Efficiency of Experimental Formulation Containing Duddingtonia flagrans and Pochonia chlamydosporia against Moniezia expansa Eggs
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining Moniezia expansa Eggs
2.2. Obtaining the Experimental Fungal Formulation
2.3. Goats Used in the Experiment
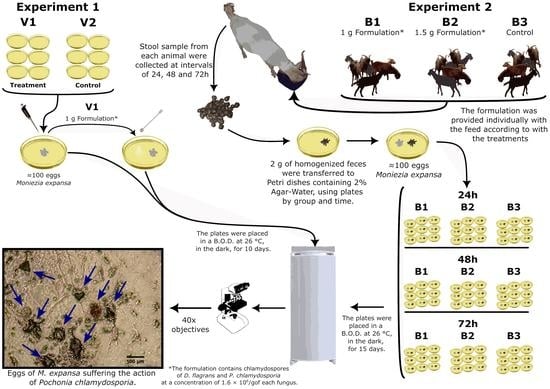
2.4. Experimental Tests
2.4.1. Experiment 1
2.4.2. Experiment 2
2.5. Statistical Analysis
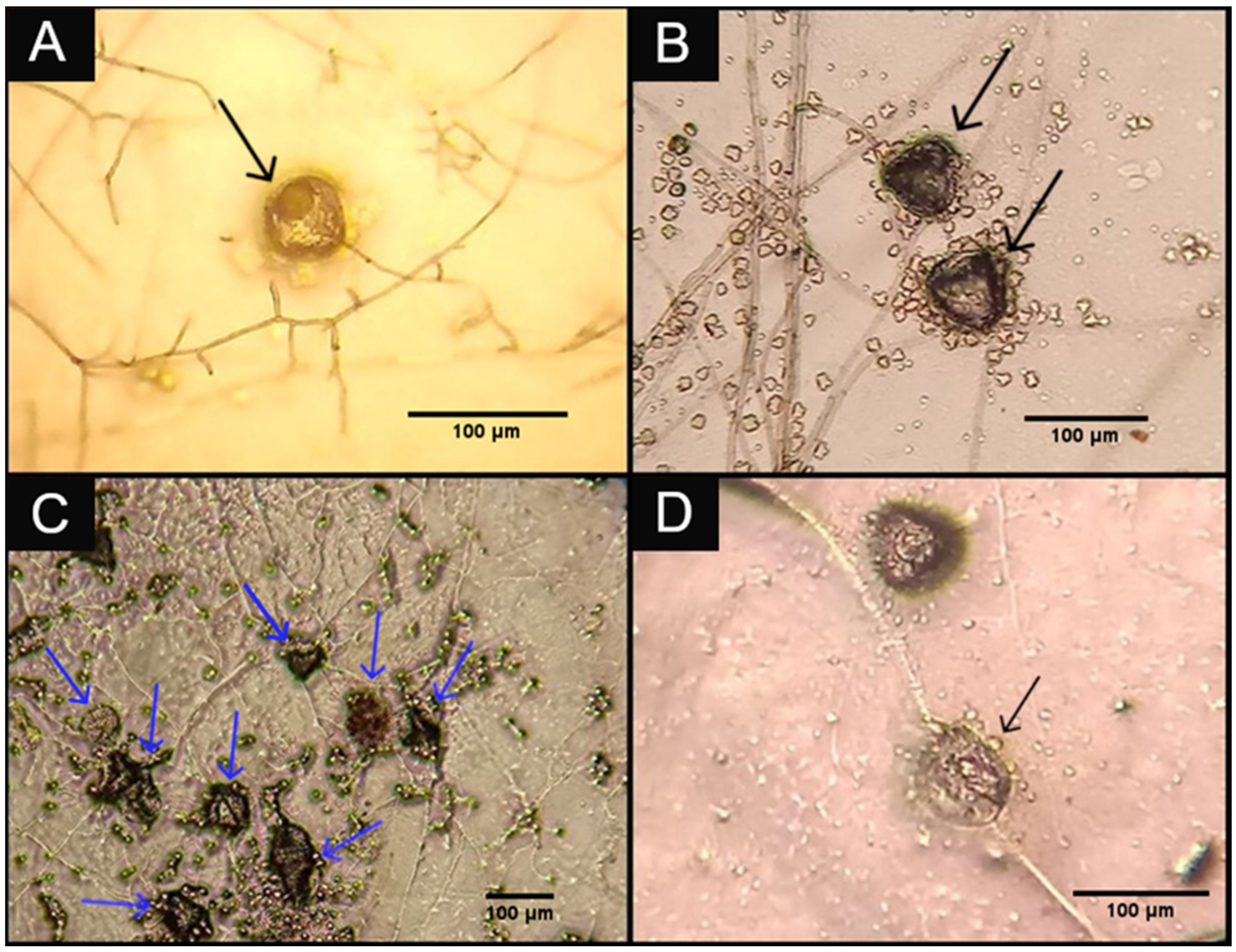
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bashtar, A.-R.; Hassanein, M.; Abdel-Ghaffar, F.; Al-Rasheid, K.; Hassan, S.; Mehlhorn, H.; AL-Mahdi, M.; Morsy, K.; Al-Ghamdi, A. Studies on Monieziasis of Sheep I. Prevalence and Antihelminthic Effects of Some Plant Extracts, a Light and Electron Microscopic Study. Parasitol. Res. 2011, 108, 177–186. [Google Scholar] [CrossRef]
- Mohamed, H.I.; Arafa, W.M.; El-Dakhly, K.M. Prevalence and Associated Risk Factors of Gastrointestinal Helminths and Coccidian Infections in Domestic Goats, Capra hircus, in Minya, Egypt. Beni-Suef Univ. J. Basic Appl. Sci. 2023, 12, 29. [Google Scholar] [CrossRef]
- Kelly, R.; Evans, M.; Sargison, N. Identifying Knowledge Gaps in Moniezia expansa Epidemiology: A Report of a Small Intestinal Torsion in a 5-Week-Old Lamb. N. Z. Vet. J. 2021, 69, 186–189. [Google Scholar] [CrossRef]
- Rahman, S.; Bulbul, K.H. Monieziosis: A Neglected Helminthic Disease in Ruminants. Anim. Dis. 2022; submitted. [Google Scholar]
- Iacob, O.C.; El-Deeb, W.M.; Paşca, S.-A.; Turtoi, A.-I. Uncommon Co-Infection Due to Moniezia expansa and Moniezia benedeni in Young Goats from Romania: Morphological and Histopathological Analysis. Ann. Parasitol. 2020, 66, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Salgado, J.A.; Santos, C.d.P. Overview of Anthelmintic Resistance of Gastrointestinal Nematodes of Small Ruminants in Brazil. Rev. Bras. Parasitol. Vet. 2016, 25, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Willy, M.L.L. Determinación de resistencia antihelmíntica (Moniezia expansa, Moniezia benedeni y Thysanosoma actioides) frente a albendazol y febendazol en ovino en tres rebaños de La Paz—Bolivia. REDVET Rev. Electrónica Vet. 2009, 10, 1–13. [Google Scholar]
- Amarante, A.F.T. Os Parasitas De Ovinos; Editora UNESP: São Paulo, Brasil, 2014; ISBN 978-85-68334-42-3. [Google Scholar]
- Kumar, G.; Selvakkumar, R. Fenbendazole and Praziquantel Resistance in Moniezia expansa in Jamunapari Goat Kids. J. Entomol. Zool. Stud. 2020, 8, 1124–1125. [Google Scholar]
- Vercruysse, J.; Charlier, J.; Dijk, J.V.; Morgan, E.R.; Geary, T.; Samson-Himmelstjerna, G.V.; Claerebout, E. Control of Helminth Ruminant Infections by 2030. Parasitology 2018, 145, 1655–1664. [Google Scholar] [CrossRef]
