Discovery of a Digenean (Cryptogonimidae) Living in a Cleft-Lipped Goby, Sicyopterus cynocephalus (Teleostei: Gobiidae) from Ranongga Island, Solomon Islands: Analysis of Multiple Ribosomal DNA Regions
Abstract
1. Introduction
2. Materials and Methods
2.1. Host and Parasite Collection
2.2. Molecular Analysis
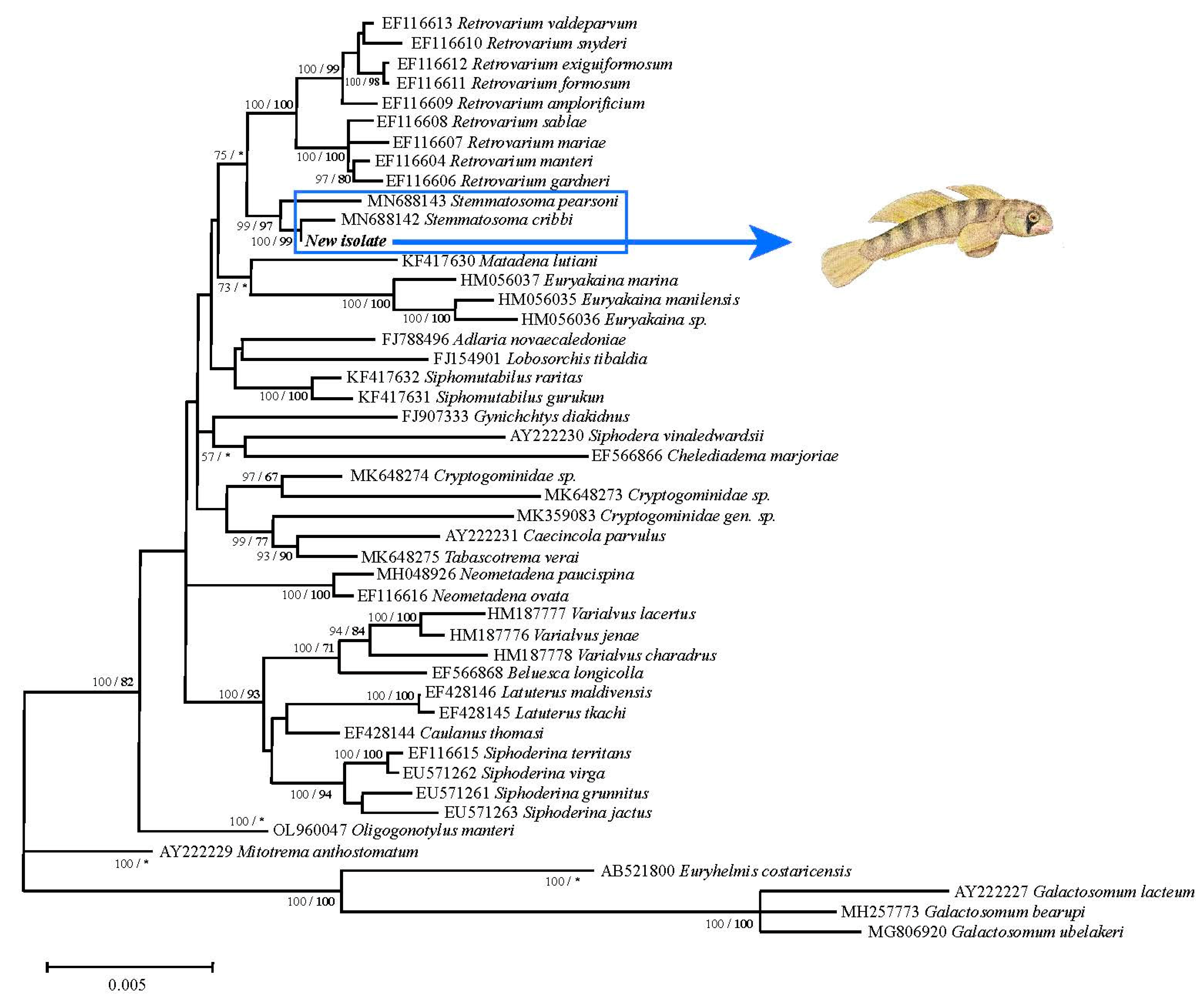
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anggraini, N.; Karyadi, B.; Ekaputri, R.Z.; Zukmadini, A.Y.; Sastiawan, R.; Anggriani, F. The population and habitat of mungkus fish (Sicyopterus cynocephalus) in Bengkenang Waters South of Bengkulu. J. Phys. Conf. Ser. 2018, 1116, 052005. [Google Scholar] [CrossRef]
- Lord, C.; Bellec, L.; Dettaï, A.; Bonillo, C.; Keith, P. Does your lip stick? Evolutionary aspects of the mouth morphology of the Indo-Pacific clinging goby of the Sicyopterus genus (Teleostei: Gobioidei: Sicydiinae) based on mitogenome phylogeny. J. Zool. Syst. Evol. Res. 2019, 57, 910–925. [Google Scholar] [CrossRef]
- Keith, P.; Lord, C.; Maeda, K. Indo-Pacific Sicydiinae Gobies: Biodiversity, Life Traits and Conservation; Société Française d’Ichtyologie: Paris, France, 2015; p. 256. [Google Scholar]
- Keith, P. Biology and ecology of amphidromous Gobiidae in the Indo-Pacific and the Caribbean regions. J. Fish Biol. 2003, 63, 831–847. [Google Scholar] [CrossRef]
- Keith, P.; Lord, C. Tropical freshwater gobies: Amphidromy as a life cycle. In The Biology of Gobies; Patzner, R.A., Van Tassell, J.L., Kovacic, M., Kapoor, B.G., Eds.; Science Publishers: St. Helier, UK, 2011; pp. 119–128. [Google Scholar]
- Lord, C.; Brun, C.; Hautecœur, M.; Keith, P. Insights on endemism: Comparison of the marine larval duration estimated by otolith microstructural analysis of three amphidromous Sicyopterus species (Gobiidae: Sicydiinae) from Vanuatu and New Caledonia. Ecol. Freshw. Fish. 2010, 19, 26–38. [Google Scholar] [CrossRef]
