Spotted Fever Group Rickettsiae in Ticks and Small Mammals from Grassland and Forest Habitats in Central Germany
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites, Sample Collection, and DNA Extraction
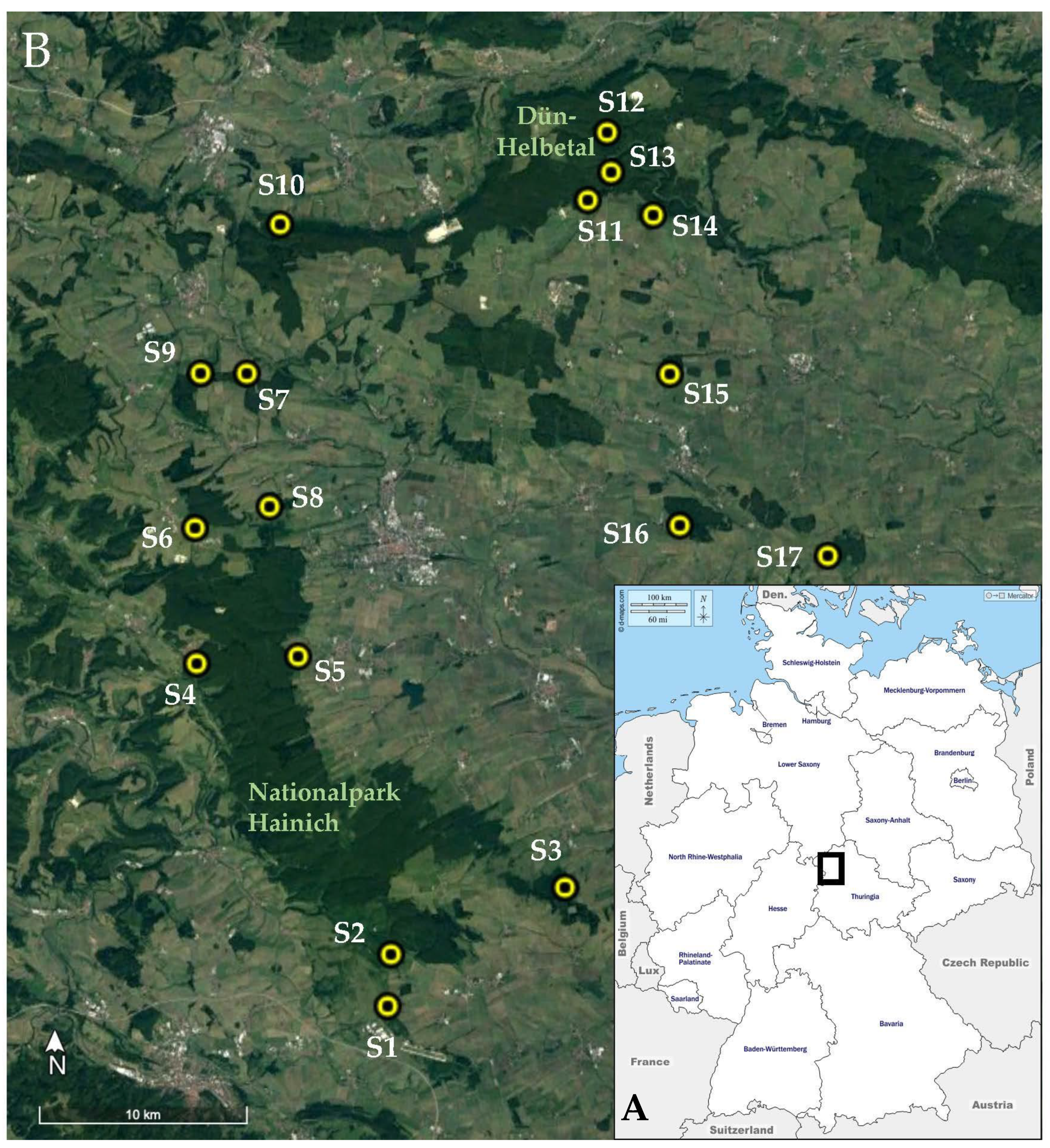
2.1.1. Study Sites
2.1.2. Tick Collection
2.1.3. Small Mammal Collection
2.2. Real-Time PCR, Conventional PCR and Sequencing
2.3. Statistical Analysis
2.4. Co-Infections of Borrelia spp. and Rickettsia spp. in Ticks and Small Mammals
3. Results
3.1. Rickettsia spp. Detection in Ticks
3.2. Rickettsia spp. Detection in Small Mammals
3.3. Co-Infection with Rickettsia spp. and Borrelia spp. in Ticks and Small Mammals
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Absolute Number of Ticks (Percentage in Each Season) | ||||
|---|---|---|---|---|
| Tick Species | Spring | Summer | Autumn | Total |
| Ixodes ricinus complex 1 | 789 (77.2%) | 203 (19.9%) | 30 (2.9%) | 1022 (100%) |
| Dermacentor reticulatus | 37 (39.8%) | 1 (1.1%) | 55 (59.1%) | 93 (100%) |
| Total | 826 (74.1%) | 204 (18.3%) | 85 (7.6%) | 1115 (100%) |
| Factor | Estimate | Standard Error | Z Value | Probability (>|z|) |
|---|---|---|---|---|
| Total | ||||
| Intercept | −2.64 | 0.66 | −4.03 | 5.67 × 10−05 *** |
| D. reticulatus vs. I. ricinus complex | −3.96 | 0.64 | −6.17 | 6.69 × 10−10 *** |
| Adult vs. Nymph | −0.63 | 0.27 | −2.33 | 0.02 * |
| Ecotone vs. Forest | −0.10 | 0.26 | −0.37 | 0.71 |
| Autumn vs. Spring | 4.75 | 0.80 | 5.93 | 3.05 × 10−09 *** |
| Autumn vs. Summer | 4.79 | 0.86 | 5.57 | 2.57 × 10−08 *** |
| Ixodes ricinus | ||||
| Intercept | −1.74 | 0.79 | −2.19 | 0.03 * |
| Adult vs. Nymph | −0.79 | 0.27 | −2.79 | 0.01 ** |
| Ecotone vs. Forest | −0.15 | 0.28 | −0.53 | 0.59 |
| Autumn vs. Spring | −0.18 | 0.77 | −0.24 | 0.81 |
| Autumn vs. Summer | 0.23 | 0.78 | 0.29 | 0.77 |
| Factor | Estimate | Standard Error | Z Value | Probability (>|z|) |
|---|---|---|---|---|
| Total | ||||
| Intercept | −4.09 | 0.66 | −6.20 | 5.78 × 10−10 *** |
| Grassland vs. Forest | 1.83 | 0.40 | 4.56 | 5.22 × 10−06 *** |
| Spring vs. summer | 0.55 | 0.34 | 1.62 | 0.11 |
| A. agrarius vs. A. flavicollis | 0.00 | 0.46 | 0.01 | 1.00 |
| A. agrarius vs. A. sylvaticus | 0.70 | 0.50 | 1.38 | 0.17 |
| A. agrarius vs. M. arvalis | 0.44 | 0.53 | 0.83 | 0.41 |
| A. agrarius vs. Cl. glareolus | −1.046 | 0.49 | −2.12 | 0.03 * |
| Apodemus flavicollis | ||||
| Intercept | −17.73 | 223.46 | −0.08 | 0.94 |
| Female vs. Male | −0.10 | 0.63 | −0.17 | 0.87 |
| Grassland vs. Forest | 16.58 | 223.46 | 0.07 | 0.94 |
| Spring vs. Summer | −0.37 | 0.58 | −0.64 | 0.52 |
| Clethrionomys glareolus | ||||
| Intercept | −1.32 | 1.70 | −0.78 | 0.44 |
| Female vs. Male | −0.42 | 1.00 | −0.42 | 0.67 |
| Grassland vs. Forest | −0.66 | 1.42 | −0.47 | 0.64 |
| Spring vs. Summer | −0.17 | 0.75 | −0.23 | 0.82 |
| Microtus arvalis | ||||
| Intercept | −5.20 | 1.30 | −3.98 | 6.8 × 10−05 *** |
| Female vs. Male | 1.06 | 0.86 | 1.23 | 0.22 |
| Grassland vs. Forest | 1.97 | 0.60 | 3.31 | 0.000948 *** |
| Spring vs. Summer | 1.28 | 1.09 | 1.18 | 0.24 |
| Species No. of Co-Infected Ticks/Total (%) | Year No. of Co-Infected Ticks/Total (%) | Season No. of Co-Infected Ticks/Total (%) | Habitat No. of Co-Infected Ticks/Total (%) | Borrelia spp. | Rickettsia spp. |
|---|---|---|---|---|---|
| I. ricinus complex 9/1094 (0.8) | 2018 5/565 (0.9) | spring 5/436 (1.1) | ecotone 3/201 (1.5) | B. valaisiana | R. helvetica |