- Do Carmo, T.A.; Mena, M.O.; Cipriano, I.d.A.; de Favare, G.M.; Guelpa, G.J.; Pinto, S.D.C.; do Amarante, A.F.T.; de Araújo, J.V.; de Soutello, R.V.G. Biological Control of Gastrointestinal Nematodes in Horses Fed with Grass in Association with Nematophagus Fungi Duddingtonia flagrans and Pochonia chlamydosporia. Biol. Control 2023, 182, 105219. [Google Scholar] [CrossRef]
- Gives, P.M.; Arellano, M.E.L.; Marcelino, L.A.; Jenkins, S.O.; Guerrero, D.R.; Várgas, G.R.; Murillo, V.E.V. The Nematophagous Fungus Duddingtonia flagrans Reduces the Gastrointestinal Parasitic Nematode Larvae Population in Faeces of Orally Treated Calves Maintained under Tropical Conditions-Dose/Response Assessment. Vet. Parasitol. 2018, 263, 66–72. [Google Scholar] [CrossRef]
- Vilela, V.L.R.; Feitosa, T.F.; Braga, F.R.; Vieira, V.D.; Lucena, S.C.d.; Araújo, J.V.d. Control of Sheep Gastrointestinal Nematodes Using the Combination of Duddingtonia flagrans and Levamisole Hydrochloride 5%. Rev. Bras. De Parasitol. Veterinária 2018, 27, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.A.; Oliveira, C.R.C.; Anjos, D.H.S.; Ornelas, É.I.D. Potencial dos fungos nematófagos Arthrobotrys sp. E Monacrosporium thaumasium para o controle de larvas de ciatostomíneos de eqüinos (Nematoda: Cyathostominae). Rev. Bras. Parasitol. Vet. 2003, 12, 53–57. [Google Scholar]
- Braga, F.R.; Araújo, J.V.; Silva, A.R.; Carvalho, R.O.; Araujo, J.M.; Campos, A.K.; Tavela, A.O. Ação in vitro dos fungos nematófagos Duddingtonia flagrans (Duddington, 1955), Monacrosporium thaumasium (Drechsler, 1937) e Pochonia chlamydosporia (Gams & Zare, 2001) sobre ovos de Eurytrema coelomaticum (Giard & Billet, 1892). Arq. Inst. Biológico 2009, 76, 131–134. [Google Scholar]
- Wang, B.-B.; Zhang, N.; Gong, P.-T.; Li, J.-H.; Yang, J.; Zhang, H.-B.; Zhang, X.-C.; Cai, K.-Z. Morphological Variability, Molecular Phylogeny, and Biological Characteristics of the Nematophagous Fungus Duddingtonia flagrans. J. Basic Microbiol. 2019, 59, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Buzatti, A.; Santos, C.P.; Fernandes, M.A.M.; Yoshitani, U.Y.; Sprenger, L.K.; Molento, M.B. Duddingtonia flagrans no controle de nematoides gastrintestinais de equinos em fases de vida livre. Arq. Bras. Med. Veterinária E Zootec. 2017, 69, 364–370. [Google Scholar] [CrossRef]
- Jobim, M.B.; Santurio, J.M.; De La Rue, M.L. Duddingtonia flagrans: Controle biológico de nematodeos de bovinos a campo. Ciênc. Rural 2008, 38, 2256–2263. [Google Scholar] [CrossRef][Green Version]