- Ebner, B.C.; Donaldson, J.A.; Allen, G.R.; Keith, P. Visual census, photographic records and the trial of a video network provide first evidence of the elusive Sicyopterus cynocephalus in Australia. Cybium 2017, 41, 117–125. [Google Scholar]
- Tigulu, I.G.; Ifuto’o, M.R.; Sheppard, S. Ridges to Reef Conservation Plan Ghizo and Kolombangara Western Province, SOLOMON Islands; WWF-Pacific Solomon Islands, World Wide Fund for Nature: Gizo, Solomon Islands, 2018; p. 60. [Google Scholar]
- Keith, P.; Boseto, D.; Lord, C. Freshwater Fish of the Solomon Islands; Société Française d’Ichtyologie: Paris, France, 2021; p. 174. [Google Scholar]
- Mathews, P.D.; Bonillo, C.; Rabet, N.; Lord, C.; Causse, R.; Keith, P.; Audebert, F. Phylogenetic analysis and characterization of a new parasitic cnidarian (Myxosporea: Myxobolidae) parasitizing skin of the giant mottled eel from the Solomon Islands. Infect. Genet. Evol. 2021, 94, 104986. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.L.; Adlard, R.D. Stemmatostoma cribbi n. sp. (Digenea: Cryptogonimidae) from Freshwater Fishes in the Wet Tropics Bioregion of Queensland, Australia. J. Parasitol. 2020, 106, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.L.; Cribb, T.H. Family Cryptogonimidae Ward, 1917. In Keys to the Trematoda; Bray, R.A., Gibson, D.I., Jones, A., Eds.; CAB International: Wallingford, UK, 2008; pp. 51–112. [Google Scholar]
- Quintana, M.G.; De Núñez, M.O. The life cycle of Pseudosellacotyla lutzi (Digenea: Cryptogonimidae), in Aylacostoma chloroticum (Prosobranchia: Thiaridae), and Hoplias malabaricus (Characiformes: Erythrinidae), in Argentina. J. Parasitol. 2014, 100, 805–811. [Google Scholar] [CrossRef]
- Kmentová, N.; Bray, R.A.; Koblmüller, S.; Artois, T.; De Keyser, E.L.; Gelnar, M.; Vanhove, M.P.M.; Georgieva, S. Uncharted digenean diversity in Lake Tanganyika: Cryptogonimids (Digenea: Cryptogonimidae) infecting endemic lates perches (Actinopterygii: Latidae). Parasit. Vectors 2020, 13, 221. [Google Scholar] [CrossRef]