| B. afzelii | |||||
| Borrelia spp. | |||||
| forest 2/235 (0.9) | B. valaisiana | ||||
| B. afzelii | |||||
| 2019 4/529 (0.8) | spring 3/386 (0.8) | ecotone 1/101 (1.0) | Borrelia spp. | ||
| forest 2/285 (0.7) | |||||
| summer 1/141 (0.7) | forest 1/22 (4.5) |
| No. of Co-Infected Animals/Total (%) | Year No. of Co-Infected Animals/Total (%) | Season No. of Co-Infected Animals/Total (%) | Habitat No. of Co-Infected Animals/Total (%) | Species No. of Co-Infected Animals/Total (%) | Borrelia spp. | Rickettsia spp. |
|---|---|---|---|---|---|---|
| 9/1167 (0.8) | 2017 1/290 (0.3) | Summer 1/290 (0.3) | Forest 1/152 (0.7) | A. flavicollis 1/72 (1.4) | Borrelia spp. | Rickettsia spp. |
| 2018 6/94 (6.4) | Spring 1/23 (4.3) | Grassland 1/12 (8.3) | M. arvalis 1/12 (8.3) | |||
| Summer 5/71 (7.0) | Grassland 1/40 (2.5) | M. arvalis 1/33 (3.0) | ||||
| Forest 4/31 (12.9) | S. araneus 2/7 (28.6) | |||||
| Cl. glareolus 2/12 (16.7) | ||||||
| 2019 2/783 (0.3) | Summer 2/638 (0.3) | Forest 2/372 (0.5) | Cl. glareolus 1/172 (0.6) | |||
| M. arvalis 1/19 (5.3) |
| Class | Season | Alpha MLE | Blaker CI (Lower) | Blaker CI (Upper) | p-Value |
|---|---|---|---|---|---|
| Arachnida (ticks) | Spring | −0.72 | −1.60 | 0.00 | 0.06 |
| Summer | −0.54 | −3.62 | 1.39 | 1.00 | |
| Autumn | ND 1 | ND 1 | ND 1 | ND 1 | |
| Mammalia (Small mammals) | Spring | 0.09 | −3.05 | 2.05 | 1.00 |
| Summer | 0.32 | −0.55 | 1.11 | 0.51 |
References
- Petney, T.N.; Pfäffle, M.P.; Skuballa, J.D. An annotated checklist of the ticks (Acari: Ixodida) of Germany. Syst. Appl. Acarol. 2012, 17, 115–170. [Google Scholar] [CrossRef]
- Gray, J.; Kahl, O.; Zintl, A. What do we still need to know about Ixodes ricinus? Ticks Tick-Borne Dis. 2021, 12, 101682. [Google Scholar] [CrossRef] [PubMed]
- Stanko, M.; Derdáková, M.; Špitalská, E.; Kazimírová, M. Ticks and their epidemiological role in Slovakia: From the past till present. Biologia 2021, 77, 1575–1610. [Google Scholar] [CrossRef] [PubMed]
- Medlock, J.M.; Hansford, K.M.; Bormane, A.; Derdakova, M.; Estrada-Peña, A.; George, J.-C.; Golovljova, I.; Jaenson, T.G.T.; Jensen, J.-K.; Jensen, P.M.; et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasites Vectors 2013, 6, 1. [Google Scholar] [CrossRef]
- Drehmann, M.; Springer, A.; Lindau, A.; Fachet, K.; Mai, S.; Thoma, D.; Schneider, C.R.; Chitimia-Dobler, L.; Bröker, M.; Dobler, G.; et al. The Spatial Distribution of Dermacentor Ticks (Ixodidae) in Germany—Evidence of a Continuing Spread of Dermacentor reticulatus. Front. Veter-Sci. 2020, 7, 578220. [Google Scholar] [CrossRef]
- Burri, C.; Schumann, O.; Schumann, C.; Gern, L. Are Apodemus spp. mice and Myodes glareolus reservoirs for Borrelia miyamotoi, Candidatus Neoehrlichia mikurensis, Rickettsia helvetica, R. monacensis and Anaplasma phagocytophilum? Ticks Tick-Borne Dis. 2014, 5, 245–251. [Google Scholar] [CrossRef]
- Kraft, R. Mäuse und Spitzmäuse in Bayern Verbreitung, Lebensraum, Bestandssituation; Ulmer: Stuttgart, Germany, 2008. [Google Scholar]
- Krawczyk, A.I.; van Duijvendijk, G.L.A.; Swart, A.; Heylen, D.; Jaarsma, R.I.; Jacobs, F.H.H.; Fonville, M.; Sprong, H.; Takken, W. Effect of rodent density on tick and tick-borne pathogen populations: Consequences for infectious disease risk. Parasites Vectors 2020, 13, 34. [Google Scholar] [CrossRef]
