Pulmonary Co-Infections Detected Premortem Underestimate Postmortem Findings in a COVID-19 Autopsy Case Series
Abstract
1. Introduction
2. Materials and Methods
2.1. Autopsies
2.2. Histopathology
2.3. Chart Review
2.4. Statistics
2.5. Pathogen Identification by PCR and Sequence-Based Taxonomic Classification
3. Results
3.1. Case-Series Demographics
3.2. Clinical Interventions
3.3. Premortem Co-Infections
3.4. Postmortem Co-Infection Findings
3.5. Comparison of Premortem and Postmortem Pulmonary Infection Findings
3.6. Factors Associated with Premortem Identification of Pulmonary Infection
3.7. Identification of Pre- and Postmortem Pulmonary Bacterial Pathogens
3.8. Pulmonary Fungal Co-Infections
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morens, D.M.; Taubenberger, J.K.; Fauci, A.S. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: Implications for pandemic influenza preparedness. J. Infect. Dis. 2008, 198, 962–970. [Google Scholar] [CrossRef]
- Klein, E.Y.; Monteforte, B.; Gupta, A.; Jiang, W.; May, L.; Hsieh, Y.H.; Dugas, A. The frequency of influenza and bacterial coinfection: A systematic review and meta-analysis. Influ. Other Respir. Viruses 2016, 10, 394–403. [Google Scholar] [CrossRef]
- Shieh, W.J.; Blau, D.M.; Denison, A.M.; Deleon-Carnes, M.; Adem, P.; Bhatnagar, J.; Sumner, J.; Liu, L.; Patel, M.; Batten, B.; et al. 2009 pandemic influenza A (H1N1): Pathology and pathogenesis of 100 fatal cases in the United States. Am. J. Pathol. 2010, 177, 166–175. [Google Scholar] [CrossRef]
- Rouze, A.; Martin-Loeches, I.; Povoa, P.; Makris, D.; Artigas, A.; Bouchereau, M.; Lambiotte, F.; Metzelard, M.; Cuchet, P.; Boulle Geronimi, C.; et al. Relationship between SARS-CoV-2 infection and the incidence of ventilator-associated lower respiratory tract infections: A European multicenter cohort study. Intensive Care Med. 2021, 47, 188–198. [Google Scholar] [CrossRef]
- Shafran, N.; Shafran, I.; Ben-Zvi, H.; Sofer, S.; Sheena, L.; Krause, I.; Shlomai, A.; Goldberg, E.; Sklan, E.H. Secondary bacterial infection in COVID-19 patients is a stronger predictor for death compared to influenza patients. Sci. Rep. 2021, 11, 12703. [Google Scholar] [CrossRef]
