Brain Tuberculosis: An Odyssey through Time to Understand This Pathology
Abstract
1. Introduction
2. Tuberculosis in Antiquity
3. Tuberculosis in the Middle Ages: The Royal Healing Touch
4. The First Clinical Observations from the 17th Century
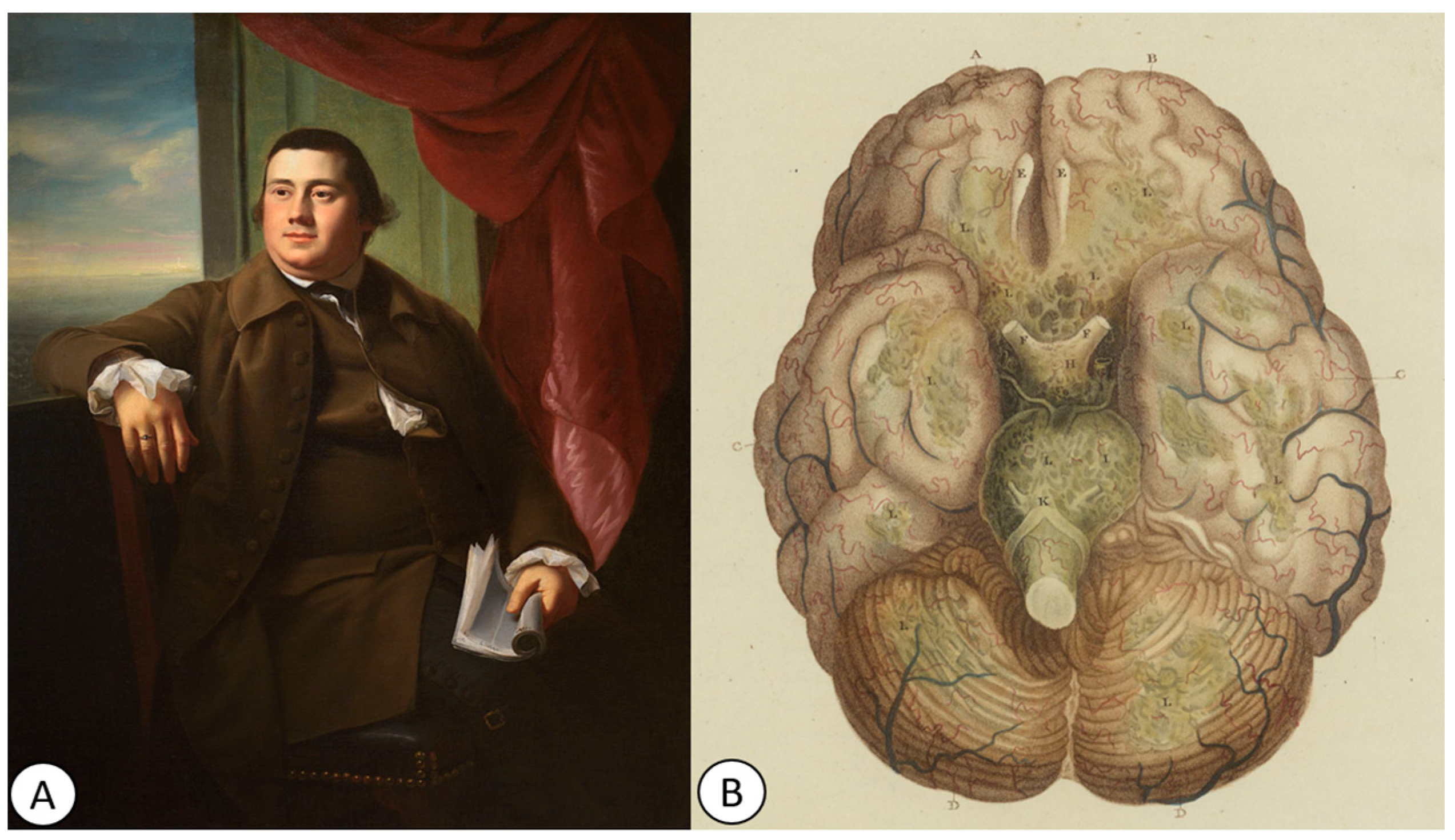
5. The 18th Century: The First Description of Tuberculous Meningitis and the First Clinical–Pathological Correlations
6. The 19th Century: Bringing to Light the Pathogenesis of Brain Tuberculosis and the Discovery of the Koch Bacillus
6.1. The First Theories of Inflammation of the Pia Mater and Arachnoid Membrane
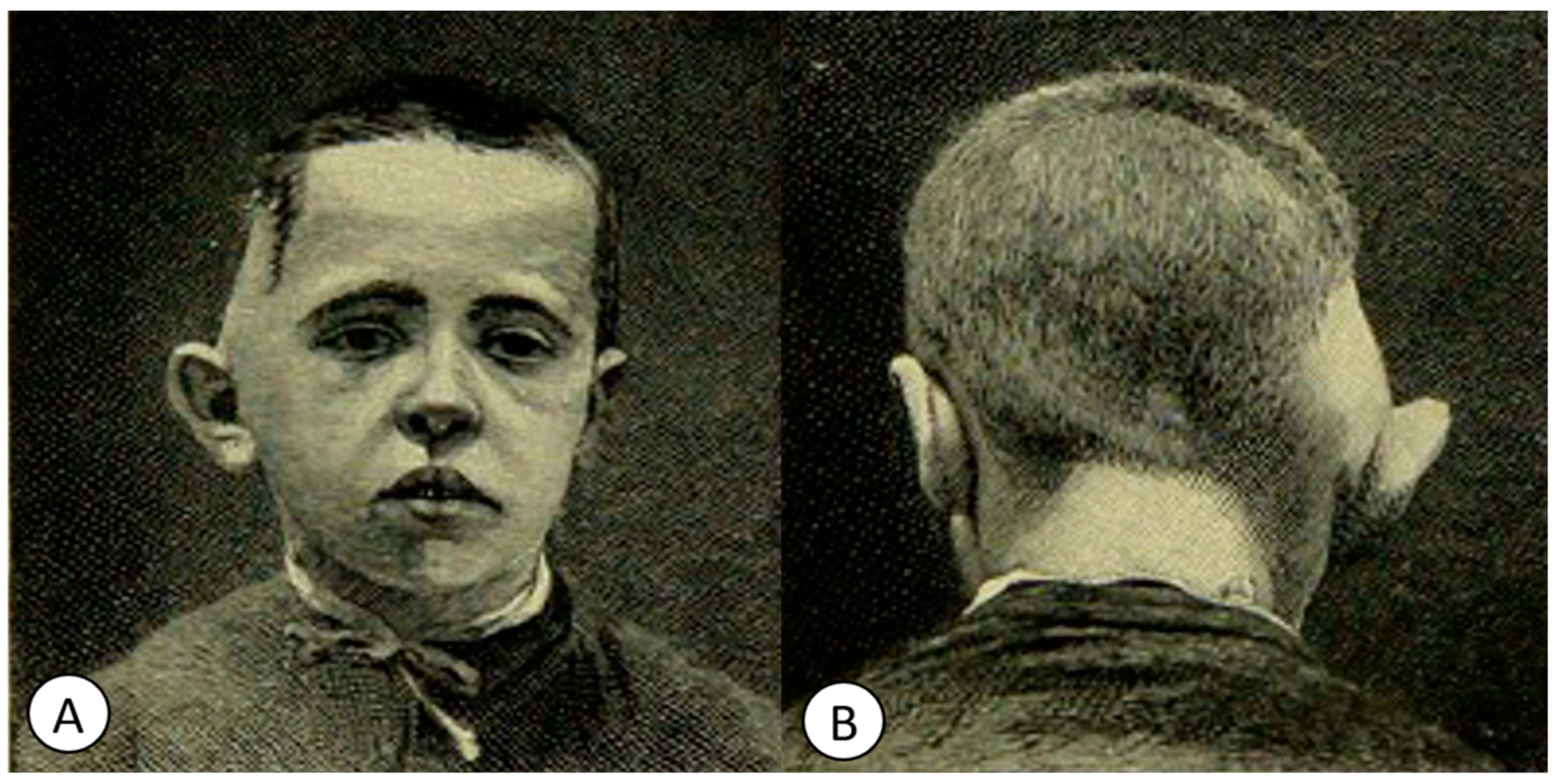
6.2. Granulomatous Meningitis
7. The First Clinical–Pathological Correlations
8. From the Cannulation Technique to the First Treatments against Tuberculosis
9. The Beginnings of Tuberculosis Treatment
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mac Donald, E.M.; Izzo, A.A. Tuberculosis Vaccine Development. In Tuberculosis-Expanding Knowledge; Ribbon, W., Ed.; InTechOpen: London, UK, 2015. [Google Scholar]
- Barberis, I.; Bragazzi, N.L.; Galluzzo, L.; Martini, M. The History of Tuberculosis: From the First Historical Records to the Isolation of Koch’s Bacillus. J. Prev. Med. Hyg. 2017, 58, E9–E12. [Google Scholar]
- Hayman, J. Mycobacterium ulcerans: An Infection from Jurassic Time? Lancet 1984, 2, 1015–1016. [Google Scholar] [CrossRef]
- Breed, R.S.; Murray, E.G.D.; Smith, N.B. Bergey’s Manual of Determinative Bacteriology; Williams & Wilkins: Baltimore, MD, USA, 1957. [Google Scholar]
- Zimmerman, M.R. Pulmonary and osseous tuberculosis in an Egyptian mummy. Bull. N. Y. Acad. Med. 1979, 55, 5. [Google Scholar]
- Morse, D.; Brothwell, D.R.; Ucko, P.J. Tuberculosis in Ancient Egypt. Am. Rev. Respir. Dis. 1964, 90, 524–541. [Google Scholar] [PubMed]
- Bedeir, S.A. Tuberculosis in Ancient Egypt. In Tuberculosis; Madkour, M.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Cave, A.J.E. The Evidence for the Incidence of Tuberculosis in Ancient Egypt. Br. J. Tuberc. 1939, 33, 142–152. [Google Scholar] [CrossRef]
- Brown, L. The Story of Clinical Pulmonary Tuberculosis; Williams & Wilkins: Baltimore, MD, USA, 1941. [Google Scholar]
- Salo, W.L.; Aufderheide, A.C.; Buikstra, J.; Holcomb, T.A. Identification of Mycobacterium tuberculosis DNA in a Pre-Columbian Peruvian Mummy. Proc. Natl. Acad. Sci. USA 1994, 91, 2091–2094. [Google Scholar] [CrossRef] [PubMed]
- Daniel, T.M. The Origins and Precolonial Epidemiology of Tuberculosis in the Americas: Can We Figure Them Out? Int. J. Tuberc. Lung Dis. 2000, 4, 395–400. [Google Scholar]