- Rodrigues, J.V.F.; Braga, F.R.; Campos, A.K.; de Carvalho, L.M.; Araujo, J.M.; Aguiar, A.R.; Ferraz, C.M.; da Silveira, W.F.; Valadão, M.C.; Oliveira, T.d.; et al. Duddingtonia flagrans Formulated in Rice Bran in the Control of Oesophagostomum spp. Intestinal Parasite of Swine. Exp. Parasitol. 2018, 184, 11–15. [Google Scholar] [CrossRef]
- Fonseca, J.D.S.; Ferreira, V.M.; Freitas, S.G.d.; Vieira, Í.S.; Araújo, J.V.d. Efficacy of a Fungal Formulation with the Nematophagous Fungus Pochonia chlamydosporia in the Biological Control of Bovine Nematodiosis. Pathogens 2022, 11, 695. [Google Scholar] [CrossRef]
- Lýsek, H.; Fassatiová, O.; Cuervo Pineda, N.; Lorenzo Hernández, N. Ovicidal Fungi in Soils of Cuba. Folia Parasitol. 1982, 29, 265–270. [Google Scholar]
- Araújo, J.M.; Araújo, J.V.; Braga, F.R.; Carvalho, R.O.; Silva, A.R.; Campos, A.K. Interaction and Ovicidal Activity of Nematophagous Fungus Pochonia chlamydosporia on Taenia saginata Eggs. Exp. Parasitol. 2009, 121, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, J.d.S.; Altoé, L.S.C.; de Carvalho, L.M.; Soares, F.E.d.F.; Braga, F.R.; de Araújo, J.V. Nematophagous Fungus Pochonia chlamydosporia to Control Parasitic Diseases in Animals. Appl. Microbiol. Biotechnol. 2023, 107, 3859–3868. [Google Scholar] [CrossRef]
- Stunkard, H.W. The Life Cycle of Moniezia expansa. Science 1937, 86, 312. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.V.; Braga, F.R.; Mendoza-de-Gives, P.; Paz-Silva, A.; Vilela, V.L.R. Recent Advances in the Control of Helminths of Domestic Animals by Helminthophagous Fungi. Parasitologia 2021, 1, 168–176. [Google Scholar] [CrossRef]
- Hoog, G.S.d.; Oorschot, C.A.N.v.; Hijwegen, T. Taxonomy of the Dactylaria Complex II. Dissoconium Gen. Nov. and Cordana Preuss. Proc. Kon. Ned. Akad. Wet. Ser. C 1983, 86, 197–206. [Google Scholar]
- Li, S.; Wang, D.; Gong, J.; Zhang, Y. Individual and Combined Application of Nematophagous Fungi as Biological Control Agents against Gastrointestinal Nematodes in Domestic Animals. Pathogens 2022, 11, 172. [Google Scholar] [CrossRef]
- Braga, F.R.; Ferraz, C.M.; da Silva, E.N.; de Araújo, J.V. Efficiency of the Bioverm® (Duddingtonia flagrans) Fungal Formulation to Control In Vivo and In Vitro of Haemonchus contortus and Strongyloides papillosus in Sheep. 3 Biotech 2020, 10, 62–66. [Google Scholar] [CrossRef]
- Braga, F.R.; Araújo, J.V.; Campos, A.K.; Araújo, J.M.; Carvalho, R.O.; Silva, A.R.; Tavela, A.O. In Vitro Evaluation of the Action of the Nematophagous Fungi Duddingtonia Flagrans, Monacrosporium Sinense and Pochonia Chlamydosporia on Fasciola Hepatica Eggs. World J. Microbiol. Biotechnol. 2008, 24, 1559–1564. [Google Scholar] [CrossRef]