- Karaba, S.M.; Jones, G.; Helsel, T.; Smith, L.L.; Avery, R.; Dzintars, K.; Salinas, A.B.; Keller, S.C.; Townsend, J.L.; Klein, E.; et al. Prevalence of Co-infection at the Time of Hospital Admission in COVID-19 Patients, A Multicenter Study. Open Forum Infect. Dis. 2021, 8, ofaa578. [Google Scholar] [CrossRef]
- Dudoignon, E.; Camelena, F.; Deniau, B.; Habay, A.; Coutrot, M.; Ressaire, Q.; Plaud, B.; Bercot, B.; Depret, F. Bacterial Pneumonia in COVID-19 Critically Ill Patients: A Case Series. Clin. Infect. Dis. 2021, 72, 905–906. [Google Scholar] [CrossRef]
- COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: A prospective cohort study. Intensive Care Med. 2021, 47, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Feldman, C.; Anderson, R. The role of co-infections and secondary infections in patients with COVID-19. Pneumonia 2021, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Brandi, N.; Ciccarese, F.; Balacchi, C.; Rimondi, M.R.; Modolon, C.; Sportoletti, C.; Capozzi, C.; Renzulli, M.; Paccapelo, A.; Castelli, A.; et al. Co-Infections and Superinfections in COVID-19 Critically Ill Patients Are Associated with CT Imaging Abnormalities and the Worst Outcomes. Diagnostics 2022, 12, 1617. [Google Scholar] [CrossRef] [PubMed]
- Bardi, T.; Pintado, V.; Gomez-Rojo, M.; Escudero-Sanchez, R.; Azzam Lopez, A.; Diez-Remesal, Y.; Martinez Castro, N.; Ruiz-Garbajosa, P.; Pestana, D. Nosocomial infections associated to COVID-19 in the intensive care unit: Clinical characteristics and outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 495–502. [Google Scholar] [CrossRef]
- Grasselli, G.; Scaravilli, V.; Mangioni, D.; Scudeller, L.; Alagna, L.; Bartoletti, M.; Bellani, G.; Biagioni, E.; Bonfanti, P.; Bottino, N.; et al. Hospital-Acquired Infections in Critically Ill Patients With COVID-19. Chest 2021, 160, 454–465. [Google Scholar] [CrossRef]
- Brandi, N.; Ciccarese, F.; Rimondi, M.R.; Balacchi, C.; Modolon, C.; Sportoletti, C.; Renzulli, M.; Coppola, F.; Golfieri, R. An Imaging Overview of COVID-19 ARDS in ICU Patients and Its Complications: A Pictorial Review. Diagnostics 2022, 12, 846. [Google Scholar] [CrossRef]