- Arriaza, B.T.; Salo, W.; Aufderheide, A.C.; Holcomb, T.A. Pre-Columbian Tuberculosis in Northern Chile: Molecular and Skeletal Evidence. Am. J. Phys. Anthropol. 1995, 98, 37–45. [Google Scholar] [CrossRef]
- Hippocrates. Book 1—Of the Epidemics. In The Genuine Works of Hippocrates; Adams, F., Ed.; The Sydenham Society: London, UK, 1849. [Google Scholar]
- Daniel, T.M. The history of tuberculosis. Respir. Med. 2006, 100, 1862–1870. [Google Scholar] [CrossRef]
- Pease, A.S. Some Remarks on the Diagnosis and Treatment of Tuberculosis in Antiquity. Isis 1940, 31, 380–393. Available online: http://www.jstor.org/stable/225758 (accessed on 8 June 2023).
- Bynum, H. Spitting Blood; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Roberts, C.A.; Buikstra, J.E. The Bioaerchaeology of Tuberculosis: A Global View on a Reemerging Disease; University of Florida Press: Gainesville, FL, USA, 2003. [Google Scholar]
- Tyler, K.L. A History of Bacterial Meningitis. In Handbook of Clinical Neurology; Aminoff, M.J., Boller, F., Swabb, D.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Herzog, H. History of Tuberculosis. Respiration 1998, 65, 5–15. [Google Scholar] [CrossRef]
- Costea, C.F.; Turliuc, Ş.; Buzdugă, C.; Cucu, A.I.; Dumitrescu, G.F.; Sava, A.; Turliuc, M.D. The History of Optic Chiasm from Antiquity to the Twentieth Century. Childs Nerv. Syst. 2017, 33, 1889–1898. [Google Scholar] [CrossRef]
- Garrison, F.H. An Introduction to the History of Medicine; Saunders: Philadelphia, PA, USA, 1913. [Google Scholar]
- Madkour, M.M.; Al-Otaibi, K.E.; Al Swailem, R. Historical Aspects of Tuberculosis. In Tuberculosis; Madkour, M.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Agarwal, Y.; Chopra, R.; Gupta, D.; Sethi, R. The Tuberculosis Timeline: Of White Plague, a Birthday Present, and Vignettes of Myriad Hues. Astrocyte 2017, 4, 7. [Google Scholar] [CrossRef]
- Barlow, F. The King’s Evil. Engl. Hist. Rev. 1980, 95, 3–27. Available online: https://www.jstor.org/stable/569080 (accessed on 10 June 2023). [CrossRef]
- Dormandy, T. The White Death: A History of Tuberculosis; New York University Press: New York, NY, USA, 2000. [Google Scholar]