- Tavela, A.O.; Araújo, J.V.; Braga, F.R.; Araujo, J.M.; Magalhães, L.Q.; Silveira, W.F.; Borges, L.A. In Vitro Association of Nematophagous Fungi Duddingtonia Flagrans (AC001), Monacrosporium Thaumasium (NF34) and Pochonia Chlamydosporia (VC1) to Control Horse Cyathostomin (Nematoda: Strongylidae). Biocontrol Sci. Technol. 2012, 22, 607–610. [Google Scholar] [CrossRef]
- Vilela, V.L.R.; Feitosa, T.F.; Braga, F.R.; de Araújo, J.V.; de Oliveira Souto, D.V.; da Silva Santos, H.E.; da Silva, G.L.L.; Athayde, A.C.R. Biological Control of Goat Gastrointestinal Helminthiasis by Duddingtonia flagrans in a Semi-Arid Region of the Northeastern Brazil. Vet. Parasitol. 2012, 188, 127–133. [Google Scholar] [CrossRef]
- Da Silveira, W.F.; de Oliveira, G.D.; Braga, F.R.; de Carvalho, L.M.; Domingues, R.R.; da Silva, L.A.; Zanuncio, J.C.; de Araújo, J.V. Predation Rate of Nematophagous Fungi after Passing through the Gastrointestinal Tract of Goats. Small Rumin. Res. 2017, 147, 101–105. [Google Scholar] [CrossRef]
- Araújo, J.M.; Braga, F.R.; Araújo, J.V.; Soares, F.E.F.; Geniêr, H.L.A. Biological Control of Taenia saginata Eggs. Helminthologia 2010, 47, 189–192. [Google Scholar] [CrossRef]
- Braga, F.R.; Silva, A.R.; Carvalho, R.O.; Araújo, J.V.; Pinto, P.S.A. Ovicidal Activity of Different Concentrations of Pochonia chlamydosporia Chlamydospores on Taenia taeniaeformis Eggs. J. Helminthol. 2011, 85, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.M.; Braga, F.R.; de Araújo, J.V.; Carvalho, R.O. Atividade dos fungos nematófagos Pochonia chlamydosporia e Paecilomyces lilacinus sobre cápsulas de ovos de Dipylidium caninum. Rev. Inst. Adolfo Lutz 2009, 68, 488–491. [Google Scholar] [CrossRef]
- Braga, F.R.; Araújo, J.V.; Araujo, J.M.; Carvalho, R.O.; Silva, A.R.; Campos, A.K.; Tavela, A.O. Ovicidal Activity of Paecilomyces lilacinus on Moniezia sp. Eggs. J. Helminthol. 2008, 82, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Braga, F.R.; Araújo, J.V. Nematophagous Fungi for Biological Control of Gastrointestinal Nematodes in Domestic Animals. Appl. Microbiol. Biotechnol. 2014, 98, 71–82. [Google Scholar] [CrossRef]
- Braga, F.R.; Araújo, J.V.; Soares, F.E.F.; Tavela, A.O.; Araujo, J.M.; Carvalho, R.O.; Fernandes, F.M.; Queiroz, J.H. Enzymatic Analysis and in Vitro Ovicidal Effect of Pochonia Chlamydosporia and Paecilomyces Lilacinus on Oxyuris Equi Eggs of Horses. Biocontrol Sci. Technol. 2012, 22, 685–696. [Google Scholar] [CrossRef]