- Miller, T.L.; Cribb, T.H. Two new cryptogonimid genera Beluesca n. gen. and Chelediadema n. gen. (Digenea: Cryptogonimidae) from tropical Indo-West Pacific Haemulidae (Perciformes). Zootaxa 2007, 1543, 45–60. [Google Scholar] [CrossRef]
- Miller, T.L.; Cribb, T.H. Dramatic phenotypic plasticity within species of Siphomutabilus n. g. (Digenea: Cryptogonimidae) from Indo-Pacific caesionines (Perciformes: Lutjanidae). Syst. Parasitol. 2013, 86, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.B.; Cutmore, S.C. Siphoderina hustoni n. sp. (Platyhelminthes: Trematoda: Cryptogonimidae) from the Maori snapper Lutjanus rivulatus (Cuvier) on the Great Barrier Reef. Syst. Parasitol. 2022, 99, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.L.; Cribb, T.H. Eight new species of Siphoderina Manter, 1934 (Digenea, Cryptogonimidae) infecting Lutjanidae and Haemulidae (Perciformes) off Australia. Acta Parasitol. 2008, 53, 344–364. [Google Scholar] [CrossRef]
- Cribb, T.H.; Bray, R.A.; Justine, J.-L.; Reimer, J.; Sasal, P.; Shirakashi, S.; Cutmore, S.C. A world of taxonomic pain: Cryptic species, inexplicable host-specificity, and host-induced morphological variation among species of Bivesicula Yamaguti, 1934 (Trematoda: Bivesiculidae) from Indo-Pacific Holocentridae, Muraenidae and Serranidae. Parasitology 2022, 149, 1–73. [Google Scholar] [CrossRef]
- Kvach, Y.; Bryjová, A.; Sasal, P.; Winkler, H.M. A revision of the genus Aphalloides (Digenea: Cryptogonimidae), parasites of European brackish water fishes. Parasitol. Res. 2017, 116, 1973–1980. [Google Scholar] [CrossRef]
- Cribb, T.H. The life cycle and morphology of Stemmatostoma pearsoni, gen. et sp. nov., with notes on the morphology of Telogaster opisthorchis Macfarlane (Digenea: Cryptogonimidae). Aust. J. Zool. 1986, 34, 279–304. [Google Scholar] [CrossRef]
- Cuadros, R.C.; Rivadeneyra, N.L.S.; Malta, J.C.O.; Serrano-Martínez, M.E.; Mathews, P.D. Morphology and surface ultrastructure of Dadaytrema oxycephala (Digenea: Cladorchiidae) with a new host record from Peruvian Amazon floodplain. Biologia 2018, 73, 569–575. [Google Scholar] [CrossRef]