- Clancy, C.J.; Schwartz, I.S.; Kula, B.; Nguyen, M.H. Bacterial Superinfections Among Persons With Coronavirus Disease 2019: A Comprehensive Review of Data From Postmortem Studies. Open Forum Infect. Dis. 2021, 8, ofab065. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-Garcia, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect. 2021, 27, 83–88. [Google Scholar] [CrossRef]
- Alanio, A.; Delliere, S.; Fodil, S.; Bretagne, S.; Megarbane, B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir. Med. 2020, 8, E48–E49. [Google Scholar] [CrossRef]
- Koehler, P.; Cornely, O.A.; Bottiger, B.W.; Dusse, F.; Eichenauer, D.A.; Fuchs, F.; Hallek, M.; Jung, N.M.; Klein, F.; Persigehl, T.; et al. COVID-19 associated pulmonary aspergillosis. Mycoses 2020, 63, 528–534. [Google Scholar] [CrossRef]
- Marr, K.A.; Platt, A.; Tornheim, J.A.; Zhang, S.X.; Datta, K.; Cardozo, C.; Garcia-Vidal, C. Aspergillosis Complicating Severe Coronavirus Disease. Emerg. Infect. Dis. 2021, 27, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Santana, M.F.; Pivoto, G.; Alexandre, M.A.A.; Baia-da-Silva, D.C.; Borba, M.G.D.; Val, F.A.; Brito-Sousa, J.D.; Melo, G.C.; Monteiro, W.M.; Souza, J.V.B.; et al. Confirmed Invasive Pulmonary Aspergillosis and COVID-19: The value of postmortem findings to support antemortem management. Rev. Soc. Bras. Med. Trop. 2020, 53, e20200401. [Google Scholar] [CrossRef] [PubMed]
- Salmanton-Garcia, J.; Sprute, R.; Stemler, J.; Bartoletti, M.; Dupont, D.; Valerio, M.; Garcia-Vidal, C.; Falces-Romero, I.; Machado, M.; de la Villa, S.; et al. COVID-19-Associated Pulmonary Aspergillosis, March-August 2020. Emerg. Infect. Dis. 2021, 27, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Kula, B.E.; Clancy, C.J.; Hong Nguyen, M.; Schwartz, I.S. Invasive mould disease in fatal COVID-19: A systematic review of autopsies. Lancet Microbe 2021, 2, e405–e414. [Google Scholar] [CrossRef] [PubMed]
- Bradley, B.T.; Maioli, H.; Johnston, R.; Chaudhry, I.; Fink, S.L.; Xu, H.; Najafian, B.; Deutsch, G.; Lacy, J.M.; Williams, T.; et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: A case series. Lancet 2020, 396, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.R.; Ramelli, S.C.; Grazioli, A.; Chung, J.Y.; Singh, M.; Yinda, C.K.; Winkler, C.W.; Sun, J.; Dickey, J.M.; Ylaya, K.; et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature 2022, 612, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Yek, C.; Warner, S.; Wiltz, J.L.; Sun, J.; Adjei, S.; Mancera, A.; Silk, B.J.; Gundlapalli, A.V.; Harris, A.M.; Boehmer, T.K.; et al. Risk Factors for Severe COVID-19 Outcomes Among Persons Aged >/=18 Years Who Completed a Primary COVID-19 Vaccination Series—465 Health Care Facilities, United States, December 2020–October 2021. MMWR Morb. Mortal Wkly. Rep. 2022, 71, 19–25. [Google Scholar] [CrossRef]
- Fernando, S.M.; Tran, A.; Cheng, W.; Klompas, M.; Kyeremanteng, K.; Mehta, S.; English, S.W.; Muscedere, J.; Cook, D.J.; Torres, A.; et al. Diagnosis of ventilator-associated pneumonia in critically ill adult patients-a systematic review and meta-analysis. Intensive Care Med. 2020, 46, 1170–1179. [Google Scholar] [CrossRef]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratala, J.; et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef]
- Lieberman, J.A.; Bryan, A.; Mays, J.A.; Stephens, K.; Kurosawa, K.; Mathias, P.C.; SenGupta, D.; Bourassa, L.; Salipante, S.J.; Cookson, B.T. High Clinical Impact of Broad-Range Fungal PCR in Suspected Fungal Sinusitis. J. Clin. Microbiol. 2021, 59, e0095521. [Google Scholar] [CrossRef]
- Lee, S.A.; Plett, S.K.; Luetkemeyer, A.F.; Borgo, G.M.; Ohliger, M.A.; Conrad, M.B.; Cookson, B.T.; Sengupta, D.J.; Koehler, J.E. Bartonella quintana Aortitis in a Man with AIDS, Diagnosed by Needle Biopsy and 16S rRNA Gene Amplification. J. Clin. Microbiol. 2015, 53, 2773–2776. [Google Scholar] [CrossRef]
- Chen, Y.C.; Eisner, J.D.; Kattar, M.M.; Rassoulian-Barrett, S.L.; Lafe, K.; Bui, U.; Limaye, A.P.; Cookson, B.T. Polymorphic internal transcribed spacer region 1 DNA sequences identify medically important yeasts. J. Clin. Microbiol. 2001, 39, 4042–4051. [Google Scholar] [CrossRef]
- Chen, Y.C.; Eisner, J.D.; Kattar, M.M.; Rassoulian-Barrett, S.L.; LaFe, K.; Yarfitz, S.L.; Limaye, A.P.; Cookson, B.T. Identification of medically important yeasts using PCR-based detection of DNA sequence polymorphisms in the internal transcribed spacer 2 region of the rRNA genes. J. Clin. Microbiol. 2000, 38, 2302–2310. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Lieberman, J.A.; Kurosawa, K.; SenGupta, D.; Cookson, B.T.; Salipante, S.J.; Busch, D. Identification of Leptotrichia goodfellowii infective endocarditis by next-generation sequencing of 16S rDNA amplicons. Cold Spring Harb. Mol. Case Stud. 2021, 7, a005876. [Google Scholar] [CrossRef] [PubMed]
- Cummings, L.A.; Kurosawa, K.; Hoogestraat, D.R.; SenGupta, D.J.; Candra, F.; Doyle, M.; Thielges, S.; Land, T.A.; Rosenthal, C.A.; Hoffman, N.G.; et al. Clinical Next Generation Sequencing Outperforms Standard Microbiological Culture for Characterizing Polymicrobial Samples. Clin. Chem. 2016, 62, 1465–1473. [Google Scholar] [CrossRef]
- Dudeck, M.A.; Weiner, L.M.; Allen-Bridson, K.; Malpiedi, P.J.; Peterson, K.D.; Pollock, D.A.; Sievert, D.M.; Edwards, J.R. National Healthcare Safety Network (NHSN) report, data summary for 2012, Device-associated module. Am. J. Infect. Control. 2013, 41, 1148–1166. [Google Scholar] [CrossRef] [PubMed]
- Metersky, M.L.; Wang, Y.; Klompas, M.; Eckenrode, S.; Bakullari, A.; Eldridge, N. Trend in Ventilator-Associated Pneumonia Rates Between 2005 and 2013. JAMA 2016, 316, 2427–2429. [Google Scholar] [CrossRef] [PubMed]
- Louie, J.; Jean, C.; Chen, T.H.; Park, S.; Ueki, R.; Harper, T.; Chmara, E.; Myers, J.; Stoppacher, R.; Catanese, C.; et al. Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1)—United States, May-August 2009. MMWR Morb. Mortal. Wkly. Rep. 2009, 58, 1071–1074. [Google Scholar]
- Schulte-Schrepping, J.; Reusch, N.; Paclik, D.; Bassler, K.; Schlickeiser, S.; Zhang, B.; Kramer, B.; Krammer, T.; Brumhard, S.; Bonaguro, L.; et al. Severe COVID-19 Is Marked by a Dysregulated Myeloid Cell Compartment. Cell 2020, 182, 1419–1440.e23. [Google Scholar] [CrossRef]
- Babouee Flury, B.; Weisser, M.; Prince, S.S.; Bubendorf, L.; Battegay, M.; Frei, R.; Goldenberger, D. Performances of two different panfungal PCRs to detect mould DNA in formalin-fixed paraffin-embedded tissue: What are the limiting factors? BMC Infect. Dis. 2014, 14, 692. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef]