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, M.Y.; Stenos, J.; Bitam, I.; Fournier, P.-E.; et al. Update on Tick-Borne Rickettsioses around the World: A Geographic Approach. Clin. Microbiol. Rev. 2013, 26, 657–702. [Google Scholar] [CrossRef]
- Kim, H.K. Rickettsia-Host-Tick Interactions: Knowledge Advances and Gaps. Infect. Immun. 2022, 90, e00621. [Google Scholar] [CrossRef]
- Merhej, V.; Raoult, D. Rickettsial evolution in the light of comparative genomics. Biol. Rev. Camb. Philos. Soc. 2010, 86, 379–405. [Google Scholar] [CrossRef]
- Hauck, D.; Jordan, D.; Springer, A.; Schunack, B.; Pachnicke, S.; Fingerle, V.; Strube, C. Transovarial transmission of Borrelia spp., Rickettsia spp. and Anaplasma phagocytophilum in Ixodes ricinus under field conditions extrapolated from DNA detection in questing larvae. Parasites Vectors 2020, 13, 176. [Google Scholar] [CrossRef]
- Raoult, D.; Roux, V. Rickettsioses as paradigms of new or emerging infectious diseases. Clin. Microbiol. Rev. 1997, 10, 694–719. [Google Scholar] [CrossRef] [PubMed]
- Socolovschi, C.; Mediannikov, O.; Raoult, D.; Parola, P. The relationship between spotted fever group Rickettsiae and Ixodid ticks. Veter-Res. 2009, 40, 34. [Google Scholar] [CrossRef] [PubMed]
- Karbowiak, G.; Biernat, B.; Stańczak, J.; Szewczyk, T.; Werszko, J. The role of particular tick developmental stages in the circulation of tick-borne pathogens affecting humans in Central Europe. 3. Rickettsiae. Ann. Parasitol. 2016, 62, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Sprong, H.; Wielinga, P.R.; Fonville, M.; Reusken, C.; Brandenburg, A.H.; Borgsteede, F.; Gaasenbeek, C.; van der Giessen, J.W. Ixodes ricinus ticks are reservoir hosts for Rickettsia helvetica and potentially carry flea-borne Rickettsia species. Parasites Vectors 2009, 2, 41. [Google Scholar] [CrossRef] [PubMed]
- Galfsky, D.; Król, N.; Pfeffer, M.; Obiegala, A. Long-term trends of tick-borne pathogens in regard to small mammal and tick populations from Saxony, Germany. Parasites Vectors 2019, 12, 131. [Google Scholar] [CrossRef]
- Wölfel, R.; Terzioglu, R.; Kiessling, J.; Wilhelm, S.; Essbauer, S.; Pfeffer, M.; Dobler, G. Rickettsia spp. in Ixodes ricinus Ticks in Bavaria, Germany. Ann. N. Y. Acad. Sci. 2006, 1078, 509–511. [Google Scholar] [CrossRef]
- May, K.; Strube, C. Prevalence of Rickettsiales (Anaplasma phagocytophilum and Rickettsia spp.) in hard ticks (Ixodes ricinus) in the city of Hamburg, Germany. Parasitol. Res. 2014, 113, 2169–2175. [Google Scholar] [CrossRef]
- Obiegala, A.; Oltersdorf, C.; Silaghi, C.; Kiefer, D.; Kiefer, M.; Woll, D.; Pfeffer, M. Rickettsia spp. in small mammals and their parasitizing ectoparasites from Saxony, Germany. Veter-Parasitol. Reg. Stud. Rep. 2016, 5, 19–24. [Google Scholar] [CrossRef]
- Tappe, J.; Strube, C. Anaplasma phagocytophilum and Rickettsia spp. infections in hard ticks (Ixodes ricinus) in the city of Hanover (Germany): Revisited. Ticks Tick-Borne Dis. 2013, 4, 432–438. [Google Scholar] [CrossRef]