- Risse, G.B. History of Western Medicine from Hippocrates to Germ Theory. In The Cambridge World History of Human Disease; Kiple, K.F., Ed.; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Johnston, W.D. Tuberculosis. In The Cambridge World History of Human Disease; Kiple, K.F., Ed.; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Gallagher, N.E. Islamic and Indian Medicine. In The Cambridge World History of Human Disease; Kiple, K.F., Ed.; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Keers, R.Y. Richard Morton (1637-98) and His Phthisiologia. Thorax 1982, 37, 26–31. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morton, R. Phthisiologia; W. & J. Innys: London, UK, 1720. [Google Scholar]
- Ruhräh, J. The History of Tuberculous Meningitis. Med. Libr. Hist. J. 1904, 2, 160–165. [Google Scholar]
- Willis, T. The London Practice of Physick, or, The Whole Practical Part of Physick Contained in the Works of Dr. Willis: Faithfully Made English, and Printed Together for the Public Good; Thomas Baffet and William Crooke: London, UK, 1685. [Google Scholar]
- Walusinski, O. History of the Concept of Tuberculous Meningitis. Eur. Neurol. 2021, 84, 61–70. [Google Scholar] [CrossRef]
- Saint-Clair, A. History of a Fever and of an Epilepsy; Ed. Med. Ess: Edinburgh, UK, 1737. [Google Scholar]
- Paisley, J.A. Hydrocephalum with Remarkable Symptoms; Ed. Med. Ess: Edinburgh, UK, 1737. [Google Scholar]
- Pott, P. Remarks on That Kind of Palsy of the Lower Limbs Which Is Frequently Found to Accompany a Curvature of the Spine; J. Johnson: London, UK, 1979. [Google Scholar]
- Burke, R.M. A Historical Chronology of Tuberculosis; Charles C Thomas: Springfield, IL, USA, 1938. [Google Scholar]
- Willan, R. On Cutaneous Diseases; J. Johnson: London, UK, 1808; Volume 1. [Google Scholar]
- Rayer, P.F.O. Traite Des Maladies Des Reins; J B Bailliere: Paris, France, 1841; pp. 1839–1841. [Google Scholar]
- Von Rokitansky, C. Handbuch Der Pathologischen Anatomie; Braumuller & Seidel: Wien, Austria, 1842; p. 628. [Google Scholar]
- Mann, R. Tuberculosis. Int. J. STD AIDS 1991, 2 (Suppl. S1), 13–19. [Google Scholar] [CrossRef]
- Brouardel, P.C.H. La Mort et La Mortesubite; J B Bailliere: Paris, France, 1895. [Google Scholar]
- Miller, J. Aural tuberculosis. Arch. Otolaryngol. Head. Neck Surg. 1934, 20, 677–692. [Google Scholar] [CrossRef]
- Petit, J.L. Hydrocéphalie Ou Tumeur Aqueuse de La Teste. Histoire de l’Académie Royale des Sciences 1718; Impr. Royale: Paris, France, 1741. [Google Scholar]
- Eschle, F. Tuberkelbacillen in Dem Ausflusse Bei Mittelohreiterungen von Phthisikern. Dtsch. Med. Wochenschr. 1883, 9, 441. [Google Scholar]
- Whytt, R. Observations on the Dropsy in the Brain; John Balfour: Edinburgh, UK, 1768. [Google Scholar]
- Pearce, J.M. Robert Whytt and the Stretch Reflex. J. Neurol. Psychiatry 1997, 62, 484. [Google Scholar] [CrossRef][Green Version]
