Joshaghani, H.; Shokohi, T.; Ahmadi, A.; Mehrbakhsh, Z. Antifungal Activity of ZnO Nanoparticles and Nystatin and Downregulation of SAP1-3 Genes Expression in Fluconazole-Resistant Candida albicans Isolates from Vulvovaginal Candidiasis. Infect. Drug Resist. 2020, 13, 385–394. [Google Scholar] [CrossRef]
- Garcia-Marin, L.E.; Juarez-Moreno, K.; Vilchis-Nestor, A.R.; Castro-Longoria, E. Highly Antifungal Activity of Biosynthesized Copper Oxide Nanoparticles against Candida albicans. Nanomaterials 2022, 12, 3856. [Google Scholar] [CrossRef]
- Martinez-Gutierrez, F.; Olive, P.L.; Banuelos, A.; Orrantia, E.; Nino, N.; Sanchez, E.M.; Ruiz, F.; Bach, H.; Av-Gay, Y. Synthesis, characterization, and evaluation of antimicrobial and cytotoxic effect of silver and titanium nanoparticles. Nanomedicine 2010, 6, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Prucek, R.; Tuček, J.; Kilianová, M.; Panáček, A.; Kvítek, L.; Filip, J.; Kolář, M.; Tománková, K.; Zbořil, R. The targeted antibacterial and antifungal properties of magnetic nanocomposite of iron oxide and silver nanoparticles. Biomaterials 2011, 32, 4704–4713. [Google Scholar] [CrossRef]
- Lara, H.H.; Romero-Urbina, D.G.; Pierce, C.; Lopez-Ribot, J.L.; Arellano-Jiménez, M.J.; Jose-Yacaman, M. Effect of silver nanoparticles on Candida albicans biofilms: An ultrastructural study. J. Nanobiotechnol. 2015, 13, 91. [Google Scholar] [CrossRef]
- Lara, H.H.; Ixtepan-Turrent, L.; Jose Yacaman, M.; Lopez-Ribot, J. Inhibition of Candida auris Biofilm Formation on Medical and Environmental Surfaces by Silver Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 21183–21191. [Google Scholar] [CrossRef]
- Hetta, H.F.; Al-Kadmy, I.M.; Khazaal, S.S.; Abbas, S.; Suhail, A.; El-Mokhtar, M.A.; Ellah, N.H.A.; Ahmed, E.A.; Abd-Ellatief, R.B.; El-Masry, E.A. Antibiofilm and antivirulence potential of silver nanoparticles against multidrug-resistant Acinetobacter baumannii. Sci. Rep. 2021, 11, 10751. [Google Scholar] [CrossRef]
- Eid, A.M.; Fouda, A.; Niedbała, G.; Hassan, S.E.-D.; Salem, S.S.; Abdo, A.M.; Hetta, H.F.; Shaheen, T.I. Endophytic Streptomyces laurentii mediated green synthesis of Ag-NPs with antibacterial and anticancer properties for developing functional textile fabric properties. Antibiotics 2020, 9, 641. [Google Scholar] [CrossRef] [PubMed]
- Gangadoo, S.; Elbourne, A.; Medvedev, A.E.; Cozzolino, D.; Truong, Y.B.; Crawford, R.J.; Wang, P.-Y.; Truong, V.K.; Chapman, J. Facile Route of Fabricating Long-Term Microbicidal Silver Nanoparticle Clusters against Shiga Toxin-Producing Escherichia coli O157:H7 and Candida auris. Coatings 2020, 10, 28. [Google Scholar] [CrossRef]
- AlJindan, R.; AlEraky, D.M. Silver Nanoparticles: A Promising Antifungal Agent against the Growth and Biofilm Formation of the Emergent Candida auris. J. Fungi 2022, 8, 744. [Google Scholar] [CrossRef]
- Malik, M.A.; Batterjee, M.G.; Kamli, M.R.; Alzahrani, K.A.; Danish, E.Y.; Nabi, A. Polyphenol-Capped Biogenic Synthesis of Noble Metallic Silver Nanoparticles for Antifungal Activity against Candida auris. J. Fungi 2022, 8, 639. [Google Scholar] [CrossRef]
- Vazquez-Munoz, R.; Arellano-Jimenez, M.J.; Lopez-Ribot, J.L. Bismuth nanoparticles obtained by a facile synthesis method exhibit antimicrobial activity against Staphylococcus aureus and Candida albicans. BMC Biomed. Eng. 2020, 2, 11. [Google Scholar] [CrossRef]