- Azagi, T.; Hoornstra, D.; Kremer, K.; Hovius, J.W.R.; Sprong, H. Evaluation of Disease Causality of Rare Ixodes ricinus-Borne Infections in Europe. Pathogens 2020, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, R.; dos Santos, M.L.; Cruz, C.; Almeida, V.; Garrote, A.R.; Ramirez, F.; Seixas, D.; Manata, M.J.; Maltez, F. Rare Case of Rickettsiosis Caused by Rickettsia monacensis, Portugal, 2021. Emerg. Infect. Dis. 2022, 28, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Buczek, W.; Koman-Iżko, A.; Buczek, A.; Bartosik, K.; Kulina, D.; Ciura, D. Spotted fever group rickettsiae transmitted by Dermacentor ticks and determinants of their spread in Europe. Ann. Agric. Environ. Med. 2020, 27, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Rovery, C.; Rolain, J.M.; Brouqui, P.; Davoust, B.; Raoult, D. Rickettsia slovaca and R. raoultii in Tick-borne Rickettsioses. Emerg. Infect. Dis. 2009, 15, 1105–1108. [Google Scholar] [CrossRef]
- Rieg, S.; Schmoldt, S.; Theilacker, C.; de With, K.; Wölfel, S.; Kern, W.V.; Dobler, G. Tick-borne lymphadenopathy (TIBOLA) acquired in Southwestern Germany. BMC Infect. Dis. 2011, 11, 167. [Google Scholar] [CrossRef]
- Špitalská, E.; Štefanidesová, K.; Kocianová, E.; Boldiš, V. Rickettsia slovaca and Rickettsia raoultii in Dermacentor marginatus and Dermacentor reticulatus ticks from Slovak Republic. Exp. Appl. Acarol. 2012, 57, 189–197. [Google Scholar] [CrossRef]
- Rizzoli, A.; Hauffe, H.C.; Carpi, G.; Vourc’h, G.I.; Neteler, M.; Rosà, R. Lyme borreliosis in Europe. Eurosurveillance 2011, 16, 19906. [Google Scholar] [CrossRef]
- Reye, A.L.; Hübschen, J.M.; Sausy, A.; Muller, C.P. Prevalence and Seasonality of Tick-Borne Pathogens in Questing Ixodes ricinus Ticks from Luxembourg. Appl. Environ. Microbiol. 2010, 76, 2923–2931. [Google Scholar] [CrossRef]
- Špitalská, E.; Boldiš, V.; Derdáková, M.; Selyemová, D.; Tarageľová, V.R. Rickettsial infection in Ixodes ricinus ticks in urban and natural habitats of Slovakia. Ticks Tick-Borne Dis. 2014, 5, 161–165. [Google Scholar] [CrossRef]
- Knoll, S.; Springer, A.; Hauck, D.; Schunack, B.; Pachnicke, S.; Strube, C. Regional, seasonal, biennial and landscape-associated distribution of Anaplasma phagocytophilum and Rickettsia spp. infections in Ixodes ticks in northern Germany and implications for risk assessment at larger spatial scales. Ticks Tick-Borne Dis. 2021, 12, 101657. [Google Scholar] [CrossRef]
- Król, N.; Obiegala, A.; Imholt, C.; Arz, C.; Schmidt, E.; Jeske, K.; Ulrich, R.G.; Rentería-Solís, Z.; Jacob, J.; Pfeffer, M. Diversity of Borrelia burgdorferi sensu lato in ticks and small mammals from different habitats. Parasites Vectors 2022, 15, 195. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Mihalca, A.D.; Petney, T.N. Ticks of Europe and North Africa: A guide to species identification; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Siuda, K. Kleszcze Polski (Acari: Ixodida): Systematyka i Rozmieszczenie; Polskie Towarzystwo Parazytologiczne: Warszawa, Poland, 1993. [Google Scholar]
- Estrada-Peña, A.; Nava, S.; Petney, T. Description of all the stages of Ixodes inopinatus n. sp. (Acari: Ixodidae). Ticks Tick-Borne Dis. 2014, 5, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Jeske, K.; Weber, S.; Pfaff, F.; Imholt, C.; Jacob, J.; Beer, M.; Ulrich, R.G.; Hoffmann, D. Molecular Detection and Characterization of the First Cowpox Virus Isolate Derived from a Bank Vole. Viruses 2019, 11, 1075. [Google Scholar] [CrossRef] [PubMed]
- Jeske, K.; Jacob, J.; Drewes, S.; Pfeffer, M.; Heckel, G.; Ulrich, R.G.; Imholt, C. Hantavirus–Leptospira coinfections in small mammals from central Germany. Epidemiol. Infect. 2021, 149, e97. [Google Scholar] [CrossRef] [PubMed]