- Peghin, M.; Vena, A.; Graziano, E.; Giacobbe, D.R.; Tascini, C.; Bassetti, M. Improving management and antimicrobial stewardship for bacterial and fungal infections in hospitalized patients with COVID-19. Ther. Adv. Infect. Dis. 2022, 9, 20499361221095732. [Google Scholar] [CrossRef]
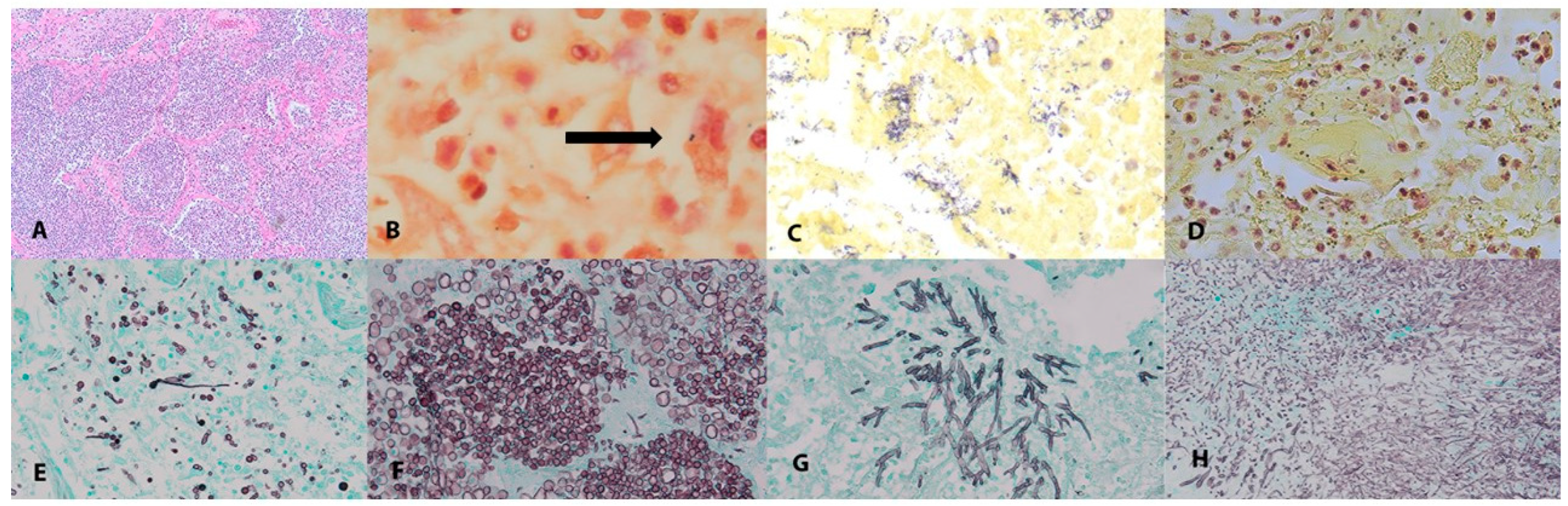
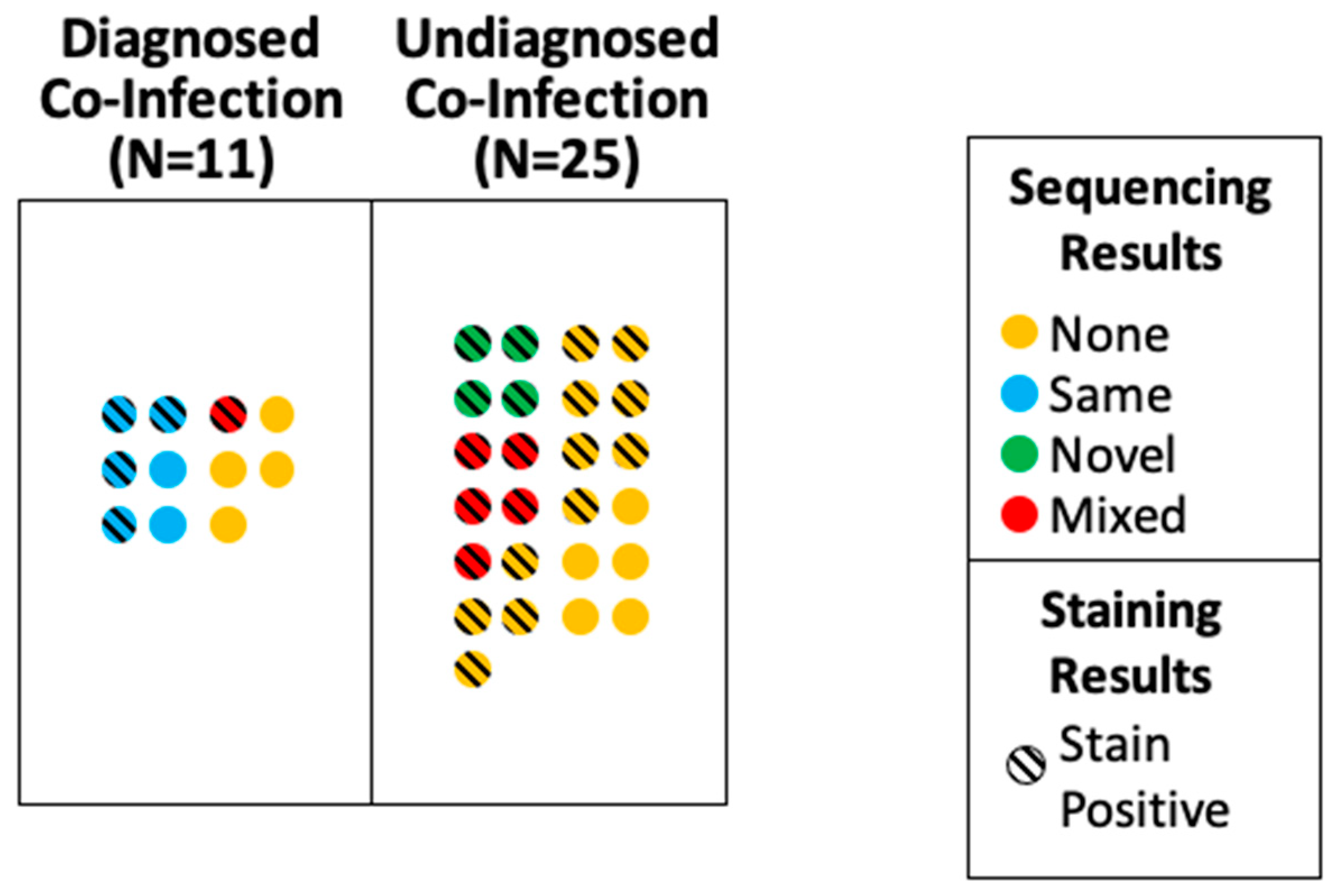
| All Cases (n = 55) | Diagnosed Pulmonary Co-Infection (n = 11) | Undiagnosed Pulmonary Co-Infection (n = 25) | No Pulmonary Co-Infection (n = 19) | p-Value | |
|---|---|---|---|---|---|
| Median age in years (IQR) | 63 (48.5,71) | 48 (41.5,64.5) | 63 (60,71) | 68 (48.5,73) | 0.0995 |
| Female (%) | 18/55 (32.7%) | 3/11 (27.3%) | 9/25 (36.0%) | 6/19 (31.6%) | 0.2187 |
| Race (%) | 0.1384 | ||||
| White (%) | 25/55 (45.5%) | 4/11 (36.4%) | 13/25 (52.0%) | 8/19 (42.1%) | |
| Black or African American (%) | 19/55 (34.5%) | 2/11 (18.2%) | 10/25 (40.0%) | 7/19 (36.8%) | |
| Hispanic Ethnicity (%) | 11/55 (20.0%) | 6/11 (54.5%) | 2/25 (8.0%) | 3/19 (15.8%) | 0.0048 |
| Median BMI (IQR) | 31.15 (25.9,36.8) | 39 (34.3,48.7) | 31 (24.3,35.4) | 28 (25.2,33.45) | 0.0368 |
| Major Comorbidities (IQR) | 2 (1,3) | 1 (1,3) | 2 (2,4) | 2 (1.5,3) | 0.3632 |
| Immunosuppression/Cancer (%) | 9/55 (16.4%) | 1/11 (9.1%) | 4/25 (11.1%) | 4/19 (21.1%) | 0.7328 |
| Pulmonary (%) | 20/55 (36.4%) | 4/11 (36.4%) | 8/25 (22.2%) | 8/19 (42.1%) | 0.4764 |
| Cardiac (%) | 33/55 (60.0%) | 3/11 (27.3%) | 19/25 (52.8%) | 11/19 (57.9%) | 0.0222 |
| Liver (%) | 3/55 (5.5%) | 0/11 (0.0%) | 2/25 (5.6%) | 1/19 (5.3%) | 0.9501 |
| Neuro (%) | 5/55 (9.1%) | 1/11 (9.1%) | 2/25 (5.6%) | 2/19 (10.5%) | 0.0834 |
| Diabetes Mellitus (%) | 23/55 (41.8%) | 3/11 (27.3%) | 13/25 (36.1%) | 7/19 (36.8%) | 0.3304 |
| CKD (%) | 9/55 (16.4%) | 0/11 (0.0%) | 5/25 (13.9%) | 4/19 (21.1%) | 0.2594 |
| Obesity (%) | 30/55 (54.5%) | 9/11 (81.8%) | 13/25 (36.1%) | 8/19 (42.1%) | 0.1027 |
| All Cases (n = 55) | Diagnosed Pulmonary Co-Infection (n = 11) | Undiagnosed Pulmonary Co-Infection (n = 25) | No Pulmonary Co-Infection (n = 19) | p-Value | |
|---|---|---|---|---|---|
| Days from onset to death (IQR) | 18 (12,32) | 48 (33,65.5) | 16 (9,19) | 23 (10,31) | 0.0009 |
| Hospital duration, days (IQR) | 12 (6,25.5) | 45 (23,59) | 8 (5,16) | 12 (4.5,26.5) | 0.0012 |