- Thapa, S.; Meyling, N.V.; Katakam, K.K.; Thamsborg, S.M.; Mejer, H. A Method to Evaluate Relative Ovicidal Effects of Soil Microfungi on Thick-Shelled Eggs of Animal-Parasitic Nematodes. Biocontrol Sci. Technol. 2015, 25, 756–767. [Google Scholar] [CrossRef]
- Ojeda-Robertos, N.F.; Torres-Acosta, J.F.; Ayala-Burgos, A.J.; Sandoval-Castro, C.A.; Valero-Coss, R.O.; Mendoza-de-Gives, P. Digestibility of Duddingtonia flagrans chlamydospores in ruminants: In Vitro and In Vivo studies. BMC Vet. Res. 2009, 5, 46–52. [Google Scholar] [CrossRef]
- Caley, J. In Vitro Hatching of the Tapeworm Moniezia expansa (Cestoda: Anoplocephalidae) and Some Properties of the Egg Membranes. Z. Parasitenk. 1975, 45, 335–346. [Google Scholar] [CrossRef]
- Lopez-Llorcz, L.V.; Olivares-Bernabeu, C.; Salinas, J.; Jansson, H.-B.; Kolattukudy, P.E. Pre-Penetration Events in Fungal Parasitism of Nematode Eggs. Mycol. Res. 2002, 106, 499–506. [Google Scholar] [CrossRef]
- Ward, E.; Kerry, B.R.; Manzanilla-López, R.H.; Mutua, G.; Devonshire, J.; Kimenju, J.; Hirsch, P.R. The Pochonia chlamydosporia Serine Protease Gene Vcp1 Is Subject to Regulation by Carbon, Nitrogen and Ph: Implications for Nematode Biocontrol. PLoS ONE 2012, 7, e35657. [Google Scholar] [CrossRef] [PubMed]
- Braga, F.R.; Araújo, J.V.; Araujo, J.M.; Frassy, L.N.; Tavela, A.O.; Soares, F.E.F.; Carvalho, R.O.; Queiroz, L.M.; Queiroz, J.H. Pochonia chlamydosporia Fungal Activity in a Solid Medium and Its Crude Extract against Eggs of Ascaridia galli. J. Helminthol. 2012, 86, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Braga, F.R.; Araujo, J.M.; Silva, A.R.; de Araújo, J.V.; Carvalho, R.O.; de Freitas Soares, F.E.; de Queiroz, J.H.; Gênier, H.L.A. Ação ovicida do extrato bruto enzimático do fungo Pochonia chlamydosporia sobre ovos de Ancylostoma sp. Rev. Soc. Bras. Med. Trop. 2011, 44, 116–118. [Google Scholar] [CrossRef]
- Castro, L.S.; Martins, I.V.F.; Tunholi-Alves, V.M.; Amaral, L.S.; Pinheiro, J.; de Araújo, J.V.; de Oliveira Monteiro, C.M.; Tunholi, V.M. Susceptibility of Embryos of Biomphalaria tenagophila (Mollusca: Gastropoda) to Infection by Pochonia chlamydosporia (Ascomycota: Sordariomycetes). Arch. Microbiol. 2022, 204, 271. [Google Scholar] [CrossRef]
- Esteves, I.; Peteira, B.; Atkins, S.D.; Magan, N.; Kerry, B. Production of Extracellular Enzymes by Different Isolates of Pochonia Chlamydosporia. Mycol. Res. 2009, 113, 867–876. [Google Scholar] [CrossRef]
- Gouveia, A.d.S.; Monteiro, T.S.A.; Valadares, S.V.; Sufiate, B.L.; de Freitas, L.G.; de Oliveira Ramos, H.J.; de Queiroz, J.H. Understanding How Pochonia chlamydosporia Increases Phosphorus Availability. Geomicrobiol. J. 2019, 36, 747–751. [Google Scholar] [CrossRef]