- Wölfel, R.; Essbauer, S.; Dobler, G. Diagnostics of tick-borne rickettsioses in Germany: A modern concept for a neglected disease. Int. J. Med. Microbiol. 2008, 298, 368–374. [Google Scholar] [CrossRef]
- Roux, V.; Raoult, D. Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (ompB). Int. J. Syst. Evol. Microbiol. 2000, 50 Pt 4, 1449–1455. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- Mainali, K.P.; Slud, E.; Singer, M.C.; Fagan, W.F. A better index for analysis of co-occurrence and similarity. Sci. Adv. 2022, 8, eabj9204. [Google Scholar] [CrossRef]
- Mainali, K.P.; Slud, E.; Mainali, M.K. Package ‘CooccurrenceAffinity’. Available online: https://cran.r-project.org/web//packages/CooccurrenceAffinity/CooccurrenceAffinity.pdf (accessed on 30 June 2023).
- Speck, S.; Kern, T.; Aistleitner, K.; Dilcher, M.; Dobler, G.; Essbauer, S. In vitro studies of Rickettsia-host cell interactions: Confocal laser scanning microscopy of Rickettsia helvetica-infected eukaryotic cell lines. PLoS Neglected Trop. Dis. 2018, 12, e0006151. [Google Scholar] [CrossRef]
- Kartashov, M.Y.; Glushkova, L.I.; Mikryukova, T.P.; Korabelnikov, I.V.; Egorova, Y.I.; Tupota, N.L.; Protopopova, E.V.; Konovalova, S.N.; Ternovoi, V.A.; Loktev, V.B. Detection of Rickettsia helvetica and Candidatus R. tarasevichiae DNA in Ixodes persulcatus ticks collected in Northeastern European Russia (Komi Republic). Ticks Tick-Borne Dis. 2017, 8, 588–592. [Google Scholar] [CrossRef]
- Rubel, F.; Brugger, K.; Chitimia-Dobler, L.; Dautel, H.; Meyer-Kayser, E.; Kahl, O. Atlas of ticks (Acari: Argasidae, Ixodidae) in Germany. Exp. Appl. Acarol. 2021, 84, 183–214. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Mahling, M.; Pfister, K. Abundance and seasonal activity of questing Ixodes ricinus ticks in their natural habitats in southern Germany in 2011. J. Vector Ecol. 2014, 39, 56–65. [Google Scholar] [CrossRef]
- Černý, J.; Lynn, G.; Hrnková, J.; Golovchenko, M.; Rudenko, N.; Grubhoffer, L. Management Options for Ixodes ricinus-Associated Pathogens: A Review of Prevention Strategies. Int. J. Environ. Res. Public Health 2020, 17, 1830. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Chmura, M.; Siuda, K. Ticks of Poland. Review of contemporary issues and latest research. Ann. Parasitol. 2012, 58, 125–155. [Google Scholar] [PubMed]
- Kohn, M.; Krücken, J.; McKay-Demeler, J.; Pachnicke, S.; Krieger, K.; von Samson-Himmelstjerna, G. Dermacentor reticulatus in Berlin/Brandenburg (Germany): Activity patterns and associated pathogens. Ticks Tick-Borne Dis. 2018, 10, 191–206. [Google Scholar] [CrossRef]
- Von Blanckenhagen, F.; Städtler, T. Small mammal communities in agricultural landscapes in Germany: Review of field data over the last decade. Julius-Kühn-Archiv 2011, 432, 75. [Google Scholar] [CrossRef]
- Hotopp, I.; Walther, B.; Fuelling, O.; Reil, D.; Hesse, C.; Below, D.A.; Imholt, C.; Jacob, J. Habitat and Season Effects on Small Mammal Bycatch in Live Trapping. Biology 2022, 11, 1806. [Google Scholar] [CrossRef]
- Boyard, C.; Vourc’h, G.; Barnouin, J. The relationships between Ixodes ricinus and small mammal species at the woodland–pasture interface. Exp. Appl. Acarol. 2008, 44, 61–76. [Google Scholar] [CrossRef]
- Hauer, S.; Ansorge, H.; Zöphel, U. Atlas der Säugetiere Sachsens: Naturschutz und Landschaftspflege; Zentraler Broschürenversand der Sächsischen Staatsregierung; Sächsisches Landesamt für Umwelt, Landschaft und Geologie: Dresden, Germany, 2009.