- Breathnach, C.S. Robert Whyttt (1714–1766): From Dropsy in the Brain to Tuberculous Meningitis. Ir. J. Med. Sci. 2014, 183, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Ford, E. Some Remarks on Hydrocephalus Internus. Lond. Med. J. 1790, 11 Pt 1, 56–67. [Google Scholar]
- Parent-Duchatelet, A.; Martinet, L. Recherches Sur L’inflammation de L’arachnoïde Cérébrale et Spinale Ou Histoire Théorique et Pratique de L’arachnitis; Chez Crevot: Paris, France, 1821. [Google Scholar]
- Senn, L. Recherches Anatomico-Pathologiques sur la Méningite Aiguë des Enfans et ses Principales Complications; Hachette Bnf: Paris, France, 1825. [Google Scholar]
- Hooper, R. The Morbid Anatomy of the Human Brain: Illustrations of the Most Frequent and Important Organic Diseases to Which That Viscus Is Subject; Longman, Rees, Orme, Brown, and Green: London, UK, 1826. [Google Scholar]
- Smith, H.V.; Daniel, P. Some Clinical and Pathological Aspects of Tuberculosis of the Central Nervous System. Tubercle 1947, 28, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Papavoine, L.N. Observations d’arachnitis Tuberculeuses. J. Hebd. Med. 1830, 6, 113–119. [Google Scholar]
- Nysten, P.H. Dictionnaire de Médecine, de Chirurgie, de Pharmacie, des Sciences Accessoires et de L’art Vétérinaire; Hippolyte-Bailliere: Paris, France, 1855. [Google Scholar]
- Mullener, E.R. Six Geneva Physicians on Meningitis. J. Hist. Med. Allied Sci. 1965, 20, 1–26. [Google Scholar] [CrossRef]
- Rufz, E. De Quelques Affections Cérébrales Observées Dans Le Service de M. Guersent, à l’hôpital Des Enfants-Malades. Arch. Gén. Méd. 1833, 1, 184–227. [Google Scholar]
- Rufz, E. Quelques Recherches Sur Les Symptômes et Sur Les Lésions Anatomiques de l’affection Décrite Sous Les Noms d’hydrocéphale Aiguë, Fièvre Cérébrale, Méningite, Méningo-Céphalite Chez Les Enfans; Thèse Paris N°42; Impr. Didot Le Jeune: Paris, France, 1835. [Google Scholar]
- Gerhard, W.W. On the Cerebral Affections of Children. Am. J. Med. Sci. 1833, 13, 313–359; 14, 99–111. [Google Scholar] [CrossRef]
- Daniel, T.M. Pioneers in Medicine and Their Impact on Tuberculosis; University of Rochester Press: Rochester, NY, USA, 2000. [Google Scholar]
- Le Diberder, V. Essai Sur l’affection Tuberculeuse Aiguë de La Pie-Mère Chez Les Adultes, Connue Chez Les Enfants Sous Le Nom d’hydrocéphale Aiguë, Fièvre Cérébrale, Méningite Granuleuse Ou Tuberculeuse; Thèse Paris N°410; Impr. Rignoux: Paris, France, 1837. [Google Scholar]
- Valleix, I. De La Méningite Tuberculeuse Chez l’adulte. Arch. Gén. Méd. 1838, 5–29. [Google Scholar]