- Dallemole-Giaretta, R.; de Freitas, L.G.; de Brito Caixeta, L.; Xavier, D.M.; Ferraz, S.; de Fátima Silva, C. Produção de clamidósporos de Pochonia chlamydosporia em diferentes substratos. Ciênc. Agrotec. 2011, 35, 314–321. [Google Scholar] [CrossRef][Green Version]
- Youssar, L.; Wernet, V.; Hensel, N.; Yu, X.; Hildebrand, H.-G.; Schreckenberger, B.; Kriegler, M.; Hetzer, B.; Frankino, P.; Dillin, A.; et al. Intercellular Communication Is Required for Trap Formation in the Nematode-Trapping Fungus Duddingtonia flagrans. PLoS Genet. 2019, 15, e1008029. [Google Scholar] [CrossRef]
- Céspedes-Gutiérrez, E.; Aragón, D.M.; Gómez-Álvarez, M.I.; Cubides-Cárdenas, J.A.; Cortés-Rojas, D.F. Survival of the Nematophagous Fungus Duddingtonia flagrans to In Vitro Segments of Sheep Gastrointestinal Tract. Exp. Parasitol. 2021, 231, 108172. [Google Scholar] [CrossRef]
- Monteiro, T.S.A.; Gouveia, A.S.; Balbino, H.M.; Morgan, T.; Grassi de Freitas, L. Chapter 34—Duddingtonia. In Beneficial Microbes in Agro-Ecology; Amaresan, N., Senthil Kumar, M., Annapurna, K., Kumar, K., Sankaranarayanan, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 683–694. ISBN 978-0-12-823414-3. [Google Scholar]
- Martins, N.S.; Dos Santos, C.C.; da Motta, S.P.; da Silva Moreira, A.; da Rosa Farias, N.A.; Ruas, J.L. Gastrointestinal Parasites in Sheep from the Brazilian Pampa Biome: Prevalence and Associated Factors. Braz. J. Vet. Med. 2022, 44, e001522. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Santos Castelo Branco de Oliveira, L.; Dias, F.G.S.; Melo, A.L.T.; de Carvalho, L.M.; Silva, E.N.; de Araújo, J.V. Bioverm® in the Control of Nematodes in Beef Cattle Raised in the Central-West Region of Brazil. Pathogens 2021, 10, 548. [Google Scholar] [CrossRef]
- de Castro, L.L.D.; Sprenger, L.K.; Madrid, I.M.; de Oliveira, F.C.; de Oliveira, P.A.; de Castro, L.M.; Berne, M.E.A.; Leite, F.P.L. Efeito in vitro e in vivo de extratos de Eugenia Uniflora Em Nematódeos Gastrintestinais de ovinos. Ciênc. Anim. Bras./Braz. Anim. Sci. 2019, 20, 1–12. [Google Scholar] [CrossRef]
- Porto Filho, J.M.; Costa, R.G.; Araújo, A.C.P.; Albuquerque Júnior, E.C.; Cunha, A.N.; Cruz, G.R.B. da Determining Anthelmintic Residues in Goat Milk in Brazil. Rev. Bras. Saúde Prod. Anim. 2019, 20, e04102019. [Google Scholar] [CrossRef]
- Fissiha, W.; Kinde, M.Z. Anthelmintic Resistance and Its Mechanism: A Review. Infect. Drug Resist. 2021, 14, 5403–5410. [Google Scholar] [CrossRef]
- Lourenco, A.; Fraga-Corral, M.; De Colli, L.; Moloney, M.; Danaher, M.; Jordan, K. Determination of the Presence of Pathogens and Anthelmintic Drugs in Raw Milk and Raw Milk Cheeses from Small Scale Producers in Ireland. LWT 2020, 130, 109347. [Google Scholar] [CrossRef]