- Dobler, G.; Wölfel, R. Typhus and Other Rickettsioses: Emerging infections in Germany. Dtsch Arztebl. Int. 2009, 106, 348–354. [Google Scholar] [CrossRef]
- Nilsson, K. Septicaemia with Rickettsia helvetica in a patient with acute febrile illness, rash and myasthenia. J. Infect. 2009, 58, 79–82. [Google Scholar] [CrossRef]
- Hildebrand, J.; Perec-Matysiak, A.; Popiołek, M.; Merta, D.; Myśliwy, I.; Buńkowska-Gawlik, K. A molecular survey of spotted fever group rickettsiae in introduced raccoons (Procyon lotor). Parasites Vectors 2022, 15, 162. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Roldan, J.A.; Manoj, R.R.S.; Latrofa, M.S.; Iatta, R.; Annoscia, G.; Lovreglio, P.; Stufano, A.; Dantas-Torres, F.; Davoust, B.; Laidoudi, Y.; et al. Role of reptiles and associated arthropods in the epidemiology of rickettsioses: A one health paradigm. PLOS Neglected Trop. Dis. 2021, 15, e0009090. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Spierling, N.G.; Heuser, E.; Kling, C.; Schmidt, S.; Rosenfeld, U.M.; Reil, D.; Imholt, C.; Jacob, J.; Ulrich, R.G.; et al. High prevalence of Rickettsia helvetica in wild small mammal populations in Germany. Ticks Tick-Borne Dis. 2018, 9, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Mysterud, A.; Byrkjeland, R.; Qviller, L.; Viljugrein, H. The generalist tick Ixodes ricinus and the specialist tick Ixodes trianguliceps on shrews and rodents in a northern forest ecosystem– a role of body size even among small hosts. Parasites Vectors 2015, 8, 639. [Google Scholar] [CrossRef]
- Mardosaitė-Busaitienė, D.; Radzijevskaja, J.; Balčiauskas, L.; Paulauskas, A. First detection of Rickettsia helvetica in small mammals in Lithuania. New Microbes New Infect. 2018, 22, 19–23. [Google Scholar] [CrossRef]
- Schex, S.; Dobler, G.; Riehm, J.; Müller, J.; Sormunen, J.J.; Penttinen, R.; Klemola, T.; Hänninen, J.; Vuorinen, I.; Laaksonen, M.; et al. Rickettsia spp. in Wild Small Mammals in Lower Bavaria, South-Eastern Germany. Vector-Borne Zoonotic Dis. 2011, 11, 493–502. [Google Scholar] [CrossRef]
- Obiegala, A.; Król, N.; Oltersdorf, C.; Nader, J.; Pfeffer, M. The enzootic life-cycle of Borrelia burgdorferi (sensu lato) and tick-borne rickettsiae: An epidemiological study on wild-living small mammals and their ticks from Saxony, Germany. Parasites Vectors 2017, 10, 115. [Google Scholar] [CrossRef]
- Stańczak, J.; Racewicz, M.; Michalik, J.; Cieniuch, S.; Sikora, B.; Skoracki, M. Prevalence of infection with Rickettsia helvetica in feeding ticks and their hosts in western Poland. Clin. Microbiol. Infect. 2009, 15 (Suppl. 2), 328–329. [Google Scholar] [CrossRef]
- Svoboda, P.; Dobler, G.; Markotić, A.; Kurolt, I.-C.; Speck, S.; Habuš, J.; Vucelja, M.; Krajinović, L.C.; Tadin, A.; Margaletić, J.; et al. Survey for Hantaviruses, Tick-Borne Encephalitis Virus, and Rickettsia spp. in Small Rodents in Croatia. Vector-Borne Zoonotic Dis. 2014, 14, 523–530. [Google Scholar] [CrossRef]
- Miťková, K.; Berthová, L.; Kalúz, S.; Kazimírová, M.; Burdová, L.; Kocianová, E. First detections of Rickettsia helvetica and R. monacensis in ectoparasitic mites (Laelapidae and Trombiculidae) infesting rodents in south-western Slovakia. Parasitol. Res. 2015, 114, 2465–2472. [Google Scholar] [CrossRef]