- Keen, W.W. Surgery of the Lateral Ventricles of the Brain. Lancet 1890, 136, 553–555. [Google Scholar] [CrossRef]
- Quincke, H.I. Die Lumbopunction Des Hydrocephalus. Berl. Klin. Wochenschr. 1891, 28. [Google Scholar]
- Frederiks, J.A.; Koehler, P.J. The First Lumbar Puncture. J. Hist. Neurosci. 1997, 6, 147–153. [Google Scholar] [CrossRef]
- Roguin, A. Rene Theophile Hyacinthe Laënnec (1781–1826): The Man Behind the Stethoscope. Clin. Med. Res. 2006, 4, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Keers, R.Y. Laennec: His Medical History. Thorax 1981, 36, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, V.D.; Tonietto, R.G.; Tonietto, V.; Laennec, R.T.H. (1781–1826). In Pioneers in Pathology; van den Tweel, J.G., Ed.; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Bishop, P.J. Laennec: A Great Student of Tuberculosis. Tubercle 1981, 62, 129–134, 147–148. [Google Scholar] [CrossRef]
- Sakula, A. Robert Koch: Centenary of the Discovery of the Tubercle Bacillus, 1882. Thorax 1982, 37, 246–251. [Google Scholar] [CrossRef]
- Itard, J.M.G. Hydrocéphale in Dictionnaire des Sciences Médicales; Panckoucke: Paris, France, 1818. [Google Scholar]
- Empis, G. De La Granulie Ou Maladie Granuleuse: Connue Sous Les Noms de Fièvre Cérébrale, de Méningite Granuleuse, d’hydrocéphale Aiguë, de Phthisie Galopante, de Tuberculisation Aiguë, Etc.; P. Asselin: Paris, France, 1865. [Google Scholar]
- Pearce, J.M. Walter Essex Wynter, Quincke, and Lumbar Puncture. J. Neurol. Neurosurg. Psychiatry 1994, 57, 179. [Google Scholar] [CrossRef]
- Quincke, H.I. Ueber Hydrocephalus. Verhandlungen Des X Congr. Für Inn. Med. (Wiesb.) 1891, 70, 321–339. [Google Scholar]
- Wentworth, A.H. Lumbar Puncture. Boston Med. Surg. J. 1897, 136, 107. [Google Scholar] [CrossRef]
- Morton, C.A. The Pathology of Tuberculous Meningitis with Reference to Its Treatment by Tapping the Subarachnoid Space of the Spinal Cord. BMJ 1891, 2, 840–841. [Google Scholar] [CrossRef]
- Wynter, W.E. Four Cases of Tubercular Meningitis in Which Paracentesis of the Theca Vertebralis Was Performed for the Relief of Fluid Pressure. Lancet 1891, 1, 981–982. [Google Scholar] [CrossRef]
- Koch, R. Die Aetiologie Der Tuberculose. Berl. Klin. Wochenschr. 1882, 19, 221–230. [Google Scholar] [CrossRef]
- Barnes, D.S. Historical perspectives on the etiology of tuberculosis. Microbes Infect. 2000, 2, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.nobelprize.org/prizes/medicine/1905/koch/facts/ (accessed on 10 July 2023).