- Ward, R.D.; Zemlak, T.S.; Innes, B.H.; Last, P.R.; Hebert, P.D. DNA barcoding Australia’s fish species. Phil. Trans. R. Soc. B. 2005, 360, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.H.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA 6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van der Mark, P.; Ayres, D.L.; Darling, A.A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Cribb, T.H.; Bray, R.A.; Olson, P.D.; Littlewood, D.T.J. Life cycle evolution in the digenea: A new perspective from phylogeny. Adv. Parasitol. 2003, 54, 198–253. [Google Scholar]
- Abdel-Gabe, R.; Abdel-Ghaffar, F.; Maher, S.; El-Mallah, A.; Al Quraishy, S.; Mehlhorn, H. Morphological re-description and phylogenetic relationship of five myxosporean species of family Myxobolidae infecting the Nile tilapia Oreochromis niloticus (Perciformes: Cichlidae). Dis. Aquat. Org. 2017, 124, 201–214. [Google Scholar] [CrossRef]
- Thatcher, V.E.; Varella, A.B. Patologia de peixes da Amazônia brasileira. 2. Um tumor maligno das brânquias relacionado com as metacercárias de um trematódeo. Acta Amaz. 1980, 10, 651–656. [Google Scholar] [CrossRef]
- Overstreet, R.M.; Curram, S.S. Defeating diplostomoid dangers in USA catfish aquaculture. Folia Parasitol. 2004, 51, 53–165. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, L.L.; Souza, G.T.; Takemoto, R.M.; Ceccarelli, P.S.; Adriano, E.A. Behavioral changes caused by Austrodiplostomum spp. in Hoplias malabaricus from the São Francisco River, Brazil. Parasitol. Res. 2014, 113, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Madrid, R.R.M.; Mertins, O.; Tavares-Dias, M.; Flores-Gonzales, A.P.; Patta, A.C.M.F.; Ramirez, C.A.B.; Rigoni, V.L.S.; Mathews, P.D. High compliance and effective treatment of fish endoparasitic infections with oral drug delivery nanobioparticles: Safety of intestinal tissue and blood parameters. J. Fish