| ICU duration, days (IQR) | 12 (5.5,23.5) | 44 (21,55.5) | 7 (2,12) | 11 (1,21.5) | 0.0006 |
| Intubation duration, days (IQR) | 11 (2.8,21.3) | 42 (20.5,55.5) | 4 (0.0,10.0) | 11 (0.0,16.0) | 0.0003 |
| Post-Mortem Interval, hours (IQR) | 24 (18.9,39.5) | 24.6 (18.6,43.5) | 24.0 (19.4,35.1) | 23.1 (16.0,46.5) | 0.8793 |
| ICU Admission (%) | 51/55 (92.7%) | 11/11 (100.0%) | 23/25 (92.0%) | 17/19 (89.5%) | 0.5542 |
| Intubated (%) | 47/55 (85.5%) | 11/11 (100.0%) | 21/25 (84.0%) | 15/19 (78.9%) | 0.2777 |
| Pressor Use (%) | 44/55 (80.0%) | 11/11 (100.0%) | 17/25 (68.0%) | 16/19 (84.2%) | 0.0712 |
| RRT (%) | 22/55 (40.0%) | 6/11 (54.5%) | 9/25 (36.0%) | 7/19 (36.8%) | 0.5446 |
| ECMO (%) | 13/55 (23.6%) | 6/11 (54.5%) | 1/25 (4.0%) | 7/19 (36.8%) | 0.0022 |
| Abnormal Chest Imaging (%) | 52/55 (94.5%) | 10/11 (90.9%) | 25/25 (100%) | 17/19 (89.7%) | 0.2629 |
| Antibiotic Use (%) | 50/55 (90.9%) | 11/11 (100.0%) | 23/25 (92.0%) | 16/19 (84.2%) | 0.0288 |
| HAP Coverage (%) | 34/55 (61.8%) | 10/11 (90.9%) | 14/25 (56.0%) | 10/19 (52.6%) | 0.0828 |
| COVID-Specific Therapies | |||||
| Steroid Use (%) | 46/55 (83.6%) | 11/11 (100.0%) | 21/25 (84.0%) | 14/19 (73.7%) | 0.1712 |
| Remdesivir (%) | 24/55 (43.6%) | 5/11 (45.5%) | 13/25 (52.0%) | 5/19 (26.3%) | 0.2229 |
| Tocilizumab (%) | 4/55 (7.3%) | 4/11 (36.4%) | 0/25 (0.0%) | 1/19 (5.3%) | 0.0017 |
| Convalescent Plasma (%) | 7/55 (12.7%) | 5/11 (45.5%) | 3/25 (12.0%) | 3/19 (15.8%) | 0.0589 |
| All Cases (n = 55) | Diagnosed Pulmonary Co-Infection (n = 11) | Undiagnosed Pulmonary Co-Infection (n = 25) | No Pulmonary Co-Infection (n = 19) | p-Value | |
|---|---|---|---|---|---|
| Any Pre-mortem Infection | 23/55 (41.8%) | 11/11 (100.0%) | 5/25 (20.0%) | 7/19 (36.8%) | <0.0001 |
| Pulmonary | 15/55 (27.3%) | 11/11 (100.0%) | 0/25 (0.0%) | 4/19 (21.1%) | <0.0001 |
| Extra-Pulmonary | 20/55 (36.4%) | 9/11 (81.8%) | 6/25 (24.0%) | 5/19 (26.3%) | 0.0021 |
| Bacteremia | 16/55 (29.1%) | 9/11 (81.8%) | 2/25 (8.0%) | 5/19 (26.3%) | <0.0001 |
| UTI | 4/55 (7.3%) | 0/11 (0.0%) | 2/25 (8.0%) | 2/19 (10.5%) | 0.5542 |
| Fungemia | 4/55 (7.3%) | 2/11 (18.2%) | 2/25 (8.0%) | 0/19 (0.0%) | 0.1781 |
| Skin/Soft Tissue Infection | 1/55 (1.8%) | 0/11 (0.0%) | 1/25 (4.0%) | 0/19 (0.0%) | 0.5427 |