- Adams, S. Tubercular Abscess of the Brain. Arch. Pédiatrie 1896, 13, 607–609. [Google Scholar]
- Wouters, E.F.M.; Hupperts, R.M.M.; Vreeling, F.W.; Greve, L.H.; Janevski, B.; Willebrand, D.; Berfelo, W.F. Successful Treatment of Tuberculous Brain Abscess. J. Neurol. 1985, 232, 118–119. [Google Scholar] [CrossRef] [PubMed]
- Gonzlez, P.R.M.; Flambertjoachim, G.; Sevilla, G.C. Tuberculous Brain Abscess. J. Neurosurg. 1980, 52, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Thiebaut, F.; Philippides, D. Tubercules de l’encephale En Forme d’abces. Neurochirurgie 1960, 6, 377–378. [Google Scholar] [PubMed]
- Macewen, W. Pyogenic Infective Diseases of the Brain and Spinal Cord: Meningitis, Abscess of the Brain, Infective Sinus Thrombosis; James Maclehose and Sons: Glasgow, UK, 1893. [Google Scholar]
- King, J.E.J. The treatment of brain abscess: A surgical technic in which the usual drainage methods are avoided. Arch. Otolaryngol. Head. Neck Surg. 1925, 1, 26–41. [Google Scholar] [CrossRef]
- Dandy, W.E. Treatment of chronic abscess of the brain by tapping: Preliminary note. JAMA 1926, 87, 1477–1478. [Google Scholar] [CrossRef]
- Sargent, P. Remarks on drainage of brain abscess. Br. Med. J. 1928, 2, 971–972. [Google Scholar] [CrossRef]
- Vincent, C. Sur Une Méthode de Traitement Des Abcès Subaigus Des Hémisphères Cérébraux: Large Décompression, Puis Ablation En Masse sans Drainage. Gaz. Méd. Fr. 1936, 43, 93–96. [Google Scholar]
- Rich, A.R.; McCordock, H.A. The Pathogenesis of Tuberculous Meningitis. Bull. Johns Hopkins Hosp. 1933, 52, 5–37. [Google Scholar]
- Price, D.S. Tuberculosis in Childhood; John Wright: Bristol, UK, 1942. [Google Scholar]
- Ferry, G. Dorothy Stopford Price and the Control of Tuberculosis in Ireland. Lancet 2019, 393, 20. [Google Scholar] [CrossRef]
- Iseman, M.D. Tuberculosis Therapy: Past, Present and Future. Eur. Respir. J. Suppl. 2002, 36, 87s–94s. [Google Scholar] [CrossRef] [PubMed]
- McSweeney, C.J. Clinical Approach to Fevers; J & A Churchill: London, UK, 1953. [Google Scholar]
- The Medical Research Council Investigation. Treatment Of Pulmonary Tuberculosis with Streptomycin and Para-Aminosalicylic Acid. Br. Med. J. 1950, 2, 1073–1085. Available online: http://www.jstor.org/stable/25358924 (accessed on 11 June 2023). [CrossRef]
- Canetti, G. The Eradication of Tuberculosis: Theoretical Problems and Practical Solutions. Tubercle 1962, 43, 301–321. [Google Scholar] [CrossRef] [PubMed]
- Mitchison, D.A. Chemotherapy of Tuberculosis: A Bacteriologist’s Viewpoint. BMJ 1965, 1, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- British Medical Research Council. Long-Term Chemotherapy in the Treatment of Chronic Pulmonary Tuberculosis with Cavitation: A Report to the Medical Research Council by Their Tuberculosis Chemotherapy Trials Committee. Tubercle 1962, 43, 201–219. [Google Scholar] [CrossRef]
- East African/British Medical Research Council. Controlled Clinical Trial of Four Short-Course (6-Month) Regimens of Chemotherapy for Treatment of Pulmonary Tuberculosis. Second Report. Lancet 1973, 1, 1331–1338. [Google Scholar]
- East-African/British Medical Research Council. Controlled Clinical Trial of Four Short-Course (6-Month) Regimens of Chemotherapy for Treatment of Pulmonary Tuberculosis. Third Report. Lancet 1974, 2, 237–240. [Google Scholar]