Dis. 2021, 44, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Mathews, P.D.; Patta, A.C.M.F.; Gonçalves, J.V.; Gama, G.S.; Garcia, I.T.S.; Mertins, O. Targeted drug delivery and treatment of endoparasites with biocompatible particles of pH-responsive structure. Biomacromolecules 2018, 19, 499–510. [Google Scholar] [CrossRef]
- Mathews, P.D.; Patta, A.C.M.F.; Madrid, R.R.M.; Ramirez, C.A.B.; Pimenta, B.V.; Mertins, O. Efficient treatment of fish intestinal parasites applying membrane-penetrating oral drug delivery nanoparticle. ACS Biomater. Sci. Eng. 2023, 9, 2911–2923. [Google Scholar] [CrossRef] [PubMed]
- Noga, E.J. Fish Disease: Diagnosis and Treatment; Blackwell Publishing Company, Ames: Hoboken, NJ, USA, 2000; pp. 173–178. [Google Scholar]
- Magro, L.; Cutmore, S.C.; Carrasson, M.; Cribb, T.H. Integrated characterisation of nine species of the Schistorchiinae (Trematoda: Apocreadiidae) from Indo-Pacific fishes: Two new species, a new genus, and a resurrected but ‘cryptic’ genus. Syst. Parasitol. 2023, 100, 381–413. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.M.; Morgan, J.A.T.; Galzin, R.; Cribb, T.H. Identical digeneans in coral reef fishes from French Polynesia and the Great Barrier Reef (Australia) demonstrated by morphology and molecules. Int. J. Parasitol. 2001, 31, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Nolan, M.J.; Cribb, T.H. The use and implications of ribosomal DNA sequencing for the discrimination of digenean species. Adv. Parasitol. 2005, 60, 101–163. [Google Scholar]
- Abdou, A.; Lord, C.; Keith, P.; Galzin, R. Phylogéographie de Neritina stumpffi Boettger, 1890 et Neritina canalis Sowerby, 1825 (Gastropoda, Cycloneritida, Neritidae). Zoosystema 2019, 41, 237–248. [Google Scholar] [CrossRef]
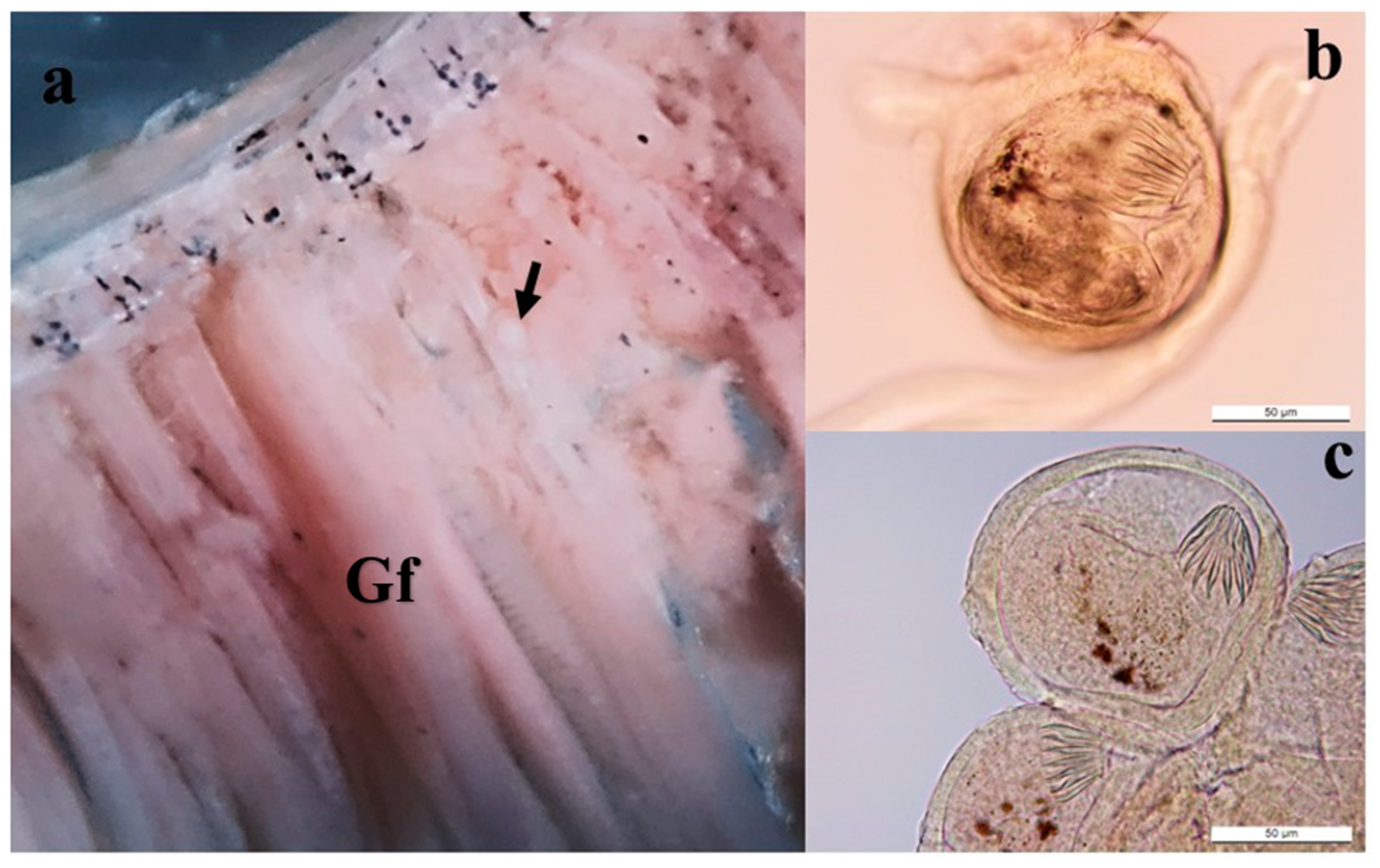
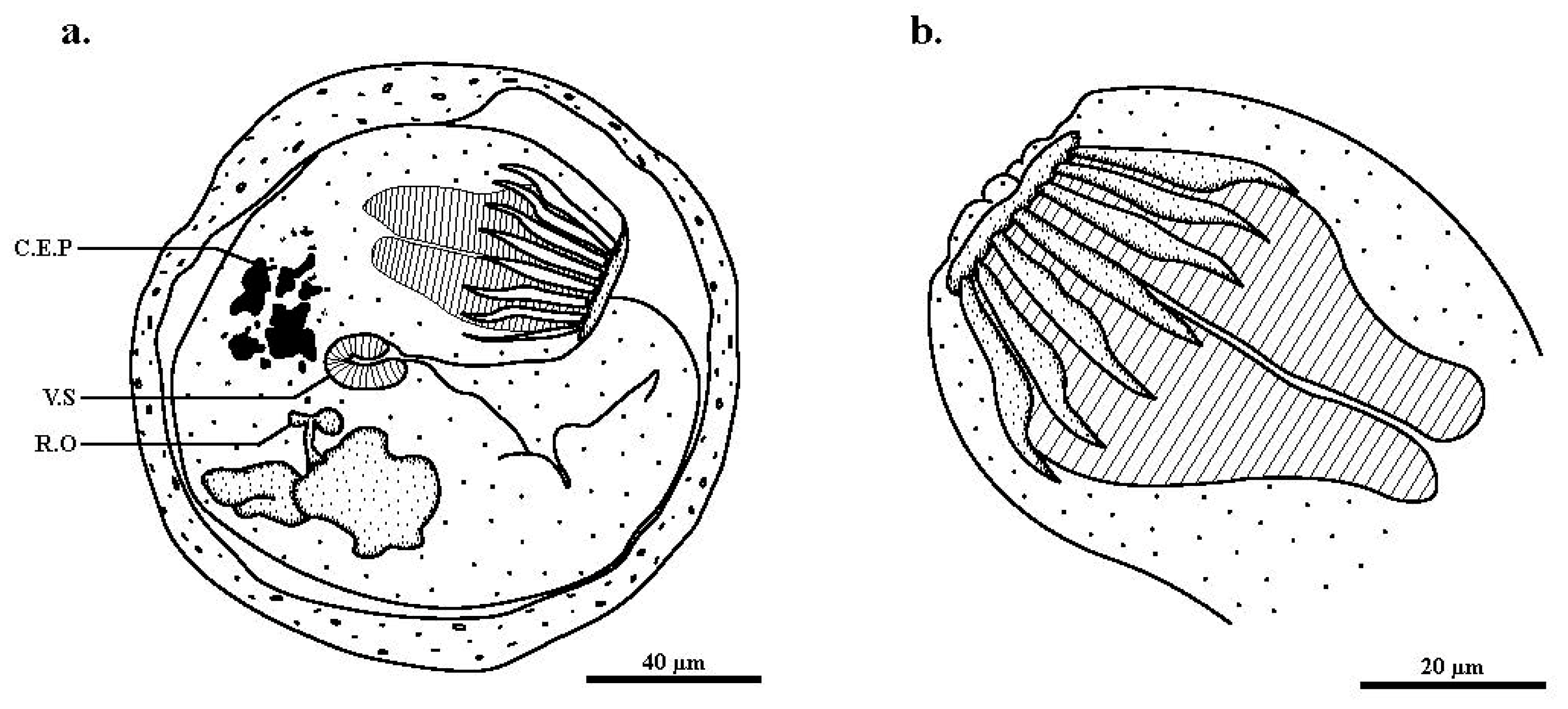
| Specimen Cyst | Encysted | Encysted | Circumoral | Oral Sucker | Ventral Sucker | |
|---|---|---|---|---|---|---|