- Martello, E.; Mannelli, A.; Grego, E.; Ceballos, L.A.; Ragagli, C.; Stella, M.C.; Tomassone, L. Borrelia burgdorferi sensu lato and spotted fever group rickettsiae in small rodents and attached ticks in the Northern Apennines, Italy. Ticks Tick-Borne Dis. 2019, 10, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Pluta, S.; Hartelt, K.; Oehme, R.; Mackenstedt, U.; Kimmig, P. Prevalence of Coxiella burnetii and Rickettsia spp. in ticks and rodents in southern Germany. Ticks Tick-Borne Dis. 2010, 1, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.-C.; Hu, Y.; Wu, T.-T.; Ma, X.-X.; Jiang, B.-G.; Jia, N.; Wang, A.-Q.; Jiang, J.-F. The Role of Ranged Horses in Eco-Epidemiology of Rickettsia raoultii Infection in China. Front. Microbiol. 2022, 12, 795500. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhao, S.; Tan, W.; Hornok, S.; Yuan, W.; Mi, L.; Wang, S.; Liu, Z.; Zhang, Y.; Hazihan, W.; et al. Rickettsiae in red fox (Vulpes vulpes), marbled polecat (Vormela peregusna) and their ticks in northwestern China. Parasites Vectors 2021, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Zeroual, F.; Leulmi, H.; Bitam, I.; Benakhla, A. Molecular evidence of Rickettsia slovaca in spleen of wild boars in northeastern Algeria. New Microbes New Infect. 2018, 24, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Stańczak, J.; Biernat, B.; Racewicz, M.; Zalewska, M.; Matyjasek, A. Prevalence of different Rickettsia spp. in Ixodes ricinus and Dermacentor reticulatus ticks (Acari: Ixodidae) in north-eastern Poland. Ticks Tick-Borne Dis. 2017, 9, 427–434. [Google Scholar] [CrossRef]
- Schorn, S.; Pfister, K.; Reulen, H.; Mahling, M.; Silaghi, C. Occurrence of Babesia spp., Rickettsia spp. and Bartonella spp. in Ixodes ricinus in Bavarian public parks, Germany. Parasites Vectors 2011, 4, 135. [Google Scholar] [CrossRef]
- Silaghi, C.; Gilles, J.; Höhle, M.; Pradel, I.; Just, F.T.; Fingerle, V.; Küchenhoff, H.; Pfister, K. Prevalence of spotted fever group rickettsiae in Ixodes ricinus (Acari: Ixodidae) in southern Germany. J. Med. Entomol. 2008, 45, 948–955. [Google Scholar] [CrossRef]
- Samoylenko, I.; Shpynov, S.; Raoult, D.; Rudakov, N.; Fournier, P.-E. Evaluation of Dermacentor species naturally infected with Rickettsia raoultii. Clin. Microbiol. Infect. 2009, 15, 305–306. [Google Scholar] [CrossRef]
- Hildebrandt, A.; Krämer, A.; Sachse, S.; Straube, E. Detection of Rickettsia spp. and Anaplasma phagocytophilum in Ixodes ricinus ticks in a region of Middle Germany (Thuringia). Ticks Tick-Borne Dis. 2010, 1, 52–56. [Google Scholar] [CrossRef]
- Blazejak, K.; Janecek, E.; Strube, C. A 10-year surveillance of Rickettsiales (Rickettsia spp. and Anaplasma phagocytophilum) in the city of Hanover, Germany, reveals Rickettsia spp. as emerging pathogens in ticks. Parasites Vectors 2017, 10, 588. [Google Scholar] [CrossRef] [PubMed]
- Kantsø, B.; Svendsen, C.B.; Jensen, P.M.; Vennestrøm, J.; Krogfelt, K.A. Seasonal and habitat variation in the prevalence of Rickettsia helvetica in Ixodes ricinus ticks from Denmark. Ticks Tick-Borne Dis. 2010, 1, 101–103. [Google Scholar] [CrossRef]
- Raulf, M.-K.; Jordan, D.; Fingerle, V.; Strube, C. Association of Borrelia and Rickettsia spp. and bacterial loads in Ixodes ricinus ticks. Ticks Tick-Borne Dis. 2018, 9, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Knoll, S.; Springer, A.; Hauck, D.; Schunack, B.; Pachnicke, S.; Fingerle, V.; Strube, C. Distribution of Borrelia burgdorferi s.l. and Borrelia miyamotoi in Ixodes tick populations in Northern Germany, co-infections with Rickettsiales and assessment of potential influencing factors. Med. Veter-Èntomol. 2021, 35, 595–606. [Google Scholar] [CrossRef]
- Blaker, H. Confidence curves and improved exact confidence intervals for discrete distributions. Can. J. Stat. 2000, 28, 783–798. [Google Scholar] [CrossRef]
| Tick Species | No. of Individuals in Ecotone Positive/Total (%) | No. of Individuals in Forest Positive/Total (%) | Total No. of Positive Individuals/Total (%) |
|---|---|---|---|
| Ixodes ricinus complex 1 | 28/304 (9.2) | 60/714 (8.4) | 88/1018 (8.6) |
| Dermacentor reticulatus | 34/74 (45.3) | 3/6 (50.0) | 37/80 (46.3) |
| Total | 62/378 (16.4) | 63/720 (8.8) | 125/1098 (11.4) |