- Xi, Y.; Zhang, W.; Qiao, R.-J.; Tang, J. Risk Factors for Multidrug-Resistant Tuberculosis: A Worldwide Systematic Review and Meta-Analysis. PLoS ONE 2022, 17, e0270003. [Google Scholar] [CrossRef]
- Seung, K.J.; Keshavjee, S.; Rich, M.L. Multidrug-Resistant Tuberculosis and Extensively Drug-Resistant Tuberculosis. Cold Spring Harb. Perspect. Med. 2015, 5, a017863. [Google Scholar] [CrossRef] [PubMed]
- Dean, A.S.; Zignol, M.; Cabibbe, A.M.; Falzon, D.; Glaziou, P.; Cirillo, D.M.; Köser, C.U.; Gonzalez-Angulo, L.Y.; Tosas-Auget, O.; Ismail, N.; et al. Prevalence and Genetic Profiles of Isoniazid Resistance in Tuberculosis Patients: A Multicountry Analysis of Cross-Sectional Data. PLoS Med. 2020, 17, e1003008. [Google Scholar] [CrossRef]
- Mulu, W.; Mekonnen, D.; Yimer, M.; Admassu, A.; Abera, B. Risk Factors for Multidrug Resistant Tuberculosis Patients in Amhara National Regional State. Afr. Health Sci. 2015, 15, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Xu, Y.; Zhang, X.; Qiu, Q.; Lei, S.; Li, J.; Yuan, W.; Song, Q.; Xu, J. Risk Factors of Multidrug-Resistant Tuberculosis in China: A Meta-Analysis. Public Health Nurs. 2019, 36, 257–269. [Google Scholar] [CrossRef]
- Hang, N.T.L.; Maeda, S.; Lien, L.T.; Thuong, P.H.; Hung, N.V.; Thuy, T.B.; Nanri, A.; Mizoue, T.; Hoang, N.P.; Cuong, V.C.; et al. Primary Drug-Resistant Tuberculosis in Hanoi, Viet Nam: Present Status and Risk Factors. PLoS ONE 2013, 8, e71867. [Google Scholar] [CrossRef]
- Andries, K.; Verhasselt, P.; Guillemont, J.; Göhlmann, H.W.H.; Neefs, J.-M.; Winkler, H.; Van Gestel, J.; Timmerman, P.; Zhu, M.; Lee, E.; et al. A Diarylquinoline Drug Active on the ATP Synthase of Mycobacterium tuberculosis. Science 2005, 307, 223–227. [Google Scholar] [CrossRef]
- Matsumoto, M.; Hashizume, H.; Tomishige, T.; Kawasaki, M.; Tsubouchi, H.; Sasaki, H.; Shimokawa, Y.; Komatsu, M. OPC-67683, a Nitro-Dihydro-Imidazooxazole Derivative with Promising Action against Tuberculosis in Vitro and in Mice. PLoS Med. 2006, 3, e466. [Google Scholar] [CrossRef]
- Diacon, A.H.; Dawson, R.; Hanekom, M.; Narunsky, K.; Venter, A.; Hittel, N.; Geiter, L.J.; Wells, C.D.; Paccaly, A.J.; Donald, P.R. Early Bactericidal Activity of Delamanid (OPC-67683) in Smear-Positive Pulmonary Tuberculosis Patients. Int. J. Tuberc. Lung Dis. Off. J. Int. Union Tuberc. Lung Dis. 2011, 15, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Gler, M.T.; Skripconoka, V.; Sanchez-Garavito, E.; Xiao, H.; Cabrera-Rivero, J.L.; Vargas-Vasquez, D.E.; Gao, M.; Awad, M.; Park, S.-K.; Shim, T.S.; et al. Delamanid for Multidrug-Resistant Pulmonary Tuberculosis. N. Engl. J. Med. 2012, 366, 2151–2160. [Google Scholar] [CrossRef]
- Conradie, F.; Diacon, A.H.; Ngubane, N.; Howell, P.; Everitt, D.; Crook, A.M.; Mendel, C.M.; Egizi, E.; Moreira, J.; Timm, J.; et al. Nix-TB Trial Team. Treatment of Highly Drug-Resistant Pulmonary Tuberculosis. N. Engl. J. Med. 2020, 382, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Pipeline|Working Group for New TB Drugs. Available online: https://www.newtbdrugs.org/pipeline/clinical (accessed on 3 August 2023).