- Lobato, V.; Rath, S.; Reyes, F.G.R. Occurrence of Ivermectin in Bovine Milk from the Brazilian Retail Market. Food Addit. Contam. 2006, 23, 668–673. [Google Scholar] [CrossRef]
- Mendoza-de Gives, P.; Braga, F.R.; de Araújo, J.V. Nematophagous Fungi, an Extraordinary Tool for Controlling Ruminant Parasitic Nematodes and Other Biotechnological Applications. Biocontrol Sci. Technol. 2022, 32, 777–793. [Google Scholar] [CrossRef]
| Mean of Intact Eggs | |||
|---|---|---|---|
| Group (n) | 24 h | 48 h | 72 h |
| B1 (9) | 59.0 a ± 16.3 (SE = 5.4) | 42.5 a ± 14.7 (SE = 4.9) | 57.7 a ± 12.6 (SE = 6.41) |
| B2 (9) | 49.5 a ± 20.10 (SE = 6.7) | 39.1 a ± 17.9 (SE = 5.9) | 46.8 a ± 18.3 (SE = 6.1) |
| B3 (9) | 115.2 b ± 21.25 (SE = 10.4) | 105.0 b ± 20.6 (SE = 6.9) | 110.4 b ± 22.47 (SE = 7.49) |
| Percentage Reduction of Eggs (%) | |||
|---|---|---|---|
| Group | 24 h | 48 h | 72 h |
| B1 | 49 a | 60 a | 48 a |
| B2 | 57 a | 63 a | 58 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, G.B.; de Almeida Moura, I.; e Silva, A.R.; de Araújo, J.V.; de Oliveira Monteiro, C.M.; dos Santos Fonseca, J.; de Oliveira, A.P.D.; de Souza Perinotto, W.M. Efficiency of Experimental Formulation Containing Duddingtonia flagrans and Pochonia chlamydosporia against Moniezia expansa Eggs. Pathogens 2023, 12, 1028. https://doi.org/10.3390/pathogens12081028
Ribeiro GB, de Almeida Moura I, e Silva AR, de Araújo JV, de Oliveira Monteiro CM, dos Santos Fonseca J, de Oliveira APD, de Souza Perinotto WM. Efficiency of Experimental Formulation Containing Duddingtonia flagrans and Pochonia chlamydosporia against Moniezia expansa Eggs. Pathogens. 2023; 12(8):1028. https://doi.org/10.3390/pathogens12081028
Chicago/Turabian StyleRibeiro, Giancarlo Bomfim, Ially de Almeida Moura, André Ricardo e Silva, Jackson Victor de Araújo, Caio Márcio de Oliveira Monteiro, Júlia dos Santos Fonseca, Ana Patrícia David de Oliveira, and Wendell Marcelo de Souza Perinotto. 2023. "Efficiency of Experimental Formulation Containing Duddingtonia flagrans and Pochonia chlamydosporia against Moniezia expansa Eggs" Pathogens 12, no. 8: 1028. https://doi.org/10.3390/pathogens12081028
APA StyleRibeiro, G. B., de Almeida Moura, I., e Silva, A. R., de Araújo, J. V., de Oliveira Monteiro, C. M., dos Santos Fonseca, J., de Oliveira, A. P. D., & de Souza Perinotto, W. M. (2023). Efficiency of Experimental Formulation Containing Duddingtonia flagrans and Pochonia chlamydosporia against Moniezia expansa Eggs. Pathogens, 12(8), 1028. https://doi.org/10.3390/pathogens12081028