| Small Mammal Species | No. of Individuals in Grassland Positive/Total (%) | No. of Individuals in Forest Positive/Total (%) | Total No. of Positive Individuals/Total (%) |
|---|---|---|---|
| Microtus arvalis | 19/377 (5.0) | 7/30 (23.3) | 26/407 (6.4) |
| Clethrionomys glareolus | 1/8 (12.5) | 18/270 (6.7) | 19/278 (6.8) |
| Apodemus flavicollis | 0/20 (0) | 37/220 (16.8) | 37/240 (15.4) |
| Apodemus sylvaticus | 1/28 (3.6) | 19/80 (23.8) | 20/108 (18.5) |
| Apodemus agrarius | 1/46 (2.2) | 7/44 (15.9) | 8/90 (8.9) |
| Sorex araneus | 0/11 (0) | 2/9 (22.2) | 2/20 (10.0) |
| Microtus agrestis | 0/8 (0) | 2/7 (28.6) | 2/15 (13.3) |
| Arvicola amphibius | 0/3 (0) | 0/1 (0) | 0/4 (0) |
| Sorex minutus | 0/2 (0) | 1/2 (50.0) | 1/4 (25.0) |
| Crocidura russula | 0/1 (0) | 0 | 0/1 (0) |
| Total | 22/504 (4.4) | 93/663 (14.0) | 115/1167 (9.9) |
| Habitat | Host Species | Sex /Life Stage | Rickettsia Species | Study Site 1 | No. of Individuals | Maximal Identity (%) | GenBank ID |
|---|---|---|---|---|---|---|---|
| ecotone | Ixodes ricinus | F | Rickettsia helvetica | S17 | 1 | 100 | MF163037 |
| 1 | 99.9 | ||||||
| S16 | 1 | 100 | |||||
| S7 | 1 | 100 | |||||
| S8 | 1 | 99.9 | |||||
| S12 | 1 | 100 | |||||
| S9 | 1 | 100 | |||||
| S5 | 1 | 100 | |||||
| M | S16 | 1 | 100 | ||||
| S9 | 1 | 100 | |||||
| 1 | 100 | ||||||
| S5 | 1 | 100 | |||||
| N | S10 | 1 | 100 | ||||
| S9 | 1 | 99.9 | |||||
| 1 | 100 | ||||||
| 1 | 99.75 | ||||||
| 1 | 100 | ||||||
| 1 | 100 | ||||||
| S7 | 1 | 99.9 | |||||
| 1 | 100 | ||||||
| S5 | 2 | 100 | |||||
| Dermacentor reticulatus | F | Rickettsia raoultii | S2 | 1 | 99.9 | KU723537 | |
| 5 | 99.9 | MG811717 | |||||
| 3 | 100 | ||||||
| M | 1 | 99.9 | MF002526 | ||||
| forest | I. ricinus | F | R. helvetica | S9 | 1 | 99.8 | MF163037 |
| 1 | 100 | ||||||
| S8 | 1 | 100 | KP866151 | ||||
| S3 | 1 | 99.5 | MF163037 | ||||
| S14 | 1 | 100 | |||||
| M | S9 | 1 | 99.8 | ||||
| 2 | 100 | ||||||
| S3 | 1 | 100 | |||||
| N | S14 | 1 | 100 | ||||
| 1 | 98 | ||||||
| S12 | 1 | 99.9 | |||||
| 1 | 100 | ||||||
| S10 | 1 | 99.8 | |||||
| S9 | 3 | 99.8 | |||||
| 15 | 100 | ||||||
| S7 | 1 | 100 | |||||
| S8 | 3 | 100 | |||||
| S5 | 2 | 100 | |||||
| S3 | 1 | 99.7 | |||||
| 9 | 100 | ||||||
| 1 | 99.9 | ||||||
| S15 | 1 | 100 | |||||
| S13 | 1 | 100 | |||||
| S6 | 1 | 100 | |||||
| D. reticulatus | F | R. raoultii | S2 | 1 | 100 | MF002526 | |
| M | S3 | 1 | 99.9 | MG811717 | |||
| forest | Apodemus flavicollis | na | R. helvetica | S15 | 1 | 100 | MF163037 |
| S13 | 1 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arz, C.; Król, N.; Imholt, C.; Jeske, K.; Rentería-Solís, Z.; Ulrich, R.G.; Jacob, J.; Pfeffer, M.; Obiegala, A. Spotted Fever Group Rickettsiae in Ticks and Small Mammals from Grassland and Forest Habitats in Central Germany. Pathogens 2023, 12, 933. https://doi.org/10.3390/pathogens12070933
Arz C, Król N, Imholt C, Jeske K, Rentería-Solís Z, Ulrich RG, Jacob J, Pfeffer M, Obiegala A. Spotted Fever Group Rickettsiae in Ticks and Small Mammals from Grassland and Forest Habitats in Central Germany. Pathogens. 2023; 12(7):933. https://doi.org/10.3390/pathogens12070933
Chicago/Turabian StyleArz, Charlotte, Nina Król, Christian Imholt, Kathrin Jeske, Zaida Rentería-Solís, Rainer G. Ulrich, Jens Jacob, Martin Pfeffer, and Anna Obiegala. 2023. "Spotted Fever Group Rickettsiae in Ticks and Small Mammals from Grassland and Forest Habitats in Central Germany" Pathogens 12, no. 7: 933. https://doi.org/10.3390/pathogens12070933
APA StyleArz, C., Król, N., Imholt, C., Jeske, K., Rentería-Solís, Z., Ulrich, R. G., Jacob, J., Pfeffer, M., & Obiegala, A. (2023). Spotted Fever Group Rickettsiae in Ticks and Small Mammals from Grassland and Forest Habitats in Central Germany. Pathogens, 12(7), 933. https://doi.org/10.3390/pathogens12070933