| Year | The Name of Contributor | The Discovery Achieved |
|---|---|---|
| 2400 BC | Egyptian mummies with spinal deformities typical of TB | |
| 400–300 BC | Hippocrates | the clinical presentation of brain tuberculosis |
| 1679 | Franciscus Sylvius (1614–1672) | uses the term tubercles |
| 1689 | Richard Morton (1637–1698) | Phthisiologia |
| 1768 | Robert Whytt (1714–1766) | describes tuberculous meningitis for the first time |
| 1779 | Percival Pott (1714–1788) | Pott’s disease of the spine |
| 1819 | Rene T H Laënnec (1781–1823) | describes the tubercles, invents the stethoscope |
| 1827 | Louis Benoît Guersant (1777–1848) | describes meningitis granuleuse |
| 1834 | Johann Lukas Schonlein (1793–1864 | introduces the term tuberculosis (tuberkulose) |
| 1882 | Robert Koch (1843–1910) | discovers tubercle bacillus, the pathogen that causes TB |
| 1889 | Walter Essex Wynter (1860–1945) | describes the cannulation technique in patients with tuberculous meningitis |
| 1890 | Friedr Neelson (1854–1898) Franz Ziehl (1859–1926) | Ziehl–Neelsen acid-fast staining |
| 1891 | Heinrich Quinke (1842–1922) | lumbar puncture |
| 1895 | Wilhelm Conrad Röentgen (1845–1923) | discovers X-rays |
| 1896 | Adam S | reports the first tubercular abscess of the brain |
| 1943 | Selman Waxman (1886–1973) | discovers streptomycin (SM) |
| 1944 | Jörgen Erik Lehman (1898–1989) | discovers the para-amino salt of salicylic acid (PAS) |
| 1948 | BMRC *, first randomized trial: SM versus PAS versus SM/PAS | |
| 1962 | Georges Canetti (1911–1971) | proposes the principle of two-phase chemotherapy |
| 1965 | Denis Anthony Mitchison (1919–2018) | recommends initial therapy with a combination of three drugs |
| 2005 | Andries et al. | discovers bedaquiline |
| 2013 | WHO | Monoresistance—resistance to one first-line anti-TB drug alone Polydrug resistance—resistance to more than one first-line anti-TB drug other than both isoniazid and rifampicin Multidrug resistance (MDR)—resistance to at least both isoniazid and rifampicin Rifampicin resistance (RR) Extensive drug resistance (XDR)—resistance to any fluoroquinolone and at least one of three second-line injectable drugs |
| 2013 | European Medicines Agency approves the use of delamanid | |
| 2019 | The first 6-month regimen was approved for the treatment of MDR and XDR TB: bedaquiline, pretomanid and linezolid | |
| Present | Working Group on New TB Drugs | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patrascu, R.E.; Cucu, A.I.; Costea, C.F.; Cosman, M.; Blaj, L.A.; Hristea, A. Brain Tuberculosis: An Odyssey through Time to Understand This Pathology. Pathogens 2023, 12, 1026. https://doi.org/10.3390/pathogens12081026
Patrascu RE, Cucu AI, Costea CF, Cosman M, Blaj LA, Hristea A. Brain Tuberculosis: An Odyssey through Time to Understand This Pathology. Pathogens. 2023; 12(8):1026. https://doi.org/10.3390/pathogens12081026
Chicago/Turabian StylePatrascu, Raluca Elena, Andrei Ionut Cucu, Claudia Florida Costea, Mihaela Cosman, Laurentiu Andrei Blaj, and Adriana Hristea. 2023. "Brain Tuberculosis: An Odyssey through Time to Understand This Pathology" Pathogens 12, no. 8: 1026. https://doi.org/10.3390/pathogens12081026
APA StylePatrascu, R. E., Cucu, A. I., Costea, C. F., Cosman, M., Blaj, L. A., & Hristea, A. (2023). Brain Tuberculosis: An Odyssey through Time to Understand This Pathology. Pathogens, 12(8), 1026. https://doi.org/10.3390/pathogens12081026