- Vazquez-Munoz, R.; Lopez, F.D.; Lopez-Ribot, J.L. Bismuth Nanoantibiotics Display Anticandidal Activity and Disrupt the Biofilm and Cell Morphology of the Emergent Pathogenic Yeast Candida auris. Antibiotics 2020, 9, 461. [Google Scholar] [CrossRef] [PubMed]
- Kamli, M.R.; Srivastava, V.; Hajrah, N.H.; Sabir, J.S.M.; Hakeem, K.R.; Ahmad, A.; Malik, M.A. Facile Bio-Fabrication of Ag-Cu-Co Trimetallic Nanoparticles and Its Fungicidal Activity against Candida auris. J. Fungi 2021, 7, 62. [Google Scholar] [CrossRef]
- De Groote, M.A.; Fang, F.C. NO inhibitions: Antimicrobial properties of nitric oxide. Clin. Infect. Dis. 1995, 21 (Suppl. S2), S162–S165. [Google Scholar] [CrossRef]
- Friedman, A.; Friedman, J. New biomaterials for the sustained release of nitric oxide: Past, present and future. Expert Opin. Drug Deliv. 2009, 6, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Jones-Carson, J.; Vazquez-Torres, A.; van der Heyde, H.C.; Warner, T.; Wagner, R.D.; Balish, E. γδ T cell-induced nitric oxide production enhances resistance to mucosal candidiasis. Nat. Med. 1995, 1, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Cruz, N.; Reitzel, R.A.; Rosenblatt, J.; Chaftari, A.-M.; Dib, R.W.; Hachem, R.; Kontoyiannis, D.P.; Raad, I.I. Nitroglycerin-Citrate-Ethanol Catheter Lock Solution Is Highly Effective for In Vitro Eradication of Candida auris Biofilm. Antimicrob. Agents Chemother. 2019, 63, e00299-00219. [Google Scholar] [CrossRef]
- Philip, S.; Kuriakose, S. Synthesis of Superparamagnetic Iron Oxide Nanoparticles Stabilized by Biocompatible Supramolecular β-Cyclodextrin for Biomedical Applications. Mater. Today Proc. 2019, 11, 1030–1035. [Google Scholar] [CrossRef]
- Liu, X.; Guo, C.; Zhuang, K.; Chen, W.; Zhang, M.; Dai, Y.; Tan, L.; Ran, Y. A recyclable and light-triggered nanofibrous membrane against the emerging fungal pathogen Candida auris. PLoS Pathog. 2022, 18, e1010534. [Google Scholar] [CrossRef]
- Wu, D.; Wang, W.; Ng, T.W.; Huang, G.; Xia, D.; Yip, H.Y.; Lee, H.K.; Li, G.; An, T.; Wong, P.K. Visible-light-driven photocatalytic bacterial inactivation and the mechanism of zinc oxysulfide under LED light irradiation. J. Mater. Chem. A 2016, 4, 1052–1059. [Google Scholar] [CrossRef]
- Moro, M.G.; de Carvalho, V.F.; Godoy-Miranda, B.A.; Kassa, C.T.; Horliana, A.C.R.T.; Prates, R.A. Efficacy of antimicrobial photodynamic therapy (aPDT) for nonsurgical treatment of periodontal disease: A systematic review. Lasers Med. Sci. 2021, 36, 1573–1590. [Google Scholar] [CrossRef]
- Zhenjun, D.; Lown, J.W. Hypocrellins and their use in photosensitization. Photochem. Photobiol. 1990, 52, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Kolge, H.; Patil, G.; Jadhav, S.; Ghormade, V. A pH-tuned chitosan-PLGA nanocarrier for fluconazole delivery reduces toxicity and improves efficacy against resistant Candida. Int. J. Biol. Macromol. 2023, 227, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Fayed, B.; Jayakumar, M.N.; Soliman, S.S.M. Caspofungin-resistance in Candida auris is cell wall-dependent phenotype and potential prevention by zinc oxide nanoparticles. Med. Mycol. 2021, 59, 1243–1256. [Google Scholar] [CrossRef]