| C. difficile colitis | 1/55 (1.8%) | 1/11 (9.1%) | 0/25 (0.0%) | 0/19 (0.0%) | 0.1304 |
| Pre-Mortem Pulmonary Culture Results (n = 11) | Postmortem Sequencing Results (n = 36) | |
|---|---|---|
| P. aeruginosa | 4 | 2 |
| K. pneumoniae | 4 | 0 |
| MSSA | 3 | 2 * |
| MRSA | 3 | |
| E. coli | 2 | 3 |
| A. baumanii | 2 | 1 |
| S. marcesens | 1 | 0 |
| K. aerogenes | 1 | 0 |
| K. oxytoca | 1 | 0 |
| P. mirabilis | 1 | 0 |
| C. albicans | 1 | 0 |
| Mycoplasma salivarium | 0 | 2 |
| Fusobacterium nucleatum | 0 | 2 |
| P. putida | 0 | 1 |
| S. pneumoniae | 0 | 1 |
| Legionella sp. | 0 | 1 |
| Prevotella melanogenica | 0 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Platt, A.P.; Bradley, B.T.; Nasir, N.; Stein, S.R.; Ramelli, S.C.; Ramos-Benitez, M.J.; Dickey, J.M.; Purcell, M.; Singireddy, S.; Hays, N.; et al. Pulmonary Co-Infections Detected Premortem Underestimate Postmortem Findings in a COVID-19 Autopsy Case Series. Pathogens 2023, 12, 932. https://doi.org/10.3390/pathogens12070932
Platt AP, Bradley BT, Nasir N, Stein SR, Ramelli SC, Ramos-Benitez MJ, Dickey JM, Purcell M, Singireddy S, Hays N, et al. Pulmonary Co-Infections Detected Premortem Underestimate Postmortem Findings in a COVID-19 Autopsy Case Series. Pathogens. 2023; 12(7):932. https://doi.org/10.3390/pathogens12070932
Chicago/Turabian StylePlatt, Andrew P., Benjamin T. Bradley, Nadia Nasir, Sydney R. Stein, Sabrina C. Ramelli, Marcos J. Ramos-Benitez, James M. Dickey, Madeleine Purcell, Shreya Singireddy, Nicole Hays, and et al. 2023. "Pulmonary Co-Infections Detected Premortem Underestimate Postmortem Findings in a COVID-19 Autopsy Case Series" Pathogens 12, no. 7: 932. https://doi.org/10.3390/pathogens12070932
APA StylePlatt, A. P., Bradley, B. T., Nasir, N., Stein, S. R., Ramelli, S. C., Ramos-Benitez, M. J., Dickey, J. M., Purcell, M., Singireddy, S., Hays, N., Wu, J., Raja, K., Curto, R., Salipante, S. J., Chisholm, C., Carnes, S., Marshall, D. A., Cookson, B. T., Vannella, K. M., ... Chertow, D. S. (2023). Pulmonary Co-Infections Detected Premortem Underestimate Postmortem Findings in a COVID-19 Autopsy Case Series. Pathogens, 12(7), 932. https://doi.org/10.3390/pathogens12070932