| Diameter | Metacercaria Length | Metacercaria Width | Spine Length | Width | Width | |
| 1 | 113 | 165 | 48 | 30 | 16 | 15 |
| 2 | 131 | 231 | 55 | 29 | 23 | NA |
| 3 | 139 | 214 | 49 | 41 | 17 | 20 |
| 4 | 179 | 250 | 63 | 39 | 19 | NA |
| 5 | 115 | 209 | 46 | 28 | 20 | NA |
| Mean | 135.4 | 213.8 | 52.2 | 33.4 | 19 | 17.5 |
| min | 113 | 165 | 46 | 28 | 16 | 15 |
| max | 179 | 250 | 63 | 41 | 23 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathews, P.D.; Rabet, N.; L. Espinoza, L.; Haÿ, V.; Bonillo, C.; Keith, P.; Lord, C.; Audebert, F. Discovery of a Digenean (Cryptogonimidae) Living in a Cleft-Lipped Goby, Sicyopterus cynocephalus (Teleostei: Gobiidae) from Ranongga Island, Solomon Islands: Analysis of Multiple Ribosomal DNA Regions. Pathogens 2023, 12, 923. https://doi.org/10.3390/pathogens12070923
Mathews PD, Rabet N, L. Espinoza L, Haÿ V, Bonillo C, Keith P, Lord C, Audebert F. Discovery of a Digenean (Cryptogonimidae) Living in a Cleft-Lipped Goby, Sicyopterus cynocephalus (Teleostei: Gobiidae) from Ranongga Island, Solomon Islands: Analysis of Multiple Ribosomal DNA Regions. Pathogens. 2023; 12(7):923. https://doi.org/10.3390/pathogens12070923
Chicago/Turabian StyleMathews, Patrick D., Nicolas Rabet, Luis L. Espinoza, Vincent Haÿ, Céline Bonillo, Philippe Keith, Clara Lord, and Fabienne Audebert. 2023. "Discovery of a Digenean (Cryptogonimidae) Living in a Cleft-Lipped Goby, Sicyopterus cynocephalus (Teleostei: Gobiidae) from Ranongga Island, Solomon Islands: Analysis of Multiple Ribosomal DNA Regions" Pathogens 12, no. 7: 923. https://doi.org/10.3390/pathogens12070923
APA StyleMathews, P. D., Rabet, N., L. Espinoza, L., Haÿ, V., Bonillo, C., Keith, P., Lord, C., & Audebert, F. (2023). Discovery of a Digenean (Cryptogonimidae) Living in a Cleft-Lipped Goby, Sicyopterus cynocephalus (Teleostei: Gobiidae) from Ranongga Island, Solomon Islands: Analysis of Multiple Ribosomal DNA Regions. Pathogens, 12(7), 923. https://doi.org/10.3390/pathogens12070923