- Marena, G.D.; Ramos, M.A.D.S.; Lima, L.C.; Chorilli, M.; Bauab, T.M. Galleria mellonella for systemic assessment of anti-Candida auris using amphotericin B loaded in nanoemulsion. Sci. Total Environ. 2022, 807, 151023. [Google Scholar] [CrossRef] [PubMed]
- Marena, G.D.; Carvalho, G.C.; Dos Santos Ramos, M.A.; Chorilli, M.; Bauab, T.M. Anti-Candida auris activity in vitro and in vivo of micafungin loaded nanoemulsions. Med. Mycol. 2022, 2, 3–61. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M.; Ożarowski, M.; Seremak-Mrozikiewicz, A.; Wolski, H.; Adamczak, A. Plant preparations and compounds with activities against biofilms formed by Candida spp. J. Fungi 2021, 7, 360. [Google Scholar] [CrossRef]
- Butassi, E.; Svetaz, L.; Carpinella, M.C.; Efferth, T.; Zacchino, S. Fungal biofilms as a valuable target for the discovery of natural products that cope with the resistance of medically important fungi—Latest findings. Antibiotics 2021, 10, 1053. [Google Scholar] [CrossRef] [PubMed]
- de Alteriis, E.; Maione, A.; Falanga, A.; Bellavita, R.; Galdiero, S.; Albarano, L.; Salvatore, M.M.; Galdiero, E.; Guida, M. Activity of Free and Liposome-Encapsulated Essential Oil from Lavandula angustifolia against Persister-Derived Biofilm of Candida auris. Antibiotics 2022, 11, 26. [Google Scholar] [CrossRef]
- Pla, L.; Santiago-Felipe, S.; Tormo-Mas, M.Á.; Ruiz-Gaitán, A.; Pemán, J.; Valentín, E.; Sancenón, F.; Aznar, E.; Martínez-Máñez, R. Oligonucleotide-capped nanoporous anodic alumina biosensor as diagnostic tool for rapid and accurate detection of Candida auris in clinical samples. Emerg. Microbes Infect. 2021, 10, 407–415. [Google Scholar] [CrossRef]
- Radhakrishnan, V.S.; Reddy Mudiam, M.K.; Kumar, M.; Dwivedi, S.P.; Singh, S.P.; Prasad, T. Silver nanoparticles induced alterations in multiple cellular targets, which are critical for drug susceptibilities and pathogenicity in fungal pathogen (Candida albicans). Int. J. Nanomed. 2018, 13, 2647–2663. [Google Scholar] [CrossRef]
- Hwang, I.S.; Lee, J.; Hwang, J.H.; Kim, K.J.; Lee, D.G. Silver nanoparticles induce apoptotic cell death in Candida albicans through the increase of hydroxyl radicals. FEBS J. 2012, 279, 1327–1338. [Google Scholar] [CrossRef]
- Khatoon, N.; Sharma, Y.; Sardar, M.; Manzoor, N. Mode of action and anti-Candida activity of Artemisia annua mediated-synthesized silver nanoparticles. J. Mycol. Medicale 2019, 29, 201–209. [Google Scholar] [CrossRef]
- Barros, D.; Pradhan, A.; Pascoal, C.; Cássio, F. Transcriptomics reveals the action mechanisms and cellular targets of citrate-coated silver nanoparticles in a ubiquitous aquatic fungus. Environ. Pollut. 2021, 268, 115913. [Google Scholar] [CrossRef] [PubMed]
- Horstmann, C.; Campbell, C.; Kim, D.S.; Kim, K. Transcriptome profile with 20 nm silver nanoparticles in yeast. FEMS Yeast Res. 2019, 19, foz003. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Ahmed, G. Silver nanoparticles damage yeast cell wall. J. Biotechnol. 2012, 3, 36–39. [Google Scholar]
- Yassin, M.T.; Mostafa, A.A.-F.; Al-Askar, A.A.; Al-Otibi, F.O. Synergistic Antifungal Efficiency of Biogenic Silver Nanoparticles with Itraconazole against Multidrug-Resistant Candidal Strains. Crystals 2022, 12, 816. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).